Abstract
Congenital disorders of the hepatic portal vasculature are rare in man but occur frequently in certain dog breeds. In dogs, there are two main subtypes: intrahepatic portosystemic shunts, which are considered to stem from defective closure of the embryonic ductus venosus, and extrahepatic shunts, which connect the splanchnic vascular system with the vena cava or vena azygos. Both subtypes result in nearly complete bypass of the liver by the portal blood flow. In both subtypes the development of the smaller branches of the portal vein tree in the liver is impaired and terminal branches delivering portal blood to the liver lobules are often lacking. The clinical signs are due to poor liver growth, development, and function. Patency of the ductus venosus seems to be a digenic trait in Irish wolfhounds, whereas Cairn terriers with extrahepatic portosystemic shunts display a more complex inheritance. The genes involved in these disorders cannot be identified with the sporadic human cases, but in dogs, the genome-wide study of the extrahepatic form is at an advanced stage. The canine disease may lead to the identification of novel genes and pathways cooperating in growth and development of the hepatic portal vein tree. The same pathways likely regulate the development of the vascular system of regenerating livers during liver diseases such as hepatitis and cirrhosis. Therefore, the identification of these molecular pathways may provide a basis for future proregenerative intervention.
Congenital portosystemic shunts and associated liver dysfunctions
Maintenance of liver mass and function is provided mostly by hepatic perfusion, especially by the quantity and quality of portal blood (van den Ingh et al. 1995). Normally, the abdominal organs connected to the splanchnic vascular bed (gastrointestinal tract, pancreas, and spleen) supply their efflux blood to the portal vein. Portal blood delivers toxins, nutrients, and bacteria absorbed from the intestine to the liver. In addition, it contains specific hepatotrophic factors like insulin, insulin-like growth factors, glucagon, and hepatocyte growth factor (van den Ingh et al. 1995), and carries 50% of the oxygen supply to the liver (Nelson et al. 2005).
Congenital portosystemic shunts (CPSS) are abnormal vascular connections made during embryonic development, which connect the portal vein directly to the vena cava or vena (hemi)azygos. Portal blood thus bypasses the liver and its functional units, the liver lobules (Vulgamott 1985; Winkler et al. 2003). Because of the importance of portal blood for the liver, portosystemic shunting has severe impact on the homeostasis and well-being of the organism. First, there is severely impaired liver growth and atrophy of the remaining hepatocytes, leading to reduced hepatic functions (Allen et al. 1999; Franchi-Abella et al. 2010; Uchino et al. 1999; Winkler et al. 2003). Second, due to portosystemic shunting the important function of the liver—to clear portal blood—cannot be performed so that toxins and metabolites reach the systemic circulation in high concentrations. Substances derived from the gastrointestinal tract and pancreas, like ammonia, aromatic amino acids, absorbed bacteria and endotoxins, hormones, and growth factors are not subjected to hepatic metabolism or presented to the liver (Nelson et al. 2005; Vulgamott 1985; Winkler et al. 2003). A major consequence is that the brain is exposed to neurotoxins, resulting in hepatic encephalopathy and eventually death (Martin 1993). The biochemical features and the associated clinical signs are similar between dogs and humans with congenital portosystemic shunts. In addition, to a large extent these are similar to those seen in advanced chronic progressive liver disease with fibrosis and cirrhosis. The main difference between chronic progressive liver disease and congenital portosystemic shunts is that the first group has portal hypertension, which is absent in the congenital diseases.
Congenital portosystemic shunts can be divided roughly into two main subtypes: intrahepatic (IHPSS) and extrahepatic shunts (EHPSS) (van den Ingh et al. 1995) (Fig. 1). Although the genetic basis of CPSS in dogs is not clear yet, many authors have demonstrated that congenital shunts are more frequently diagnosed in purebred dogs and that a number of breeds are predisposed to it (Hunt 2004; Tobias 2003), which indicates an inherited basis for this disease (Meyer and Rothuizen 1991; van Straten et al. 2005). An equal frequency of affected males and females was generally reported (Hunt 2004; van Straten et al. 2005). In addition, EHPSS and IHPSS were very rarely seen in the same breed (Hunt 2004; Krotscheck et al. 2007; Martin 1993; Tobias and Rohrbach 2003; Vulgamott 1985; Winkler et al. 2003). Intrahepatic shunts were diagnosed mainly in large dog breeds and extrahepatic shunts in the smaller and toy breeds (Tobias and Rohrbach 2003; Tisdall et al. 1994). CPSS in humans has been classified as being a rare disease (Stringer 2008).
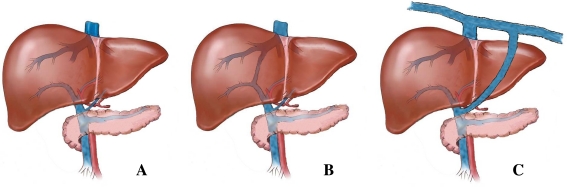
Fig. 1.
Overview of the anatomy of a normal liver and of livers with intra- and extrahepatic portosystemic shunts. a No connection of blood vessels in the liver is seen within a normal liver resulting in a blood flow through the hepatic sinusoids. b In case of PSS, blood bypasses the liver sinusoids and is therefore not subjected to hepatic metabolism. The intrahepatic shunt represents an abnormal connection of the portal vein with the systemic circulation, which is seen inside the liver. c In the case of an extrahepatic shunt, the aberrant connection is located outside the liver
Congenital portosystemic shunts in man and comparison with dogs
The same subtypes of intrahepatic and extrahepatic shunts which are known in dogs have been described in humans. From several literature reviews and case reports, the disease appears to be more prevalent in the Japanese population. In total, there have been 173 human cases of EHPSS reported (Caruso et al. 2010; Franchi-Abella et al. 2010; Kobayashi et al. 2010; Konstas et al. 2010; Lautz et al. 2011; Newman et al. 2010; Ohno et al. 2008) and 89 human cases of IHPSS (Ferrero et al. 2010; Franchi-Abella et al. 2010; Kamimatsuse et al. 2010; Konstas et al. 2010; Ohno et al. 2008; Schierz et al. 2011; Stringer 2008; Tsai et al. 2009; Uchino et al. 1999; Yoshimoto et al. 2004). In human EHPSS, a classification has been made based on the presence or absence of portal blood flow (Abernethy 1793). Doppler ultrasound measurement of portal flow to the liver proximal to the diversion of the shunting vessel has shown that in dogs as well, cases with and cases without hepatopetal blood flow do exist. Some affected dogs even have a reversed hepatofugal portal blood flow away from the liver (Szatmari et al. 2004a, c).
The histological features of the liver are identical in humans and dogs with intrahepatic or extrahepatic portosystemic shunt. The findings include absence of portal veins in small portal tracts, absent or hypoplastic portal veins in medium-sized and larger portal tracts, tortuosity, increased number and hypertrophy of arterioles in the portal areas, and atrophy of hepatocytes (Baade et al. 2006; Cullen et al. 2006; Lisovsky et al. 2011).
With respect to clinical presentation and methods to diagnose the disease, there are no essential differences between man and dog. The most prominent clinical signs relate to brain dysfunction caused by hepatic encephalopathy in man (Ali et al. 2010; Alonso-Gamarra et al. 2011; Murray et al. 2003) and dog (Maddison 1992). This syndrome is caused by neurotoxins bypassing the liver and the blood brain barrier. The pathogenesis of the encephalopathy is multifactorial in both species, but hyperammonemia is a common major factor. In man and in dogs the severe brain dysfunctions caused by hepatic encephalopathy are transient and can be completely resolved upon successful surgical closure of the congenital shunt (Alonso-Gamarra et al. 2011; Hunt 2004; Kummeling et al. 2004).
Phenotyping
Dogs can be diagnosed starting at 6 weeks of age. A globally used screening method measures increased pre- and postprandial serum bile acid levels (Kerr and van Doorn 1999). This test, however, gives abnormal results for many different liver diseases and is sensitive, but not specific, for congenital and acquired portosystemic shunting (Gerritzen-Bruning et al. 2006). Alternatively, screening can be performed by measuring basal plasma ammonia levels (Meyer et al. 1995; Sterczer et al. 1999; van Steenbeek et al. 2009). In Irish wolfhounds, however, a congenital urea cycle enzyme deficiency may also cause hyperammonemia (Zandvliet and Rothuizen 2007). In all other dog breeds, like in humans, the fasting blood ammonia concentration and the rectal ammonia tolerance test are the most sensitive and specific tools to detect portosystemic shunting (Gerritzen-Bruning et al. 2006). In all cases, the diagnosis of the shunt and the precise subtype has to be confirmed by direct demonstration of the shunting vessel. This can be achieved by ultrasonography, computed tomography, or surgery (Bertolini 2010; Szatmari et al. 2004b). The diagnostic criteria used in veterinary and human medicine are essentially identical. Hepatic encephalopathy, increased plasma ammonia concentrations, and a decreased ammonia tolerance indicate the likelihood of portosystemic shunting. The diagnosis in both species is confirmed and the subtype of the shunt is assessed with ultrasonography or computed tomography (Alonso-Gamarra et al. 2011; Bertolini 2010; Szatmari et al. 2004b). The use of a plasma bile acid measurement as a liver function test, which is also used in the diagnostic procedure in veterinary medicine, is rarely used in human medicine. This reflects a difference in established traditions between the two professions rather than a difference between the types of shunts in man and dog.
Canine intrahepatic portosystemic shunt
An intrahepatic portosystemic shunt (IHPSS) is caused by incomplete closure of the ductus venosus. The ductus venosus is an embryonic vessel connecting the vena porta with the vena cava, ensuring blood flow from the placenta directly to vital organs without traversing liver sinusoids. This vessel should close within the first few days after birth. The moment of closure slightly fluctuates between species (Lohse and Suter 1977). In dogs, the closure occurs within 6–9 days (Lamb and Burton 2004). IHPSS is diagnosed almost exclusively in large-sized purebred dogs (Hunt 2004; Rothuizen and van den Ingh 1982) and a predisposition to IHPSS is suggested for Irish wolfhounds (Kerr and van Doorn 1999; Meyer et al. 1995), Australian Cattle dogs (Tisdall et al. 1994), Old English Sheepdogs (Lamb and White 1998), and Labrador and Golden retrievers (Tobias and Rohrbach 2003; van den Ingh et al. 1995). The shunt can be anatomically positioned at the left or right side or centrally in the liver. Epidemiologic factors influencing the position of the shunt have been surveyed using case reports from the United States and Australia. Significant association was found between IHPSS location and country of origin (P = 0.048), breed (P = 0.025), and sex (P = 0.016) (Krotscheck et al. 2007).
Heritability of IHPSS in Irish wolfhounds
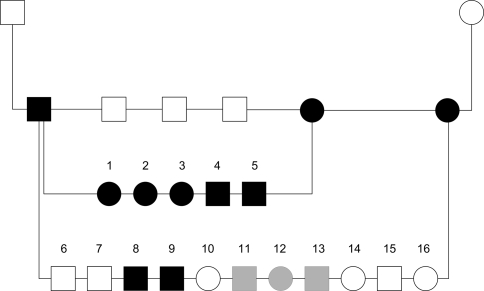
The clear familial distribution for IHPSS in Irish wolfhounds indicates a hereditary basis (Meyer et al. 1995; Ubbink et al. 1998). Between 1984 and 1992 the entire Dutch Irish wolfhound population was screened for IHPSS by measuring ammonia levels in blood and performing subsequent ultrasonography on dogs with hyperammonemia. The observed incidence increased over the years concomitant with increased inbreeding (Meyer et al. 1995). A litter of Irish wolfhounds was presented to the Utrecht University Clinic for Companion Animals for screening of IHPSS. Both parents were unaffected, and their offspring consisted of three affected and three unaffected dogs. We studied the mode of inheritance by test matings between an affected sire and two affected bitches after surgery and maturation. The matings resulted in one litter of 5 affecteds only and a litter of 11, with 5 affected and 6 unaffected pups (Fig. 2). The unaffected offspring in the second litter minimizes the possibility of a simple monogenic recessive disorder. The presence of both left- and right-divisional IHPSS suggests that the position is not genetically determined. The results indicate a genetic background with possibly a digenic mode of inheritance (van Steenbeek et al. 2009). In our model, two loci interact to cause the phenotype; at least three of the four alleles at the loci would be mutant in affected dogs.
Fig. 2.
Pedigree of test matings of Irish wolfhounds with intrahepatic shunt. An affected male was mated with two affected sisters. Squares represent males, circles represent females. Solid symbols are affected dogs and open symbols represent unaffected pups. Pups with uncertain phenotypes are filled gray (reprinted with permission from John Wiley and Sons; van Steenbeek et al. 2009)
Canine extrahepatic portosystemic shunt
Whereas intrahepatic shunts are derived from pre-existing embryonic connections, extrahepatic shunts must be considered as developmental anomalies. They represent abnormal functional communication between the embryonic vitelline veins, which form the entire extrahepatic portal system, and the cardinal venous system, which contributes to all nonportal abdominal veins. This connection results in a shunting vessel between the portal vein or its contributors, such as the left gastric, splenic, mesenteric veins, or the gastroduodenal vein, and the caudal vena cava or (hemi)azygos vein. Because the extrahepatic portal vein develops from different parts of the vitelline vein, and the vena cava and vena (hemi)azygos develop from the embryonic cardinal vein, connections between the cardinal and vitelline systems could not occur during any phase of embryonic development (Payne et al. 1990).
Extrahepatic shunts occur in small dog breeds with a predisposition in Cairn terriers (van Straten et al. 2005), Yorkshire terriers (Tobias 2003; Tobias and Rohrbach 2003), Jack Russell terriers (Hunt 2004), Dachshunds (van den Ingh et al. 1995), miniature schnauzers, Havanese, Dandie Dinmont terriers (Tobias and Rohrbach 2003), and Maltese (Tisdall et al. 1994).
Heritability of EHPSS in breeds of small dogs
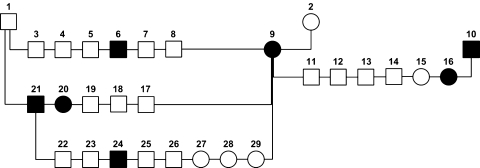
A pedigree analysis of affected Cairn terriers born in The Netherlands between 1990 and 2001 was performed to study the genetics of EHPSS (van Straten et al. 2005). A total of 6,367 pups were screened for shunts by measuring venous ammonia concentrations at an age of 6 weeks. Prevalences of 1.9–5.9% in three breeding lines were significantly higher than the prevalence in the entire population (0.58%), indicating a hereditary basis for this disease. Three test matings were performed (Fig. 3). A successfully operated on female was mated with her unaffected father, an affected son, and an unrelated affected male. Four of the 19 pups (21%) born from these matings were affected, providing further evidence that EHPSS is a genetic disorder. The mode of inheritance is most likely polygenic and there seems to be no sex effect (van Straten et al. 2005).
Fig. 3.
Pedigree of test matings with Cairn terriers with extrahepatic shunt. An affected female (#9) was used three times, with her unaffected father (#1), with her affected son (#21), and with an unrelated affected male (#10). Squares represent males, circles represent females. Solid symbols are affected dogs and open symbols are healthy dogs (reprinted with permission from John Wiley and Sons; van Straten et al. 2005)
EHPSS can be classified in two subtypes: porta cava and porta (hemi)azygos shunts. We have surveyed a large number of cases (F. G. van Steenbeek, unpublished) and found that both subtypes occur in all ten predisposed breeds. On average, 23% connected with the vena (hemi)azygos and 67% connected with the vena cava. The presence of both types in all predisposed breed populations indicated a common genetic basis. The erroneous development of the portosystemic connection between the vitelline system and the vena cava or the vena (hemi)azygos is most likely due to modulating factors at specific time points during embryogenesis. The cava and azygos veins are formed through several transformations of the cardinal system (Payne et al. 1990).
Molecular genetic studies in our lab support the above conclusions (F. G. van Steenbeek, unpublished). A genome-wide association study (GWAS) performed on Cairn terriers resulted in three genomic regions strongly associated with EHPSS.
Candidate genes for congenital portosystemic shunts
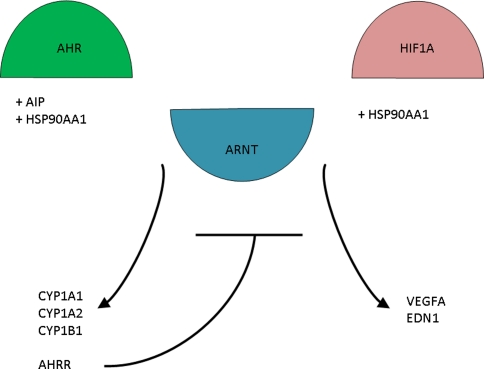
Serendipitously, a mouse model was found that had an intrahepatic shunt as seen in breeds of large dogs and in man. This knockout mouse is homozygous for a deletion in the aryl hydrocarbon receptor gene (AHR) (Lahvis et al. 2000). More recently, it was demonstrated that AHR signaling in vascular endothelial cells is necessary for developmental closure of the ductus venosus. In contrast, AHR signaling in hepatocytes is necessary to generate detoxifying responses to dioxin exposure of the liver (Walisser et al. 2005). AHR activates transcription of several cytochrome P-450 subtype genes (Fig. 4) by forming a heterodimer with aryl hydrocarbon receptor nuclear translocator [ARNT, also known as hypoxia-inducible factor 1, beta subunit (HIF1B)) (Mimura and Fujii-Kuriyama 2003). A unique cytochrome P-450 system functions in the closure of the ductus venosus by its contractile effect on the sphincter region in lambs (Adeagbo et al. 1990). Which cytochrome P-450 subtype is responsible for this contraction is still to be determined but CYP1A1 and CYP1A2 were recently excluded (Nukaya et al. 2009).
Fig. 4.
The relationship between the AHR pathway and the HIF1A pathway. Both pathways share ARNT and HSP90AA1 as key regulators
ARNT is known for its role in angiogenesis under hypoxia when it dimerizes with hypoxia inducible factor 1α (HIF1A). Under hypoxic conditions, ARNT regulates hypoxia-activated transcription in hepatocytes (Wood et al. 1996). ARNT−/− mouse embryos die in utero around E10.5 due to extreme vascular defects (Maltepe et al. 1997). This impaired vascularization appears to be affected by decreased expression of vascular endothelial growth factor A (VEGFA). Activation of endothelial VEGFA combined with inactivation of stromal transforming growth factor-β was reported as being essential for AHR-mediated angiogenesis (Roman et al. 2009), suggesting an interaction between the AHR pathway and the HIF1A pathway.
Hypomorphic ARNT knockout mice appeared to display identical phenotypical alterations as those of AHR knockout mice (Walisser et al. 2004). When a hepatocyte-specific deletion of ARNT was applied, no shunting was observed; hence, hepatocyte ARNT does not function in AHR-mediated hepatovascular development (Nukaya et al. 2010b).
Another protein that has a central role in both pathways is heat shock protein 90 kDa alpha (cytosolic), class A member 1 (HSP90AA1). HSP90AA1 was found to form a cytosolic complex in hepatocytes with AHR (Perdew 1988), but it also appears to be an essential regulator in HIF1A activation (Minet et al. 1999). Therefore, HSP90AA1 could very well be the gene connecting AHR with vascularization.
Aryl hydrocarbon receptor interacting protein (AIP) was also found to play an important role in closure of the ductus venosus. Construction of AIP (−/−) mice resulted in 83% of the mice having intrahepatic shunting (Lin et al. 2008). Hepatocyte-specific knockout mice showed that AIP has an important role in the maintenance of cytosolic AHR levels as well as in regulation of both CYP1B1 and the aryl hydrocarbon receptor repressor. Striking though was that CYP1A1 and CYP1A2 expression was not altered, suggesting the presence of other AHR-responsive genes (Nukaya et al. 2010a).
The AHR pathway is a strong candidate pathway for involvement in canine and human IHPSS. In mice, all homozygous AHR knockouts have IHPSS, whereas the mode of inheritance in dogs is expected to be more complex. In addition, the mouse model has quite a complex phenotype, with multiorgan lesions that do not occur in dogs or humans with IHPSS.
Closure of the ductus arteriosus, the fetal shunt connecting the pulmonary artery with the aorta that allows blood to bypass the unexpanded lungs, is physiologically comparable to closure of the ductus venosus in the liver. Closure of the ductus arteriosus appears to be mediated by the cytochrome P-450 gene CYP3A13 and the gene coding for endothelin-1 (Baragatti et al. 2011). These findings could correspond with a model of inheritance of defective vessel closures involving a low number of genes.
In summary, the above genes and pathways have been shown to be involved in IHPSS in mice and should be considered important candidates for the human and canine variants, but their role remains to be elucidated.
General medical relevance of genes and pathways causing portosystemic shunts
Congenital forms of portosystemic shunt in children are rare, with only 173 reported cases. Therefore, it is unlikely that the molecular basis can be elucidated by study of human cases. As illustrated above, purebred dogs display the same types of congenital portosystemic shunts as seen in man, with the same consequences with respect to clinical signs, pathophysiology, and liver pathology. The inbreeding of dog populations has led to an increased incidence of genetic diseases which remained incidental in the panmictic human populations. The complex genetic background of portosystemic shunts may well be unraveled in dogs. This may not only reveal new genes and pathways involved in vascular embryogenesis, but also important candidate genes for the human forms of the disease. The role of the candidate genes for the intrahepatic congenital shunts discussed above could also become clear.
The importance of these genes and pathways may, however, be much broader. Chronic progressive liver disease is a frequent and important health problem in man and dog. Recent studies have shown that the pathogenetic pathways of chronic fibrotic liver disease leading to cirrhosis are identical in man and dog (Ijzer et al. 2010; Schotanus et al. 2009; Spee et al. 2007). Chronic liver diseases are characterized by progressive fibrosis, ultimately leading to dissection of the normal lobular structure of the liver and ending in irreversible cirrhosis (Calvaruso et al. 2008; Matsuzaki 2009; Thenappan et al. 2010; Torok 2008; Spee et al. 2006). Nowadays, the only possible treatment is liver transplantation. Apart from fibrosis, progression of these diseases is characterized by decreased regeneration to compensate for the concomitant loss of functional liver epithelium. The normally huge capacity of the liver to regenerate by expansion of the hepatocyte compartment becomes lost in chronic fibrotic disease, regardless of the etiology (Hartmann et al. 2011; Nakajima et al. 2006; Wege and Brummendorf 2007; Wege et al. 2003; Wiemann et al. 2002). This is in part compensated for by adult stem cell activation, but this safeguard is usually too small and occurs too late (Fausto 2004; Ijzer et al. 2010; Roskams et al. 2003; Schotanus et al. 2009; Spee et al. 2010). The third component of chronic deterioration of liver function in progressive liver disease is the ongoing impairment of portal liver perfusion (Fernandez et al. 2009; Treiber et al. 2005; Yokoyama et al. 2007). The triangle of lost regeneration, fibrosis leading to cirrhosis, and impaired vascularization determines the vicious cycle of chronic progressive liver disease ending in the need for transplantation.
In both man and dog the presence of a congenital portosystemic shunt, either intra- or extrahepatic, is associated with deranged formation and growth of the smallest intrahepatic branches of the portal vein tree. In the veterinary literature this has been referred to as portal vein hypoplasia (van den Ingh et al. 1995; Zwingenberger and Shofer 2007) or microvascular dysplasia (Allen et al. 1999). There is one case report of a dog with three congenital vascular malformations combined: congenital portosystemic shunt, hypoplasia of the intrahepatic portal tree, and intrahepatic arteriovenous fistula (Favier et al. 2004). As described above, we have observed that the different subtypes of congenital extrahepatic porta cava and porta (hemi)azygos shunts exist in similar ratios in all affected dog breeds. This indicates that a limited number of genes regulate the complex formation of the splanchnic vascular bed and associated portal venous system. It is anticipated that elucidation of these genes and the associated pathways will also give insight into the pathologic vascular derangements that are an essential part of the pathogenesis of chronic progressive liver disease. This may also provide new ways to intervene in these presently incurable diseases.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abernethy J. Account of two instances of uncommon formation in the viscera of the human body. Philos Trans R Soc Lond B Biol Sci. 1793;83:295–299. [Google Scholar]
- Adeagbo AS, Breen CA, Cutz E, Lees JG, Olley PM, Coceani F. Lamb ductus venosus: evidence of a cytochrome P-450 mechanism in its contractile tension. J Pharmacol Exp Ther. 1990;252:875–879. [PubMed] [Google Scholar]
- Ali S, Stolpen AH, Schmidt WN. Portosystemic encephalopathy due to mesoiliac shunt in a patient without cirrhosis. J Clin Gastroenterol. 2010;44:381–383. doi: 10.1097/MCG.0b013e3181aae51b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen L, Stobie D, Mauldin GN, Baer KE. Clinicopathologic features of dogs with hepatic microvascular dysplasia with and without portosystemic shunts: 42 cases (1991–1996) J Am Vet Med Assoc. 1999;214:218–220. [PubMed] [Google Scholar]
- Alonso-Gamarra E, Parron M, Perez A, Prieto C, Hierro L, Lopez-Santamaria M. Clinical and radiologic manifestations of congenital extrahepatic portosystemic shunts: a comprehensive review. Radiographics. 2011;31:707–722. doi: 10.1148/rg.313105070. [DOI] [PubMed] [Google Scholar]
- Baade S, Aupperle H, Grevel V, Schoon HA. Histopathological and immunohistochemical investigations of hepatic lesions associated with congenital portosystemic shunt in dogs. J Comp Pathol. 2006;134:80–90. doi: 10.1016/j.jcpa.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Baragatti B, Ciofini E, Scebba F, Angeloni D, Sodini D, Luin S, Ratto GM, Ottaviano V, Pagni E, Paolicchi A, Nencioni S, Coceani F. Cytochrome P-450 3A13 and endothelin jointly mediate ductus arteriosus constriction to oxygen in mice. Am J Physiol Heart Circ Physiol. 2011;300:H892–H901. doi: 10.1152/ajpheart.00907.2010. [DOI] [PubMed] [Google Scholar]
- Bertolini G. Acquired portal collateral circulation in the dog and cat. Vet Radiol Ultrasound. 2010;51:25–33. doi: 10.1111/j.1740-8261.2009.01616.x. [DOI] [PubMed] [Google Scholar]
- Calvaruso V, Maimone S, Gatt A, Tuddenham E, Thursz M, Pinzani M, Burroughs AK. Coagulation and fibrosis in chronic liver disease. Gut. 2008;57:1722–1727. doi: 10.1136/gut.2008.150748. [DOI] [PubMed] [Google Scholar]
- Caruso S, Riva S, Spada M, Luca A, Gridelli B. Congenital extrahepatic portosystemic shunts (CEPS) type Ib: MDCT finding. Dig Liver Dis. 2010;42:593. doi: 10.1016/j.dld.2009.02.053. [DOI] [PubMed] [Google Scholar]
- Cullen JM, van den Ingh TSGAM, Bunch SE, Rothuizen J, Washabau RJ, Desmet VJ. WSAVA Standards for clinical and histological diagnosis. St. Louis: WSAVA Liver Disease Standardization Group; 2006. pp. 41–59. [Google Scholar]
- Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- Favier RP, Szatmari V, Rothuizen J. Multiple congenital portal vein anomalies in a dog. Vet Rec. 2004;154:604–605. doi: 10.1136/vr.154.19.604. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Ferrero GB, Porta F, Biamino E, Mussa A, Garelli E, Chiappe F, Veltri A, Silengo MC, Gennari F. Remittent hyperammonemia in congenital portosystemic shunt. Eur J Pediatr. 2010;169:369–372. doi: 10.1007/s00431-009-1031-z. [DOI] [PubMed] [Google Scholar]
- Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J, Riou JY, Pariente D, Gauthier F, Jacquemin E, Bernard O. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010;51:322–330. doi: 10.1097/MPG.0b013e3181d9cb92. [DOI] [PubMed] [Google Scholar]
- Gerritzen-Bruning MJ, van den Ingh TS, Rothuizen J. Diagnostic value of fasting plasma ammonia and bile acid concentrations in the identification of portosystemic shunting in dogs. J Vet Intern Med. 2006;20:13–19. doi: 10.1111/j.1939-1676.2006.tb02818.x. [DOI] [PubMed] [Google Scholar]
- Hartmann D, Srivastava U, Thaler M, Kleinhans KN, N’kontchou G, Scheffold A, Bauer K, Kratzer RF, Kloos N, Katz SF, Song Z, Begus-Nahrmann Y, Kleger A, von Figura G, Strnad P, Lechel A, Gunes C, Potthoff A, Deterding K, Wedemeyer H, Ju Z, Song G, Xiao F, Gillen S, Schrezenmeier H, Mertens T, Ziol M, Friess H, Jarek M, Manns MP, Beaugrand M, Rudolph KL. Telomerase gene mutations are associated with cirrhosis formation. Hepatology. 2011;53:1608–1617. doi: 10.1002/hep.24217. [DOI] [PubMed] [Google Scholar]
- Hunt GB. Effect of breed on anatomy of portosystemic shunts resulting from congenital diseases in dogs and cats: a review of 242 cases. Aust Vet J. 2004;82:746–749. doi: 10.1111/j.1751-0813.2004.tb13233.x. [DOI] [PubMed] [Google Scholar]
- Ijzer J, Schotanus BA, Vander Borght S, Roskams TA, Kisjes R, Penning LC, Rothuizen J, van den Ingh TS. Characterisation of the hepatic progenitor cell compartment in normal liver and in hepatitis: an immunohistochemical comparison between dog and man. Vet J. 2010;184:308–314. doi: 10.1016/j.tvjl.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Kamimatsuse A, Onitake Y, Kamei N, Tajima G, Sakura N, Sueda T, Hiyama E. Surgical intervention for patent ductus venosus. Pediatr Surg Int. 2010;26:1025–1030. doi: 10.1007/s00383-010-2662-x. [DOI] [PubMed] [Google Scholar]
- Kerr MG, van Doorn T. Mass screening of Irish wolfhound puppies for portosystemic shunts by the dynamic bile acid test. Vet Rec. 1999;144:693–696. doi: 10.1136/vr.144.25.693. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Niwa T, Kirikoshi H, Fujita K, Yoneda M, Saito S, Nakajima A. Clinical classification of congenital extrahepatic portosystemic shunts. Hepatol Res. 2010;40:585–593. doi: 10.1111/j.1872-034X.2010.00667.x. [DOI] [PubMed] [Google Scholar]
- Konstas AA, Digumarthy SR, Avery LL, Wallace KL, Lisovsky M, Misdraji J, Hahn PF. Congenital portosystemic shunts: Imaging findings and clinical presentations in 11 patients. Eur J Radiol. 2010;80(2):175–181. doi: 10.1016/j.ejrad.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Krotscheck U, Adin CA, Hunt GB, Kyles AE, Erb HN. Epidemiologic factors associated with the anatomic location of intrahepatic portosystemic shunts in dogs. Vet Surg. 2007;36:31–36. doi: 10.1111/j.1532-950X.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- Kummeling A, Van Sluijs FJ, Rothuizen J. Prognostic implications of the degree of shunt narrowing and of the portal vein diameter in dogs with congenital portosystemic shunts. Vet Surg. 2004;33:17–24. doi: 10.1111/j.1532-950x.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, Bentz M, Southard J, Bradfield CA. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci USA. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb CR, Burton CA. Doppler ultrasonographic assessment of closure of the ductus venosus in neonatal Irish wolfhounds. Vet Rec. 2004;155:699–701. doi: 10.1136/vr.155.22.699. [DOI] [PubMed] [Google Scholar]
- Lamb CR, White RN. Morphology of congenital intrahepatic portacaval shunts in dogs and cats. Vet Rec. 1998;142:55–60. doi: 10.1136/vr.142.3.55. [DOI] [PubMed] [Google Scholar]
- Lautz TB, Tantemsapya N, Rowell E, Superina RA. Management and classification of type II congenital portosystemic shunts. J Pediatr Surg. 2011;46:308–314. doi: 10.1016/j.jpedsurg.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Lin BC, Nguyen LP, Walisser JA, Bradfield CA. A hypomorphic allele of aryl hydrocarbon receptor-associated protein-9 produces a phenocopy of the AHR-null mouse. Mol Pharmacol. 2008;74:1367–1371. doi: 10.1124/mol.108.047068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisovsky M, Konstas AA, Misdraji J. Congenital extrahepatic portosystemic shunts (Abernethy malformation): a histopathologic evaluation. Am J Surg Pathol. 2011;35:1381–1390. doi: 10.1097/PAS.0b013e3182230ce4. [DOI] [PubMed] [Google Scholar]
- Lohse CL, Suter PF. Functional closure of the ductus venosus during early postnatal life in the dog. Am J Vet Res. 1977;38:839–844. [PubMed] [Google Scholar]
- Maddison JE. Hepatic encephalopathy. Current concepts of the pathogenesis. J Vet Intern Med. 1992;6:341–353. doi: 10.1111/j.1939-1676.1992.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- Martin RA. Congenital portosystemic shunts in the dog and cat. Vet Clin North Am Small Anim Pract. 1993;23:609–623. doi: 10.1016/s0195-5616(93)50309-1. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K. Modulation of TGF-beta signaling during progression of chronic liver diseases. Front Biosci. 2009;14:2923–2934. doi: 10.2741/3423. [DOI] [PubMed] [Google Scholar]
- Meyer HP, Rothuizen J. Congenital portosystemic shunts (PSS) in dogs are a genetic disorder. Tijdschr Diergeneeskd. 1991;116(Suppl 1):80S–81S. [PubMed] [Google Scholar]
- Meyer HP, Rothuizen J, Ubbink GJ, van den Ingh TS. Increasing incidence of hereditary intrahepatic portosystemic shunts in Irish wolfhounds in The Netherlands (1984 to 1992) Vet Rec. 1995;136:13–16. doi: 10.1136/vr.136.1.13. [DOI] [PubMed] [Google Scholar]
- Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003;1619:263–268. doi: 10.1016/S0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, Michiels C. Hypoxia-induced activation of HIF-1: role of HIF-1alpha-Hsp90 interaction. FEBS Lett. 1999;460:251–256. doi: 10.1016/S0014-5793(99)01359-9. [DOI] [PubMed] [Google Scholar]
- Murray CP, Yoo SJ, Babyn PS. Congenital extrahepatic portosystemic shunts. Pediatr Radiol. 2003;33:614–620. doi: 10.1007/s00247-003-1002-x. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Moriguchi M, Katagishi T, Sekoguchi S, Nishikawa T, Takashima H, Kimura H, Minami M, Itoh Y, Kagawa K, Tani Y, Okanoue T. Premature telomere shortening and impaired regenerative response in hepatocytes of individuals with NAFLD. Liver Int. 2006;26:23–31. doi: 10.1111/j.1478-3231.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- Nelson RW, Couto CG, King C, Ashby K, editors. Manual of small animal internal medicine. Philadelphia: Elsevier Mosby; 2005. [Google Scholar]
- Newman B, Feinstein JA, Cohen RA, Feingold B, Kreutzer J, Patel H, Chan FP. Congenital extrahepatic portosystemic shunt associated with heterotaxy and polysplenia. Pediatr Radiol. 2010;40:1222–1230. doi: 10.1007/s00247-009-1508-y. [DOI] [PubMed] [Google Scholar]
- Nukaya M, Moran S, Bradfield CA. The role of the dioxin-responsive element cluster between the Cyp1a1 and Cyp1a2 loci in aryl hydrocarbon receptor biology. Proc Natl Acad Sci USA. 2009;106(12):4923–4928. doi: 10.1073/pnas.0809613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukaya M, Lin BC, Glover E, Moran SM, Kennedy GD, Bradfield CA. The aryl hydrocarbon receptor-interacting protein (AIP) is required for dioxin-induced hepatotoxicity but not for the induction of the Cyp1a1 and Cyp1a2 genes. J Biol Chem. 2010;285:35599–35605. doi: 10.1074/jbc.M110.132043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukaya M, Walisser JA, Moran SM, Kennedy GD, Bradfield CA. Aryl hydrocarbon receptor nuclear translocator in hepatocytes is required for aryl hydrocarbon receptor-mediated adaptive and toxic responses in liver. Toxicol Sci. 2010;118:554–563. doi: 10.1093/toxsci/kfq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T, Muneuchi J, Ihara K, Yuge T, Kanaya Y, Yamaki S, Hara T. Pulmonary hypertension in patients with congenital portosystemic venous shunt: a previously unrecognized association. Pediatrics. 2008;121:e892–e899. doi: 10.1542/peds.2006-3411. [DOI] [PubMed] [Google Scholar]
- Payne JT, Martin RA, Constantinescu GM. The anatomy and embryology of portosystemic shunts in dogs and cats. Semin Vet Med Surg (Small Anim) 1990;5:76–82. [PubMed] [Google Scholar]
- Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988;263:13802–13805. [PubMed] [Google Scholar]
- Roman AC, Carvajal-Gonzalez JM, Rico-Leo EM, Fernandez-Salguero PM. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J Biol Chem. 2009;284:25135–25148. doi: 10.1074/jbc.M109.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- Rothuizen J, van den Ingh TS. Rectal ammonia tolerance test in the evaluation of portal circulation in dogs with liver disease. Res Vet Sci. 1982;33:22–25. [PubMed] [Google Scholar]
- Schierz IA, La Placa S, Giuffre M, Montalbano G, Lenzo M, Corsello G. Transient hepatic nodular lesions associated with patent ductus venosus in preterm infants. Am J Perinatol. 2011;28:177–180. doi: 10.1055/s-0030-1265829. [DOI] [PubMed] [Google Scholar]
- Schotanus BA, van den Ingh TS, Penning LC, Rothuizen J, Roskams TA, Spee B. Cross-species immunohistochemical investigation of the activation of the liver progenitor cell niche in different types of liver disease. Liver Int. 2009;29:1241–1252. doi: 10.1111/j.1478-3231.2009.02024.x. [DOI] [PubMed] [Google Scholar]
- Spee B, Arends B, van den Ingh TS, Brinkhof B, Nederbragt H, Ijzer J, Roskams T, Penning LC, Rothuizen J. Transforming growth factor beta-1 signalling in canine hepatic diseases: new models for human fibrotic liver pathologies. Liver Int. 2006;26:716–725. doi: 10.1111/j.1478-3231.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- Spee B, Arends B, van den Ingh TS, Roskams T, Rothuizen J, Penning LC. Major HGF-mediated regenerative pathways are similarly affected in human and canine cirrhosis. Comp Hepatol. 2007;6:8. doi: 10.1186/1476-5926-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spee B, Carpino G, Schotanus BA, Katoonizadeh A, Vander Borght S, Gaudio E, Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59:247–257. doi: 10.1136/gut.2009.188367. [DOI] [PubMed] [Google Scholar]
- Sterczer A, Meyer HP, Boswijk HC, Rothuizen J. Evaluation of ammonia measurements in dogs with two analysers for use in veterinary practice. Vet Rec. 1999;144:523–526. doi: 10.1136/vr.144.19.523. [DOI] [PubMed] [Google Scholar]
- Stringer MD. The clinical anatomy of congenital portosystemic venous shunts. Clin Anat. 2008;21:147–157. doi: 10.1002/ca.20574. [DOI] [PubMed] [Google Scholar]
- Szatmari V, Rothuizen J, van den Ingh TS, van Sluijs FJ, Voorhout G. Ultrasonographic findings in dogs with hyperammonemia: 90 cases (2000–2002) J Am Vet Med Assoc. 2004;224:717–727. doi: 10.2460/javma.2004.224.717. [DOI] [PubMed] [Google Scholar]
- Szatmari V, Rothuizen J, Voorhout G. Standard planes for ultrasonographic examination of the portal system in dogs. J Am Vet Med Assoc. 2004;224(713–716):698–699. doi: 10.2460/javma.2004.224.713. [DOI] [PubMed] [Google Scholar]
- Szatmari V, van Sluijs FJ, Rothuizen J, Voorhout G. Ultrasonographic assessment of hemodynamic changes in the portal vein during surgical attenuation of congenital extrahepatic portosystemic shunts in dogs. J Am Vet Med Assoc. 2004;224:395–402. doi: 10.2460/javma.2004.224.395. [DOI] [PubMed] [Google Scholar]
- Thenappan A, Li Y, Kitisin K, Rashid A, Shetty K, Johnson L, Mishra L. Role of transforming growth factor beta signaling and expansion of progenitor cells in regenerating liver. Hepatology. 2010;51:1373–1382. doi: 10.1002/hep.23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall PL, Hunt GB, Bellenger CR, Malik R. Congenital portosystemic shunts in Maltese and Australian cattle dogs. Aust Vet J. 1994;71:174–178. doi: 10.1111/j.1751-0813.1994.tb03382.x. [DOI] [PubMed] [Google Scholar]
- Tobias KM. Determination of inheritance of single congenital portosystemic shunts in Yorkshire terriers. J Am Anim Hosp Assoc. 2003;39:385–389. doi: 10.5326/0390385. [DOI] [PubMed] [Google Scholar]
- Tobias KM, Rohrbach BW. Association of breed with the diagnosis of congenital portosystemic shunts in dogs: 2, 400 cases (1980–2002) J Am Vet Med Assoc. 2003;223:1636–1639. doi: 10.2460/javma.2003.223.1636. [DOI] [PubMed] [Google Scholar]
- Torok NJ. Recent advances in the pathogenesis and diagnosis of liver fibrosis. J Gastroenterol. 2008;43:315–321. doi: 10.1007/s00535-008-2181-x. [DOI] [PubMed] [Google Scholar]
- Treiber G, Csepregi A, Malfertheiner P. The pathophysiology of portal hypertension. Dig Dis. 2005;23:6–10. doi: 10.1159/000084720. [DOI] [PubMed] [Google Scholar]
- Tsai A, Paltiel HJ, Sena LM, Kim HB, Fishman SJ, Alomari AI. Neonatal hemochromatosis and patent ductus venosus: clinical course and diagnostic pitfalls. Pediatr Radiol. 2009;39:823–827. doi: 10.1007/s00247-009-1294-6. [DOI] [PubMed] [Google Scholar]
- Ubbink GJ, van de Broek J, Meyer HP, Rothuizen J. Prediction of inherited portosystemic shunts in Irish Wolfhounds on the basis of pedigree analysis. Am J Vet Res. 1998;59:1553–1556. [PubMed] [Google Scholar]
- Uchino T, Matsuda I, Endo F. The long-term prognosis of congenital portosystemic venous shunt. J Pediatr. 1999;135:254–256. doi: 10.1016/S0022-3476(99)70031-4. [DOI] [PubMed] [Google Scholar]
- van den Ingh TS, Rothuizen J, Meyer HP. Circulatory disorders of the liver in dogs and cats. Vet Q. 1995;17:70–76. doi: 10.1080/01652176.1995.9694536. [DOI] [PubMed] [Google Scholar]
- van Steenbeek FG, Leegwater PA, van Sluijs FJ, Heuven HC, Rothuizen J. Evidence of inheritance of intrahepatic portosystemic shunts in Irish Wolfhounds. J Vet Intern Med. 2009;23:950–952. doi: 10.1111/j.1939-1676.2009.0319.x. [DOI] [PubMed] [Google Scholar]
- van Straten G, Leegwater PA, de Vries M, van den Brom WE, Rothuizen J. Inherited congenital extrahepatic portosystemic shunts in Cairn terriers. J Vet Intern Med. 2005;19:321–324. doi: 10.1111/j.1939-1676.2005.tb02701.x. [DOI] [PubMed] [Google Scholar]
- Vulgamott JC. Portosystemic shunts. Vet Clin North Am Small Anim Pract. 1985;15:229–242. doi: 10.1016/s0195-5616(85)50013-3. [DOI] [PubMed] [Google Scholar]
- Walisser JA, Bunger MK, Glover E, Harstad EB, Bradfield CA. Patent ductus venosus and dioxin resistance in mice harboring a hypomorphic Arnt allele. J Biol Chem. 2004;279:16326–16331. doi: 10.1074/jbc.M400784200. [DOI] [PubMed] [Google Scholar]
- Walisser JA, Glover E, Pande K, Liss AL, Bradfield CA. Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types. Proc Natl Acad Sci USA. 2005;102:17858–17863. doi: 10.1073/pnas.0504757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H, Brummendorf TH. Telomerase activation in liver regeneration and hepatocarcinogenesis: Dr. Jekyll or Mr. Hyde? Curr Stem Cell Res Ther. 2007;2:31–38. doi: 10.2174/157488807779317062. [DOI] [PubMed] [Google Scholar]
- Wege H, Chui MS, Le HT, Strom SC, Zern MA. In vitro expansion of human hepatocytes is restricted by telomere-dependent replicative aging. Cell Transplant. 2003;12:897–906. doi: 10.3727/000000003771000138. [DOI] [PubMed] [Google Scholar]
- Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, Flemming P, Franco S, Blasco MA, Manns MP, Rudolph KL. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- Winkler JT, Bohling MW, Tillson DM, Wright JC, Ballagas AJ. Portosystemic shunts: diagnosis, prognosis, and treatment of 64 cases (1993–2001) J Am Anim Hosp Assoc. 2003;39:169–185. doi: 10.5326/0390169. [DOI] [PubMed] [Google Scholar]
- Wood SM, Gleadle JM, Pugh CW, Hankinson O, Ratcliffe PJ. The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. Studies in ARNT-deficient cells. J Biol Chem. 1996;271:15117–15123. doi: 10.1074/jbc.271.48.30392. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Nagino M, Nimura Y. Mechanism of impaired hepatic regeneration in cholestatic liver. J Hepatobiliary Pancreat Surg. 2007;14:159–166. doi: 10.1007/s00534-006-1125-1. [DOI] [PubMed] [Google Scholar]
- Yoshimoto Y, Shimizu R, Saeki T, Harada T, Sugio Y, Nomura S, Tanaka H. Patent ductus venosus in children: a case report and review of the literature. J Pediatr Surg. 2004;39:E1–E5. doi: 10.1016/j.jpedsurg.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Zandvliet MM, Rothuizen J. Transient hyperammonemia due to urea cycle enzyme deficiency in Irish wolfhounds. J Vet Intern Med. 2007;21:215–218. doi: 10.1111/j.1939-1676.2007.tb02951.x. [DOI] [PubMed] [Google Scholar]
- Zwingenberger AL, Shofer FS. Dynamic computed tomographic quantitation of hepatic perfusion in dogs with and without portal vascular anomalies. Am J Vet Res. 2007;68:970–974. doi: 10.2460/ajvr.68.9.970. [DOI] [PubMed] [Google Scholar]