Abstract
ST6GalNAcI is a sialyltransferase responsible for the synthesis of sialyl Tn (sTn) antigen which is expressed in a variety of adenocarcinomas including gastric cancer especially in advanced cases, but the roles of ST6GalNAcI and sTn in cancer progression are largely unknown. We generated sTn-expressing human gastric cancer cells by ectopic expression of ST6GalNAcI to evaluate metastatic ability of these cells and prognostic effect of ST6GalNAcI and sTn in a mouse model, and identified sTn carrier proteins to gain insight into the function of ST6GalNAcI and sTn in gastric cancer progression. A green fluorescent protein-tagged human gastric cancer cell line was transfected with ST6GalNAcI to produce sTn-expressing cells, which were transplanted into nude mice. STn-positive gastric cancer cells showed higher intraperitoneal metastatic ability in comparison with sTn-negative control, resulting in shortened survival time of the mice, which was mitigated by anti-sTn antibody administration. Then, sTn-carrying proteins were immunoprecipitated from culture supernatants and lysates of these cells, and identified MUC1 and CD44 as major sTn carriers. It was confirmed that MUC1 carries sTn also in human advanced gastric cancer tissues. Identification of sTn carrier proteins will help understand mechanisms of metastatic phenotype acquisition of gastric cancer cells by ST6GalNAcI and sTn.
Keywords: CD44, Gastric cancer, Glycoprotein, Mouse, MUC1, Peritoneal metastasis, Sialyl Tn, ST6GalNAcI
Introduction
Sialyl Tn (sTn) antigen, one of the most well-known cancer-associated glycan structures, which is expressed in a large proportion of gastric, colorectal, ovarian, breast, pancreatic and other adenocarcinoma tissues, and is detected in sera and/or other body fluids of patients with these cancers [1–9]. STn expression is correlated with poor prognosis of patients [1–4, 7, 10], and thus it is used as a serum or tissue marker for these tumors. In gastric cancer, sTn expression is also correlated with peritoneal metastasis [11, 12], a major cause of recurrence, which is thought to originate from intraperitoneal free tumor cells. Thus, sTn is viewed as a favorable target for detection of intraperitoneal free tumor cells and peritoneal micrometastases, as well as prediction of prognosis of patients, although only recently developed, RT-PCR-based detection methods [13–15] are the most sensitive for this purpose to date.
STn is synthesized by transferring a sialic acid in an α2,6-linkage to an N-acetylgalactosamine linked to a serine or threonine residue (Tn antigen) by ST6GalNAcI [16]. Since the expression of sTn is correlated with, or induced by, the expression of ST6GalNAcI in some colorectal, gastric, and breast cancer cell lines [16–18], the emergence of sTn is thought to be partly due to aberrant expression of ST6GalNAcI, with or without concomitant decrease or loss of other glycosyltransferases which compete with ST6GalNAcI for their substrate [19].
Positive correlations of sTn expression with cancer aggressiveness and poor prognosis of the patients have provoked great interest in the functional analyses on sTn. Induction of sTn in a mouse mammary carcinoma cell line led to morphological changes, impaired proliferation, and decreased migration on fibronectin and hyaluronic acid strata [20]. A human gastric cancer cell line showed decreased cell–cell aggregation, elevated adhesion and migration activity on ECM proteins, and increased invasive capability in in vitro assay using Matrigel, all of which were blocked by an anti-sTn mAb, HB-STn [21]. Further, transplantation of an ST6GalNAcI-transfected, sTn-positive human breast cancer cell line into mice showed increased tumorigenicity and tumor size in comparison to mock transfectants [22]. However, the molecular mechanisms underlying these phenotypic changes of the cells still need to be elucidated.
In addition, the roles of sTn and ST6GalNAcI in peritoneal metastasis of gastric cancer are not well understood. Previously, we established a convenient in vivo monitoring system for micrometastases in nude mice using GFP-tagged human gastric cancer cell lines [23, 24]. In the present study, we applied this in vivo monitoring system to elucidate the roles of ST6GalNAcI and sTn in gastric cancer peritoneal metastasis. We showed that ectopic expression of ST6GalNAcI in a gastric cancer cell line induced surface expression of sTn and resulted in enhanced intraperitoneal metastasis and tumor growth, and shortened survival time, the latter of which was mitigated by administration of anti-sTn mAb. We also found that the major carrier proteins for sTn in these cells are MUC1 and CD44, suggesting the possible involvement of these sTn-carrying glycoproteins in acquisition of this metastatic phenotype by these gastric cancer cells.
Materials and methods
Cell lines
A GFP-tagged human gastric cancer cell line, GCIY-EGFP [23], a derivative subline of GCIY which had been originally obtained from the RIKEN Cell Bank (Tsukuba, Japan), and its transfectants were maintained in RPMI1640 (Sigma, St. Louis, MO) supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA) in a humidified 5% CO2 incubator at 37°C.
DNA transfection and isolation of sTn-expressing cells
To obtain sTn-expressing GCIY-EGFP cells [23], cells were transfected with an ST6GalNAc I-expressing vector, pXLS [16], using Lipofectamine 2,000 reagent (Invitrogen). Transfectants were selected with geneticin (G418; 0.6 mg/ml, Sigma) in RPMI1640 supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Three days after transfection, cells were harvested and stained for sTn with anti-sTn mAb, B72.3, and allophycocyanin (APC)-labeled goat anti-mouse Ig (BD Biosciences, San Diego, CA). Cells positive for both APC and GFP were collected by a FACS (FACSaria; BD Biosciences), as sTn-expressing cells (GCIY/6L) and maintained in RPMI1640 supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. As a negative control, GCIY-EGFP cells were transfected with pXSS, which expressed a truncated inactive form of ST6GalNAc I [16], and the transfectants were selected with geneticin in RPMI1640 supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin (GCIY/6S).
Mice
Seven- to eight-week-old male athymic nude mice of the KSN strain were purchased from Shizuoka Laboratory Animal Center (Hamamatsu, Japan) and kept under specific pathogen-free conditions at Aich Cancer Center Research Institute.
Peritoneal metastasis assay and anti-sTn antibody treatment
As described previously [23], exponentially growing cells were harvested with trypsin/EDTA (Invitrogen), washed and resuspended in Hanks’ balanced salt solution. Cell suspensions containing 4 × 106 GCIY/6L or GCIY/6S cells were injected into the peritoneal cavity of the recipient mice. Peritoneal metastasis was externally monitored in living mice under illumination of blue light twice a week until the end point. Some of the recipients were sacrificed at 5 weeks after injection to clearly visualize intraperitoneal distribution of cancer cells and the resected tumor masses were measured.
For antibody treatment, the recipient mice were intraperitoneally injected with 4 × 106 GCIY/6L cells. Then, one milligram of purified anti-sTn mAb B72.3 or control mouse IgG (Sigma) was intraperitoneally injected twice a week for 3 weeks (total 6 times) starting from 2 days after injection of tumor cells.
All animal experiments were conducted according to the ethical guidelines for animal experiments of the institutions which met the standard as defined by the UKCCR (UK Co-ordinating Committee on Cancer Research) guidelines and the safety guidelines for DNA manipulation experiments.
Preparation of anti-sialyl Tn mAb, B72.3
The B72.3 hybridoma cell line [25, 26] was obtained from ATCC. The hybridoma cells were adapted to serum-free medium (Hybridoma-SFM; Invitrogen) to remove proteins from FBS in the normal media. The culture supernatants were then collected and B72.3 mAb was affinity-purified with protein G or protein A Sepharose beads (GE Healthcare, Buckinghamshire, UK).
Immunoprecipitation
For sTn, purified B72.3 mAb was conjugated covalently to the Sepharose beads with CNBr-activated Sepharose 4B (GE Healthcare). For MUC1 and CD44 pull-downs, anti-MUC1 mAb VU4H5, and anti-CD44 polyclonal antibody H-300 (Santa Cruz Biotechnology, Santa Cruz, CA), were immobilized to beads in the Protein G Sepharose 4 Fast Flow kit (GE Healthcare). Cells in culture were grown to near confluency, and culture supernatants were collected and centrifuged to obtain clear supernatants. Cells on dishes or frozen tissue sections were lysed with RIPA buffer (50 mM Tris–HCl, pH7.3, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1%SDS) containing protease inhibitor cocktail (Complete Mini, EDTA-free; Roche Diagnostics GmbH, Mannheim, Germany) and centrifuged to prepare clear lysates. These cell culture supernatants and lysates were mixed with antibody-fixed Sepharose beads and rotated overnight at 4°C. After extensive washing with RIPA buffer, bound proteins were extracted from the precipitates by incubating in SDS sample buffer at 98°C for 5 min and then subjected to SDS-PAGE.
Western blotting
Protein samples were applied to SDS-PAGE and separated proteins were transferred onto polyvinylidene difluoride membranes (Immune-Blot PVDF; Bio-Rad Laboratories, Hercules, CA). After blocking with 5% skim milk in Tris-buffered saline containing 0.1% Tween20 (TBS-T), the blot was incubated with the primary antibody appropriately diluted in TBS-T and then with the HRP-conjugated secondary antibody. Detection of the proteins of interest was done by the Western Lightning Chemiluminescence Reagent Plus (PerkinElmer, Boston, MA).
Protein identification by mass spectrometry
Protein samples were applied to SDS-PAGE and the gels were silver-stained with SilverQuest Silver Staining Kit (Invitrogen). Protein bands were excised and destained, followed by reduction and alkylation. Then the proteins were in-gel digested with sequencing grade modified trypsin (Promega, Madison, WI). The tryptic peptides were extracted, absorbed to the C18 resin packed in a ZipTip (Millipore, Bedford, MA), and eluted with elution buffer (50% acetonitrile, 0.1% trifluoroacetic acid (TFA)). After mixing with equal volume of CHCA (α-cyano-4-hydroxycinnamic acid) matrix solution (10 mg/ml CHCA dissolved in 50% acetonitrile, 0.1% TFA), eluted peptides were spotted onto an Anchorchip sample target plate and analyzed by ultraflex MALDI-TOF/TOF mass spectrometer (Bruker Daltonics, Bremen, Germany). The MS-Fit database search engine of the ProteinProspector web site was used for protein identification.
Clinical samples
Gastric cancer tissues were collected from patients with advanced gastric cancer who had completed a written informed consent at Aichi Cancer Center Hospital after Institutional Review Board approval. All experiments using human tissues were conducted after the approval by the institutional ethics committees at Aichi Cancer Center and National Institute of Advanced Industrial Science and Technology (AIST).
Statistical analysis
Survival period were analyzed by the Kaplan–Meier method and compared using the log-rank test. The statistical significance of difference in metastatic tumor weight between groups was determined by applying Student’s t test. Differences in the incidence between the groups were analyzed by Fisher’s exact test. Significant differences were considered as P < 0.05.
Results
Induction of sTn-expression in a gastric cancer cell line transfected with ST6GalNAcI
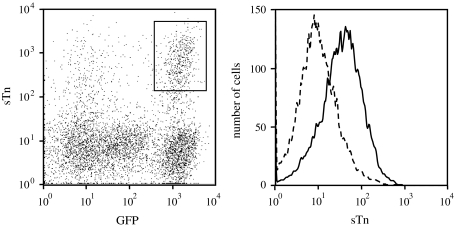
A number of human gastric cancer cell lines have been established to date. However, gastric cancer cell lines with endogenous expression of sTn have not yet been established, although sTn expression is often observed in gastric cancer tissues. Thus, we introduced the ST6GalNAcI expression vector pXLS [16] into a GFP-tagged gastric cancer cell line, GCIY-EGFP [23], to drive sTn synthesis in the cells. Flow cytometric analysis showed that a subpopulation positive for both GFP and sTn emerged (Fig. 1, left, enclosed by a rectangle). These cells were resorted by FACS and expanded as sTn-expressing GCIY-EGFP cells (GCIY/6L), which express sTn on the cell surface at a moderate level as a whole (Fig. 1, right). As a negative control, GCIY-EGFP cells were transfected with pXSS, which encoded a truncated inactive form of ST6GalNAcI [16], and the transfectants were selected with geneticin and expanded as sTn-negative GCIY/6S cells (data not shown).
Fig. 1.
Establishment of an sTn-expressing human gastric cancer cell line. An GFP-tagged human gastric cancer cell line, GCIY-EGFP, was transfected with the ST6GalNAcI-expression vector, pXLS, and analyzed by a flow cytometer for sTn and GFP dual expression. A cell population positive for both sTn and GFP is enclosed by a rectangle and termed as GCIY/6L (left). GCIY/6L cells were sorted by a cell sorter and re-analyzed by a flow cytometer for sTn expression (right, a solid line) together with a negative control (a dotted line)
GCIY/6L cells show high metastatic ability and poor outcome when transplanted in nude mice
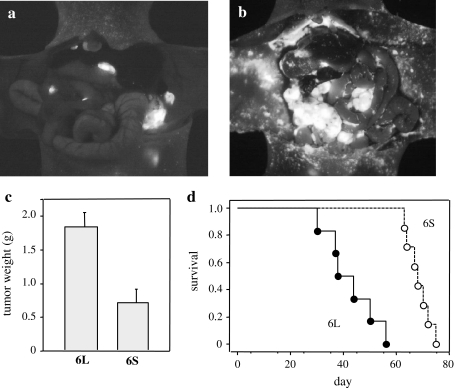
To evaluate the effects of ST6GalNAcI and sTn expression on in vivo metastatic ability, GCIY/6L and GCIY/6S cells were injected into the abdominal cavity of athymic nude mice (4 × 106 cells per mouse). At 5 weeks after injection, the mice were sacrificed to visualize the peritoneal metastasis under blue light illumination and the tumor masses were weighed. In mice injected with GCIY/6L, a larger number of metastatic foci were formed on the peritoneum in comparison to GCIY/6S (Fig. 2a, b), indicating that ST6GalNAcI expression enhances attachment of the cancer cells to the peritoneum. Total tumor weights in the peritoneal cavity were also significantly larger in mice injected with GCIY/6L (Fig. 2c), suggesting cancer cell growth promotion by ST6GalNAcI, as well as peritoneum attachment of cancer cells. In addition, survival time of mice was significantly shorter for GCIY/6L-transplanted mice than GCIY/6S-transplanted ones (Fig. 2d). These results clearly demonstrate that ST6GalNAcI expression enhances in vivo metastatic ability and mortality of the recipient mice in this assay system. To date, sTn synthesis is the only function of ST6GalNAcI as an enzyme [16], thus it is likely that this phenotypic change is due to induced expression of sTn, although unknown function of ST6GalNAcI might be involved.
Fig. 2.
Increased metastasis and mortality in GCIY/6L-transplanted mice. a, b Recipient nude mice were i.p. injected with 4 × 106 GCIY/6L or GCIY/6S cells, and were subjected to laparotomy to visualize metastatic cells under UV light at 5 weeks after transplantation. The number of metastatic foci was definitively larger in GCIY/6L-transplanted mice (b) than in GCIY/6S-transplanted mice (a). c At this time point, peritoneal tumor masses were resected and weighed. GCIY/6L-derived tumor masses were significantly larger in weight than those of GCIY/6S. P < 0.005; n = 7 (GCIY/6L) and n = 5 (GCIY/6S). Bars represent standard errors (SE). d Overall survival of the recipient mice as depicted by the Kaplan-Meier method. Survival time was significantly shorter for GCIY/6L-transplanted mice (closed circles and a solid line) than for GCIY/6S-transplanted mice (open circles and a dotted lines). P < 0.0005; n = 6 (GCIY/6L) and n = 7 (GCIY/6S)
Effects of B72.3 administration on metastasis, tumor growth, and survival time of the recipient mice
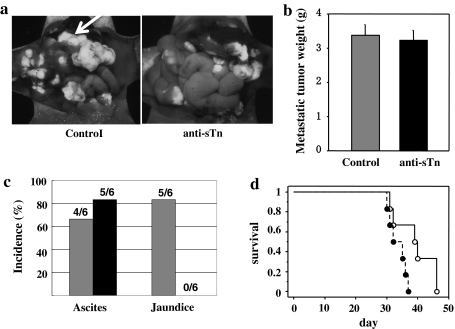
To confirm the involvement of sTn in the peritoneal metastasis in this model, we tried to suppress the peritoneal metastasis of GCIY/6L and to prolong survival time of the mice by administration of anti-sTn mAb, B72.3. One milligram of purified B72.3, or purified mouse IgG was injected into the peritoneal cavity of the recipient mice twice a week for 3 weeks after injection of GCIY/6L cells (4 × 106 per mouse). As shown in Fig. 3a, the number and the sizes of peritoneal metastatic foci tended to be smaller in B72.3-treated mice than in control mice. In accordance with this observation, incidence of jaundice due to the obstruction of biliary tract, which is associated with the progression of tumor invasion, was significantly lower in B72.3-treated mice than controls, while incidence of ascites accumulation was not different between the two groups (Fig. 3c). However, we did not detect significant difference in metastatic tumor weights between B72.3-treated and control groups in this experimental condition (Fig. 3b). Nevertheless, survival time of the mice was significantly longer for B72.3-treated mice than controls (Fig. 3d). These results suggest possible involvement of sTn in the augmented metastatic property of gastric cancer cells in this mouse model.
Fig. 3.
Effects of B72.3 administration on metastatic ability and survival of the recipient mice. a GFP-fluorescence view of peritoneal metastasis. Intraperitoneal dissemination such as peri-bile duct invasion (arrow), a cause of jaundice was reduced by B72.3 treatment. b Metastatic tumor weights excised from control mouse IgG-treated and B72.3-treated mice. c The incidence of jaundice was significantly lower in B72.3-treated mice than in IgG-treated control. *P < 0.05. d Overall survival of the GCIY/6L-transplanted recipient mice as depicted by the Kaplan-Meier method. The recipients administered with B72.3 (open circles) survived significantly longer than those with control mouse IgG (closed circles). P < 0.05; n = 6
MUC1 and CD44 are major carriers of sTn on GCIY/6L
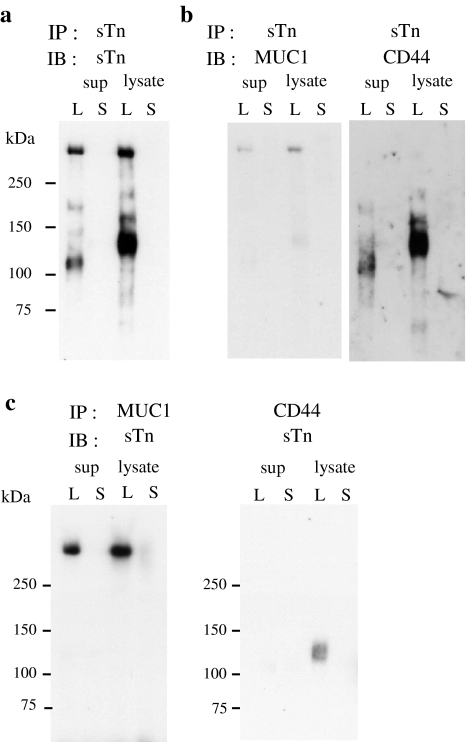
To gain insight into the molecular mechanisms of enhancement of peritoneal metastasis by sTn, we determined the carrier protein(s) of sTn. sTn carrier proteins were immunoprecipitated with B72.3 from the culture supernatants and the cell lysates of GCIY/6L and GCIY/6S cells, and subjected to western blot analysis with the same mAb. From the GCIY/6L supernatant, 2 major bands of ~110 and >250 kDa were detected along with some minor bands (Fig. 4a). Also from the GCIY/6L cell lysate, 2 major bands of ~120 and >250 kDa, as well as a few minor bands, were found (Fig. 4a). For the ~120 kDa band found in the GCIY/6L lysate, a specific mass spectrum was obtained from the silver-stained gel slice and found to be derived from CD44 (data not shown). Western blot analysis using CD44-specific polyclonal antibody confirmed this result (Fig. 4b, right). In addition, a faint CD44 signal of ~110 kDa was also detected in the GCIY/6L supernatant by long exposure (data not shown), which is thought to be a truncated form of CD44. These results indicate that CD44 is one of the major sTn carriers in GCIY/6L cells. On the contrary, no significant mass spectrum was obtained from the bands of >250 kDa in both the supernatant and the lysate (data not shown). This may be due to resistance of these molecules to trypsin digestion conferred by dense glycosylation. Their large size, putative heavy glycosylation, and preferential recognition of consecutive sTn cluster by B72.3 [27], suggest that these molecules may be mucins. To confirm this idea, the blot used above was examined for mucin expression by re-probing with a series of anti-mucin antibodies. We found that only anti-MUC1 mAb reacted to the same-sized bands as the sTn signals at >250 kDa (Fig. 4b, left), but the antibodies to other mucins (MUC2, 3, 4. 5AC, 5B and 16) did not (data not shown), indicating that MUC1 is the other major sTn carrier in GCIY/6L cells. By the reciprocal immunoprecipitation-western blot analyses further confirmed that CD44 and MUC1 carry sTn (Fig. 4c). In summary, these results clearly demonstrate that MUC1 and CD44 are the major carriers of sTn in GCIY/6L cells.
Fig. 4.
Identification of sTn carrier proteins. a Cell lysates and culture supernatants of GCIY/6L (L) and GCIY/6S (S) were immunoprecipitated with B72.3 and analyzed by western blot with the same mAb. b The blot used in (a) was stripped and re-probed with anti-MUC1 mAb (left) or anti-CD44 polyclonal antibody (right). c Cell lysates and culture supernatants of GCIY/6L (L) and GCIY/6S (S) were immunoprecipitated with anti-MUC1 mAb (left) or anti-CD44 polyclonal antibody, and analyzed by western blot using B72.3
MUC1 carries sTn in human gastric cancer tissues
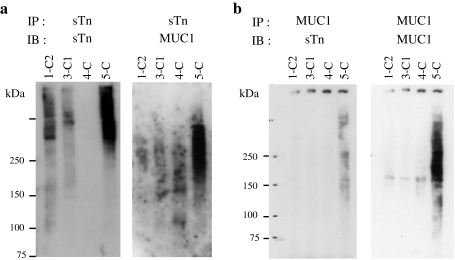
In GCIY/6L cells, sTn expression was obtained by ectopic expression of ST6GalNAcI. Like GCIY, currently available gastric cancer cell lines do not express endogenous sTn, although sTn expression is often detected in gastric cancer tissues, indicating that in vitro culture and/or cloning processes affect the expression status of ST6GalNAcI and sTn. Thus, sTn carrier proteins in human gastric cancer tissues may differ from those identified in GCIY/6L. To determine whether MUC1 and CD44 carry sTn also in human, frozen gastric cancer tissues from 4 cases were sliced and lysed in RIPA buffer to obtain tissue lysates. After centrifugation, clear supernatants were subjected to immunoprecipitation-western blot analysis using B72.3. Broad sTn bands at high molecular weights were detected in 3 of 4 cases (Fig. 5a, left). When the blot was re-probed with anti-MUC1 mAb, the MUC1-specific band was detected only in one case (5-C), but not in the others (Fig. 5a, right). In case of 5-C, the MUC1-specific diffuse band overlapped with but was clearly distinct from the sTn diffuse bands (Fig. 5a). This difference may result from the recognition preference of the mAb for un- or under-glycosylated MUC1 tandem repeats. When the same tissue lysates were immunoprecipitated with anti-MUC1 mAb, sTn-specific signals were detected at approximately the same size range as in the MUC1 immunoprecipitation (Fig. 5b). In contrast, CD44 was not detected in any of these cases (data not shown). These results indicate that MUC1 is an sTn carrier in some advanced human gastric cancer tissues.
Fig. 5.
Immunoprecipitation-western blot analysis of human gastric cancer tissue lysates for sTn-carrying MUC1. a Lysates from 4 advanced gastric cancer tissues were immunoprecipitated with B72.3 and subjected to western blot analysis with the same mAb (left), then re-probed with anti-MUC1 mAb (right). b The same lysates were immunoprecipitated with anti-MUC1 mAb and subjected to western blot analysis using B72.3 (left), then re-probed with anti-MUC1 mAb (right)
Discussion
In this study, we established ST6GalNAcI transfectant of gastric cancer cells with surface expression of sTn and provided the first demonstration of ST6GalNAcI and sTn involvement in intraperitoneal metastasis in a mouse model. Our results suggest that glycoform alteration of carrier proteins to sTn may be involved in the enhanced peritoneal metastasis observed in our animal model. The mechanisms of this enhancement in metastasis are not entirely clear, but may include accelerated cell proliferation, enhanced migratory activity, altered adhesiveness to target matrices or cells, and/or decreased apoptotic activity. These possibilities are supported by the reports showing that expression of sTn induced phenotypic change of the cells in vitro and in vivo [18–22]. In fact, we observed a larger number of metastatic foci in GCIY/6L cell-transplanted mice, indicating enhancement of cancer cell attachment to the peritoneum.
In addition, we found that a large proportion of sTn is carried by MUC1 and CD44 in GCIY/6L cells, suggesting that glycoform alteration of these molecules or unidentified carrier proteins to sTn may be involved in the enhanced peritoneal metastasis observed in our animal model.
MUC1 is a membrane-bound mucin and enhanced expression is detected in many types of epithelial and non-epithelial tumors [28]. It has been reported that MUC1 expression level or content is positively correlated with the extent of cancer progression or disease stage [29, 30]. In addition, overexpression of MUC1 confers tumorigenic potential on the cells [31–33]. Although the molecular basis of MUC1 tumorigenicity is not clearly known, phenotypic changes of MUC1-overexpressing cells are thought to be partly due to steric hindrance to the interaction between cell adhesion molecules by its protruding structure above the cell surface and by its dense negative charges from sialic acids on the termini of a large number of O-glycans [34, 35]. In addition, glycosylation patterns of MUC1 are also changed during tumor formation. In breast cancer cell lines, MUC1 O-glycans are mostly core 1-based or Tn antigen, in contrast to normal mammary glands which express core 2-based glycans on MUC1 [36]. Modification of MUC1 with sTn was reported in gastric and breast cancer cell lines transfected with ST6GalNAcI [21, 22], and in pancreatic and colon cancer cell lines in which exogenous FLAG-tagged MUC1 was introduced [37]. In the former two cases [21, 22], alteration of cellular characteristics was observed, although molecules other than MUC1 were also modified with sTn. In our report, MUC1 modification with sTn was concomitant with enhancement of peritoneal metastatic activity, suggesting that sTn modification of MUC1 was involved in this process. It is not known how sTn modification of MUC1 causes such a phenotypic change, however, two possible mechanisms may be involved. First, glycoform change of MUC1 may alter conformation of the peptide backbone as previously reported [38, 39]. Second, structural change in glycan may cause changes in interaction with other molecules such as lectins, i.e. loss of interaction with one lectin and gain of interaction with another, although sTn-recognizing endogenous lectins have not yet been identified to date. Although further studies are required to clarify these questions, sTn-MUC1 may be a target molecule for gastric cancer cell detection.
CD44 is a type I transmembrane glycoprotein involved in cell–cell and cell–matrix interactions and cancer metastasis through interaction with extracellular matrix molecules [40]. Involvement of CD44 in metastasis was first reported by Gunthert & colleagues [41]. In the report, a variant of CD44 was expressed almost exclusively in metastatic tissues and cancer cell lines, and the expression of this variant converted a non-metastatic cell line to metastatic. It was also reported that the variant-specific anti-CD44 antibody treatment blocked metastasis [42]. Although the mechanisms by which CD44 variants affect metastasis are not yet fully understood, interacting molecules such as ERM proteins (ezrin, radixin, moesin), which regulate cell motility and shape [43] and bind to CD44 cytoplasmic tail in active states [44, 45], are thought to be involved. Besides alternative splicing, aberrant glycosylation of CD44 also affects cellular phenotypes such as tumorigenicity [46, 47]. It was reported that CD44 carries sTn in a human breast cancer cell line transfected with ST6GalNAcI, although the roles of sTn-carrying CD44 in enhanced subcutaneous tumor growth have not yet been elucidated [22]. As also shown by our results, enhancement of intraperitoneal metastatic activity was parallel with sTn-modification of CD44. These results suggest possible roles of sTn-modified CD44 in tumorigenicity and intraperitoneal metastasis in vivo, as well as those of MUC1. However, sTn-modified CD44 was not detected in gastric cancer tissues in the clinical samples used here. This may be because the glycosylation pathway of CD44 may differ from that of MUC1 in tumor cells in gastric cancer tissues or due to the small sample size in our study, thus, extensive studies with much more gastric cancer cases may be necessary to detect sTn-modified CD44 in human tissues.
In this study, we observed prognostic improvement of recipient mice by repeated injection of anti-sTn mAb (Fig. 3), suggesting anti-tumor and anti-metastatic effects of this mAb. Reduced incidence of jaundice by the antibody-treatment may support this idea. However, no significant difference in total tumor weights was observed between anti-sTn mAb-treated and control IgG-treated mice at necropsy in this experimental condition. The results also suggest that administration dose of the mAb and dosing protocol should be improved. Or the role of sTn in the metastatic process may still remain partial, which leads to the observed limited effects of mAb administration. For example unknown functions of ST6GalNAcI may be involved through mechanisms other than sTn synthesis.
In summary, this experimental model will provide a valuable system to study roles of ST6GalNAcI and sTn in gastric cancer cell properties including metastasis.
Acknowledgments
This study was supported in part by Medical Glyco Project of New Energy and Industrial Technology Development Organization (NEDO) of Japan.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- EDTA
Ethylenediaminetetraacetic acid
- GFP
Green fluorescent protein
- FACS
Fluorescence-activated cell sorter
- FBS
Fetal bovine serum
- GFP
Green fluorescent protein
- mAb
Monoclonal antibody
- RT-PCR
Reverse transcription-polymerase chain reaction
- sTn
Sialyl Tn
References
- 1.Werther JL, Rivera-MacMurray S, Bruckner H, et al. Mucin-associated sialosyl-Tn antigen expression in gastric cancer correlates with an adverse outcome. Br J Cancer. 1994;69(3):613–616. doi: 10.1038/bjc.1994.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werther JL, Tatematsu M, Klein R, et al. Sialosyl-Tn antigen as a marker of gastric cancer progression: an international study. Int J Cancer. 1996;69(3):193–199. doi: 10.1002/(SICI)1097-0215(19960621)69:3<193::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Ma XC, Terata N, Kodama M, et al. Expression of sialyl-Tn antigen is correlated with survival time of patients with gastric carcinomas. Eur J Cancer. 1993;29A(13):1820–1823. doi: 10.1016/0959-8049(93)90529-O. [DOI] [PubMed] [Google Scholar]
- 4.Itzkowitz SH, Bloom EJ, Kokal WA, et al. Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer. 1990;66(9):1960–1966. doi: 10.1002/1097-0142(19901101)66:9<1960::AID-CNCR2820660919>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 5.Guadagni F, Roselli M, Cosimelli M, et al. Correlation between tumor-associated glycoprotein 72 mucin levels in tumor and serum of colorectal patients as measured by the quantitative CA 72–4 immunoassay. Cancer Res. 1996;56(22):5293–5298. [PubMed] [Google Scholar]
- 6.Ghazizadeh M, Ogawa H, Sasaki Y, et al. Mucin carbohydrate antigens (T, Tn, and sialyl-Tn) in human ovarian carcinomas: relationships with histopathology and prognosis. Hum Pathol. 1997;28(8):960–966. doi: 10.1016/S0046-8177(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H, Terao T, Kawashima Y. Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J Clin Oncol. 1992;10(1):95–101. doi: 10.1200/JCO.1992.10.1.95. [DOI] [PubMed] [Google Scholar]
- 8.Cho SH, Sahin A, Hortobagyi GN, et al. Sialyl-Tn antigen expression occurs early during human mammary carcinogenesis and is associated with high nuclear grade and aneuploidy. Cancer Res. 1994;54(24):6302–6305. [PubMed] [Google Scholar]
- 9.Schuessler MH, Pintado S, Welt S, et al. Blood group and blood-group-related antigens in normal pancreas and pancreas cancer: enhanced expression of precursor type 1, Tn and sialyl-Tn in pancreas cancer. Int J Cancer. 1991;47(2):180–187. doi: 10.1002/ijc.2910470204. [DOI] [PubMed] [Google Scholar]
- 10.Dabelsteen E. Cell surface carbohydrates as prognostic markers in human carcinomas. J Pathol. 1996;179(4):358–369. doi: 10.1002/(SICI)1096-9896(199608)179:4<358::AID-PATH564>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Kakeji Y, Maehara Y, Morita M, et al. Correlation between sialyl Tn antigen and lymphatic metastasis in patients with Borrmann type IV gastric carcinoma. Br J Cancer. 1995;71(1):191–195. doi: 10.1038/bjc.1995.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagoe T, Sawai T, Tsuji T, et al. Predictive factors for preoperative serum levels of sialyl Lewis(x), sialyl Lewis(a) and sialyl Tn antigens in gastric cancer patients. Anticancer Res. 2002;22(1A):451–458. [PubMed] [Google Scholar]
- 13.Koga S, Kaibara N, Iitsuka Y, et al. Prognostic significance of intraperitoneal free cancer cells in gastric cancer patients. J Cancer Res Clin Oncol. 1984;108(2):236–238. doi: 10.1007/BF00402474. [DOI] [PubMed] [Google Scholar]
- 14.Kodera Y, Nakanishi H, Yamamura Y, et al. Prognostic value and clinical implications of disseminated cancer cells in the peritoneal cavity detected by reverse transcriptase-polymerase chain reaction and cytology. Int J Cancer. 1998;79(4):429–433. doi: 10.1002/(SICI)1097-0215(19980821)79:4<429::AID-IJC20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi H, Kodera Y, Yamamura Y, et al. Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric-cancer patients with real-time RT-PCR on the lightcycler. Int J Cancer. 2000;89(5):411–417. doi: 10.1002/1097-0215(20000920)89:5<411::AID-IJC3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Ikehara Y, Kojima N, Kurosawa N, et al. Cloning and expression of a human gene encoding an N-acetylgalactosamine-α2, 6-sialyltransferase (ST6GalNAc I): a candidate for synthesis of cancer-associated sialyl-Tn antigens. Glycobiology. 1999;9(11):1213–1224. doi: 10.1093/glycob/9.11.1213. [DOI] [PubMed] [Google Scholar]
- 17.Marcos NT, Pinho S, Grandela C, et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64(19):7050–7057. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 18.Julien S, Krzewinski-Recchi MA, Harduin-Lepers A, et al. Expression of sialyl-Tn antigen in breast cancer cells transfected with the human CMP-Neu5Ac: GalNAc α2, 6-sialyltransferase (ST6GalNAc I) cDNA. Glycoconj J. 2001;18(11–12):883–893. doi: 10.1023/A:1022200525695. [DOI] [PubMed] [Google Scholar]
- 19.Brockhausen I, Yang J, Lehotay M, et al. Pathways of mucin O-glycosylation in normal and malignant rat colonic epithelial cells reveal a mechanism for cancer-associated Sialyl-Tn antigen expression. Biol Chem. 2001;382(2):219–232. doi: 10.1515/BC.2001.029. [DOI] [PubMed] [Google Scholar]
- 20.Clément M, Rocher J, Loirand G, et al. Expression of sialyl-Tn epitopes on β1 integrin alters epithelial cell phenotype, proliferation and haptotaxis. J Cell Sci. 2004;117(Pt21):5059–5069. doi: 10.1242/jcs.01350. [DOI] [PubMed] [Google Scholar]
- 21.Pinho S, Marcos NT, Ferreira B, et al. Biological significance of cancer-associated sialy-Tn antigen: modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007;249(2):157–170. doi: 10.1016/j.canlet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Julien S, Adriaenssens E, Ottenberg K, et al. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumorigenicity. Glycobiology. 2006;16(1):54–64. doi: 10.1093/glycob/cwj033. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi H, Mochizuki Y, Kodera Y, et al. Chemosensitivity of peritoneal micrometastases as evaluated using a green fluorescence protein (GFP)-tagged human gastric cancer cell line. Cancer Sci. 2003;94(1):112–118. doi: 10.1111/j.1349-7006.2003.tb01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoyama H, Nakanishi H, Kodera Y, et al. Biological significance of isolated tumor cells and micrometastasis in lymph nodes evaluated using a green fluorescent protein-tagged human gastric cancer cell line. Clin Cancer Res. 2006;12(2):361–368. doi: 10.1158/1078-0432.CCR-05-1963. [DOI] [PubMed] [Google Scholar]
- 25.Colcher D, Hand PH, Nuti M, et al. A spectrum of monoclonal antibodies reactive with human mammry tumor cells. Proc Natl Acad Sci USA. 1981;78(5):3199–3203. doi: 10.1073/pnas.78.5.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kjeldsen T, Clausen H, Hirohashi S, et al. Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2–6 α-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Res. 1988;48(8):2214–2220. [PubMed] [Google Scholar]
- 27.Zhang S, Walberg LA, Ogata S, et al. Immune sera and monoclonal antibodies define two configurations for the sialyl Tn tumor antigen. Cancer Res. 1995;55(15):3364–3368. [PubMed] [Google Scholar]
- 28.Denda-Nagai K, Irimura T. MUC1 in carcinoma-host interactions. Glycoconj J. 2000;17(7–9):649–658. doi: 10.1023/A:1011039013134. [DOI] [PubMed] [Google Scholar]
- 29.Nakamori S, Ota DM, Cleary KR, et al. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology. 1994;106(2):353–361. doi: 10.1016/0016-5085(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 30.Walsh MD, Hohn BG, Thong W, et al. Mucin expression by transitional cell carcinomas of the bladder. Br J Urol. 1994;73(3):256–262. doi: 10.1111/j.1464-410X.1994.tb07514.x. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder JA, Masri AA, Adriance MC, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene. 2004;23(34):5739–5747. doi: 10.1038/sj.onc.1207713. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Liu D, Chen D, et al. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22(38):6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satoh S, Hinoda Y, Hayashi T, et al. Enhancement of metastatic properties of pancreatic cancer cells by MUC1 gene encoding an anti-adhesion molecule. Int J Cancer. 2000;88(4):507–518. doi: 10.1002/1097-0215(20001115)88:4<507::AID-IJC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell–cell adhesion by the membrane–associated mucin episialin/MUC1. Mol Biol Cell. 1996;7(4):565–577. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ligtenberg MJ, Buijs F, Vos HL, et al. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992;52(8):2318–2324. [PubMed] [Google Scholar]
- 36.Lloyd KO, Burchell J, Kudryashov V, et al. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J Biol Chem. 1996;271(52):33325–33334. doi: 10.1074/jbc.271.52.33317. [DOI] [PubMed] [Google Scholar]
- 37.Burdick MD, Harris A, Reid CJ, et al. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J Biol Chem. 1997;272(39):24198–24202. doi: 10.1074/jbc.272.39.24198. [DOI] [PubMed] [Google Scholar]
- 38.Kinarsky L, Suryanarayanan G, Prakash O, et al. Conformational studies on the MUC1 tandem repeat glycopeptides: implication for the enzymatic O-glycosylation of the mucin protein core. Glycobiology. 2003;13(12):929–939. doi: 10.1093/glycob/cwg109. [DOI] [PubMed] [Google Scholar]
- 39.Dziadek S, Griesinger C, Kunz H, et al. Synthesis and structural model of an α(2, 6)-sialyl-T glycosylated MUC1 eicosapeptide under physiological conditions. Chem Eur J. 2006;12(19):4981–4993. doi: 10.1002/chem.200600144. [DOI] [PubMed] [Google Scholar]
- 40.Marhaba R, Zöller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35(3):211–231. doi: 10.1023/B:HIJO.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 41.Günthert U, Hofmann M, Rudy W, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65(1):13–24. doi: 10.1016/0092-8674(91)90403-L. [DOI] [PubMed] [Google Scholar]
- 42.Seiter S, Arch R, Reber S, et al. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993;177(2):443–455. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Natl Rev Mol Cell Biol. 2002;3(8):586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 44.Tsukita S, Oishi K, Sato N, et al. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126(2):391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yonemura S, Hirao M, Doi Y, et al. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol. 1998;140(4):885–895. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Labarrière N, Piau JP, Otry C, et al. H blood group antigen carried by CD44 V modulates tumorigenicity of rat colon carcinoma cells. Cancer Res. 1994;54(23):6275–6281. [PubMed] [Google Scholar]
- 47.Goupille C, Hallouin F, Meflah K, et al. Increase of rat colon carcinoma cells tumorigenicity by α(1–2) fucosyltransferase gene transfection. Glycobiology. 1997;7(2):221–229. doi: 10.1093/glycob/7.2.221. [DOI] [PubMed] [Google Scholar]