Abstract
Hereditary forms of copper toxicosis exist in man and dogs. In man, Wilson’s disease is the best studied disorder of copper overload, resulting from mutations in the gene coding for the copper transporter ATP7B. Forms of copper toxicosis for which no causal gene is known yet are recognized as well, often in young children. Although advances have been made in unraveling the genetic background of disorders of copper metabolism in man, many questions regarding disease mechanisms and copper homeostasis remain unanswered. Genetic studies in the Bedlington terrier, a dog breed affected with copper toxicosis, identified COMMD1, a gene that was previously unknown to be involved in copper metabolism. Besides the Bedlington terrier, a number of other dog breeds suffer from hereditary copper toxicosis and show similar phenotypes to humans with copper storage disorders. Unlike the heterogeneity of most human populations, the genetic structure within a purebred dog population is homogeneous, which is advantageous for unraveling the molecular genetics of complex diseases. This article reviews the work that has been done on the Bedlington terrier, summarizes what was learned from studies into COMMD1 function, describes hereditary copper toxicosis phenotypes in other dog breeds, and discusses the opportunities for genome-wide association studies on copper toxicosis in the dog to contribute to the understanding of mammalian copper metabolism and copper metabolism disorders in man.
Introduction
The trace element copper plays an essential role in a variety of biological processes, including mitochondrial respiration, antioxidant defense, neurotransmitter synthesis, connective tissue formation, pigmentation, and iron metabolism. However, it is extremely toxic when present in excessive amounts. Therefore, copper concentrations in the body are tightly regulated (de Romana et al. 2011). The importance of proper functioning of its homeostatic regulation is illustrated by the genetic disorders Menkes disease (OMIM #309400) and Wilson’s disease (OMIM #277900), that result from mutations in genes coding for the homologous copper-transporting P-type ATPases ATP7A and ATP7B, respectively.
Dietary copper uptake takes place in the small intestine (Mason 1979), where CTR1 (Zhou and Gitschier 1997) and possibly CTR2 (van den Berghe et al. 2007) and DMT1 (Gunshin et al. 1997) can facilitate copper uptake into enterocytes. Copper is transported from the enterocytes into the portal circulation by ATP7A that is located at the basal membrane of the enterocyte under high copper conditions (Pase et al. 2004). In the blood, copper is bound to small molecules such as histidine and to serum proteins like α2-macroglobulin and albumin (Moriya et al. 2008) for transport to the liver, the primary site of copper storage (Liu et al. 2007; McArdle et al. 1990; Weiss and Linder 1985).
Copper enters the hepatocytes via CTR1 (Kim et al. 2009) and is sequestered by small molecules like metallothionein (Coyle et al. 2002) and glutathione (Freedman et al. 1989) in the cytosol. Specialized copper chaperones shuttle copper to their destination molecules. CCS shuttles copper to SOD1, which participates in oxidative stress defense (Culotta et al. 1997). COX17 is the copper chaperone for the cytochrome C oxidase, which resides in the mitochondrial inner membrane and plays a critical role in the electron transport chain for cellular respiration (Amaravadi et al. 1997).
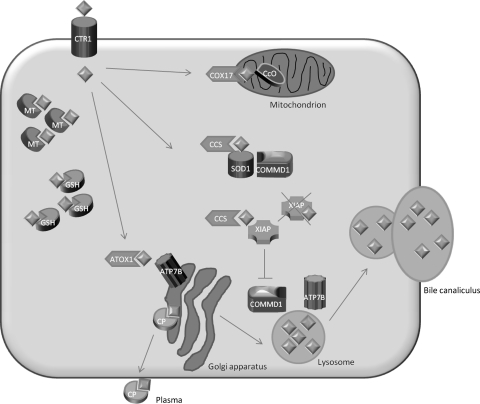
The copper chaperone ATOX1 (Klomp et al. 1997) delivers copper to ATP7B that is located in the trans-Golgi compartment (Hamza et al. 1999; Larin et al. 1999; van Dongen et al. 2004). Here, copper is necessary for the formation of holo-ceruloplasmin, which is subsequently secreted into the blood (Yanagimoto et al. 2011). In addition, ATP7B facilitates the excretion of excess copper into the bile (Cater et al. 2006) (Fig. 1).
Fig. 1.
Model of hepatocyte copper metabolism. Copper (diamonds) enters the cell via copper transporter 1 (CTR1) and is sequestered in the cytoplasm by the small molecules metallothionein (MT) and glutathione (GSH). Shuttling of copper to the destination molecules takes place via copper chaperones. COX17 shuttles copper to the cytochrome C oxidase (CcO) in the mitochondria. CCS is the chaperone for superoxide dismutase (SOD1). Recently, COMMD1 was shown to interact with SOD1 and this interaction requires CCS-mediated copper incorporation in SOD1. ATOX1 transports copper to ATP7B in the trans-Golgi network, where incorporation of copper in apo-ceruloplasmin (CP) takes place. Holo-ceruloplasmin is subsequently excreted in the plasma. The precise mechanism for export of excess copper in the bile is not completely resolved, but it is hypothesized that ATP7B and COMMD1 mediate fusion of copper-loaded vesicular compartments to the apical membrane. Furthermore, COMMD1 may play a role in the maintenance of ATP7B. XIAP can inhibit COMMD1 by promoting its degradation, resulting in cellular copper accumulation. XIAP itself can receive copper from CCS, and copper binding of XIAP results in its degradation and decrease in caspase inhibition, which may result in enhanced apoptosis
Mutations in ATP7B can result in Wilson’s disease (WD), an autosomal recessive disorder (Bull et al. 1993; Tanzi et al. 1993) characterized by copper accumulation in the liver, brain, and cornea (Gitlin 2003). Clinical signs manifest in the form of hepatic, neurologic, or psychiatric impairment and often become evident in people in the second or third decades of life (Merle et al. 2007) (Table 1). The disease incidence is estimated to be 1 in 30,000 (Schilsky 1996). A wide variety of mutations in ATP7B have been described (http://www.wilsondisease.med.ualberta.ca/database.asp) and most patients are compound heterozygotes (Kenney and Cox 2007). There is a lack of correlation between the genotype and the phenotype, and individuals carrying the same mutation can show distinct clinical signs, which poses major difficulties for diagnosing the disease (Riordan and Williams 2001; Senzolo et al. 2007). Other genes or environmental influences are thought to modify clinical expression of the disease but have not yet been identified (de Bie et al. 2007a).
Table 1.
Comparison of phenotypes between different forms of copper toxicosis in man and dogs
| WD | ICC | ETIC | ICT | Bedlington | Labrador | Dobermann | WHW terrier | Dalmatian | |
|---|---|---|---|---|---|---|---|---|---|
| Gene | ATP7B | UK | UK | UK | COMMD1 | UK | UK | UK | UK |
| Mode of inheritance | Autosomal recessive | UK | UK | UK | Autosomal recessive | Complex | Complex | UK | UK |
| Sex predisposition | No | Male | No | No | No | Female | Female | No | No |
| Age of onset | Adolescence | Early childhood | Early childhood | Early childhood | Adolescence-middle age | Adolescence-middle age | Adolescence-middle age | Middle age-older dogs | Adolescence-middle age |
| Liver pathology | Cirrhosis | Cirrhosis | Cirrhosis | Cirrhosis | Cirrhosis | Cirrhosis | Cirrhosis | Cirrhosis | Cirrhosis |
| Reported liver copper increase compared to reference value | 10× | 100× | UK | 50× | 50× | 10× | 10× | 20× | 20× |
| Neurological impairment | Yes | No | No | No | No | No | No | No | No |
| Ceruloplasmin | Decreased | Normal or raised | UK | Normal or raised | Normal or raised | UK | UK | UK | UK |
| Dietary influence | Minor | Major | Major | Suspected | Minor | Major | UK | UK | UK |
UK unknown
Normal values in liver copper for humans are <50 μg/g dwl and for dogs <400 μg/g dwl
Non-Wilsonian forms of hepatic copper toxicosis in man that often occur early in childhood include Indian childhood cirrhosis (ICC) (Tanner 1998), endemic Tyrolean infantile cirrhosis (ETIC) (Muller et al. 1996), and idiopathic copper toxicosis (ICT) (Scheinberg and Sternlieb 1996) (Table 1). Although an increased incidence can occur in certain populations, overall these diseases are rare. Genetic defects in these forms of copper toxicosis have not been identified yet, but consanguinity and high dietary copper intake are reported to be involved in the disease pathogenesis, pointing toward a genetic cause modified by environmental factors.
Besides in man, copper storage disorders have also been identified in other mammals, including dogs. Hereditary canine copper toxicosis is identified with a high incidence in a number of purebred dog populations, including the Bedlington terrier (Hardy et al. 1975), Skye terrier (Haywood et al. 1988), West Highland White terrier (Thornburg et al. 1986), Dalmatian (Webb et al. 2002), Dobermann (Mandigers et al. 2004), and Labrador retriever (Hoffmann et al. 2006). Although the disease is characterized by copper accumulation in the liver leading to inflammation and eventually liver cirrhosis in all breeds, phenotypic differences in the magnitude of copper accumulation, sex predisposition, and severity of the disease exist between breeds (Table 1). One of the best studied copper storage disorders in dogs is Bedlington terrier copper toxicosis (BTCT). The identification of the causal mutation in COMMD1 in this breed (van De Sluis et al. 2002) was a breakthrough in the understanding of mammalian copper homeostasis and the use of purebred dogs to identify disease-causing genes. No mutations are currently known in the other affected breeds, suggesting that there are more, currently unidentified, genes involved in canine copper homeostasis.
Recently, the purebred dog has emerged as a powerful model to study genetic diseases because of the unique population structure (Tsai et al. 2007). With the availability of the complete DNA sequence of the canine genome and new techniques for high-throughput genotyping and DNA sequencing, opportunities are now open to perform genome-wide association studies in dogs. The high incidence of copper storage disorders in certain dog breeds, the resemblance with the human phenotypes, the apparent complex etiology, and the possibility to study dietary copper intake make copper toxicosis a promising phenotype for genetic studies in the dog.
Here we review the unraveling of the genetics of Bedlington terrier copper toxicosis and how it contributed to the gain in knowledge of the functional aspects of COMMD1. Furthermore, we describe copper toxicosis phenotypes in several dog breeds and discuss the opportunities and possible pitfalls of genome-wide association studies in canine copper storage disorders for the detection of new genes underlying copper homeostasis disorders.
Discovery of the copper toxicosis gene in the Bedlington terrier
The appearance of a progressive form of chronic hepatitis accompanied by high liver copper values in the Bedlington terrier was first described in the United States (Hardy et al. 1975). Subsequently, Bedlington terrier copper toxicosis (BTCT) was recognized in Australia (Studdert 1982) and Europe (Eriksson 1983; Kelly et al. 1984; Meulenaar et al. 1983; Sewelius 1986; Wilsdorf et al. 1985). The prevalence was very high, ranging from 25 to 46% in different Bedlington terrier populations (Herrtage et al. 1987; Ubbink et al. 2000).
Familial predisposition and an increased incidence of a disease in a closed population, such as the Bedlington terrier breed, indicate a hereditary etiology. Test matings confirmed this assumption and showed an autosomal recessive inheritance pattern (Johnson et al. 1980; Owen and Ludwig 1982). Selective breeding by excluding affected dogs that were diagnosed based on a liver biopsy led to a decrease in incidence of BTCT. In the well-documented Dutch Bedlington terrier population, the incidence dropped dramatically from 46 to 11% (Ubbink et al. 2000). However, because carrier dogs could not be identified by a liver biopsy, there was a need for a DNA test for eradication of the disease.
A search for the causal gene was initiated by several research groups, resulting in the exclusion of genes coding for the copper transporter ATP7B (Dagenais et al. 1999; van de Sluis et al. 1999), metal transporter ATP6H (Nanji et al. 2001), copper transporters CTR1 and CTR2 (van de Sluis et al. 1999) and the copper chaperone ATOX1 (Dagenais et al. 1999; Nanji and Cox 1999) as candidates for BTCT based on mapping criteria or resequencing efforts. The application of the first whole-genome linkage study with microsatellite markers in dogs led to the detection of linkage between the microsatellite marker C04107 and BTCT (Yuzbasiyan-Gurkan et al. 1997). Two alleles were present in the Bedlington terrier population, and allele 2 cosegregated with BTCT. The frequency of the disease-associated allele was very high in the European, American, and Australian populations and varied from 0.31 to 0.5 (Holmes et al. 1998; Lee et al. 2007; Rothuizen et al. 1999; Yuzbasiyan-Gurkan et al. 1997). Whereas implementation of the microsatellite marker test in the breeding programs was an important step forward in decreasing the disease incidence within the Bedlington terrier populations, the search for the causal gene continued. A positional cloning strategy identified a large genomic deletion of 39.7 kb encompassing exon 2 of the originally named MURR1 gene (Forman et al. 2005; van De Sluis et al. 2002). This MURR1 gene was previously not known to be involved in copper metabolism, and copper-binding motifs in the predicted protein product were not recognized in this stage. Upon discovery of nine other proteins related to the MURR1 protein, the gene was renamed into Copper Metabolism gene MURR1 containing Domain 1 (COMMD1) (Burstein et al. 2005). All ten proteins are characteristic for the COMM domain, which seems to be necessary for the interaction among the COMMD proteins as well as for the interaction with other proteins (Burstein et al. 2005; Chang et al. 2011; de Bie et al. 2007b; Drevillon et al. 2011; Maine et al. 2007; Narindrasorasak et al. 2007; Thoms et al. 2010; van de Sluis et al. 2007a, 2009).
Neither full-length nor truncated COMMD1 protein was detectable in liver homogenates of affected Bedlington terriers, suggesting that COMMD1 exon 2 deletion results in a complete loss of function of COMMD1 (Klomp et al. 2003). In retrospect, the C04107 microsatellite marker was positioned within the COMMD1 gene in intron 1, 13.5 kb upstream of the exon 2 deletion (Forman et al. 2005).
Although in the majority of cases C04107 allele 2 was linked to copper toxicosis, linkage of allele 1 to the disease phenotype was reported as well (Haywood et al. 2001; Holmes et al. 1998; Yuzbasiyan-Gurkan et al. 1997). In an American Bedlington terrier pedigree, it was confirmed that C04107 allele 1 was linked to the exon 2 deletion, implying that direct analysis for the exon 2 deletion would be the only reliable genetic test for copper toxicosis in the Bedlington (Favier et al. 2005; Lee et al. 2007; van de Sluis et al. 2003). The presence of the new haplotype in the American Bedlington terriers raised the question whether this haplotype had a different genetic origin, or occurred due to a recombination event between the microsatellite marker and the exon 2 deletion (van de Sluis et al. 2003).
Remarkably, affected Bedlington terriers that were heterozygous for the exon 2 deletion or had two copies of the normal exon 2 were identified in Finnish and Australian populations. In these populations, a transition near the 5′ splice site mutation of COMMD1 exon 2 was found. However, no effect of this C-to-A transition on splicing was noted by analysis of cDNA, and no association between this mutation and BTCT could be established (Coronado et al. 2003; Hyun et al. 2004). In these dogs, another mutation may be responsible for the observed disease phenotype.
In the search for modifier genes of BTCT, the gene coding for the copper transporter ATP7B was investigated by DNA sequencing in a pedigree that did not show complete cosegregation between the COMMD1 deletion and BTCT. Eleven polymorphisms were identified, two of which affected the encoded protein. One missense mutation in exon 21 resulted in an amino acid change from arginine to glutamine at a highly conserved position. However, all investigated Bedlington terriers were homozygous for this mutation and therefore no correlation with BTCT in this pedigree could be established (Coronado et al. 2008).
In conclusion, careful evaluation of the copper toxicosis phenotype in the Bedlington terrier and genetic mapping studies led to the discovery of the COMMD1 gene, which was previously unknown to be involved in copper metabolism. This illustrates the relevance of studying spontaneous disease phenotypes to unravel important gene functions. The homozygous state for the exon 2 deletion in COMMD1 causes copper toxicosis in Bedlington terriers. Identification of the causal mutation led to an enormous decrease in the disease frequency in Bedlington terrier populations and was very beneficial for the breed. However, the presence of one or two normal copies of COMMD1 exon 2 does not exclude copper toxicosis in subpopulations of Bedlington terriers. In these dogs copper toxicosis may be explained by unidentified mutations in regulatory elements of COMMD1 or in an unidentified gene.
Molecular function of COMMD1
COMMD1 specifically binds Cu(II), for which the binding site is located in the exon 2 product that is deleted in affected Bedlington terriers (Narindrasorasak et al. 2007). Direct biochemical evidence for involvement of COMMD1 in cellular copper metabolism was provided by the observation of copper accumulation after RNAi knockdown of COMMD1 in canine, human, and murine cell lines (Burstein et al. 2004; Miyayama et al. 2010; Spee et al. 2007).
The deficient copper excretion into the bile in the Bedlington terrier (Su et al. 1982a) suggests a function for COMMD1 in copper excretion. Affected Bedlington terriers show massive copper accumulation in the hepatocytic lysosomes, which, in combination with the observation that COMMD1 localizes to a vesicular compartment, led to the hypothesis that COMMD1 facilitates degranulation of the lysosomal content into the bile (de Bie et al. 2005; Klomp et al. 2003) (Fig. 1).
Interestingly, COMMD1 was found to interact with the amino terminus of ATP7B (Lutsenko and Petris 2003; Voskoboinik et al. 2002), suggesting that COMMD1 may cooperate with ATP7B by facilitating copper transport from the trans-Golgi network (TGN) to the canalicular membrane of the hepatocytes for biliary excretion. As ceruloplasmin levels in affected Bedlington terriers are normal (Su et al. 1982b), copper transport to the Golgi compartment seems to be unaltered. Copper-induced translocation of ATP7B from the TGN to dispersed vesicles was not impaired by depletion of COMMD1, which indicates indeed that COMMD1 plays a role later in the process of copper excretion (Weiss et al. 2008). Facilitation of recruitment of ATP7B from the vesicles back to the TGN in low-copper conditions may be another role of COMMD1 in the regulation of efficient copper efflux by ATP7B (Miyayama et al. 2010).
In addition, COMMD1 was found to stabilize the ATP7B protein and may be involved in its quality control by promoting degradation of newly synthesized and incorrectly folded ATP7B proteins (de Bie et al. 2007b). Intriguingly, this interaction increased when ATP7B was mutated, indicating that COMMD1 may contribute to the molecular basis of WD.
Apart from its role in ATP7B functioning, COMMD1 has several other roles in copper homeostasis. It also binds ATP7A, the homolog of ATP7B which is defective in copper deficiency disorders in man (Kaler 2011). Interestingly, binding of COMMD1 to mutant ATP7A partially restored protein expression, subcellular localization, and copper-exporting activities (Vonk et al. 2011).
Recently, it was discovered that COMMD1 can bind to SOD1 and plays a role in the maturation and activation of this protein (Fig. 1). RNAi-mediated knockdown of COMMD1 expression resulted in a significant induction of SOD1 activity and a consequent decrease in superoxide anion concentrations, whereas overexpression of COMMD1 exerts the opposite effect (Vonk et al. 2010).
As is shown above, COMMD1 has many functions in the regulation of copper metabolism, but the regulation of COMMD1 itself is not yet completely understood.
Intracellular trafficking of many copper-binding proteins is regulated by intracellular copper levels (van den Berghe and Klomp 2010); however, this does not seem to be the case for COMMD1, as subcellular localization of COMMD1 is not influenced by intracellular copper levels (Klomp et al. 2003).
Besides copper-dependent transcriptional regulation (Muller et al. 2007), different forms of ubiquitination were found to be important for COMMD1 regulation. XIAP (Burstein et al. 2004; Maine et al. 2009), HSCARG (Lian and Zheng 2009), Clusterin (Zoubeidi et al. 2010), and ARF (Huang et al. 2008) were identified as COMMD1 interacting proteins regulating several components of this process.
A physiological role for the interaction between COMMD1 and XIAP was supported by the fact that increased levels of XIAP expression induced copper accumulation in several cell models, and XIAP deficiency in mice led to decreased hepatic copper concentration (Burstein et al. 2004). Interestingly, copper itself specifically binds to the cysteine residues of the XIAP protein and is delivered to XIAP via CCS (Brady et al. 2010) (Fig. 1). The binding of copper results in a conformational change of XIAP that induces an increased intracellular degradation and impairs the ability to inhibit caspases, thus lowering the apoptotic threshold (Mufti et al. 2006). This phenomenon sheds new light on the pathogenesis of copper-associated hepatitis, which starts with copper accumulation followed by hepatocellular apoptosis. Oxidative stress induced by free copper may not be the only trigger, as has been the general belief; this may have implications for therapeutic interventions.
Ubiquitous expression of COMMD1 in a number of different cell types indicates a more pleiotropic function of COMMD1 than copper metabolism alone (Klomp et al. 2003). Indeed, COMMD1 was found to be involved in many different cellular processes, including sodium metabolism (Biasio et al. 2004; Chang et al. 2011; Ke et al. 2010), regulation of NFκB (Burstein et al. 2005; Maine and Burstein 2007), and HIF1a-mediated transcription (van de Sluis et al. 2007b, 2009, 2010).
Upon identification of COMMD1 in the Bedlington terrier, many new functions of COMMD1 were discovered and more knowledge has been gained about the function and regulation of this interesting protein. Although the entire function of COMMD1 in copper homeostasis is not completely resolved yet, recent data indicate that it at least plays a role in the functioning and stability of ATP7B. This may indicate that human Wilson’s disease and canine COMMD1-deficient copper toxicosis partly share their disease mechanism through disturbance of ATP7B-mediated copper export from hepatocytes.
COMMD1 in human copper toxicosis
The non-Wilsonian forms of copper toxicosis—ICC, ETIC, and ICT—resemble the hepatic form of Wilson’s disease, but in contrast there is no neurological involvement and the age of onset is often early in childhood (Table 1). In these diseases, consanguinity and high dietary copper intake are suggested to play a role in the pathogenesis (Muller et al. 1996; Scheinberg and Sternlieb 1996; Tanner 1998). Since a direct role of ATP7B mutations had been excluded and the phenotype of humans with ICC, ETIC, or ICT resembles that of BTCT, COMMD1 was tested as a candidate gene. In two small studies of 23 and 3 cases, respectively, no correlation between mutations and phenotype could be established (Coronado et al. 2005; Muller et al. 2003).
In Wilson’s disease there is a wide variety of mutations in ATP7B. The clinical presentation in its hepatic or neurological form is highly variable, even among patients with the same mutation, which led several research groups to propose that this variation may be subject to other modulating genes (Riordan and Williams 2001; Schaefer et al. 1999; Thomas et al. 1995). As COMMD1 is known to interact with ATP7B and both proteins work in conjunction copper excretion, COMMD1 is an interesting candidate modifier in patients with Wilson’s disease with an atypical presentation or in whom no or only one mutation was detected. Several research groups screened their WD patient cohorts for mutations in COMMD1. Heterozygosity of a silent missense mutation c.492 GAT > GAC (Asp164Asp) in COMMD1 was reported to be possibly associated with an earlier onset of neurological manifestation of Wilson’s disease (Stuehler et al. 2004); however, whereas this mutation was observed in other cohorts as well, an association with the phenotype could not be confirmed (Gupta et al. 2010; Lovicu et al. 2006; Weiss et al. 2006). Several other mutations in COMMD1 were detected, but none of them was significantly correlated with variations of the disease phenotype (Coronado et al. 2005; Hayashi et al. 2007; Lovicu et al. 2006; Weiss et al. 2006; Wu et al. 2006).
Recently, a new mutation in COMMD1 was described in a patient with Wilson’s disease. This nonsynonymous change, c.521 ACG → ATG; Thr174Met, resided in the recently identified NES (Nuclear Export Signal) region. The patient carrying this mutation was a compound heterozygote for WD mutations and exhibited extremely high urinary copper levels. In this case, the COMMD1 mutation may have contributed to exaggeration of the disease phenotype (Gupta et al. 2010).
Studies aiming to find COMMD1 mutations to explain the variability in WD are, in general, difficult to perform for two reasons. First, the disease is rare and therefore recruitment of a large enough cohort is a challenge. Secondly, WD cohorts are heterogeneous with respect to mutations in the ATP7B gene and the clinical presentation. This makes it difficult to establish a relationship between variations in COMMD1 and clinical manifestation of WD.
In conclusion, although there are indications that COMMD1 may be a modifying factor in human disorders of copper metabolism, it does not seem to have a major role. Thus far, unknown genes active in copper homeostasis may be responsible for the observed disease phenotypes.
Copper storage diseases in dogs
In addition to copper toxicosis in the Bedlington terrier, hereditary copper-associated liver disease has also been described in other dog breeds such as the Dobermann (Mandigers et al. 2004), the West Highland White terrier (Thornburg et al. 1986), and the Dalmatian (Webb et al. 2002). More incidental reports of copper-storage-related hepatitis are available for the Anatolian shepherd (Bosje et al. 2003) and the Skye terrier (Haywood et al. 1988). Recently, the Labrador retriever, which forms one of the largest purebred dog populations worldwide, was documented to have an inherited form of copper-associated hepatitis (Hoffmann et al. 2006). In addition, results from a large survey of liver copper concentrations in dogs (Thornburg et al. 1990) and results from a retrospective review on dogs diagnosed with primary hepatitis (Poldervaart et al. 2009) suggest that there may be more dog breeds in which high liver copper levels and copper-associated hepatitis are present.
Histologically, copper toxicosis in different dog breeds shows many similarities. Accumulation of copper precedes inflammatory changes in the liver and always starts in the centrolobular regions of the liver lobules (zone 3). Around the central vein branches, multifocal regions with increased copper develop, first in the hepatocytes which then become apoptotic and are phagocytized, after which part of the copper is concentrated in the Kupffer cells. The disease is characterized by progressive inflammation, necrosis, and bridging fibrosis between centrolobular areas, eventually leading to irreversible liver cirrhosis (Fig. 2). Cholestasis can be present in very advanced stages of the disease but is never the main histological finding. This is also underscored by blood tests which show that in copper toxicosis the liver enzyme alanine-aminotransferase is often much more increased than alkaline phosphatase, indicating hepatocellular rather than cholestatic liver disease. Clinical signs can result from acute severe liver failure or end-stage cirrhosis and include lethargy, anorexia, vomiting, icterus, ascites, and hepatoencephalopathy. In some breeds, acute hemolytic crisis due to a massive release of copper into the circulation is recognized. As in humans, treatment with the copper chelators D-penicillamine and 2,3,2-tetramine is effective in decreasing liver copper levels in dogs (Allen et al. 1987; Hoffmann et al. 2009; Mandigers et al. 2005; Twedt et al. 1988). Administration of zinc acetate or zinc gluconate is described to have beneficial effects in decoppering and in maintenance therapy (Brewer et al. 1992; Hoffmann 2009; Hoffmann et al. 2009; Hoogenraad and Rothuizen 1986).
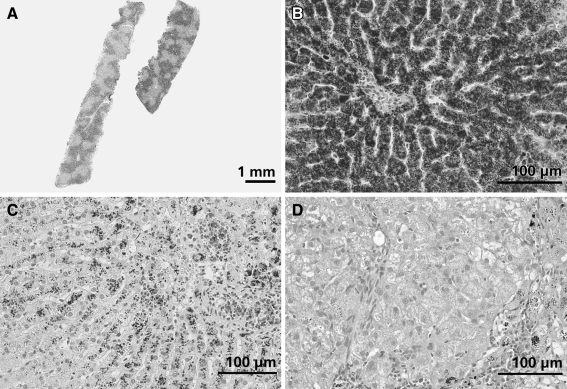
Fig. 2.
Histological appearance of copper-associated hepatitis in different dog breeds. Slides are stained with rubeanic acid and hematoxylin counterstain. a Liver biopsy of a female Bedlington terrier clearly showing centrolobular distribution of copper. b Liver biopsy of a female Bedlington terrier, 3 years of age with a liver copper value of 11,500 μg/g dwl copper. Massive amounts of copper granules are visible mainly in hepatocytes but also in Kupffer cells. The central vein is located in the middle of the picture. c Liver biopsy of a female Labrador, 5 years of age with a liver copper concentration of 2,360 μg/g dwl. Copper granules are present in hepatocytes and macrophages in the centrolobular area. The centrolobular region is characterized by loss of hepatocytes, mild fibrosis, and moderate numbers of lymphocytes and plasma cells. d Liver biopsy of a female Dobermann, 6 years of age with a liver copper value of 1,700 μg/g dwl. The centrolobular area (bottom right of the picture) is characterized by mild fibrosis with multifocal accumulation of macrophages containing lipofuscin pigment and copper granules. Furthermore, this area shows moderate infiltration with lymphocytes. Hepatocytes in the centrolobular region contain moderate amounts of copper granules
Although there are many similarities in copper toxicosis phenotypes between breeds, differences exist in clinical presentation and liver copper levels, as outlined in the following subsections and summarized in Table 1.
Bedlington terrier
Although there are some reports of atypical copper toxicosis in Bedlington terriers, a homozygous COMMD1 exon 2 deletion is causative for BTCT in the majority of dogs (van De Sluis et al. 2002). Impaired biliary copper excretion leads to a massive accumulation of copper in the liver, which is the highest that is recognized in any dog breed. Copper levels as high as 2,000 μg/g dwl are already recognized in 1 year old dogs; however, often no histological signs of hepatitis are present then (Su et al. 1982b; Twedt et al. 1979). Hepatitis develops around 2–5 years of age and the dogs become clinically ill. Successful treatment is possible with D-penicillamine. Without treatment, the hepatic copper level tends to increase over time and can reach values of 5,000 μg/g dwl. In some cases extremely high liver copper levels of 15,000 μg/g dwl have been reported. A tendency toward a decrease in liver copper levels is present in old animals or in advanced stages of liver cirrhosis (Twedt et al. 1979).
West Highland White terrier
Hepatitis associated with hepatic copper accumulation was first reported in this breed in the Unites States (Thornburg et al. 1986). Later, the same authors reported on a larger group of 71 dogs, of which many were related (Thornburg et al. 1996). The disease had a clear familial distribution, and when two affected dogs were mated, all dogs in the offspring showed increased liver copper values, indicating a hereditary background. Of the 71 cases investigated by Thornburg et al., 44 had a highly increased copper concentration with an equal distribution over both sexes. Copper levels do not reach the extremely high values seen in the Bedlington terrier (the highest value reported was 6,800 μg/g dwl); however, the majority of affected West Highland White terriers has copper concentrations around 2,000 μg/g dwl.
Dobermann
Dobermanns have been reported to have a very severe form of hepatitis and cirrhosis, which is seen almost exclusively in females and often has a fatal course within weeks or a few months after diagnosis. Reports from the U.S. (Thornburg 1998), Finland (Speeti et al. 1998), and the Netherlands (Mandigers et al. 2004, 2007; Spee et al. 2005; van den Ingh et al. 1988) describe increased copper concentrations and a predominant monocellular infiltrate in the liver of affected Dobermanns. In the Dutch population, a random sample of 15% of a cohort of 3-year-old Dobermanns was followed over time. In 6% of these dogs, copper-associated subclinical hepatitis was present and liver copper levels increased to 1,000 μg/g dwl over time. The etiologic role of copper was demonstrated by the dramatic improvement upon treatment with D-penicillamine and the associated normalization of copper concentrations (Mandigers et al. 2005). Mandigers et al. (2007) also demonstrated that the biliary excretion of intravenously injected 64Cu tends to be decreased in affected Dobermanns.
MHC class II antigen expression was detected in hepatocytes in cases of Dobermann hepatitis, but not in control tissue (Speeti et al. 2003). Therefore, Dobermann hepatitis was suggested to be an autoimmune disease. Induction of MHC class II antigen expression in nonlymphatic cells can also be induced by toxins, like copper. Homozygosity for DLA-DRB1*00601 of the dog leukocyte antigen (DLA) system genotype was found to be associated with an increased risk for Dobermann hepatitis in Finnish Dobermanns (Dyggve et al. 2011). Dobermann hepatitis behaves quite differently compared with other copper storage diseases with respect to the very severe prognosis when left untreated, the strong female predisposition, and the relatively mild increase in hepatic copper levels associated with severe disease. Female predisposition is a common feature for autoimmune diseases both in humans and in dogs. Possibly, there is a combined role for copper accumulation and autoimmune deregulation in the pathogenesis of Dobermann hepatitis.
Currently, genome-wide association studies followed by next-generation sequencing of associated regions and RNA sequencing efforts are being performed in the combined Dutch and Finnish cohorts of Dobermann hepatitis liver samples within the LUPA initiative (Lequarre et al. 2011).
Dalmatian
One report from the U.S. (Webb et al. 2002) has convincingly demonstrated that the Dalmatian has an inherited copper storage disease causing hepatitis and liver cirrhosis. Early case reports (Cooper et al. 1997; Noaker et al. 1999) already had indicated the presence of a copper storage disease in the American population. The mean hepatic copper concentrations that were reported ranged from 650 to 9,424 μg/g dwl. In the liver biopsies, necroinflammatory alterations were present in regions with copper-laden hepatocytes and Kupffer cells. Several cases in the Netherlands and Germany have been diagnosed (J. Rothuizen, personal communication), so that this disease is also present in European Dalmatians. However, currently reliable incidence estimates are lacking. There seems to be no sex predisposition in this breed.
Labrador retriever
An increased incidence of chronic hepatitis was reported in the Labrador retriever previously (Andersson and Sevelius 1991). However, Hoffmann et al. (2006) were the first to demonstrate an association of increased liver copper levels and hepatitis in this breed in Dutch Labrador retrievers. Soon thereafter, copper-associated hepatitis in the American Labrador retriever population was recognized as well (Shih et al. 2007; Smedley et al. 2009). There is a strong female predisposition and breeding bitches in the postpartum period have an increased risk for clinical illness. Hormones or an increased stress on the liver during pregnancy and lactation may influence deterioration of the liver function; however, no evidence for this hypothesis currently exists. Copper-accumulating traits in the Labrador retriever show a heritability of up to 85% (Hoffmann et al. 2008). Involvement of environmental factors in the disease pathogenesis was proven by the fact that dietary management with a low-copper diet was effective in preventing progression of the disease (Hoffmann et al. 2009). Unpublished results demonstrated that the disease is polygenic and the Labrador form of copper storage disease might become a good example of the power of canine populations to resolve complex genetic diseases (J. Rothuizen, personal communication).
Opportunities and pitfalls in genetic studies into canine copper storage disorders
Discovery of the COMMD1 gene in the Bedlington terrier was an enormous step forward in the diagnosis of affected and carrier dogs by use of a DNA test. The implementation of this test in the selection of breeding dogs led to dramatic decrease in the number of affected puppies that were born. In addition, the subsequent functional studies have shed a new light on the regulation of mammalian copper metabolism.
However, several questions remain unanswered. A minority of Bedlington terriers is affected with copper toxicosis but do not have the homozygous COMMD1 exon 2 deletion. In addition, the role of COMMD1 as a modifier gene in Wilson’s disease was not clearly established, and no causal mutations for non-Wilsonian forms of copper toxicosis have been detected thus far. These phenomena are a reflection of the complex regulation of copper metabolism and it is likely that other as yet unidentified genes may be at play.
In the preceding subsections we summarized the forms of copper accumulation that are well documented in different dog breeds. The phenotypes in the dogs have some resemblance with those of human copper storage disorders. For example, copper accumulation in the liver and response to D-penicillamine therapy are features that are both shared among all human and canine forms of copper toxicosis. The age of onset of the clinical signs in dogs is comparable with the general age of onset of Wilson’s disease, i.e., adolescence or middle age.
In non-Wilsonian forms of copper toxicosis, a strong influence of dietary copper intake on the expression of the disease phenotype is noticed; the same strong effect is seen in Labrador retrievers. On the other hand, a change to a low-copper diet did not halt disease progression in Bedlington terriers (R. Favier, personal communication).
In dogs, no neurological impairments have been noticed, although behavioral changes have been seen in Dobermanns, Labradors, and Bedlington terriers. More research is needed in order to conclude if copper accumulation in the brain may influence these behavioral changes.
In the search for new genes involved in copper metabolism, genome-wide association studies in dog breeds with a high prevalence of copper toxicosis could make a valuable contribution. Unlike the heterogeneity of most human populations, the structure of dog breed populations is homogeneous, which is advantageous for unraveling the molecular genetics of complex diseases (Karlsson and Lindblad-Toh 2008; Wilbe et al. 2010). For this reason, the dog was one of the first mammals whose genome was sequenced to a high-quality level (Lindblad-Toh et al. 2005). As a consequence of breeding practices and population bottlenecks, linkage disequilibrium (LD) in the dog genome extends over distances that are up to 100 times longer than in the human genome and the number of haplotype variants in a breed is small (Lindblad-Toh et al. 2005; Sutter et al. 2004). This means that relative to human studies, genotyping of a limited number of SNPs in small patient and control groups suffices for a genome-wide association study. For this purpose, a 170K SNP array has been developed by Illumina (San Diego, CA, USA) in collaboration with the LUPA consortium (Lequarre et al. 2011). Large LD blocks in dogs may be a drawback in pinpointing the location of the gene of interest; however, fine mapping across breeds is one way to overcome this problem (Karlsson et al. 2007).
In addition to genetic homogeneity, the copper toxicosis phenotype within breeds is also much more homogeneous compared to, for example, WD phenotypes, which make a correct diagnosis more feasible. No biomarkers for copper status exist in dogs; therefore, a liver biopsy is always needed to establish the diagnosis of copper toxicosis. This is beneficial for genetic studies because a precise copper quantification as well as a careful histological description of the liver biopsy is often available. In addition, the availability of liver tissue opens the opportunity for transcriptomics and proteomics studies in order to gain insight into disease pathogenesis and the effect of gene deregulations. Another important factor that can be controlled for in dog populations is dietary copper intake. Most privately kept dogs are fed a kibble diet in which copper concentrations are relatively stable and copper intake can be estimated more precisely than in humans.
There are some pitfalls when applying genome-wide association studies in canine copper toxicosis and they have to be taken into account in study design and data analysis. As stressed before, correct phenotyping is of utmost importance in the design of a genetic study. Phenocopies can occur as a result of liver copper accumulation due to reduced bile flow and this has to be distinguished from primary copper accumulation resulting from a genetic defect. In primary forms of copper accumulation, copper is localized around the central veins in the liver lobule, whereas copper accumulation due to cholestasis is present in hepatocytes in the periportal areas. In advanced stages of copper toxicosis, when liver cirrhosis is present, the architecture of the liver is disturbed and localization of copper within the liver lobe becomes a challenge. Also, in advanced, untreated cases, liver copper levels may actually decrease due to replacement of hepatocytes with fibrotic tissue and regenerative nodules that have not yet accumulated copper. In these cases, it may become difficult to distinguish between chronic hepatitis due to primary copper toxicosis and idiopathic chronic hepatitis. In conclusion, for correct phenotyping an experienced veterinary pathologist and a reliable method for quantitative copper determination are indispensable.
In the data analysis of a genome-wide association study, it is important to look for population substructuring and encrypted relatedness in the dog sample as this can cause false positive association signals. The use of mixed models in the data analyses, for example, as implemented in the software GenABEL (Aulchenko et al. 2007), can elegantly correct for underlying population or family structure. In addition, the use of this kind of model has the advantage that traits, e.g., liver copper level, can be analyzed quantitatively and that modifying factors such as age of onset, sex, and dietary copper intake can be implemented in the analysis.
There is a high level of conservation of copper metabolism genes over species; therefore, it is likely that genetic studies into canine copper toxicosis will contribute to an increased knowledge into mammalian copper metabolism and human copper storage diseases. It is clear that upon identification of new copper metabolism-associated genes in purebred dog populations, the translational step to human disease phenotypes needs to be made. Functional studies to test the implications of mutations are indispensable and cohorts of human patients will need to be tested for involvement of the new genes. Therefore, a good collaboration between canine and human research groups is of utmost importance.
Concluding remarks
The discovery of the COMMD1 gene through genetic studies in Bedlington terrier copper toxicosis has led to a great increase in knowledge about the regulation of mammalian copper metabolism. However, several questions with respect to the etiology of copper toxicosis in both man and dogs remain to be answered. The treasury of purebred dog populations for genetic studies is expected to reveal many new details of copper homeostasis in the coming years and will be beneficial to both man and dog.
Acknowledgments
The authors thank Dr. Bart van de Sluis for critical reading of the manuscript and providing useful comments and Dr. Guy Grinwis for assistance with Fig. 2. We thank Waltham Centre for Pet Nutrition and LUPA for financial support of the studies into copper toxicosis in dogs that were performed at Utrecht University.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Allen KG, Twedt DC, Hunsaker HA. Tetramine cupruretic agents: a comparison in dogs. Am J Vet Res. 1987;48:28–30. [PubMed] [Google Scholar]
- Amaravadi R, Glerum DM, Tzagoloff A. Isolation of a cDNA encoding the human homolog of COX17, a yeast gene essential for mitochondrial copper recruitment. Hum Genet. 1997;99:329–333. doi: 10.1007/s004390050367. [DOI] [PubMed] [Google Scholar]
- Andersson M, Sevelius E. Breed, sex and age distribution in dogs with chronic liver disease: a demographic study. J Small Anim Pract. 1991;32:1–5. doi: 10.1111/j.1748-5827.1991.tb00844.x. [DOI] [Google Scholar]
- Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- Biasio W, Chang T, McIntosh CJ, McDonald FJ. Identification of Murr1 as a regulator of the human delta epithelial sodium channel. J Biol Chem. 2004;279:5429–5434. doi: 10.1074/jbc.M311155200. [DOI] [PubMed] [Google Scholar]
- Bosje JT, van den Ingh TS, Fennema A, Rothuizen J. Copper-induced hepatitis in an Anatolian shepherd dog. Vet Rec. 2003;152:84–85. doi: 10.1136/vr.152.3.84. [DOI] [PubMed] [Google Scholar]
- Brady GF, Galban S, Liu X, Basrur V, Gitlin JD, Elenitoba-Johnson KS, Wilson TE, Duckett CS. Regulation of the copper chaperone Ccs by Xiap-mediated ubiquitination. Mol Cell Biol. 2010;30(8):1923–1936. doi: 10.1128/MCB.00900-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Dick RD, Schall W, Yuzbasiyan-Gurkan V, Mullaney TP, Pace C, Lindgren J, Thomas M, Padgett G. Use of zinc acetate to treat copper toxicosis in dogs. J Am Vet Med Assoc. 1992;201:564–568. [PubMed] [Google Scholar]
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- Burstein E, Ganesh L, Dick RD, van De Sluis B, Wilkinson JC, Klomp LW, Wijmenga C, Brewer GJ, Nabel GJ, Duckett CS. A novel role for XIAP in copper homeostasis through regulation of MURR1. EMBO J. 2004;23:244–254. doi: 10.1038/sj.emboj.7600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, Maine GN, Wilkinson JC, Mayo MW, Duckett CS. COMMD proteins, a novel family of structural and functional homologs of MURR1. J Biol Chem. 2005;280:22222–22232. doi: 10.1074/jbc.M501928200. [DOI] [PubMed] [Google Scholar]
- Cater MA, La Fontaine S, Shield K, Deal Y, Mercer JF. ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology. 2006;130:493–506. doi: 10.1053/j.gastro.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Chang T, Ke Y, Ly K, McDonald FJ. COMMD1 regulates the delta epithelial sodium channel (deltaENaC) through trafficking and ubiquitination. Biochem Biophys Res Commun. 2011;411:506–511. doi: 10.1016/j.bbrc.2011.06.149. [DOI] [PubMed] [Google Scholar]
- Cooper VL, Carlson MP, Jacobson J, Schneider NR. Hepatitis and increased copper levels in a dalmatian. J Vet Diagn Invest. 1997;9:201–203. doi: 10.1177/104063879700900217. [DOI] [PubMed] [Google Scholar]
- Coronado VA, Damaraju D, Kohijoki R, Cox DW. New haplotypes in the Bedlington terrier indicate complexity in copper toxicosis. Mamm Genome. 2003;14:483–491. doi: 10.1007/s00335-002-2255-3. [DOI] [PubMed] [Google Scholar]
- Coronado VA, Bonneville JA, Nazer H, Roberts EA, Cox DW. COMMD1 (MURR1) as a candidate in patients with copper storage disease of undefined etiology. Clin Genet. 2005;68:548–551. doi: 10.1111/j.1399-0004.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- Coronado VA, O’Neill B, Nanji M, Cox DW. Polymorphisms in canine ATP7B: candidate modifier of copper toxicosis in the Bedlington terrier. Vet J. 2008;177:293–296. doi: 10.1016/j.tvjl.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- Dagenais SL, Guevara-Fujita M, Loechel R, Burgess AC, Miller DE, Yuzbasiyan-Gurkan V, Brewer GJ, Glover TW. The canine copper toxicosis locus is not syntenic with ATP7B or ATX1 and maps to a region showing homology to human 2p21. Mamm Genome. 1999;10:753–756. doi: 10.1007/s003359901085. [DOI] [PubMed] [Google Scholar]
- de Bie P, van de Sluis B, Klomp L, Wijmenga C. The many faces of the copper metabolism protein MURR1/COMMD1. J Hered. 2005;96:803–811. doi: 10.1093/jhered/esi110. [DOI] [PubMed] [Google Scholar]
- de Bie P, Muller P, Wijmenga C, Klomp LW. Molecular pathogenesis of Wilson and Menkes disease: correlation of mutations with molecular defects and disease phenotypes. J Med Genet. 2007;44:673–688. doi: 10.1136/jmg.2007.052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bie P, van de Sluis B, Burstein E, van de Berghe PV, Muller P, Berger R, Gitlin JD, Wijmenga C, Klomp LW. Distinct Wilson’s disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B. Gastroenterology. 2007;133:1316–1326. doi: 10.1053/j.gastro.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Romana DL, Olivares M, Uauy R, Araya M. Risks and benefits of copper in light of new insights of copper homeostasis. J Trace Elem Med Biol. 2011;25:3–13. doi: 10.1016/j.jtemb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Drevillon L, Tanguy G, Hinzpeter A, Arous N, de Becdelievre A, Aissat A, Tarze A, Goossens M, Fanen P. COMMD1-mediated ubiquitination regulates CFTR trafficking. PLoS One. 2011;6:e18334. doi: 10.1371/journal.pone.0018334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyggve H, Kennedy LJ, Meri S, Spillmann T, Lohi H, Speeti M. Association of Doberman hepatitis to canine major histocompatibility complex II. Tissue Antigens. 2011;77:30–35. doi: 10.1111/j.1399-0039.2010.01575.x. [DOI] [PubMed] [Google Scholar]
- Eriksson J. Copper toxicosis in Bedlington terriers. Acta Vet Scand. 1983;24:148–152. doi: 10.1186/BF03546743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier RP, Spee B, Penning LC, Brinkhof B, Rothuizen J. Quantitative PCR method to detect a 13-kb deletion in the MURR1 gene associated with copper toxicosis and HIV-1 replication. Mamm Genome. 2005;16:460–463. doi: 10.1007/s00335-004-2457-2. [DOI] [PubMed] [Google Scholar]
- Forman OP, Boursnell MEG, Dunmore BJ, Stendall N, Van De Sluis B, Fretwell N, Jones C, Wijmenga C, Rothuizen J, Van Oost BA, Holmes NG, Binns MM, Jones P. Characterization of the COMMD1 (MURR1) mutation causing copper toxicosis in Bedlington terriers. Anim Genet. 2005;36:497–501. doi: 10.1111/j.1365-2052.2005.01360.x. [DOI] [PubMed] [Google Scholar]
- Freedman JH, Ciriolo MR, Peisach J. The role of glutathione in copper metabolism and toxicity. J Biol Chem. 1989;264:5598–5605. [PubMed] [Google Scholar]
- Gitlin JD. Wilson disease. Gastroenterology. 2003;125:1868–1877. doi: 10.1053/j.gastro.2003.05.010. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Gupta A, Chattopadhyay I, Mukherjee S, Sengupta M, Das SK, Ray K. A novel COMMD1 mutation Thr174Met associated with elevated urinary copper and signs of enhanced apoptotic cell death in a Wilson Disease patient. Behav Brain Funct. 2010;6:33. doi: 10.1186/1744-9081-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza I, Schaefer M, Klomp LW, Gitlin JD. Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis. Proc Natl Acad Sci USA. 1999;96:13363–13368. doi: 10.1073/pnas.96.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RM, Stevens JB, Stowe CM. Chronic progressive hepatitis in Bedlington Terriers associated with elevated liver copper concentrations. Minnesota Vet. 1975;15:13–24. [Google Scholar]
- Hayashi H, Wakusawa S, Yano M, Okada T. Genetic background of Japanese patients with adult-onset storage diseases in the liver. Hepatol Res. 2007;37:777–783. doi: 10.1111/j.1872-034X.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- Haywood S, Rutgers HC, Christian MK. Hepatitis and copper accumulation in Skye terriers. Vet Pathol. 1988;25:408–414. doi: 10.1177/030098588802500602. [DOI] [PubMed] [Google Scholar]
- Haywood S, Fuentealba IC, Kemp SJ, Trafford J. Copper toxicosis in the Bedlington terrier: a diagnostic dilemma. J Small Anim Pract. 2001;42:181–185. doi: 10.1111/j.1748-5827.2001.tb01799.x. [DOI] [PubMed] [Google Scholar]
- Herrtage ME, Seymour CA, White RAS, Small GM, Wight DGD. Inherited copper toxicosis in the Bedlington terrier: the prevalence in asymptomatic dogs. J Small Anim Pract. 1987;28:1141–1151. doi: 10.1111/j.1748-5827.1987.tb01338.x. [DOI] [Google Scholar]
- Hoffmann G. Copper-associated liver diseases. Vet Clin North Am Small Anim Pract. 2009;39:489–511. doi: 10.1016/j.cvsm.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Hoffmann G, Van Den Ingh TS, Bode P, Rothuizen J. Copper-associated chronic hepatitis in Labrador Retrievers. J Vet Intern Med. 2006;20:856–861. doi: 10.1111/j.1939-1676.2006.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann G, Heuven HC, Leegwater PA, Jones PG, van den Ingh TS, Bode P, Rothuizen J. Heritabilities of copper-accumulating traits in Labrador retrievers. Anim Genet. 2008;39:454. doi: 10.1111/j.1365-2052.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann G, Jones PG, Biourge V, van den Ingh TS, Mesu SJ, Bode P, Rothuizen J. Dietary management of hepatic copper accumulation in Labrador Retrievers. J Vet Intern Med. 2009;23:957–963. doi: 10.1111/j.1939-1676.2009.0352.x. [DOI] [PubMed] [Google Scholar]
- Holmes NG, Herrtage ME, Ryder EJ, Binns MM. DNA marker C04107 for copper toxicosis in a population of Bedlington terriers in the United Kingdom. Vet Rec. 1998;142:351–352. doi: 10.1136/vr.142.14.351. [DOI] [PubMed] [Google Scholar]
- Hoogenraad TU, Rothuizen J (1986) Compliance in Wilson’s disease and in copper toxicosis of Bedlington Terriers. Lancet II:170 [DOI] [PubMed]
- Huang Y, Wu M, Li HY. Tumor suppressor ARF promotes non-classic proteasome-independent polyubiquitination of COMMD1. J Biol Chem. 2008;283:11453–11460. doi: 10.1074/jbc.M708544200. [DOI] [PubMed] [Google Scholar]
- Hyun C, Lavulo LT, Filippich LJ. Evaluation of haplotypes associated with copper toxicosis in Bedlington Terriers in Australia. Am J Vet Res. 2004;65:1573–1579. doi: 10.2460/ajvr.2004.65.1573. [DOI] [PubMed] [Google Scholar]
- Johnson GF, Sternlieb I, Twedt DC, Grushoff PS, Scheinberg IH. Inheritance of copper toxicosis in Bedlington Terriers. Am J Vet Res. 1980;41:1865–1866. [PubMed] [Google Scholar]
- Kaler SG. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat Rev Neurol. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson EK, Lindblad-Toh K. Leader of the pack: gene mapping in dogs and other model organisms. Nat Rev Genet. 2008;9:713–725. doi: 10.1038/nrg2382. [DOI] [PubMed] [Google Scholar]
- Karlsson EK, Baranowska I, Wade CM, Salmon Hillbertz NH, Zody MC, Anderson N, Biagi TM, Patterson N, Pielberg GR, Kulbokas EJ, 3rd, Comstock KE, Keller ET, Mesirov JP, von Euler H, Kampe O, Hedhammar A, Lander ES, Andersson G, Andersson L, Lindblad-Toh K. Efficient mapping of mendelian traits in dogs through genome-wide association. Nat Genet. 2007;39:1321–1328. doi: 10.1038/ng.2007.10. [DOI] [PubMed] [Google Scholar]
- Ke Y, Butt AG, Swart M, Liu YF, McDonald FJ. COMMD1 downregulates the epithelial sodium channel through Nedd4–2. Am J Physiol Renal Physiol. 2010;298:F1445–F1456. doi: 10.1152/ajprenal.00257.2009. [DOI] [PubMed] [Google Scholar]
- Kelly DF, Haywood S, Bennett AM. Copper toxicosis in Bedlington Terriers in the United Kingdom. J Small Anim Pract. 1984;25:293–298. doi: 10.1111/j.1748-5827.1984.tb03392.x. [DOI] [Google Scholar]
- Kenney SM, Cox DW. Sequence variation database for the Wilson disease copper transporter, ATP7B. Hum Mutat. 2007;28:1171–1177. doi: 10.1002/humu.20586. [DOI] [PubMed] [Google Scholar]
- Kim H, Son HY, Bailey SM, Lee J. Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G356–G364. doi: 10.1152/ajpgi.90632.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp LW, Lin SJ, Yuan DS, Klausner RD, Culotta VC, Gitlin JD. Identification and functional expression of HAH1, a novel human gene involved in copper homeostasis. J Biol Chem. 1997;272:9221–9226. doi: 10.1074/jbc.272.14.9221. [DOI] [PubMed] [Google Scholar]
- Klomp AE, van de Sluis B, Klomp LW, Wijmenga C. The ubiquitously expressed MURR1 protein is absent in canine copper toxicosis. J Hepatol. 2003;39:703–709. doi: 10.1016/S0168-8278(03)00380-5. [DOI] [PubMed] [Google Scholar]
- Larin D, Mekios C, Das K, Ross B, Yang AS, Gilliam TC. Characterization of the interaction between the Wilson and Menkes disease proteins and the cytoplasmic copper chaperone, HAH1p. J Biol Chem. 1999;274:28497–28504. doi: 10.1074/jbc.274.40.28497. [DOI] [PubMed] [Google Scholar]
- Lee SA, Fillipich LJ, Hyun C. Prevalence of the exon 2 deletion of the COMMD1 gene in Australian Bedlington terriers. J Genet. 2007;86:289–291. doi: 10.1007/s12041-007-0039-2. [DOI] [PubMed] [Google Scholar]
- Lequarre AS, Andersson L, Andre C, Fredholm M, Hitte C, Leeb T, Lohi H, Lindblad-Toh K, Georges M. LUPA: a European initiative taking advantage of the canine genome architecture for unravelling complex disorders in both human and dogs. Vet J. 2011;189:155–159. doi: 10.1016/j.tvjl.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Lian M, Zheng X. HSCARG regulates NF-kappaB activation by promoting the ubiquitination of RelA or COMMD1. J Biol Chem. 2009;284:17998–18006. doi: 10.1074/jbc.M809752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas EJ 3rd, Zody MC, Mauceli E, Xie X, Breen M, Wayne RK, Ostrander EA, Ponting CP, Galibert F, Smith DR, DeJong PJ, Kirkness E, Alvarez P, Biagi T, Brockman W, Butler J, Chin CW, Cook A, Cuff J, Daly MJ, DeCaprio D, Gnerre S, Grabherr M, Kellis M, Kleber M, Bardeleben C, Goodstadt L, Heger A, Hitte C, Kim L, Koepfli KP, Parker HG, Pollinger JP, Searle SM, Sutter NB, Thomas R, Webber C, Baldwin J, Abebe A, Abouelleil A, Aftuck L, Ait-Zahra M, Aldredge T, Allen N, An P, Anderson S, Antoine C, Arachchi H, Aslam A, Ayotte L, Bachantsang P, Barry A, Bayul T, Benamara M, Berlin A, Bessette D, Blitshteyn B, Bloom T, Blye J, Boguslavskiy L, Bonnet C, Boukhgalter B, Brown A, Cahill P, Calixte N, Camarata J, Cheshatsang Y, Chu J, Citroen M, Collymore A, Cooke P, Dawoe T, Daza R, Decktor K, DeGray S, Dhargay N, Dooley K, Dooley K, Dorje P, Dorjee K, Dorris L, Duffey N, Dupes A, Egbiremolen O, Elong R, Falk J, Farina A, Faro S, Ferguson D, Ferreira P, Fisher S, FitzGerald M, Foley K, Foley C, Franke A, Friedrich D, Gage D, Garber M, Gearin G, Giannoukos G, Goode T, Goyette A, Graham J, Grandbois E, Gyaltsen K, Hafez N, Hagopian D, Hagos B, Hall J, Healy C, Hegarty R, Honan T, Horn A, Houde N, Hughes L, Hunnicutt L, Husby M, Jester B, Jones C, Kamat A, Kanga B, Kells C, Khazanovich D, Kieu AC, Kisner P, Kumar M, Lance K, Landers T, Lara M, Lee W, Leger JP, Lennon N, Leuper L, LeVine S, Liu J, Liu X, Lokyitsang Y, Lokyitsang T, Lui A, Macdonald J, Major J, Marabella R, Maru K, Matthews C, McDonough S, Mehta T, Meldrim J, Melnikov A, Meneus L, Mihalev A, Mihova T, Miller K, Mittelman R, Mlenga V, Mulrain L, Munson G, Navidi A, Naylor J, Nguyen T, Nguyen N, Nguyen C, Nguyen T, Nicol R, Norbu N, Norbu C, Novod N, Nyima T, Olandt P, O’Neill B, O’Neill K, Osman S, Oyono L, Patti C, Perrin D, Phunkhang P, Pierre F, Priest M, Rachupka A, Raghuraman S, Rameau R, Ray V, Raymond C, Rege F, Rise C, Rogers J, Rogov P, Sahalie J, Settipalli S, Sharpe T, Shea T, Sheehan M, Sherpa N, Shi J, Shih D, Sloan J, Smith C, Sparrow T, Stalker J, Stange-Thomann N, Stavropoulos S, Stone C, Stone S, Sykes S, Tchuinga P, Tenzing P, Tesfaye S, Thoulutsang D, Thoulutsang Y, Topham K, Topping I, Tsamla T, Vassiliev H, Venkataraman V, Vo A, Wangchuk T, Wangdi T, Weiand M, Wilkinson J, Wilson A, Yadav S, Yang S, Yang X, Young G, Yu Q, Zainoun J, Zembek L, Zimmer A, Lander ES (2005) Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438:803–819 [DOI] [PubMed]
- Liu N, Lo LS, Askary SH, Jones L, Kidane TZ, Nguyen TTM, Goforth J, Chu Y, Vivas E, Tsai M, Westbrook T, Linder MC. Transcuprein is a macroglobulin regulated by copper and iron availability. J Nutr Biochem. 2007;18:597–608. doi: 10.1016/j.jnutbio.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu M, Dessi V, Lepori MB, Zappu A, Zancan L, Giacchino R, Marazzi MG, Iorio R, Vegnente A, Vajro P, Maggiore G, Marcellini M, Barbera C, Kostic V, Farci AM, Solinas A, Altuntas B, Yuce A, Kocak N, Tsezou A, De Virgiliis S, Cao A, Loudianos G. The canine copper toxicosis gene MURR1 is not implicated in the pathogenesis of Wilson disease. J Gastroenterol. 2006;41:582–587. doi: 10.1007/s00535-006-1807-0. [DOI] [PubMed] [Google Scholar]
- Lutsenko S, Petris MJ. Function and regulation of the mammalian copper-transporting ATPases: insights from biochemical and cell biological approaches. J Membr Biol. 2003;191:1–12. doi: 10.1007/s00232-002-1040-6. [DOI] [PubMed] [Google Scholar]
- Maine GN, Burstein E. COMMD proteins and the control of the NF kappa B pathway. Cell Cycle. 2007;6:672–676. doi: 10.4161/cc.6.6.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine GN, Mao X, Komarck CM, Burstein E. COMMD1 promotes the ubiquitination of NF-kappaB subunits through a cullin-containing ubiquitin ligase. EMBO J. 2007;26:436–447. doi: 10.1038/sj.emboj.7601489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine GN, Mao X, Muller PA, Komarck CM, Klomp LW, Burstein E. COMMD1 expression is controlled by critical residues that determine XIAP binding. Biochem J. 2009;417:601–609. doi: 10.1042/BJ20080854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandigers PJ, van den Ingh TS, Bode P, Teske E, Rothuizen J. Association between liver copper concentration and subclinical hepatitis in Doberman Pinschers. J Vet Intern Med. 2004;18:647–650. doi: 10.1111/j.1939-1676.2004.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Mandigers PJ, van den Ingh TS, Bode P, Rothuizen J. Improvement in liver pathology after 4 months of D-penicillamine in 5 Doberman Pinschers with subclinical hepatitis. J Vet Intern Med. 2005;19:40–43. doi: 10.1111/j.1939-1676.2005.tb02656.x. [DOI] [PubMed] [Google Scholar]
- Mandigers PJ, Bode P, van Wees AM, van den Brom WE, van den Ingh TS, Rothuizen J. Hepatic 64Cu excretion in Dobermanns with subclinical hepatitis. Res Vet Sci. 2007;83:204–209. doi: 10.1016/j.rvsc.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Mason KE. A conspectus of research on copper metabolism and requirements of man. J Nutr. 1979;109:1979–2066. doi: 10.1093/jn/109.11.1979. [DOI] [PubMed] [Google Scholar]
- McArdle HJ, Gross SM, Danks DM, Wedd AG. Role of albumin’s copper binding site in copper uptake by mouse hepatocytes. Am J Physiol. 1990;258:G988–G991. doi: 10.1152/ajpgi.1990.258.6.G988. [DOI] [PubMed] [Google Scholar]
- Merle U, Schaefer M, Ferenci P, Stremmel W. Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: a cohort study. Gut. 2007;56:115–120. doi: 10.1136/gut.2005.087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenaar H, van den Ingh TS, Rothuizen J. Copper storage in the liver, an inherited problem in Bedlington Terriers. Tijdschr Diergeneeskd. 1983;108:916–919. [PubMed] [Google Scholar]
- Miyayama T, Hiraoka D, Kawaji F, Nakamura E, Suzuki N, Ogra Y. Roles of COMM-domain-containing 1 in stability and recruitment of the copper-transporting ATPase in a mouse hepatoma cell line. Biochem J. 2010;429:53–61. doi: 10.1042/BJ20100223. [DOI] [PubMed] [Google Scholar]
- Moriya M, Ho Y, Grana A, Nguyen L, Alvarez A, Jamil R, Ackland ML, Michalczyk A, Hamer P, Ramos D, Kim S, Mercer JFB, Linder MC. Copper is taken up efficiently from albumin and α2-macroglobulin by cultured human cells by more than one mechanism. Am J Physiol Cell Physiol. 2008;295:C708–C721. doi: 10.1152/ajpcell.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufti AR, Burstein E, Csomos RA, Graf PC, Wilkinson JC, Dick RD, Challa M, Son JK, Bratton SB, Su GL, Brewer GJ, Jakob U, Duckett CS. XIAP is a copper binding protein deregulated in Wilson’s disease and other copper toxicosis disorders. Mol Cell. 2006;21:775–785. doi: 10.1016/j.molcel.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Muller T, Feichtinger H, Berger H, Muller W. Endemic Tyrolean infantile cirrhosis: an ecogenetic disorder. Lancet. 1996;347:877–880. doi: 10.1016/S0140-6736(96)91351-3. [DOI] [PubMed] [Google Scholar]
- Muller T, van de Sluis B, Zhernakova A, van Binsbergen E, Janecke AR, Bavdekar A, Pandit A, Weirich-Schwaiger H, Witt H, Ellemunter H, Deutsch J, Denk H, Muller W, Sternlieb I, Tanner MS, Wijmenga C. The canine copper toxicosis gene MURR1 does not cause non-Wilsonian hepatic copper toxicosis. J Hepatol. 2003;38:164–168. doi: 10.1016/S0168-8278(02)00356-2. [DOI] [PubMed] [Google Scholar]
- Muller P, van Bakel H, van de Sluis B, Holstege F, Wijmenga C, Klomp LW. Gene expression profiling of liver cells after copper overload in vivo and in vitro reveals new copper-regulated genes. J Biol Inorg Chem. 2007;12:495–507. doi: 10.1007/s00775-006-0201-y. [DOI] [PubMed] [Google Scholar]
- Nanji MS, Cox DW. The copper chaperone Atox1 in canine copper toxicosis in Bedlington terriers. Genomics. 1999;62:108–112. doi: 10.1006/geno.1999.5983. [DOI] [PubMed] [Google Scholar]
- Nanji M, Coronado VA, Cox DW. ATP6H, a subunit of vacuolar ATPase involved in metal transport: evaluation in canine copper toxicosis. Mamm Genome. 2001;12:617–621. doi: 10.1007/s00335-001-2059-1. [DOI] [PubMed] [Google Scholar]
- Narindrasorasak S, Kulkarni P, Deschamps P, She YM, Sarkar B. Characterization and copper binding properties of human COMMD1 (MURR1) Biochemistry. 2007;46:3116–3128. doi: 10.1021/bi0620656. [DOI] [PubMed] [Google Scholar]
- Noaker LJ, Washabau RJ, Detrisac CJ, Heldmann E, Hendrick MJ (1999) Copper associated acute hepatic failure in a dog. J Am Vet Med Assoc 214:1502–1506, 1495 [PubMed]
- Owen CA, Jr, Ludwig J. Animal model of human disease. Inherited copper toxicosis in Bedlington terriers. Wilson’s disease (hepatolenticular degeneration) Am J Pathol. 1982;106:432–434. [PMC free article] [PubMed] [Google Scholar]
- Pase L, Voskoboinik I, Greenough M, Camakaris J. Copper stimulates trafficking of a distinct pool of the Menkes copper ATPase (ATP7A) to the plasma membrane and diverts it into a rapid recycling pool. Biochem J. 2004;378:1031–1037. doi: 10.1042/BJ20031181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldervaart JH, Favier RP, Penning LC, van den Ingh TS, Rothuizen J. Primary hepatitis in dogs: a retrospective review (2002–2006) J Vet Intern Med. 2009;23:72–80. doi: 10.1111/j.1939-1676.2008.0215.x. [DOI] [PubMed] [Google Scholar]
- Riordan SM, Williams R. The Wilson’s disease gene and phenotypic diversity. J Hepatol. 2001;34:165–171. doi: 10.1016/S0168-8278(00)00028-3. [DOI] [PubMed] [Google Scholar]
- Rothuizen J, Ubbink GJ, van Zon P, Teske E, van den Ingh TS, Yuzbasiyan-Gurkan V. Diagnostic value of a microsatellite DNA marker for copper toxicosis in West-European Bedlington terriers and incidence of the disease. Anim Genet. 1999;30:190–194. doi: 10.1046/j.1365-2052.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Hopkins RG, Failla ML, Gitlin JD. Hepatocyte-specific localization and copper-dependent trafficking of the Wilson’s disease protein in the liver. Am J Physiol. 1999;276:G639–G646. doi: 10.1152/ajpgi.1999.276.3.G639. [DOI] [PubMed] [Google Scholar]
- Scheinberg IH, Sternlieb I. Wilson disease and idiopathic copper toxicosis. Am J Clin Nutr. 1996;63:842S–845S. doi: 10.1093/ajcn/63.5.842. [DOI] [PubMed] [Google Scholar]
- Schilsky ML. Wilson disease: genetic basis of copper toxicity and natural history. Semin Liver Dis. 1996;16:83–95. doi: 10.1055/s-2007-1007221. [DOI] [PubMed] [Google Scholar]
- Senzolo M, Loreno M, Fagiuoli S, Zanus G, Canova D, Masier A, Russo FP, Sturniolo GC, Burra P. Different neurological outcome of liver transplantation for Wilson’s disease in two homozygotic twins. Clin Neurol Neurosurg. 2007;109:71–75. doi: 10.1016/j.clineuro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Sewelius E. Copper toxicosis in Bedlington Terriers. Svensk Veterinartidning. 1986;38:198–203. [Google Scholar]
- Shih JL, Keating JH, Freeman LM, Webster CRL. Chronic hepatitis in Labrador Retrievers: Clinical presentation and prognostic factors. J Vet Intern Med. 2007;21:33–39. doi: 10.1111/j.1939-1676.2007.tb02925.x. [DOI] [PubMed] [Google Scholar]
- Smedley R, Mullaney T, Rumbeiha W. Copper-associated hepatitis in Labrador Retrievers. Vet Pathol. 2009;46:484–490. doi: 10.1354/vp.08-VP-0197-S-FL. [DOI] [PubMed] [Google Scholar]
- Spee B, Mandigers PJ, Arends B, Bode P, van den Ingh TS, Hoffmann G, Rothuizen J, Penning LC. Differential expression of copper-associated and oxidative stress related proteins in a new variant of copper toxicosis in Doberman pinschers. Comp Hepatol. 2005;4:3. doi: 10.1186/1476-5926-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spee B, Arends B, van Wees AM, Bode P, Penning LC, Rothuizen J. Functional consequences of RNA interference targeting COMMD1 in a canine hepatic cell line in relation to copper toxicosis. Anim Genet. 2007;38:168–170. doi: 10.1111/j.1365-2052.2007.01580.x. [DOI] [PubMed] [Google Scholar]
- Speeti M, Eriksson J, Saari S, Westermarck E. Lesions of subclinical doberman hepatitis. Vet Pathol. 1998;35:361–369. doi: 10.1177/030098589803500505. [DOI] [PubMed] [Google Scholar]
- Speeti M, Stahls A, Meri S, Westermarck E. Upregulation of major histocompatibility complex class II antigens in hepatocytes in Doberman hepatitis. Vet Immunol Immunopathol. 2003;96:1–12. doi: 10.1016/S0165-2427(03)00134-X. [DOI] [PubMed] [Google Scholar]
- Studdert VP. Inherited copper toxicosis in Bedlington terriers. Aust Vet J. 1982;59:128. doi: 10.1111/j.1751-0813.1982.tb02753.x. [DOI] [PubMed] [Google Scholar]
- Stuehler B, Reichert J, Stremmel W, Schaefer M. Analysis of the human homologue of the canine copper toxicosis gene MURR1 in Wilson disease patients. J Mol Med. 2004;82:629–634. doi: 10.1007/s00109-004-0557-9. [DOI] [PubMed] [Google Scholar]
- Su LC, Owen CA, Jr, Zollman PE, Hardy RM. A defect of biliary excretion of copper in copper-laden Bedlington terriers. Am J Physiol. 1982;243:G231–G236. doi: 10.1152/ajpgi.1982.243.3.G226. [DOI] [PubMed] [Google Scholar]
- Su LC, Ravanshad S, Owen CA, Jr, McCall JT, Zollman PE, Hardy RM. A comparison of copper-loading disease in Bedlington terriers and Wilson’s disease in humans. Am J Physiol. 1982;243:G226–G230. doi: 10.1152/ajpgi.1982.243.3.G226. [DOI] [PubMed] [Google Scholar]
- Sutter NB, Eberle MA, Parker HG, Pullar BJ, Kirkness EF, Kruglyak L, Ostrander EA. Extensive and breed-specific linkage disequilibrium in Canis familiaris. Genome Res. 2004;14:2388–2396. doi: 10.1101/gr.3147604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner MS. Role of copper in Indian childhood cirrhosis. Am J Clin Nutr. 1998;67:1074S–1081S. doi: 10.1093/ajcn/67.5.1074S. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- Thomas GR, Forbes JR, Roberts EA, Walshe JM, Cox DW. The Wilson disease gene: spectrum of mutations and their consequences. Nat Genet. 1995;9:210–217. doi: 10.1038/ng0295-210. [DOI] [PubMed] [Google Scholar]
- Thoms HC, Loveridge CJ, Simpson J, Clipson A, Reinhardt K, Dunlop MG, Stark LA. Nucleolar targeting of RelA(p65) is regulated by COMMD1-dependent ubiquitination. Cancer Res. 2010;70:139–149. doi: 10.1158/0008-5472.CAN-09-1397. [DOI] [PubMed] [Google Scholar]
- Thornburg LP. Histomorphological and immunohistochemical studies of chronic active hepatitis in Doberman Pinschers. Vet Pathol. 1998;35:380–385. doi: 10.1177/030098589803500507. [DOI] [PubMed] [Google Scholar]
- Thornburg LP, Shaw D, Dolan M, Raisbeck M, Crawford S, Dennis GL, Olwin DB. Hereditary copper toxicosis in West Highland white terriers. Vet Pathol. 1986;23:148–154. doi: 10.1177/030098588602300207. [DOI] [PubMed] [Google Scholar]
- Thornburg LP, Rottinghaus G, McGowan M, Kupka K, Crawford S, Forbes S. Hepatic copper concentrations in purebred and mixed-breed dogs. Vet Pathol. 1990;27:81–88. doi: 10.1177/030098589002700202. [DOI] [PubMed] [Google Scholar]
- Thornburg LP, Rottinghaus G, Dennis G, Crawford S. The relationship between hepatic copper content and morphologic changes in the liver of West Highland White Terriers. Vet Pathol. 1996;33:656–661. doi: 10.1177/030098589603300604. [DOI] [PubMed] [Google Scholar]
- Tsai KL, Clark LA, Murphy KE. Understanding hereditary diseases using the dog and human as companion model systems. Mamm Genome. 2007;18:444–451. doi: 10.1007/s00335-007-9037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twedt DC, Sternlieb I, Gilbertson SR. Clinical, morphologic, and chemical studies on copper toxicosis of Bedlington Terriers. J Am Vet Med Assoc. 1979;175:269–275. [PubMed] [Google Scholar]
- Twedt DC, Hunsaker HA, Allen KG. Use of 2, 3, 2-tetramine as a hepatic copper chelating agent for treatment of copper hepatotoxicosis in Bedlington terriers. J Am Vet Med Assoc. 1988;192:52–56. [PubMed] [Google Scholar]
- Ubbink GJ, Van den Ingh TS, Yuzbasiyan-Gurkan V, Teske E, Van de Broek J, Rothuizen J. Population dynamics of inherited copper toxicosis in Dutch Bedlington terriers (1977–1997) J Vet Intern Med. 2000;14:172–176. doi: 10.1892/0891-6640(2000)014<0172:pdoict>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- van de Sluis BJ, Breen M, Nanji M, van Wolferen M, de Jong P, Binns MM, Pearson PL, Kuipers J, Rothuizen J, Cox DW, Wijmenga C, van Oost BA. Genetic mapping of the copper toxicosis locus in Bedlington terriers to dog chromosome 10, in a region syntenic to human chromosome region 2p13–p16. Hum Mol Genet. 1999;8:501–507. doi: 10.1093/hmg/8.3.501. [DOI] [PubMed] [Google Scholar]
- van de Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet. 2002;11:165–173. doi: 10.1093/hmg/11.2.165. [DOI] [PubMed] [Google Scholar]
- van de Sluis B, Peter AT, Wijmenga C. Indirect molecular diagnosis of copper toxicosis in Bedlington terriers is complicated by haplotype diversity. J Hered. 2003;94:256–259. doi: 10.1093/jhered/esg030. [DOI] [PubMed] [Google Scholar]
- van de Sluis B, Groot AJ, Wijmenga C, Vooijs M, Klomp LW. COMMD1: a novel protein involved in the proteolysis of proteins. Cell Cycle. 2007;6:2091–2098. doi: 10.4161/cc.6.17.4646. [DOI] [PubMed] [Google Scholar]
- van de Sluis B, Muller P, Duran K, Chen A, Groot AJ, Klomp LW, Liu PP, Wijmenga C. Increased activity of hypoxia-inducible factor 1 is associated with early embryonic lethality in Commd1 null mice. Mol Cell Biol. 2007;27:4142–4156. doi: 10.1128/MCB.01932-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sluis B, Groot AJ, Vermeulen J, van der Wall E, van Diest PJ, Wijmenga C, Klomp LW, Vooijs M. COMMD1 promotes pVHL and O2-independent proteolysis of HIF-1alpha via HSP90/70. PLoS One. 2009;4:e7332. doi: 10.1371/journal.pone.0007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sluis B, Mao X, Zhai Y, Groot AJ, Vermeulen JF, van der Wall E, van Diest PJ, Hofker MH, Wijmenga C, Klomp LW, Cho KR, Fearon ER, Vooijs M, Burstein E. COMMD1 disrupts HIF-1alpha/beta dimerization and inhibits human tumor cell invasion. J Clin Invest. 2010;120:2119–2130. doi: 10.1172/JCI40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe PV, Klomp LW. Posttranslational regulation of copper transporters. J Biol Inorg Chem. 2010;15:37–46. doi: 10.1007/s00775-009-0592-7. [DOI] [PubMed] [Google Scholar]
- van den Berghe PV, Folmer DE, Malingre HE, van Beurden E, Klomp AE, van de Sluis B, Merkx M, Berger R, Klomp LW. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J. 2007;407:49–59. doi: 10.1042/BJ20070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ingh TS, Rothuizen J, Cupery R. Chronic active hepatitis with cirrhosis in the Doberman pinscher. Vet Q. 1988;10:84–89. doi: 10.1080/01652176.1988.9694154. [DOI] [PubMed] [Google Scholar]
- van Dongen EM, Klomp LW, Merkx M. Copper-dependent protein–protein interactions studied by yeast two-hybrid analysis. Biochem Biophys Res Commun. 2004;323:789–795. doi: 10.1016/j.bbrc.2004.08.160. [DOI] [PubMed] [Google Scholar]
- Vonk WI, Wijmenga C, Berger R, van de Sluis B, Klomp LW. Cu, Zn superoxide dismutase maturation and activity are regulated by COMMD1. J Biol Chem. 2010;285:28991–29000. doi: 10.1074/jbc.M110.101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk WI, de Bie P, Wichers CG, van den Berghe PV, van der Plaats R, Berger R, Wijmenga C, Klomp LW, van de Sluis B (2011) The copper-transporting capacity of ATP7A mutants associated with Menkes disease is ameliorated by COMMD1 as a result of improved protein expression. Cell Mol Life Sci. DOI 10.1007/s00018-011-0743-1 [DOI] [PMC free article] [PubMed]
- Voskoboinik I, Camakaris J, Mercer JF. Understanding the mechanism and function of copper P-type ATPases. Adv Protein Chem. 2002;60:123–150. doi: 10.1016/S0065-3233(02)60053-1. [DOI] [PubMed] [Google Scholar]
- Webb CB, Twedt DC, Meyer DJ. Copper-associated liver disease in Dalmatians: a review of 10 dogs (1998–2001) J Vet Intern Med. 2002;16:665–668. doi: 10.1892/0891-6640(2002)016<0665:cldida>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Weiss KC, Linder MC. Copper transport in rats involving a new plasma protein. Am J Physiol. 1985;249:E77–E88. doi: 10.1152/ajpendo.1985.249.1.E77. [DOI] [PubMed] [Google Scholar]
- Weiss KH, Merle U, Schaefer M, Ferenci P, Fullekrug J, Stremmel W. Copper toxicosis gene MURR1 is not changed in Wilson disease patients with normal blood ceruloplasmin levels. World J Gastroenterol. 2006;12:2239–2242. doi: 10.3748/wjg.v12.i14.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss KH, Lozoya JC, Tuma S, Gotthardt D, Reichert J, Ehehalt R, Stremmel W, Fullekrug J. Copper-induced translocation of the Wilson disease protein ATP7B independent of Murr1/COMMD1 and Rab7. Am J Pathol. 2008;173:1783–1794. doi: 10.2353/ajpath.2008.071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbe M, Jokinen P, Truve K, Seppala EH, Karlsson EK, Biagi T, Hughes A, Bannasch D, Andersson G, Hansson-Hamlin H, Lohi H, Lindblad-Toh K. Genome-wide association mapping identifies multiple loci for a canine SLE-related disease complex. Nat Genet. 2010;42:250–254. doi: 10.1038/ng.525. [DOI] [PubMed] [Google Scholar]
- Wilsdorf G, Willer S, Burghardt T. Preliminary investigation of hereditary copper toxicosis in Bedlington Terriers. Monatsh Veterinarmed. 1985;40:377–379. [Google Scholar]
- Wu ZY, Zhao GX, Chen WJ, Wang N, Wan B, Lin MT, Murong SX, Yu L. Mutation analysis of 218 Chinese patients with Wilson disease revealed no correlation between the canine copper toxicosis gene MURR1 and Wilson disease. J Mol Med. 2006;84:438–442. doi: 10.1007/s00109-005-0036-y. [DOI] [PubMed] [Google Scholar]
- Yanagimoto C, Harada M, Kumemura H, Abe M, Koga H, Sakata M, Kawaguchi T, Terada K, Hanada S, Taniguchi E, Ninomiya H, Ueno T, Sugiyama T, Sata M. Copper incorporation into ceruloplasmin is regulated by Niemann-Pick C1 protein. Hepatol Res. 2011;41:484–491. doi: 10.1111/j.1872-034X.2011.00788.x. [DOI] [PubMed] [Google Scholar]
- Yuzbasiyan-Gurkan V, Blanton SH, Cao Y, Ferguson P, Li J, Venta PJ, Brewer GJ. Linkage of a microsatellite marker to the canine copper toxicosis locus in Bedlington terriers. Am J Vet Res. 1997;58:23–27. [PubMed] [Google Scholar]
- Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA. 1997;94:7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubeidi A, Ettinger S, Beraldi E, Hadaschik B, Zardan A, Klomp LW, Nelson CC, Rennie PS, Gleave ME. Clusterin facilitates COMMD1 and I-kappaB degradation to enhance NF-kappaB activity in prostate cancer cells. Mol Cancer Res. 2010;8:119–130. doi: 10.1158/1541-7786.MCR-09-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]