Abstract
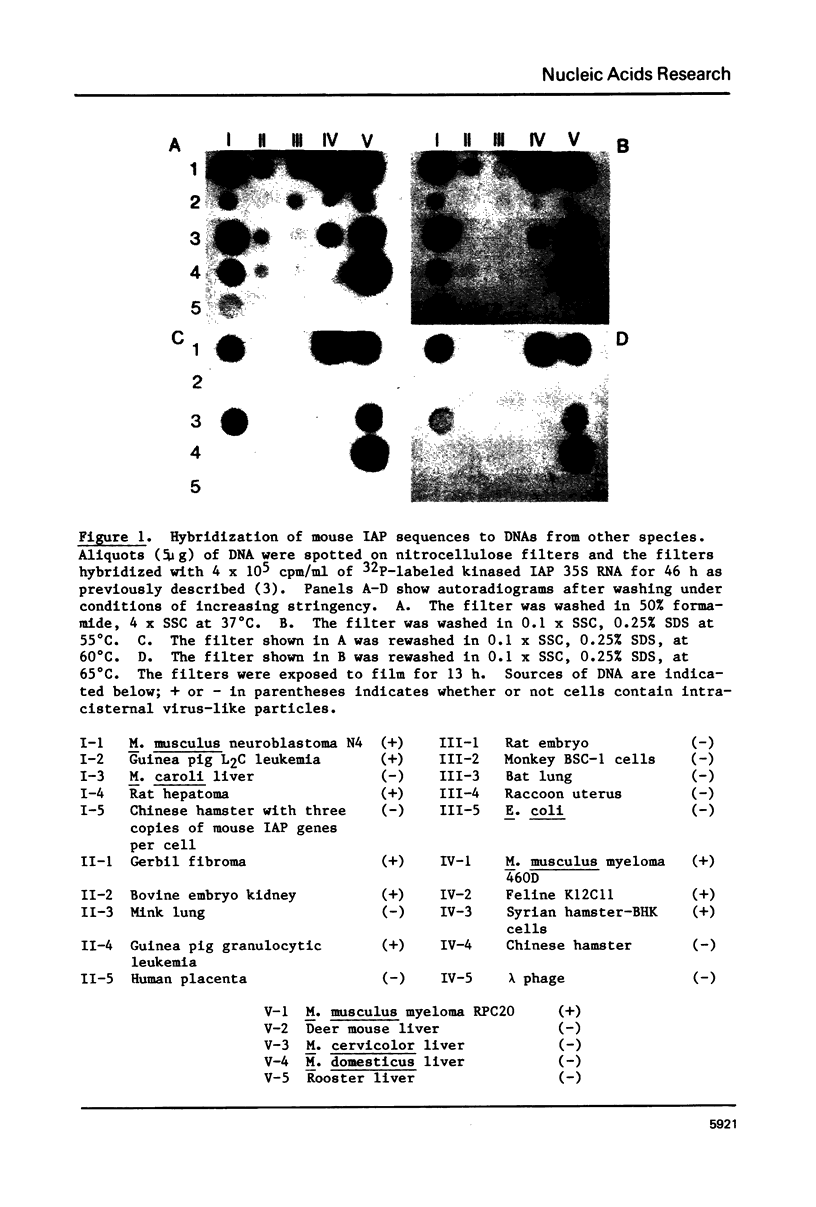
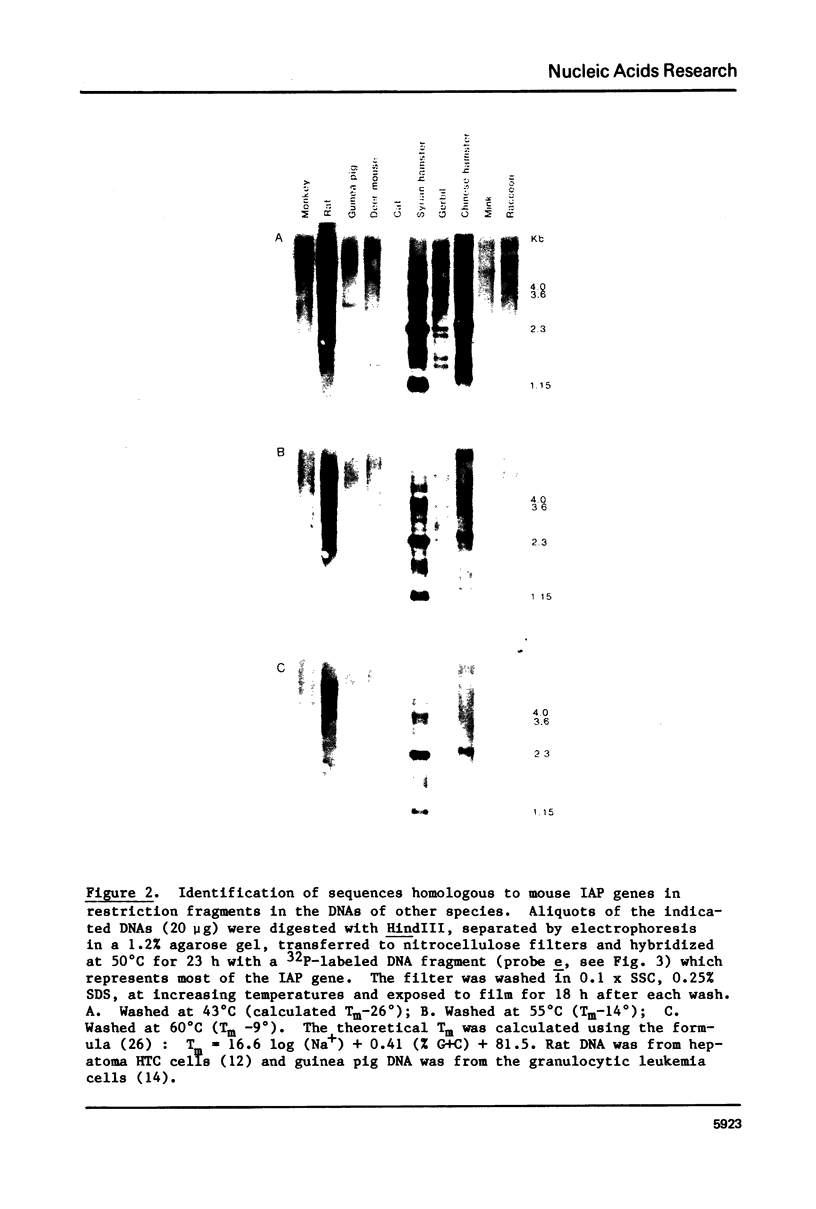
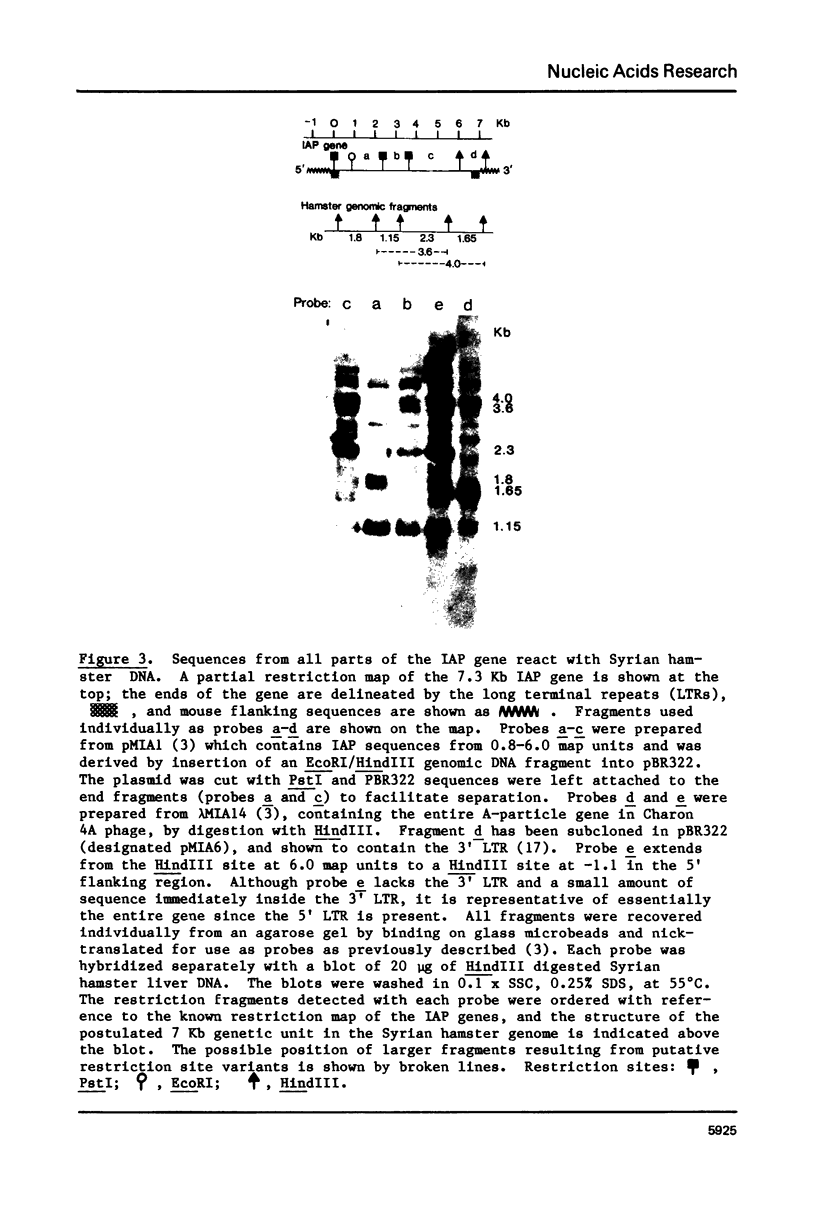
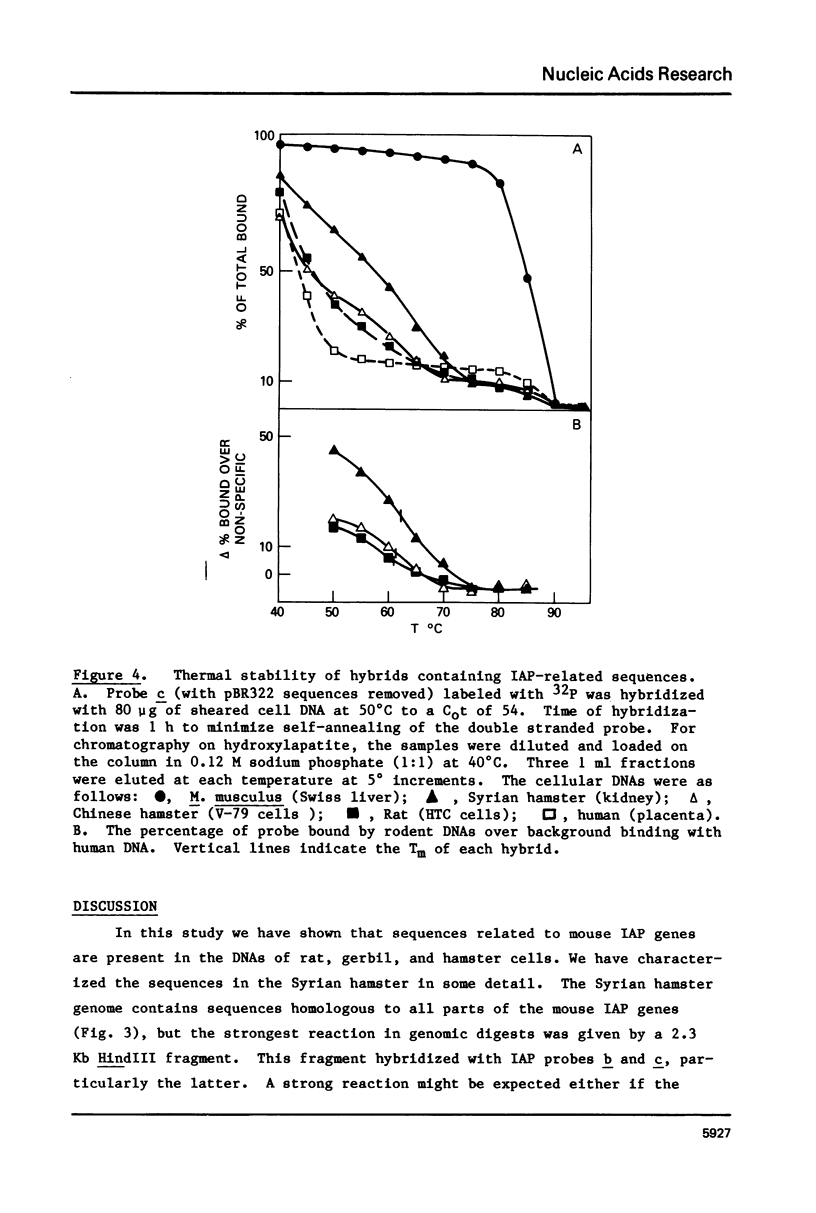
The genome of M. musculus contains many copies of DNA sequences homologous to the 35S RNA of intracisternal type-A particles (IAP) (1,2). A major class of IAP genes has been identified and isolated from a mouse library in Charon 4A (3). Cloned mouse IAP genes were used as probes to study homologous sequences in the DNA of other species. Sequences related to mouse IAP genes were detected in the DNAs from a variety of animal cells. DNAs from rat, gerbil, and hamster cells all gave strong reactions which could be localized to discrete restriction fragments on genomic blots. The reaction of Syrian hamster DNA was particularly strong. Fragments derived from different parts of the IAP gene all reacted with Syrian hamster DNA, and the reactive restriction fragments in the Syrian hamster DNA could be ordered with reference to the known restriction map of the IAP genes. The data suggest that sequences related to mouse IAP genes make up a 7 Kb unit in the Syrian hamster genome. Since the majority of the hamster sequences are quite divergent from those in the mouse, the ease with which they are detected suggests that they must be reiterated in the hamster genome.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Callahan R., Sherr C. J., Chapman V., Todaro G. J. Two distinct endogenous type C viruses isolated from the asian rodent Mus cervicolor: conservation of virogene sequences in related rodent species. J Virol. 1977 Mar;21(3):849–862. doi: 10.1128/jvi.21.3.849-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biczysko W., Pienkowski M., Solter D., Koprowski H. Virus particles in early mouse embryos. J Natl Cancer Inst. 1973 Sep;51(3):1041–1050. doi: 10.1093/jnci/51.3.1041. [DOI] [PubMed] [Google Scholar]
- Black V. H. Virus particles in primordial germ cells of fetal guinea pigs. J Natl Cancer Inst. 1974 Feb;52(2):545–551. doi: 10.1093/jnci/52.2.545. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Szollosi D. Intracisternal A particles in ova and preimplantation stages of the mouse. Nat New Biol. 1973 May 16;243(124):91–93. [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Sherr C. J., Schidlovsky G., Todaro G. J. A new class of genetically transmitted retravirus isolated from Mus cervicolor. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3579–3583. doi: 10.1073/pnas.73.10.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase D. G., Pikó L. Expression of A- and C-type particles in early mouse embryos. J Natl Cancer Inst. 1973 Dec;51(6):1971–1975. doi: 10.1093/jnci/51.6.1971. [DOI] [PubMed] [Google Scholar]
- Cole M. D., Ono M., Huang R. C. Terminally redundant sequences in cellular intracisternal A-particle genes. J Virol. 1981 May;38(2):680–687. doi: 10.1128/jvi.38.2.680-687.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Mage M. G., Hsu C. K., Himmelhoch S. R., Smith G. H. Transplantable granulocytic leukemia in strain 13 guinea pigs. Cancer Res. 1978 Jan;38(1):130–136. [PubMed] [Google Scholar]
- Feldman D. G., Gross L. Electron microscopic study of the guinea pig leukemia virus. Cancer Res. 1970 Nov;30(11):2702–2711. [PubMed] [Google Scholar]
- Heine U. I., Kramarsky B., Wendel E., Suskind R. G. Enhanced proliferation of endogenous virus in Chinese hamster cells associated with microtubules and the mitotic apparatus of the host cell. J Gen Virol. 1979 Jul;44(1):45–55. doi: 10.1099/0022-1317-44-1-45. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Leuders K. K., Ozer H. L., Wivel N. A. Some structural and antigenic properties of intracisternal A particles occurring in mouse tumors (complement fixation-immunodiffusion-neuroblastoma-plasma-cell tumor). Proc Natl Acad Sci U S A. 1972 Jan;69(1):218–222. doi: 10.1073/pnas.69.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K., Scolnick E. M. Nucleotide sequence relationship between intracisternal type A particles of Mus musculus and an endogenous retrovirus (M432) of Mus cervicolor. J Virol. 1978 Oct;28(1):66–74. doi: 10.1128/jvi.28.1.66-74.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Smith L. A., Lueders K. K. Intracisternal A-particle genes in Mus musculus: a conserved family of retrovirus-like elements. Mol Cell Biol. 1981 Mar;1(3):216–227. doi: 10.1128/mcb.1.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Genetic individuality of intracisternal A-particles of Mus musculus. J Virol. 1979 Apr;30(1):225–231. doi: 10.1128/jvi.30.1.225-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Intracisternal A-particle genes: identification in the genome of Mus musculus and comparison of multiple isolates from a mouse gene library. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3571–3575. doi: 10.1073/pnas.77.6.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Mussgay M., Reczko E., Ahl R. Demonstration of virus-like particles in a bovine cell line. J Gen Virol. 1969 Apr;4(3):445–447. doi: 10.1099/0022-1317-4-3-445. [DOI] [PubMed] [Google Scholar]
- Nadel E., Banfield W., Burstein S., Tousimis A. J. Virus particles associated with strain 2 guinea pig leukemia (L2C/N-B). J Natl Cancer Inst. 1967 Jun;38(6):979–981. [PubMed] [Google Scholar]
- Ono M., Cole M. D., White A. T., Huang R. C. Sequence organization of cloned intracisternal A particle genes. Cell. 1980 Sep;21(2):465–473. doi: 10.1016/0092-8674(80)90483-3. [DOI] [PubMed] [Google Scholar]
- Opler S. R. Observations on a new virus associated with guinea pig leukemia: preliminary note. J Natl Cancer Inst. 1967 May;38(5):797–800. [PubMed] [Google Scholar]
- Paterson B. M., Segal S., Lueders K. K., Kuff E. L. RNA associated with murine intracisternal type A particles codes for the main particle protein. J Virol. 1978 Jul;27(1):118–126. doi: 10.1128/jvi.27.1.118-126.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Straus N. A. Relatedness of mouse satellite deoxyribonucleic acid to deoxyribonucleic acid of various Mus species. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3546–3550. doi: 10.1073/pnas.70.12.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Shipman C., Jr, Vander Weide G. C., Ma B. I. Prevalence of type R virus-like particles in clones of BHK-21 cells. Virology. 1969 Aug;38(4):707–710. doi: 10.1016/0042-6822(69)90192-5. [DOI] [PubMed] [Google Scholar]
- Sobis H., Vandeputte M. Viruslike particles in hamster embryos, fetuses, and tumors. J Natl Cancer Inst. 1978 Sep;61(3):891–895. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Cholon J. J. Intracisternal type A particles and properties of a continuous cell line originating from a gerbil fibroma. Proc Soc Exp Biol Med. 1971 Apr;136(4):1107–1110. doi: 10.3181/00379727-136-35439. [DOI] [PubMed] [Google Scholar]
- Weber H. W., Geddes A., Stellwagen R. H. Induction of intracisternal type A particles by 5-bromo-2'-deoxyuridine in rat hepatoma cells. J Natl Cancer Inst. 1978 Apr;60(4):919–923. doi: 10.1093/jnci/60.4.919. [DOI] [PubMed] [Google Scholar]