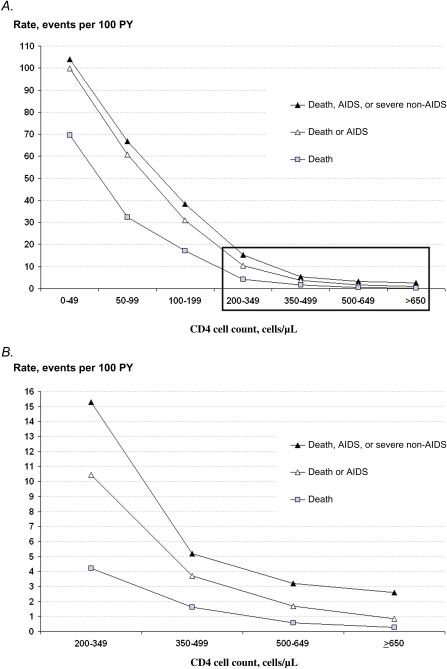
We followed antiretroviral-untreated HIV-infected adults in West Africa. In the ≥650, 500–649, 350–499, and 200–349 cells/μL CD4 cell count strata, the rates of AIDS or death were 0.9, 1.7, 3.7, and 10.4 per 100 person-years, respectively. Tuberculosis and visceral bacterial diseases were the most frequent events.
Abstract
Background. In Western Europe, North America, and Australia, large cohort collaborations have been able to estimate the short-term CD4 cell count–specific risk of AIDS or death in untreated human immunodeficiency virus (HIV)–infected adults with high CD4 cell counts. In sub–Saharan Africa, these CD4 cell count–specific estimates are scarce.
Methods. From 1996 through 2006, we followed up 2 research cohorts of HIV-infected adults in Côte d’Ivoire. This included follow-up off antiretroviral therapy (ART) across the entire spectrum of CD4 cell counts before the ART era, and only in patients with CD4 cell counts >200 cells/μL once ART became available. Data were censored at ART initiation. We modeled the CD4 cell count decrease using an adjusted linear mixed model. CD4 cell count–specific rates of events were obtained by dividing the number of first events occurring in a given CD4 cell count stratum by the time spent in that stratum.
Results. Eight hundred sixty patients were followed off ART over 2789 person-years (PY). In the ≥650, 500–649, 350–499, 200–349, 100–199, 50–99, and 0–49 cells/μL CD4 cell count strata, the rates of AIDS or death were 0.9, 1.7, 3.7, 10.4, 30.9, 60.8, and 99.9 events per 100 PY, respectively. In patients with CD4 cell counts ≥200 CD4 cells/μL, the most frequent AIDS-defining disease was tuberculosis (decreasing from 4.0 to 0.6 events per 100 PY for 200–349 and ≥650 cells/μL, respectively), and the most frequent HIV non-AIDS severe diseases were visceral bacterial diseases (decreasing from 9.1 to 3.6 events per 100 PY).
Conclusions. Rates of AIDS or death, tuberculosis, and invasive bacterial diseases are substantial in patients with CD4 cell counts ≥200 cells/μL. Tuberculosis and bacterial diseases should be the most important outcomes in future trials of early ART in sub–Saharan Africa.
CD4 lymphocyte count is one of the most important determinants of human immunodeficiency virus (HIV)–related morbidity and mortality. In Western Europe, North America, and Australia, large cohort collaborations have been able to estimate the short-term CD4 cell count–specific risk of AIDS or death in untreated HIV-infected adults across a wide spectrum of CD4 cell counts [1, 2]. On the basis of these estimates, recent models suggested that CD4 cell counts of 350 cells/μL was the minimum threshold to start antiretroviral treatment (ART) but that there might be benefits to starting earlier [3, 4]. Several trials of early ART are ongoing to address the latter issue.
In sub–Saharan Africa, knowledge about HIV-related morbidity and mortality in untreated patients comes from a limited number of studies. Most were cross-sectional hospital-based studies [5, 6]; very few were longitudinal [7, 8]. Of the latter, some were performed in the 1990s, when CD4 cell counts were not available, and most of the others described morbidity based on baseline and not follow-up CD4 cell counts [9–11]. Data are especially lacking in patients with high CD4 cell counts.
Because of the incompleteness of existing data, it remains unclear whether HIV infection treatment guidelines should be region-specific. There is extensive evidence showing that some infectious diseases, such as tuberculosis, are more frequent in sub–Saharan Africa than in Europe or in the United States, both in the general population and in the HIV-infected population [12–17]. However, because CD4 cell count–specific rates of these diseases have not been estimated across the entire spectrum of CD4 cell counts before the ART era, the question of whether patients with similar CD4 cell counts should have different guidelines, depending on the region in which they live, has not been clearly addressed. This is particularly true when it comes to the crucial question of when to start ART.
In this study, we describe the CD4 cell count–specific rates of morbidity and mortality across the full spectrum of CD4 cell counts in 2 cohorts of untreated HIV-infected adults. These cohort studies were funded by the French Agence Nationale de Recherche sur le SIDA (ANRS) in Côte d’Ivoire.
METHODS
Patients and Follow-up
We collected data from 2 large cohort studies in Côte d’Ivoire. Both cohorts started at the end of the 1990s, before ART was available in Africa, and were followed up by the same team and with similar procedures. In both cohorts, CD4 cell counts were measured every 6 months in all patients, and documentation of morbidity was the primary goal.
The cohorts have been described elsewhere. In summary, the ANRS 1203 study was followed up in Abidjan from 1996 through 2003. It was a prevalent cohort of adults who were already HIV-infected when they were enrolled and for whom the date of seroconversion was unknown [18]. The ANRS 1220 study is a cohort of adult HIV-infected blood donors whose date of seroconversion was estimated. Follow-up started in 1997 at the National Blood Bank of Abidjan and is ongoing [19].
The 2 cohorts were followed up similarly, and had in common (1) the objective of documenting morbidity, (2) CD4 cell count tests every 6 months, (3) the conditions of access to care, with all tests and treatment free of charge, (4) the types of recorded morbidities and standardized definitions, and (5) tracing procedures for patients lost to follow-up.
Recorded Morbidities
In both cohorts, the following events were systematically recorded: all World Health Organization (WHO) stages 2, 3, or 4 diseases or Centers for Disease Control and Prevention (CDC) stage 3 defining diseases; malaria; bacterial and fungal mucocutaneous infections; bacterial and parasitic enteritis; and all other morbidity events that had at least 1 of the following criteria: fever of >38°C, at least 1 day in the hospital (including day-care hospital), or leading to death. Furthermore, when a disease was classified at a WHO or CDC stage on the basis of a recurrent episode (eg, upper respiratory tract infection) or of an episode lasting >1 month (eg, unexplained fever or unexplained diarrhea), first episodes and episodes lasting <1 month were also recorded. Each episode of morbidity was reviewed by an event documentation committee (standardized criteria are shown in Supplementary Appendix 1 for the most frequent diseases).
Treatment
In the ANRS 1220 cohort, all patients received cotrimoxazole regardless of CD4 cell count, even patients at WHO stage 1. In the ANRS 1203 cohort, patients at WHO stages 2, 3, or 4 received cotrimoxazole, irrespective of CD4 cell count, and patients at WHO stage 1 received cotrimoxazole only if their CD4 cell count dropped to <500 cells/μL. In both cohorts, some patients initiated ART once ART became available in Abidjan, following WHO criteria.
Statistical Analysis
In this study, we included only patient-time receiving cotrimoxazole and prior to starting ART. We considered the following CD4 cell count strata: 0–49, 50–99, 100–199, 200–349, 350–499, 500–649, and ≥650 cells/μL. We estimated CD4 cell count–specific rates of mortality and each morbidity group by dividing the number of events that occurred in a given CD4 cell count stratum by the cumulative time spent in the same stratum, expressed in person-years (PY). In the literature, CD4 cell count–specific rates are frequently estimated using 6-month periods after the date of the latest CD4 cell count [2, 20]. In such analyses, events and follow-up time that occur >6 months after a CD4 cell count are omitted. In sub–Saharan Africa, such omissions would likely lead to underestimated death rates, because terminally ill patients are frequently transported to their village by their family to receive care at home. Consequently, deaths frequently occur >6 months after the last available CD4 cell count, even though they are appropriately documented in the databases through tracing procedures [21]. Thus, to determine the CD4 cell count at the onset of a given event and the time spent in a given stratum, we modeled the CD4 cell count decrease at the individual level by use of a linear mixed model adjusted for CD4 cell count evolution by whether or not people died. We confirmed that this approach was suitable for the data by drawing 2 curves for the distribution of individual CD4 cell counts at each follow-up time for patients remaining in care: the distribution of simulated individual CD4 cell counts over time (as estimated by the model), and the distribution of the actual CD4 cell counts measured every 6 months. As shown in Supplementary Appendix 2, the curves fit each other well. For a given CD4 cell count–specific morbidity or mortality rate, follow-up within the stratum was censored at the onset of the first event, death, time of ART initiation, or date of last contact with the clinic.
RESULTS
Patients and Follow-up
Eight hundred sixty patients were included in the analysis, of whom 94% were HIV-1 positive, 5% had dual HIV-1 and HIV-2 positivity, and 1% were HIV-2 positive. Their baseline characteristics are shown in Table 1. These patients were followed up while receiving cotrimoxazole and without ART for 2788 PY (mean follow-up per patient, 35.9 months). During this follow-up, 262 patients died (33%). The mean number of available CD4 cell counts was 6 per patient.
Table 1.
Characteristics of Participants and Follow-up of ANRS 1203 and 1220 Cohorts, Abidjan, Côte d’Ivoire
| Characteristic | No. (%) of Participants(N = 860) |
| Primo-CI ANRS 1220 cohort | 246 |
| Cotrame ANRS 1203 cohort | 614 |
| Female sex | 522 (61) |
| Age, years, median (IQR) | 30 (25–36) |
| Baseline CD4 cell count, cells/μL, mean (SD) | 392 (265) |
| Baseline WHO stage | |
| 1 | 266 (31) |
| 2 | 227 (26) |
| 3 | 294 (34) |
| 4 | 71 (8) |
| Overall follow-up, months, median (IQR) | 35.9 (18.0–59.0) |
| No. of CD4 cell counts per patient, median (IQR) | 6 (3–9) |
| Follow-up in CD4 cell count stratum, person-years | |
| >650 cells/μL | 358 |
| 501–650 cells/μL | 359 |
| 351–500 cells/μL | 612 |
| 201–350 cells/μL | 759 |
| 101–200 cells/μL | 408 |
| 51–100 cells/μL | 151 |
| 0–50 cells/μL | 141 |
| Status at study termination | |
| Alive without ART | 293 (34) |
| Lost to follow-up before ART initiation | 73 (8) |
| Censored at ART initiation | 232 (24) |
| Dead before ART initiation | 262 (33) |
Data are no. (%) of participants, unless otherwise indicated.
Abbreviations: ANRS, French Agence Nationale de Recherche sur le SIDA; ART, antiretroviral therapy; IQR, interquartile range; SD, standard deviation; WHO, World Health Organization.
Clinical Events
Table 2 shows the overall number of episodes documented for each specific and nonspecific disease. During follow-up, 5267 episodes of morbidity were recorded. Of these, 696 were WHO stage 2, 1034 WHO stage 3, 283 WHO stage 4, and 338 AIDS-defining episodes. Within the episodes of WHO stages 3 or 4 or AIDS-defining diseases, the most frequent were oral candidiasis (n = 676), invasive bacterial diseases (n = 129), esophageal candidiasis (n = 110), tuberculosis (n = 84), prolonged unexplained fever and/or diarrhea (n = 63), and nontuberculous mycobacteriosis (n = 31). The 84 episodes of tuberculosis included 55 pulmonary cases and 29 disseminated cases. The 254 episodes of invasive bacterial diseases were 90 enteritis cases, 59 isolated bacteremia cases, 50 pneumonia cases, 45 invasive urogenital infections, and 10 infections in other locations. Of these invasive bacterial disease episodes, 39% had positive blood culture, 86% were first episodes, and 14% were recurrent episodes. Of the 5267 recorded morbidity episodes, 923 required hospital admission. Of the hospital admissions, 39% were due to nonspecific symptoms (unexplained fever, diarrhea, or pulmonary or neurologic symptoms), 23% to bacterial diseases, 7% to malaria, and 6% to tuberculosis.
Table 2.
WHO Stages 2–4 Diseases and Diseases With Hospital Admission or Bacteremia in ANRS 1203 and 1220 Cohorts, Abidjan, Côte d’Ivoire
| Classification, No. (%) of Episodes |
|||||||
| Disease | No. of Episodes Overall | No. (%) of Episodes With Hospital Admission | No. (%) of Episodes With Bacteremia | WHO Stage 2 | WHO Stage 3 | WHO Stage 4 | AIDS |
| Mycobacterial diseases | |||||||
| Tuberculosis | 84 | 54 (6) | 4 (3) | 0 (0) | 55 (5) | 29 (10) | 84 (24) |
| Nontuberculous mycobacteriosis | 31 | 28 (3) | 31 (23) | 0 (0) | 0 (0) | 31 (10) | 31 (9) |
| Bacterial diseases | |||||||
| Pneumonia | 50 | 38 (4) | 14 (10) | 0 (0) | 41 (4) | 9 (3) | 9 (3) |
| Isolated bacteremia | 59 | 41 (4) | 59 (43) | 0 (0) | 49 (5) | 10 (3) | 10 (3) |
| Pyelonephritis | 24 | 20 (2) | 2 (1) | 0 (0) | 24 (2) | 0 (0) | 0 (0) |
| Prostatitis | 10 | 6 (1) | 1 (1) | 0 (0) | 10 (1) | 0 (0) | 0 (0) |
| Orchitis/ epididymitis | 7 | 3 (<1) | 0 (0) | 0 (0) | 7 (1) | 0 (0) | 0 (0) |
| Salpingitis | 4 | 3 (<1) | 0 (0) | 0 (0) | 4 (<1) | 0 (0) | 0 (0) |
| Bacterial enteritis | 90 | 46 (5) | 17 (12) | 0 (0) | 88 (9) | 2 (1) | 2 (1) |
| Other bacterial invasive diseasea | 10 | 8 (1) | 4 (3) | 0 (0) | 10 (1) | 0 (0) | 0 (0) |
| Otitis | 104 | 11 (1) | 0 (0) | 35 (5) | 0 (0) | 0 (0) | 0 (0) |
| Sinusitis | 119 | 26 (3) | 4 (3) | 43 (6) | 4 (1) | 0 (0) | 0 (0) |
| Mucocutaneous infections | 242 | 8 (1) | 1 (1) | 0 (0) | 2 (<1) | 0 (0) | 0 (0) |
| Fungal diseases | |||||||
| Oral candidiasis | 676 | 1 (<1) | 0 (0) | 0 (0) | 676 (66) | 0 (0) | 0 (0) |
| Vaginal candidiasis | 390 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Esophageal candidiasis | 110 | 7 (1) | 0 (0) | 0 (0) | 0 (0) | 110 (37) | 110 (31) |
| Cryptococcosis | 15 | 15 (2) | 0 (0) | 0 (0) | 0 (0) | 15 (5) | 15 (4) |
| Histoplasmosis | 1 | 1 (<1) | 0 (0) | 0 (0) | 0 (0) | 1 (<1) | 1 (<1) |
| Angular cheilitis | 135 | 1 (<1) | 0 (0) | 135 (20) | 0 (0) | 0 (0) | 0 (0) |
| Fungal nail infections | 68 | 0 (0) | 0 (0) | 68 (10) | 0 (0) | 0 (0) | 0 (0) |
| Other fungal skin infections | 454 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Virologic diseases | |||||||
| Herpes zoster virus infection | 149 | 12 (1) | 0 (0) | 149 (22) | 0 (0) | 0 (0) | 0 (0) |
| Genital HSV infection | 173 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 18 (6) | 18 (5) |
| Visceral HSV or CMV infection | 8 | 4 (<1) | 0 (0) | 0 (0) | 0 (0) | 8 (3) | 8 (3) |
| Parasitic diseases | |||||||
| Toxoplasmosis | 6 | 6 (1) | 0 (0) | 0 (0) | 0 (0) | 6 (2) | 6 (2) |
| Pneumocystosis | 1 | 1 (<1) | 0 (0) | 0 (0) | 0 (0) | 1 (<1) | 1 (<1) |
| Cryptosporidiosis | 14 | 10 (1) | 0 (0) | 0 (0) | 0 (0) | 14 (5) | 14 (4) |
| Nonclassifying parasitic enteritis | 59 | 11 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Isosporosis | 11 | 10 (1) | 0 (0) | 0 (0) | 0 (0) | 12 (4) | 12 (3) |
| Malaria | 129 | 65 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nonspecific diseases | |||||||
| Unexplained diarrheab | 736 | 144 (16) | 0 (0) | 0 (0) | 39 (4) | 3 (1) | 3 (1) |
| Unexplained feverc | 770 | 184 (20) | 0 (0) | 0 (0) | 24 (2) | 4 (1) | 4 (1) |
| Unexplained pneumonia | 12 | 11 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unexplained central neurological | 25 | 24 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pruritic papular eruption | 212 | 0 (0) | 0 (0) | 220 (32) | 0 (0) | 0 (0) | 0 (0) |
| Oral leucoplakia | 1 | 0 (0) | 0 (0) | 0 (0) | 1 (<1) | 0 (0) | 0 (0) |
| Oral ulcerations | 146 | 0 (0) | 0 (0) | 46 (7) | 0 (0) | 0 (0) | 0 (0) |
| Hospitalization, other cause | 121 | 121 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Malignancies | 11 | 3 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (3) | 10 (3) |
| Overall | 5267 | 923 (100) | 137 (100) | 696 (100) | 1034 (100) | 283 (100) | 338 (100) |
Abbreviations: CMV, cytomegalovirus; HSV, herpes simplex virus; WHO, World Health Organization.
Other invasive bacterial diseases include any other visceral bacterial diseases (meningitis, endocarditis, liver abscess, etc) and nonvisceral bacterial disease with bacteremia.
Unexplained diarrhea includes 2 groups: unexplained diarrhea for ≤1 month and unexplained diarrhea for >1 month. Only episodes of the latter group are classified as WHO stages 3 or 4. Thus, of the 736 episodes of unexplained diarrhea, only 42 (39 + 3) were classified WHO stages 3 or 4, meaning they lasted >1 month. The remaining 694 episodes lasted ≤1 month and did not fall into any WHO stage.
Unexplained fever includes 2 groups: unexplained fever for ≤1 month and unexplained fever for >1 month. Only episodes of the latter group are classified as WHO stages 3 or 4. Of the 770 episodes of unexplained fever, only 28 (24 + 4) were classified WHO stages 3 or 4, meaning they lasted >1 month. The remaining 742 episodes lasted ≤1 month and did not fall into any WHO stage.
CD4 Cell Count–Specific Rates of Diseases
Figure 1 shows the CD4 cell count–specific rates of death; AIDS or death; and AIDS, death, or severe non-AIDS diseases; the 95% confidence intervals (CIs) are shown in Supplementary Appendix 3. As expected, the rates of AIDS or death were highest in patients with CD4 cell counts <200 cells/μL, ranging from 30.9 events per 100 PY in the 100–199 cells/μL stratum to 99.9 events per 100 PY in the 0–49 cells/μL stratum. Although they were lower, the rates were still substantial in patients with CD4 cell counts ≥200 cells/μL, reaching 10.4 events per 100 PY in the 200–349 cells/μL stratum, 3.7 events per 100 PY in the 350–499 cells/μL stratum, 1.7 events per 100 PY in the 500–649 cells/μL stratum, and 0.9 events per 100 PY in the ≥650 cells/μL stratum.
Figure 1.
A, CD4 cell count–specific rates of mortality. B, CD4 cell count–specific rates of mortality for CD4 cell counts >200 cells/μL (inset in panel A). Severe non-AIDS includes the following illnesses: severe bacterial diseases (ie: bacterial diseases of any location with bacteremia, and the following visceral bacterial diseases: pneumonia, isolated bacteremia, pyelonephritis, prostatitis, orchiepididymitis, salpingitis, meningitis, endocarditis); and non–AIDS-defining cancers.
Abbreviation: PY, person-years.
In CD4 cell count strata of ≥200 cells/μL, the most frequent WHO stages 3 or 4 or AIDS-defining diseases were invasive bacterial diseases, oral candidiasis, and tuberculosis (Table 3; 95% CIs are given in Supplementary Appendix 3).
Table 3.
CD4-Specific Rate of Morbidity. ANRS 1203 and 1220 Cohorts, Abidjan, Côte d’Ivoire
| Rate According to Current CD4 Cell Count Stratum, Events/100 PY |
|||||||
| Disease | ≥650 cells/μL | 500–649 cells/μL | 350–499 cells/μL | 200–349 cells/μL | 100–199 cells/μL | 50–99 cells/μL | <50 cells/μL |
| WHO stage 4, overall | 0.0 | 0.8 | 1.5 | 5.6 | 13.3 | 33.1 | 46.7 |
| Tuberculosis, disseminated a | 0 | 0.3 | 0.3 | 1.9 | 1.6 | 2.9 | 1.6 |
| Recurrent bacterial pneumonia | 0 | 0 | 0.2 | 0.3 | 1.0 | 0.7 | 0 |
| Recurrent NTS bacteremia | 0 | 0 | 0 | 0.3 | 0.8 | 0.0 | 1.6 |
| Nontuberculous mycobacteriosis | 0 | 0 | 0.2 | 0.5 | 0.5 | 5.8 | 13.3 |
| Cryptococcosis | 0 | 0 | 0 | 0.4 | 0.5 | 2.2 | 6.5 |
| Toxoplasmosis | 0 | 0 | 0 | 0.1 | 0.3 | 2.1 | 1.7 |
| Cryptosporidiosis, microsporidiosis | 0 | 0 | 0.3 | 0.3 | 0.5 | 3.0 | 2.6 |
| Malignancy (Kaposi sarcoma or lymphoma) | 0 | 0 | 0.2 | 0.4 | 0.3 | 1.4 | 2.4 |
| Isosporosis | 0 | 0.3 | 0 | 0.1 | 1.6 | 2.2 | 0 |
| Visceral HSV or CMV infection | 0 | 0 | 0 | 0.3 | 0.8 | 1.5 | 0.8 |
| Esophageal candidiasis | 0 | 0.3 | 0.5 | 1.4 | 6.1 | 19.5 | 33.3 |
| Chronic genital HSV infection | 0 | 0 | 0 | 0.5 | 0.8 | 2.9 | 1.7 |
| Wasting syndrome | 0 | 0 | 0 | 0.3 | 0.3 | 0 | 1.6 |
| WHO stage 3, overall | 6.7 | 7.4 | 8.0 | 19.7 | 48.2 | 88.9 | 101.4 |
| Tuberculosis, pulmonarya | 0.6 | 0.6 | 0.5 | 2.0 | 4.8 | 7.4 | 2.5 |
| Invasive bacterial diseases, overall | 3.6 | 3.5 | 3.1 | 9.1 | 17.1 | 18.9 | 16.9 |
| Bacterial enteritis | 1.7 | 2.0 | 1.5 | 2.9 | 6.2 | 6.6 | 9.1 |
| Pneumonia | 0.3 | 0.6 | 0.3 | 2.5 | 3.7 | 1.4 | 1.6 |
| Isolated bacteremia | 0.3 | 0.6 | 0.2 | 2.3 | 5.0 | 6.5 | 2.4 |
| Urogenital infectionb | 1.1 | 0 .3 | 0.8 | 1.4 | 3.1 | 4.3 | 3.2 |
| Infection with any other organ involvementc | 0.0 | 0.0 | 0.3 | 0.5 | 0.8 | 0.0 | 0.8 |
| Unexplained diarrhea for >1 month | 0 | 0 | 0.2 | 0.4 | 2.1 | 6.1 | 11.3 |
| Unexplained fever for >1 month | 0.6 | 0 | 0.3 | 0.9 | 0.5 | 2.2 | 6.1 |
| Oral candidiasis | 3.0 | 4.0 | 4.4 | 12.6 | 32.0 | 72.8 | 89.0 |
| WHO stage 2, overall | 7.3 | 9.6 | 9.3 | 19.5 | 39.9 | 60.3 | 58.1 |
| Herpes zoster virus infection | 3.3 | 4.4 | 2.2 | 7.4 | 11.1 | 6.6 | 2.4 |
| Angular cheilitis | 1.1 | 1.4 | 1.3 | 3.6 | 8.5 | 15.9 | 11.3 |
| Fungal nail infections | 1.5 | 1.4 | 1.9 | 1.9 | 5.7 | 2.9 | 3.3 |
| Pruritic papular eruption | 2.1 | 1.4 | 2.5 | 6.0 | 13.7 | 22.1 | 35.4 |
| Recurrent oral ulcerations | 0.3 | 0 | 0.8 | 1.4 | 2.1 | 4.3 | 2.4 |
| Recurrent otitis or sinusitis | 0.3 | 0.8 | 1.2 | 1.9 | 4.8 | 10.3 | 3.2 |
| Nonclassifying diseases | |||||||
| Oral ulcerations, first episode | 2.4 | 0.8 | 2.6 | 5.0 | 7.1 | 3.6 | 6.5 |
| Otitis or sinusitis, first episode | 3.3 | 5.3 | 3.4 | 6.5 | 8.8 | 7.3 | 6.6 |
| Sinusitis, first episode | 0.6 | 3.2 | 1.5 | 2.9 | 4.8 | 3.6 | 2.4 |
| Otitis, first episode | 2.6 | 2.0 | 1.8 | 1.8 | 3.3 | 3.7 | 4.0 |
| Bacterial mucocutaneous infectionsd | 3.3 | 4.7 | 5.7 | 7.0 | 11.4 | 13.8 | 10.2 |
| Nonclassifying fungal skin infectionse | 7.7 | 4.2 | 7.0 | 12.3 | 24.0 | 28.3 | 26.6 |
| Acute genital HSV infection | 2.6 | 2.6 | 3.6 | 5.2 | 9.3 | 5.1 | 10.7 |
| Nonclassifying parasitic enteritisf | 0.9 | 0.3 | 1.3 | 2.1 | 3.2 | 2.9 | 7.1 |
| Malaria, any episode | 4.8 | 3.5 | 4.0 | 3.8 | 5.8 | 4.3 | 6.5 |
| Malaria with hospital admission | 1.4 | 1.1 | 1.7 | 2.3 | 3.1 | 3.6 | 4.8 |
| Nonspecific illness with hospital admission | |||||||
| Unexplained fever for ≤1 month | 2.4 | 4.2 | 4.2 | 6.3 | 8.2 | 10.4 | 12.1 |
| Unexplained diarrhea for ≤1 month | 2.1 | 2.0 | 0.7 | 3.3 | 6.2 | 15.1 | 18.3 |
| Unexplained central neurological symptomsg | 0 | 0.3 | 0.2 | 0.4 | 1.3 | 2.8 | 8.1 |
| Unexplained pneumoniag | 0 | 0.3 | 0.2 | 0.3 | 1.0 | 0 | 3.2 |
| Any other disease with hospital admissionh | 3.4 | 2.3 | 2.0 | 4.2 | 6.2 | 9.5 | 11.4 |
Abbreviations: CMV, cytomegalovirus; HSV, herpes simplex virus; NTS, nontyphoidal salmonella; PY, person-years; WHO, World Health Organization.
The overall rates of tuberculosis (any location) were as follows: CD4 cell count ≥650 cells/μL, 0.6 events per 100 PY; 500–649 cells/μL, 0.8 events per 100 PY; 350–499 cells/μL, 0.8 evens per 100 PY; 200–349 cells/μL, 4.0 events per 100 PY; 100–199 cells/μL, 6.5 events per 100 PY; 50–99 cells/μL, 10.6 events per 100 PY; <50 cells/μL, 4.1 events per 100 PY.
Includes bacterial pyelonephritis, prostatitis orchiepididymitis, and salpingitis.
Includes any other visceral bacterial diseases (eg, meningitis or endocarditis) and nonvisceral bacterial disease (eg, sinusitis, otitis, or mucocutaneous abscess) with bacteremia.
Includes mucocutaneous abscess and furunculosis.
Includes intertrigo, dermatophytoses, pityriasis versicolor, and tinea capita.
Includes anguillulosis, giardiasis, ankylostomiasis, intestinal bilharziosis, ascariasis, and amebiasis.
See Supplementary Appendix 1 for definitions.
Hospital admission due to any diseases not listed elsewhere in this table.
DISCUSSION
In this study, we describe for the first time in Africa the CD4 cell count–specific rates of morbidity and mortality across the full spectrum of CD4 cell counts in HIV-infected patients receiving cotrimoxazole but no ART. We derived these estimates from 2 long-term research cohorts in which patients had free access to transportation and care, including diagnosis and treatment, before the ART era.
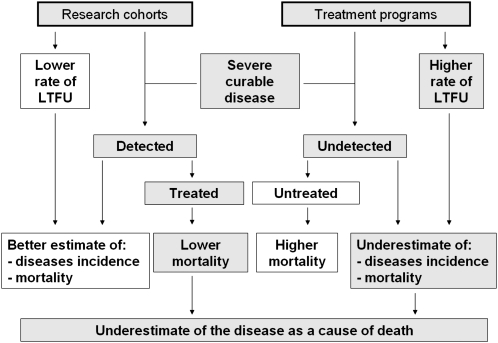
Facilities are limited in most low-resource settings. Many patients followed under routine conditions can afford neither the tests required to document morbidity nor the medications to treat it. In this context, cohorts with free access to care are likely to generate better estimates of morbidity than are routine program databases. On the other hand, participants in cohorts with free access to tests and medications are also less likely to die, because of appropriate and more rapid diagnosis and treatment. As a result, the spectrum we describe likely reflects with reasonable accuracy the full spectrum of HIV-related morbidity in untreated HIV-infected patients in Côte d’Ivoire, while underestimating mortality from these diseases in this population (Figure 2).
Figure 2.
Differences between estimates of severe curable morbidity (severe bacterial diseases and tuberculosis) and mortality from these diseases under research conditions and under treatment program conditions.
Abbreviation: LTFU, lost to follow-up.
These data support the following points. First, although mortality (4.2 events per 100 PY) and rates of AIDS or death (10.4 events per 100 PY) in patients with CD4 cell counts of 200–349 cells/μL are lower than in those with CD4 cell counts of <200 cells/μL, the absolute rates are still high. This is consistent with data previously reported from South Africa (death rate, 8.0 events per 100 PY; rate of AIDS or death, 14.0 events per 100 PY) [8] and Haiti [22], suggesting that mortality in adults at CD4 cell counts of 200–349 cells/μL is high in some low-resource settings compared with rates reported from Western Europe (CHIC cohort death rate, 0.58 events per 100 PY; CHIC cohort AIDS or death rate, 4.91 events per 100 PY; Eurosida death rate, 2.7 events per 100 PY; Eurosida AIDS rate, 6.1 events per 100 PY) [2, 20]. The novelty of the data here is what happens at CD4 cell counts above these values, with 2 important findings: that rates of death or AIDS in patients with CD4 cell counts of 350–499 cells/μL remain substantial (death rate, 1.6 events per 100 PY; rate of AIDS or death, 3.7 events per 100 PY) compared with rates among HIV-infected adults with CD4 cell counts of 350–499 cells/μL reported from Western Europe (UK Collaborative HIV Cohort [CHIC] study death rate, 0.32 events per 100 PY; CHIC cohort AIDS or death rate, 2.49 events per 100 PY) [2, 20] and with death rates from causes other than HIV infection or AIDS in the overall population (ie, HIV-infected and noninfected individuals) of sub–Saharan African adults aged 15–59 years (global and regional burden of disease and risk factor estimates from 2001, 0.6 deaths per 100 PY) [23]; and that there is a trend toward decreasing rates of mortality with increasing CD4 cell counts, even above 350 cells/μL.
Second, tuberculosis and invasive bacterial diseases are clearly the most frequent severe diseases in patients with higher CD4 cell counts. The CD4 cell count–specific rates of tuberculosis in this study are lower than those reported from South Africa [7, 24] but far higher than those reported from France [25] and Great Britain [26]. For invasive bacterial diseases, it is not possible to compare our rates to those in other settings, because studies that reported CD4 cell count–specific rates of bacterial morbidity focused only on AIDS-defining recurrent episodes [25, 26]. By doing so, they did not capture the importance of first episodes, which in our study accounted for 85% of the overall number of episodes of invasive bacterial diseases.
A key difference in the way in which high- and low-resource countries consider tuberculosis and bacterial diseases is that in rich countries these diseases are increasingly classified as nonsevere events, even when they are AIDS-defining diseases [27]. In sub–Saharan Africa, autopsy studies have shown that tuberculosis and bacterial diseases are the 2 most frequent causes of death in HIV-infected adults [12–17], even though they are easier to diagnose and treat than many other types of opportunistic infections. This is due to a combination of 3 factors: poor access to healthcare facilities, poor access to specific diagnostic tests, and poor access to treatment [28]. Because of the first 2 factors, WHO estimates that only 50% of active tuberculosis cases are diagnosed in sub–Saharan Africa [29]. As a consequence of all 3 factors, not only is the frequency of active tuberculosis underestimated in most HIV infection care programs, but mortality from tuberculosis is also likely to be underestimated. This underestimation is seen in routine programs, because most patients with undiagnosed tuberculosis die without tuberculosis being identified as the cause of death, and in cohort studies with clear follow-up, because diagnosed patients receive adequate treatment (Figure 2).
There are several limitations to this study. First is the lack of facilities to diagnose some diseases. As in most studies in Africa, even in cohort studies with the primary objective of documenting morbidity, rates of all diseases are underestimated, but some of them are underestimated to a greater extent because their diagnosis requires sophisticated diagnostic tools. Our data showing that the rate of nonspecific diseases that lead to hospital admissions is high, and that this rate increases with decreasing CD4 cell counts, illustrate this fact. In this study, we wanted to give a comprehensive overview of the entire spectrum of morbidity in our cohorts, so we recorded and reported all specific and nonspecific episodes in order to provide a clear idea of what diagnoses could have been missed. All nonspecific diseases are not equivalent in terms of their likelihood of being missed. Diarrhea lasting ≤1 month and fever lasting ≤1 month are very unlikely to be undiagnosed AIDS-defining diseases, because they resolve spontaneously either without specific treatment or with empiric antibacterial or antimalarial treatment. Thus, some episodes of these groups may be undiagnosed bacterial diseases, undiagnosed malaria, or undiagnosed non-AIDS parasitic or viral diarrhea, rather than undiagnosed AIDS-defining diseases. In contrast, diarrhea lasting >1 month, fever lasting >1 month, unexplained pneumonia, and unexplained central nervous system symptoms could cover a wide range of undiagnosed AIDS diseases, including severe viral diseases. However, these groups are far smaller than the groups with diarrhea lasting ≤1 month and fever lasting ≤1 month, and these events are almost all restricted to CD4 cell count strata of <350 cells/μL.
Another limitation is censoring due to loss to follow-up and ART initiation, both of which could have been informative. The consequence of such informative censoring is likely to be an underestimation of deaths rather than an overestimation, because death rates are more likely to be higher in patients lost to follow-up, and because patients who start ART at CD4 cell counts of >200 cells/μL are likely to have more advanced clinical stages of disease than patients with >200 cells/μL who remain off ART. Had these patients not started treatment, they would have had higher risks of death. This, together with the discussion above regarding lower mortality from curable disease in cohort studies compared with treatment programs, lead us to believe that our results should be considered as lower bound estimates. The third limitation is that we merged databases from 2 different studies. Although both studies used the same follow-up procedures and the same standardized definitions, we cannot rule out some heterogeneity in the results. Finally, another limitation is the sample size, which is smaller than collaborative cohorts in industrialized countries. The mortality we found in our study has wide confidence intervals compared with those in such cohorts. Future collaborations of cohorts of untreated patients in low-resource settings should be developed to refine such estimates.
In conclusion, we draw 2 key messages from these findings. First, the very concept of HIV non-AIDS–defining diseases, which is crucial to the current debate over when to start ART, does not cover the same spectrum in sub–Saharan Africa as in Western Europe, North America, and Australia. Our study shows that rates of tuberculosis and non-AIDS infectious diseases are substantial in patients with high CD4 cell counts. Previous studies have consistently shown that tuberculosis and bacterial diseases were the most important cause of death in HIV-infected adults in sub–Saharan Africa [12–16]. Deaths related to tuberculosis or bacterial diseases could be avoided by 2 means: improving diagnosis and treatment, and decreasing incidence. Starting ART earlier than currently recommended could help achieve the latter. Thus, the ongoing and future trials of early ART in sub–Saharan Africa should carefully document these diseases and consider them as major outcomes.
Second, because of this spectrum of illnesses, and because of the difference in access to care, trials exploring the benefits and risks of initiating ART at very high CD4 cell counts make more sense in low-resource settings similar to Côte d’Ivoire than in rich countries. Within the context of low-resource settings, starting ART very early might make even more sense in rural settings, which have limited facilities to diagnose tuberculosis and invasive bacterial diseases than in urban settings, where these diseases can be diagnosed and treated more easily.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We are indebted to all patients who participated in this trial. We also gratefully acknowledge the valuable contributions of the Centre de Diagnostic et de Recherche sur le SIDA (CeDReS), Centre de Prise en Charge et de Formation (CePReF), Centre National de Transfusion Sanguine (CNTS), CEPAC International, and INSERM U897 teams.
Members of the ANRS 1203 and 1220 Study Groups
Clinical care in Abidjan, Côte d’Ivoire. CNTS: Hugues Abé, Nicole Abé, Yao Abo, Emma Amon Anini, Isidore Bohouo, Ali Coulibaly, Issouf Coulibaly, Vafoungbé Diabaté, Lambert Dohoun, Daniel Ekra, Gwenola Gourvellec, Charlotte Huet, Jules Hyda, Mamadou Konaté, Seidou Konate, Reine Koré, Charlotte Lewden, Albert Minga, Minata Ouattara, Marie-Julie N’Dri, Sayouba Ouedraogo, Abdelh Sidibé, and Liliane Siransy. CePReF: Xavier Anglaret, Amani Anzian, Edwidge Cissé, Nicole Dakoury-Dogbo, Christine Danel, Joachim Gnokoro, Marie-Cécile Kassi, Mamadou Kone, Bertin Kouadio, Yao Kouakou, Georgette Labibi, Jean Lehou, Eugène Messou, Thérèse N’Dri-Yoman, Marie-Pascale Nogbout, Catherine Seyler, Amah-Cécile Tchehy, Siaka Toure, Moussa Traore, and Marcel Zaho.
Biology, Abidjan, Côte d’Ivoire. CeDReS, CHU de Treichville: Dominique Bonard, Patrice Combe, Arlette Emieme, André Inwoley, Hervé Menan, Timothée Ouassa, François Rouet, Thomas-d’Aquin Toni, Ramatou Toure, and Vincent Yapo.
Epidemiology and biostatistics, Bordeaux, France. INSERM U897: Delphine Gabillard and Roger Salamon.
Members of the ANRS 12222 Study Group
Steering committee. Xavier Anglaret, Robert Colebunders, François Dabis, Joseph Drabo, Serge Eholié, Delphine Gabillard, Pierre-Marie Girard, Karine Lacombe, Christian Laurent, Vincent Le Moing, and Charlotte Lewden.
Other representatives of participating studies. Gérard Allou, Clarisse Amani-Bossé, Divine Avit, Aida Benalycherif, Pierre de Beaudrap, Patrick Coffie, Eric Delaporte, Lise Denoeud, Serge Diagbouga, Didier Koumavi Ekouevi, Jean-François Etard, Sabrina Eymard-Duvernay, Isabelle Fournier-Nicolle, Hervé Hien, Issouf Konate, Sinata Koulla-Shiro, Valériane Leroy, Olivier Marcy, Nicolas Meda, Eitel Mpoudi-Ngolé, Philippe Msellati, Boubacar Nacro, Nicolas Nagot, Ibra Ndoye, Abdoulaye Ouédraogo, Men Pagnaroat, Roger Salamon, Vonthanak Saphonn, Olivier Segeral, Philippe Van de Perre, and Marcel Zannou.
Financial support.
This work was supported by the French Agence Nationale de Recherche sur le SIDA et les hépatites virales, Paris, France (grants ANRS 1203, ANRS 1220, and ANRS 12222) and the US National Institute of Allergy and Infectious Diseases (grant NIAID AI058736).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Phillips A, Pezzotti P. Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug–naive individuals and those treated in the monotherapy era. AIDS. 2004;18:51–8. doi: 10.1097/00002030-200401020-00006. [DOI] [PubMed] [Google Scholar]
- 2.Phillips AN, Gazzard B, Gilson R, et al. Rate of AIDS diseases or death in HIV-infected antiretroviral therapy–naive individuals with high CD4 cell count. AIDS. 2007;21:1717–21. doi: 10.1097/QAD.0b013e32827038bf. [DOI] [PubMed] [Google Scholar]
- 3.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Oosterhout JJ, Laufer MK, Graham SM, et al. A community-based study of the incidence of trimethoprim-sulfamethoxazole-preventable infections in Malawian adults living with HIV. J Acquir Immune Defic Syndr. 2005;39:626–31. [PubMed] [Google Scholar]
- 6.Grant AD, Djomand G, Smets P, et al. Profound immunosuppression across the spectrum of opportunistic disease among hospitalized HIV-infected adults in Abidjan, Cote d’Ivoire. AIDS. 1997;11:1357–64. doi: 10.1097/00002030-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 8.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368:1254–9. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 9.Anglaret X, Chêne G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d’Ivoire: a randomised trial. Lancet. 1999;353:1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 10.Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–75. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 11.Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–34. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 12.Lucas SB, Hounnou A, Peacock C, et al. The mortality and pathology of HIV infection in a west African city. AIDS. 1993;7:1569–79. doi: 10.1097/00002030-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Rana FS, Hawken MP, Mwachari C, et al. Autopsy study of HIV-1-positive and HIV-1–negative adult medical patients in Nairobi, Kenya. J Acquir Immune Defic Syndr. 2000;24:23–9. doi: 10.1097/00126334-200005010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Corbett EL, Churchyard GJ, Charalambos S, et al. Morbidity and mortality in South African gold miners: impact of untreated disease due to human immunodeficiency virus. Clin Infect Dis. 2002;34:1251–8. doi: 10.1086/339540. [DOI] [PubMed] [Google Scholar]
- 15.Ansari NA, Kombe AH, Kenyon TA, et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis. 2002;6:55–63. [PubMed] [Google Scholar]
- 16.Martinson NA, Karstaedt A, Venter WD, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007;21:2043–50. doi: 10.1097/QAD.0b013e3282eea47f. [DOI] [PubMed] [Google Scholar]
- 17.MacPherson P, Moshabela M, Martinson N, Pronyk P. Mortality and loss to follow-up among HAART initiators in rural South Africa. Trans R Soc Trop Med Hyg. 2009;103:588–93. doi: 10.1016/j.trstmh.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Anglaret X, Messou E, Ouassa T, et al. Pattern of bacterial diseases in a cohort of HIV-1 infected adults receiving cotrimoxazole prophylaxis in Abidjan, Cote d’Ivoire. AIDS. 2003;17:575–84. doi: 10.1097/00002030-200303070-00013. [DOI] [PubMed] [Google Scholar]
- 19.Minga A, Danel C, Abo Y, et al. Progression to WHO criteria for antiretroviral therapy in a 7-year cohort of adult HIV-1 seroconverters in Abidjan, Cote d’Ivoire. Bull World Health Organ. 2007;85:116–23. doi: 10.2471/BLT.06.032292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–9. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 21.Anglaret X, Toure S, Gourvellec G, et al. Impact of vital status investigation procedures on estimates of survival in cohorts of HIV-infected patients from sub–Saharan Africa. J Acquir Immune Defic Syndr. 2004;35:320–3. doi: 10.1097/00126334-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 22.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191:150–8. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 25.Yazdanpanah Y, Chene G, Losina E, et al. Incidence of primary opportunistic infections in two human immunodeficiency virus–infected French clinical cohorts. Int J Epidemiol. 2001;30:864–71. doi: 10.1093/ije/30.4.864. [DOI] [PubMed] [Google Scholar]
- 26.Mocroft A, Youle M, Phillips AN, et al. The incidence of AIDS-defining illnesses in 4883 patients with human immunodeficiency virus infection. Royal Free/Chelsea and Westminster Hospitals Collaborative Group. Arch Intern Med. 1998;158:491–7. doi: 10.1001/archinte.158.5.491. [DOI] [PubMed] [Google Scholar]
- 27.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–8. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harries AD, Zachariah R, Corbett EL, et al. The HIV-associated tuberculosis epidemic—when will we act? Lancet. 2010;375:1906–19. doi: 10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]
- 29. WHO. Global tuberculosis control—epidemiology, strategy, financing. WHO Report 2009. WHO/HTM/TB/2009.411. Available at: http://www.who.int/tb/publications/global_report/2009/en/. Accessed 13 October 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.