Two doses of AS03B-adjuvanted pandemic influenza vaccine may be sufficient to maintain seroprotection across 2 influenza seasons. Administration of trivalent influenza vaccine to children who previously received 2 doses of pandemic influenza vaccine is safe and is immunogenic for the H1N1 strain.
Abstract
Background. We investigated antibody persistence in children 1 year after 2 doses of either an AS03B-adjuvanted split-virion or nonadjuvanted whole-virion monovalent pandemic influenza vaccine and assessed the immunogenicity and reactogenicity of a subsequent dose of trivalent influenza vaccine (TIV).
Methods. Children previously immunized at age 6 months to 12 years in the original study were invited to participate. After a blood sample was obtained to assess persistence of antibody against swine influenza A/H1N1(2009) pandemic influenza, children received 1 dose of 2010/2011 TIV, reactogenicity data were collected for 7 days, and another blood sample was obtained 21 days after vaccination.
Results. Of 323 children recruited, 302 received TIV. Antibody persistence (defined as microneutralization [MN] titer ≥1:40) 1 year after initial vaccination was significantly higher in the AS03B-adjuvanted compared with the whole-virion vaccine group, 100% (95% confidence interval [CI], 94.1%–100%) vs 32.4% (95% CI, 21.5%–44.8%) in children immunized <3 years old and 96.9% (95% CI, 91.3%–99.4%) vs 65.9% (95% CI, 55.3%–75.5%) in those 3–12 years old at immunization, respectively (P < .001 for both groups). All children receiving TIV had post-vaccination MN titers ≥1:40. Although TIV was well tolerated in all groups, reactogenicity in children <5 years old was slightly greater in those who originally received AS03B-adjuvanted vaccine.
Conclusions. This study provides serological evidence that 2 doses of AS03B-adjuvanted pandemic influenza vaccine may be sufficient to maintain protection across 2 influenza seasons. Administration of TIV to children who previously received 2 doses of either pandemic influenza vaccine is safe and is immunogenic for the H1N1 strain.
During the first and second waves of infection in the 2009–2010 A/H1N1(2009) influenza pandemic, there were at least 18337 deaths globally [1, 2]. The United Kingdom Department of Health purchased 2 pandemic monovalent influenza vaccines (a nonadjuvanted cell culture-derived whole-virion vaccine and an AS03B-adjuvanted egg culture-derived split-virion vaccine) that were used during the national pandemic influenza vaccination program. In 2009, we compared the safety and immunogenicity of a 2-dose regimen of these vaccines in 943 children aged 6 months to 12 years old. We found the AS03B-adjuvanted vaccine more immunogenic, especially in younger children (microneutralization [MN] titer ≥1:40 in children under 3 years of age was 98.2% vs 80.1%) [3].
In the initial stage of the 2009 pandemic, at least 25000 children at increased clinical risk from influenza infection received a 2-dose regimen of monovalent H1N1 vaccine (R. Pebody, personal communication) [4, 5]. Subsequently, the United Kingdom recommended single-dose immunization for all children aged 6 months to 5 years after review of early reactogenicity data [3, 6]. Analyses have since demonstrated the AS03B-adjuvanted vaccine to be effective against the pandemic H1N1 strain [7–10], even after a single dose in young children [11]. However, there are no published data that assess the persistence of antibody in children immunized against the novel H1N1 virus to help guide future pandemic vaccination policy [12].
Concerns have arisen over both the reactogenicity and immunogenicity of the seasonal trivalent influenza vaccine (TIV) in those who have previously received monovalent pandemic vaccine, but to date this has not been studied in a clinical trial [13–15]. Recent analyses have shown significantly lower immunogenicity to monovalent pandemic influenza vaccines in those who had received previous TIV, but it is unknown whether the reverse is true [13–15].
This follow-on study investigated both the persistence of anti-H1N1 antibody 1 year after immunization with the pandemic vaccines and the immunogenicity of the H1N1 component and reactogenicity of a subsequent dose of TIV in these children.
METHODS
Study Design
This was a multicenter, open-label, phase IV study following on from a randomized trial. At visit one, a blood sample assessed persistence of antibodies to H1N1. A single, 0.5-mL intramuscular dose of TIV was administered into the deltoid. A second blood sample was taken 21 days (−7 to +14 days) later days later. For those not wishing to receive the study vaccine, parents could consent to the first blood test alone. For 7 days after administration of TIV, parents used daily diary cards to record the axillary temperature, injection site reactions, systemic symptoms, and use of antipyretic medication (Supplementary Table 1). All medical consultations between study immunization and the second study visit were recorded. Serum analysis was undertaken centrally, using standard methods [3, 16, 17]. The study was registered at ClinicalTrials.gov (NCT01239537), conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, and approved by the UK Medicines and Healthcare Products Regulatory Agency (EudraCT No. 2010-022817-24), the Oxfordshire Research Ethics Committee A (10/H0604/81), and local National Health Service (NHS) organizations.
Participants
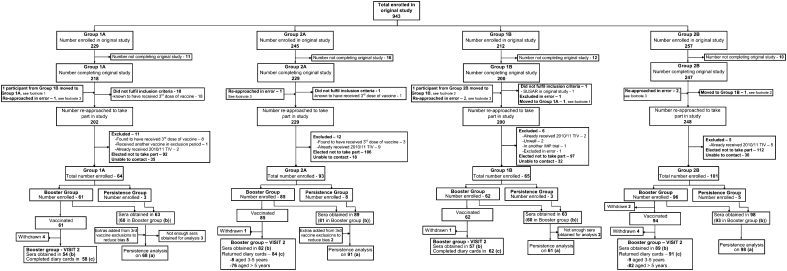
Between 11 November and 2 December 2010, 323 children who completed the original study [3] and were now between 17 months and 13 years old were recruited at 5 UK sites. Inclusion, exclusion, and temporary exclusion criteria are shown in Supplementary Table 2A and for the original study in Supplementary Table 2B. The original study recruited healthy children; however, high-risk children, such as asthmatics, were not excluded unless considered immunosuppressed. Allocation was to the same group as in the original study, in which they had been stratified for age (<3 or ≥3 years old) and randomized to receive either pandemic vaccine (consort diagram, Figure 1) [3]. All vaccinated children received a single dose of the TIV (Fluarix™; GlaxoSmithKline). Parents or guardians gave written informed consent, and children aged ≥7 years gave verbal assent.
Figure 1.
Enrollment and follow-up of study participants. Groups were allocated as in the original study, first split into those receiving either the whole-virion or the AS03B-adjuvanted vaccine and then further divided into those <3 and ≥3–12 years of age at the time of the original study [3]. Annotations (a), (b), and (c) highlight the participants on whom analysis was performed: (a) indicates persistence of antibody levels; (b) and (c), immunogenicity and reactogenicity after trivalent influenza vaccine (TIV) analysis, respectively. 1In the original study, this participant accidently received the incorrect vaccine [3] (whole-virion instead of AS03B-adjuvanted vaccine, as per randomization) and was therefore moved to the whole-virion group for follow-on in this study. 2Participant was mistakenly put into the older age group in the original study but was <3 years of age by 1 day, should have been in the younger age group, and was moved there for analysis in this follow-on study [3]. 3Six participants who did not complete all of the study procedures in the original study and therefore should have been excluded from this follow-on study were approached in error [3].
Vaccine
The TIV Fluarix™ (GlaxoSmithKline Biologicals) contained inactivated split-virion influenza virus propagated in fertilized hens’ eggs with 15 μg of hemagglutinin antigen for each strain in a 0.5-mL dose [18]. The 3 World Health Organization-recommended strains were A/California/7/2009 (H1N1)-derived strain, A/Perth/16/2009 (H3N2)-like strain, and B/Brisbane/60/2008 [18, 19].
Study End Points
The primary end points were as follows: (1) persistence of microneutralizing antibody titers against pandemic virus, measured as the percentage of children with MN titers ≥1:40 at 1 year compared with 3 weeks after 2 doses of pandemic monovalent influenza vaccine [3]; (2) immunogenicity of TIV against pandemic virus 3 weeks after vaccination, measured as the percentage of children who seroconverted and had a postvaccination MN titer ≥1:40 or hemagglutination inhibition (HI) titer ≥1:32 (against H1N1 strain) or who were seropositive before vaccination and had a 4-fold increase in titer; and (3) reactogenicity after TIV, measured as the percentage of children experiencing fever, local reactions, and nonfebrile systemic symptoms within 7 days after vaccination. Different systemic symptoms were solicited in those under and over 5 years to reflect differences in ability to articulate symptoms (Supplementary Table 1; secondary end points, Supplementary Table 3).
Statistical Analysis
Data were analyzed in groups based on a child’s vaccine allocation and original study age [3]. For analysis of reactogenicity, age was divided into <5 and ≥5 years of age at the time of TIV. Proportions with titers above MN and HI thresholds, with 4-fold changes and with adverse reactions were calculated with exact 95% confidence intervals (CIs) in each group. Differences between groups were assessed using Fisher’s exact test. To assess 4-fold changes from before to after TIV, samples were further titrated if necessary. Geometric mean titers (GMTs) and fold changes were calculated for HI with 95% CIs. Post-TIV samples with high MN titers were not diluted further to obtain precise titers (unnecessary for study end points), and therefore GMT data are not presented. Where results were above or below assay limits they were set to double or half the limit, respectively. Normal error regression on logged titers was used to compare groups. Reverse cumulative distribution curves were constructed to describe the titer distributions in each group. Comparisons were made between pandemic vaccine groups in which ages could be combined as well as between pandemic vaccine groups within age groups. Significance was set at 5%; analysis was by modified intention to treat and was performed using Stata software, version 10.0 (StataCorp).
Sample Size
A target recruitment rate of 40% would give ∼90 participants for each vaccine group aged <3 and 100 for each aged ≥3. This would give precision (95% CI) around a proportion of 80% of 70%–88% and a proportion of 80% that would be detected as different from 95% (80% power, 5% significance). In the original study, children who did not seroconvert after 2 doses were offered an additional vaccine dose. Those who received it were excluded from this study [3]. To compensate for possible resulting bias, antibody titers measured after 2 doses from an appropriate number of these subjects selected at random were included in the analysis.
RESULTS
Of 943 children enrolled in the original study, 894 were eligible for enrollment, of whom 323 were recruited (36%) (Figure 1) [3]. Three hundred four provided a sample for antibody persistence, of whom 302 received TIV (2 withdrew before receipt of TIV), and 19 took part in the persistence study alone.
Persistence of Antibody Against A/H1N1(2009) Virus
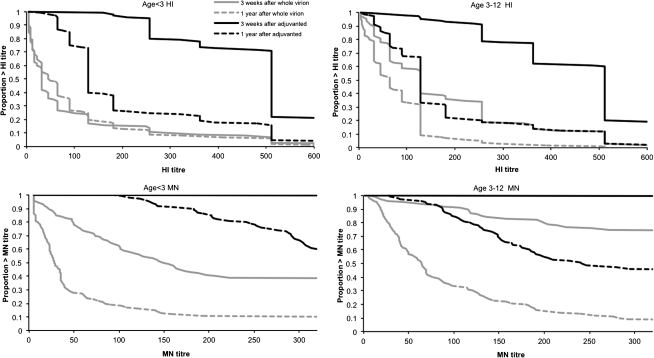
The mean interval from second dose of pandemic vaccine to persistence blood test was 387 days (range, 365–429 days). Three hundred eighteen samples were available for analysis (Figure 1 and Supplementary Table 4A The persistence of antibody levels 1 year after vaccination, defined by MN titer ≥1:40, was significantly higher in the AS03B-adjuvanted vaccine group than the whole-virion vaccine group, particularly in the younger age group (P < .001 in both age groups) (Table 1). In the original study, 100% of children in both age groups given AS03B-adjuvanted vaccine had an MN titer ≥1:40 3 weeks after vaccination (95% CIs, 94.1%–100% and 96.3%–100% in the younger and older groups, respectively). One year after vaccination, these figures were 100% (95% CI, 94.1%–100%) and 96.9% (95% CI, 91.3%–99.4%). In those originally given whole-virion vaccine, 82.4% (95% CI, 71.2%–90.5%) and 94.5% (95% CI, 87.6%–98.2%) had an MN titer ≥1:40 3 weeks after the initial vaccination, but by 1 year these had fallen to 32.4% (95% CI, 21.5%–44.8%) and 65.9% (95% CI, 55.3%–75.5%) in the younger and older groups, respectively (Table 1). These findings are illustrated by the reverse cumulative distribution curves (Figure 2).
Table 1.
Antibody Persistence (by Microneutralization [MN] and Hemagglutination Inhibition [HI] Titers) 3 Weeks and 1 Year After 2 Doses of a Nonadjuvanted Whole-Virion or an AS03B-Adjuvanted Split-Virion Monovalent Pandemic Influenza Vaccine, by Age Group
| MN ≥40 |
MN Change Between Time Points |
HI ≥32 |
HI Change Between Time Points |
|||||||
| Age Group, ya | Total No. | 3 wk After 2nd Pandemic Dose | 1 y Laterb | From ≥40 to <40 | From <40 to ≥40 | MN 1 y Later, Geometric Mean (95% CI)b | 3 wk After 2nd Pandemic Dose | 1 y Laterb | From ≥32 to <32 | From <32 to ≥32 |
| Whole-Virion Vaccine | ||||||||||
| <3 | 68 | 56 (82.4) | 22 (32.4) | 35 | 1 | 33.6 (23.8–47.5) | 40 (58.8) | 43 (63.2) | 8 | 11 |
| 3–12 | 91 | 86 (94.5) | 60 (65.9) | 26 | 0 | 66.9 (53.1–84.2) | 82 (90.1) | 72 (79.1) | 14 | 4 |
| All | 159 | 142 (89.3) | 82 (51.6) | 61 | 1 | 49.8 (40.7–61.0) | 122 (76.7) | 115 (72.3) | 22 | 15 |
| AS03B-Adjuvanted Vaccine | ||||||||||
| <3 | 61 | 61 (100) | 61 (100) | 0 | 0 | 411.9 (332.5–510.2) | 61 (100) | 60 (98.4) | 1 | 0 |
| 3–12 | 98 | 98 (100) | 95 (96.9) | 3 | 0 | 287.6 (230.5–358.9) | 98 (100) | 95 (96.9) | 3 | 0 |
| All | 159 | 159 (100) | 156 (98.1) | 3 | 0 | 330.1 (281.3–387.4) | 159 (100) | 155 (97.5) | 4 | 0 |
Unless otherwise specified, data represent No. (%) of children.
Abbreviation: CI, confidence interval.
Age groups based on age at original study [3].
P < .001 for all comparisons between 1-year values for whole-virion and AS03B-adjuvanted vaccines.
Figure 2.
Hemagglutination inhibition (HI) and microneutralization (MN) titer reverse cumulative distribution (RCD) curves 3 weeks and 1 year after pandemic vaccination by age and vaccine. (Note: MN titers are shown only to >320, the analysis end point for serum samples in the follow-on study.)
The proportion of children with titers above the putative protective threshold were comparable by both HI and MN titers (HI ≥1:32 and MN ≥1:40), except in the serum samples from those in the younger age group who had received the whole-virion vaccine, in which the percentage of children with an HI ≥1:32 increased from 58.8% (95% CI, 46.2%–70.6%) 3 weeks after initial vaccination to 63.2% (95% CI, 50.7%–74.6%) at 1 year (Table 1 and Figure 2). The HI titers of 11 of 68 younger and 4 of 91 older children in the whole-virion group were low (<1:32) 3 weeks after the second dose of pandemic vaccine but higher (≥1:32) 1 year later (Table 1); however, MN titers did not rise in these children (Table 1). When considering only children who responded well to the initial whole-virion vaccination (HI titer ≥1:32 at 3 weeks), the percentages at 1 year with HI titers ≥1:32 dropped consistently from 100% in both age groups to 80% (95% CI, 64.4%–90.9%) and 82.9% (95% CI, 73%–90.3%) in the younger and older groups, respectively (Supplementary Table 5).
Immunogenicity of Trivalent Influenza Vaccine
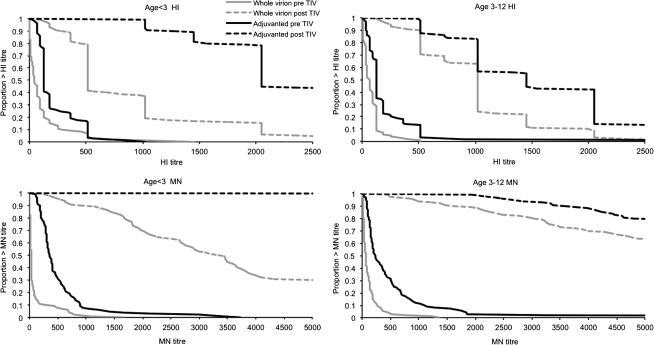
Three hundred two children received the 2010/2011 TIV (Figure 1 and Supplementary Table 4B). Across all groups the average HI GMT increased by 10.7 to 16.7 fold increase in HI GMT (Table 2). All children had a post-vaccination MN ≥1:40 and an HI titer ≥1:32 (Table 2). However, HI GMTs were significantly higher (P < .001) in those who initially received AS03B-adjuvanted vaccine compared with those receiving whole-virion vaccine, and these data are illustrated by the reverse cumulative distribution curves (Table 2 and Figure 3).
Table 2.
Immunogenicity of the H1N1 Component of Trivalent Influenza Vaccine (TIV) After a Single Dose (by Microneutralization [MN] and Hemagglutination Inhibition [HI] Titers) 1 Year After 2 Doses of Nonadjuvanted Whole-Virion or AS03B-Adjuvanted Split-Virion Monovalent Pandemic Influenza Vaccine, by Age Group
| MN ≥40 |
HI ≥32 |
HI GMT (95% CI) |
|||||||
| Age Group, ya | Before TIV 2010–2011 | 3 wk After TIV 2010–2011 | ≥4-Fold Rise in MN Titer | Before TIV 2010–2011 | 3 Weeks After TIV 2010–2011 | ≥4-Fold Rise in HI Titer | Pre-TIV 2010–2011 | 3 wk After TIV 2010–2011b | Fold Change |
| Whole-Virion Vaccine | |||||||||
| <3 | 21/60 (35.0) | 54/54 (100) | 51/53 (96.2) | 42/60 (70.0) | 54/54 (100) | 43/53 (81.1) | 50.2 (34.0–74.2) | 661.9 (524.9–834.6) | 12.6 (8.3–19.1) |
| 3–12 | 52/81 (64.2) | 82/82 (100) | 77/78 (98.7) | 64/81 (79.0) | 82/82 (100) | 73/78 (93.6) | 49.7 (38.6–64.1) | 846.6 (733.0–977.9) | 16.7 (12.8–21.7) |
| All | 73/141 (51.8) | 136/136 (100) | 128/131 (97.7) | 106/141 (75.2) | 136/136 (100) | 116/131 (88.5) | 49.9 (40.1–62.1) | 767.8 (676.6–871.3) | 14·9 (11.8–18.7) |
| AS03B-Adjuvanted Vaccine | |||||||||
| <3 | 60/60 (100) | 57/57 (100) | 56/56 (100) | 59/60 (98.3) | 57/57 (100) | 52/56 (92.9) | 159.4 (127.5–199.3) | 2611.9 (2238.1–3048.0) | 16.2 (12.2–21.4) |
| 3–12 | 91/93 (97.8) | 89/89 (100) | 82/88 (93.2) | 90/93 (96.8) | 89/89 (100) | 76/88 (86.4) | 131.4 (105.9–163.0) | 1425.8 (1244.9–1632.9) | 10.7 (8.5–13.7) |
| All | 151/153 (98.7) | 146/146 (100) | 138/144 (95.8) | 149/153 (97.4) | 146/146 (100) | 128/144 (88.9) | 141.7 (121.2–165.8) | 1805.9 (1614.3–2020.3) | 12.6 (10.5–15.1) |
Unless otherwise specified, data represent No. with MN or HI values above cutoff/total No. (%).
Abbreviations: CI, confidence interval; GMT, geometric mean titer.
Age groups based on age at original study [3].
P < .001 for all comparisons of HI GMTs 3-weeks after 2010/11 TIV between vaccine groups.
Figure 3.
Hemagglutination inhibition (HI) and microneutralization (MN) titer reverse cumulative distribution curves before and after trivalent influenza vaccine (TIV) by age and vaccine. (Note: MN titers are shown to >5120, the analysis end point for serum samples in the follow-on study.)
Reactogenicity of Trivalent Influenza Vaccine
Diaries were returned for 295 children (Figure 1). There were no serious adverse reactions. Redness and severe local symptoms were more frequent in children <5 years old who had previously received the AS03B-adjuvanted vaccine than the whole-virion vaccine (P < .05) (Supplementary Table 6). For all other solicited local and systemic symptoms, there were no significant differences between the vaccine groups. There were 2 significant differences (P < .05) between age groups: pain was reported more frequently in those >5 years of age, and fever ≥38°C was reported more frequently in those <5 years of age, regardless of preceding pandemic vaccine (Supplementary Tables 6 and 7).
Long-Term Safety Monitoring
No solicited adverse events (ie, influenzalike illness, adverse events of special interest) were reported to have occurred in the year after receipt of either pandemic influenza vaccine (Supplementary Table 8).
DISCUSSION
Persistence of Antibody
Almost all children who received the AS03B-adjuvanted vaccine retained MN titers ≥1:40 1 year later, compared with 52% of those previously immunized with whole-virion vaccine (Table 1). Other studies have shown persistence of anti-H1N1 titer at 6 months after AS03A-adjuvanted vaccine [15, 20]. However, the pediatric study used the full adult dose (0.5 mL) [15], not the half dose recommended since September 2009 [21], and the data do not extend to a second influenza season (AS03A and AS03B differ only in that “B” denotes pediatric adjuvant dose). A recent analysis of the vaccine effectiveness of either monovalent pandemic influenza vaccination in 2009/2010, TIV in 2010/2011, or both in protecting against A/H1N1 (2009) virus during the winter of 2010–2011 suggested that single-dose pandemic vaccine protection may not last across seasons, although only 20% of those studied were children [22]. These data are supported by a recent Canadian study showing that only 46% of children maintained protective levels of antibody 1 year after receiving a single dose of AS03B-adjuvanted pandemic influenza vaccine [23]. Our data suggest that a 2-dose regimen of AS03B-adjuvanted pandemic vaccine may have been more effective across influenza seasons.
In the initial stages of a pandemic, there is high likelihood of both a limited supply of pandemic antigen and logistical difficulties in administering a 2-dose immunization regimen. However, the lower antibody responses with a single dose reported by some [23] may limit protection for a second wave compared with the 2-dose regimen reported here. In a subsequent pandemic, policymakers might therefore plan to initially give a single dose of an adjuvanted influenza vaccine to all age groups for the first wave, which is of proven effectiveness [7–11], followed by a booster dose of the vaccine at the time of the second pandemic wave, thus providing capacity during the first wave and time for vaccine production for the subsequent booster dose.
Although there is less published data, MN titers are likely to be a better correlate of protection against influenza than HI titers [24]. In the younger vaccine group who received the whole-virion vaccine in 2009, the proportion with MN titers ≥1:40 fell from 82.4% 3 weeks post-vaccination to 32.4% at 1 year. Unexpectedly, the proportion with HI titers ≥1:32 remained comparable (58.8%–63.2%) during the same period (Figure 2 and Table 1). This disparity between MN and HI titers was not seen in other groups or in previous studies [3, 16, 25, 26]. Although the higher HI titers are perhaps most likely to have been caused by wild-type H1N1 infection in those who had a previously poor response to the initial vaccine course (Table 1), it is difficult to propose a biologically plausible explanation as to why their MN titers decreased. Although no influenza-like illnesses were reported, it is possible that these were mild or subclinical H1N1 infections, a possibility compatible with national seroepidemiological evidence that showed 26% of >5-year-olds and 53% of 5–14-year-olds were infected in the second wave [26]. Differences between MN and HI trends could also occur because MN measures a broader range of antibodies that neutralize the virus, whereas HI measures a limited set of epitopes involved in hemagglutination. One possible explanation is that, in some vaccinated children, responses to hemagglutinin predominate on exposure to infection. Further work will be required to assess the correlation between MN titers and protection from influenza.
Immunogenicity of Seasonal Trivalent Influenza Vaccine
Findings of other studies have suggested that prior receipt of TIV may reduce the immunogenicity of pandemic monovalent influenza vaccines [14, 15]. Despite no control group, our data do not suggest that the reverse is true. In all groups, the GMTs were higher than those seen 3 weeks after the original vaccination course. A higher anti-H1N1 titer before TIV was associated with a higher titer after vaccination (data not shown). In the younger age group, previous receipt of an AS03B-adjuvanted vaccine seems to enhance the serological response to a dose of a nonadjuvanted vaccine 1 year later, even allowing for the prevaccination titers (P = .03) (data not shown). This result could be explained by an enhanced priming mechanism in the adjuvanted vaccine group (Figure 3). Therefore, it will be informative to determine whether there was any effect of vaccine group on responses to the nonpandemic influenza strains included in the TIV.
Reactogenicity of Seasonal Trivalent Influenza Vaccine
Mild reactogenicity was seen in all groups after TIV. Four children <5 years of age had a temperature of ≥39°C, 1 in the AS03B-adjuvanted and 3 in the whole-virion vaccine groups. No febrile convulsions were reported.
Long-Term Safety Monitoring
No adverse events of special interest occurred in the year after pandemic vaccination, although the study was not powered to detect rare events. Despite recent European reports of narcolepsy in children and young adults who received AS03B-adjuvanted vaccine [27, 28], there has been no UK signal detected from passive surveillance [29].
Limitations and Applicability
First, the main limitation was lower recruitment than anticipated, possibly owing to low public anxiety regarding influenza during the recruitment window. Despite this, the demographics were similar between follow-on and original studies (Supplementary Table 9), apart from comparatively fewer <3-year-old children being recruited in the follow-on study and one site recruiting less well than in the original study. Although this loss of power is relevant when interpreting differences between groups, the resultant reduction in detectable difference is not marked (∼3%), and actual differences seen between the vaccine groups were generally large. Second, we have currently assessed only the immunogenicity of the H1N1 component of TIV. Assessment of the other 2 TIV strains is planned when funding is available. The relevance of our results to adjuvanted polyvalent vaccines remains uncertain. Third, owing to funding limitations, this study was limited to the children in our original study, who all received 2 doses of pandemic influenza vaccine rather than the 1-dose regimen subsequently recommended for the AS03B-adjuvanted vaccine in the national program for healthy children in the United Kingdom [3, 6]. The majority of children in the United Kingdom who were vaccinated with adjuvanted pandemic vaccine received only 1 dose, and they are likely to have lower antibody persistence [30].
Eighty-one percent of the children known to have died in the 2009/2010 UK winter season from pandemic H1N1 infection were in clinical risk groups [31]. More than 25000 at-risk children received a 2-dose vaccination regimen similar to that received by our cohort (R. Pebody, personal communication) [4]. While our original study did not specifically recruit at-risk children (although some were included, such as asthmatics), our data are directly applicable to the at-risk children who are most likely to die of pandemic H1N1 infection.
CONCLUSION
Children who received 2 doses of AS03B-adjuvanted pandemic influenza vaccine showed persistence of antibody above putative protective thresholds 1 year after vaccination compared with only approximately half of those who received the whole-virion vaccine. It is safe to administer trivalent seasonal influenza vaccine to children who received a 2-dose regimen of the pandemic influenza vaccines used in the United Kingdom in 2009, and this increased children’s antibody levels against the H1N1 component of the vaccine. This study provides serological evidence that a 2-dose regimen of vaccination with an AS03B-adjuvanted pandemic influenza vaccine may be sufficient to maintain protection across 2 influenza seasons. Pandemic planners may consider initially administering a single dose of adjuvanted pandemic vaccine to allow higher population coverage from limited dose supply, followed by a booster dose in the next season if the same strain is circulating.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Authors’ contributions.
A. J. P., M. D. S., E. M., S. N. F., A. F., P. T. H., R. T., N. A., K. H., P. d. W., and W. T. W. designed the study with critical input from all authors. A.J.P. was chief investigator. M. D. S., S. N. F., A. F., P. T. H., and R. T. were local principal investigators. N. A. was responsible for statistical analysis, K.H. was responsible for laboratory analysis, and P. K. was responsible for data management, all with oversight and guidance by E. M. (lead investigator). B. T., T. M. J., and C. J. were project managers. S. K. was responsible for data monitoring, and J. C. was responsible for the computer database. W. T. W., P.d.W., C. O., M. C., L. M., C. H., P. M., C. J., T. M. J., R. T., P. T. H., S. N. F., M. D. S., A. F., and A. J. P. enrolled patients and/or contributed to data collection. A. J. P., M. D. S., E. M., S. N. F., A. F., P. T. H., R. T., N. A., K. H., P. d. W., and W. T. W. were involved in data interpretation. W. T. W. and S. N. F. drafted this report, which was subsequently reviewed by all authors. All authors have seen the final submitted report and agree with its contents. All authors had full access to all the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments.
We are grateful to all children and parents and who participated in the study; and all the physicians, nurses, and administrative support staff who assisted with study visits; E. Plested (Oxford), the Southampton University Hospitals NHS Trust R&D Office and Child Health Computer Departments of Primary Care Trusts in Oxford, Southampton, Bristol, Exeter, and London (Wandsworth).
Financial support.
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (project number 10/111/01) and will be published in full in Health Technology Assessment. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS or the Department of Health. This study was also supported by the NIHR Oxford Comprehensive Biomedical Research Centre, NIHR Southampton Respiratory Biomedical Research Unit and the NIHR Thames Valley, Hampshire and Isle of Wight, South London and Western Comprehensive Local Research Networks, and the South West and London & South East NIHR Medicines for Children Local Research Network.
Potential conflicts of interest.
Vaccines used in the original study were manufactured by GlaxoSmithKline and Baxter, both of which donated the vaccine but had no role in study planning or conduct. The vaccine used in this follow-on study was manufactured by GlaxoSmithKline, from whom it was purchased. A. J. P., A. F., M. D. S., P. T. H., and S. N. F. act as chief or principal investigators for clinical trials conducted on behalf of their respective NHS trusts and/or universities, sponsored by vaccine manufacturers, but receive no personal payments from them. K.H. has been an investigator for clinical trials sponsored by vaccine manufacturers but received no personal payments from them. A. J. P., A. F., M. D. S., P. T. H., and S.N.F. have participated in advisory boards for vaccine manufacturers but receive no personal payments for this work. M. D. S., S.N.F., P. T. H., K. H., and A. F. have received financial assistance from vaccine manufacturers to attend conferences. All grants and honoraria are paid into accounts within the respective NHS trusts or universities or to independent charities.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Pandemic (H1N1) 2009-update 109. Available at: http://www.who.int/csr/don/2010_07_16/en/index.html. Accessed 30 November 2011.
- 2.Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr. 2010 doi: 10.1371/currents.RRN1153. RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waddington CS, Walker WT, Oeser C, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ. 2010;340:c2649. doi: 10.1136/bmj.c2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi M, Peabody R. Pandemic H1N1 (swine) influenza vaccine uptake amongst patient groups in primary care in England 2009/10. Available at: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_121014.pdf. Accessed 30 November 2011.
- 5. Department of Health. Influenza (updated 19 January 2011). Immunisation against infectious disease—the “green book” 2007. Department of Health, London: UK Dept of Health 2011.
- 6.Dalton I. A (H1N1) swine flu influenza: phase two of the vaccination programme: children over 6 months and under 5 years. Available at: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_110180.pdf. Accessed 30 November 2011.
- 7.Wichmann O, Stocker P, Poggensee G, et al. Pandemic influenza A(H1N1) 2009 breakthrough infections and estimates of vaccine effectiveness in Germany 2009–2010. Euro Surveill. 2010;15:1–4. [PubMed] [Google Scholar]
- 8.Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Vaccine effectiveness in pandemic influenza - primary care reporting (VIPER): an observational study to assess the effectiveness of the pandemic influenza A (H1N1)v vaccine. Health Technol Assess. 2010;14:313–46. doi: 10.3310/hta14340-05. [DOI] [PubMed] [Google Scholar]
- 9.Van Buynder PG, Dhaliwal JK, Van Buynder JL, et al. Protective effect of single-dose adjuvanted pandemic influenza vaccine in children. Influenza Other Respi Viruses. 2010;4:171–8. doi: 10.1111/j.1750-2659.2010.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skowronski DM, Janjua NZ, De Serres G, et al. Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009. BMJ. 2011;342:c7297. doi: 10.1136/bmj.c7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews N, Waight P, Yung CF, Miller E. Age-specific effectiveness of an oil-in-water adjuvanted pandemic (H1N1) 2009 vaccine against confirmed infection in high risk groups in England. J Infect Dis. 2011;203:32–9. doi: 10.1093/infdis/jiq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson J, Peabody G. Pandemic influenza vaccines. BMJ. 2011;342:d545. doi: 10.1136/bmj.d545. [DOI] [PubMed] [Google Scholar]
- 13.Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis. 2010;51:668–77. doi: 10.1086/655830. [DOI] [PubMed] [Google Scholar]
- 14.Andrews NJ, Walker WT, Finn A, et al. Predictors of immune response and reactogenicity to AS03B-adjuvanted split virion and non-adjuvanted whole virion H1N1 (2009) pandemic influenza vaccines. Vaccine. 2011;29:7913–9. doi: 10.1016/j.vaccine.2011.08.076. [DOI] [PubMed] [Google Scholar]
- 15. GlaxoSmithKline. Result summary for 113572. Available at: http://www.gsk-clinicalstudyregister.com/result_detail.jsp?protocolId=113572&studyId=945383E4-24EB-4745-BE5B-B2B9654518AC&compound=H1N1+Pandemic+Influenza+Vaccine. Accessed 30 November 2011.
- 16.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis JS, Zambon MC. Molecular analysis of an outbreak of influenza in the United Kingdom. Eur J Epidemiol. 1997;13:369–72. doi: 10.1023/A:1007391222905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. GlaxoSmithKline. Fluarix—summary of product characteristics. Available at: http://www.medicines.org.uk/emc/medicine/2038/SPC/Fluarix. Accessed 30 November 2011.
- 19. World Health Organization. Recommended viruses for influenza vaccines for use in the 2010–2011 northern hemisphere influenza season. Available at: http://www.who.int/immunization/sage/3_Recommendation.pdf. Accessed 30 November 2011.
- 20.Nicholson KG, Abrams KR, Batham S, et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis. 2011;11:91–101. doi: 10.1016/S1473-3099(10)70296-6. [DOI] [PubMed] [Google Scholar]
- 21. GlaxoSmithKline. Pandemrix. Summary of product characteristics. Available at: http://www.medicines.org.uk/emc/medicine/22352/SPC/Pandemrix+suspension+and+emulsion+for+emulsion+for+injection/. Accessed 30 November 2011.
- 22.Pebody R, Hardelid P, Fleming D, et al. Effectiveness of seasonal 2010/11 and pandemic influenza A(H1N1)2009 vaccines in preventing influenza infection in the United Kingdom: mid-season analysis 2010/11. Euro Surveill. 2011;16:1–6. [PubMed] [Google Scholar]
- 23.Gilca V, De Serres G, Hamelin M, et al. Antibody persistence and response to 2010–11 trivalent influenza vaccine one year after a single dose of 2009 AS03-adjuvanted pandemic H1N1 vaccine in children. The Hague: European Society of Pediatric Infectious Disease; 2011. [DOI] [PubMed] [Google Scholar]
- 24.Katz J, Hancock K, Veguilla V, et al. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. Morbidity Mortality Weekly Rev. 2009;58:521–4. [PubMed] [Google Scholar]
- 25.Center for Disease Control and Prevention. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009;58:521–4. [PubMed] [Google Scholar]
- 26.Hardelid P, Andrews NJ, Hoschler K, et al. Assessment of baseline age-specific antibody prevalence and incidence of infection to novel influenza A/H1N1 2009. Health Technol Assess. 2010;14:115–92. doi: 10.3310/hta14550-03. [DOI] [PubMed] [Google Scholar]
- 27. The National institute for Health and Welfare. Increased risk of narcolepsy observed among children and adolescents vaccinated with Pandemrix. Available at: http://www.thl.fi/en_US/web/en/pressrelease?id=24103. Accessed 30 November 2011.
- 28. European Medicines Agency. European Medicines Agency reviews further data on narcolepsy and possible association with Pandemrix. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2011/02/news_detail_001211.jsp&mid=WC0b01ac058004d5c1&murl=menus/news_and_events/news_and_events.jsp&jsenabled=true. Accessed 30 Nov 2011.
- 29. UK Medicines and Healthcare products Regulatory Agency (MHRA). MHRA public assessment report—Swine flu vaccines and antiviral medicines: UK post- pandemic safety review—February 2011. 2011.
- 30.Scheifele DW, Ward BJ, Dionne M, et al. Evaluation of adjuvanted pandemic H1N1(2009) influenza vaccine after one and two doses in young children. Pediatr Infect Dis J. 2011;30:402–7. doi: 10.1097/INF.0b013e3182068f33. [DOI] [PubMed] [Google Scholar]
- 31.Sachedina N, Donaldson LJ. Paediatric mortality related to pandemic influenza A H1N1 infection in England: an observational population-based study. Lancet. 2010;376:1846–52. doi: 10.1016/S0140-6736(10)61195-6. [DOI] [PubMed] [Google Scholar]