Abstract
The equine superficial digital flexor tendon (SDFT) has a graded distribution of collagen fibril diameters, with predominantly small-diameter fibrils in the region of the myotendinous junction (MTJ), a gradual increase in large-diameter fibrils toward the osteotendinous junction (OTJ), and a mixture of small- and large-diameter fibrils in the middle metacarpal (MM) region. In this study, we investigated the ultrastructure of the SDFT, to correlate the spatial relationship of the collagen fibrils with the graded distribution. The surface-to-surface distances of pairs of fibrils were found to be almost constant over the entire tendon. However, the center-to-center distances varied according to fibril diameter. Decorin is the predominant proteoglycan in normal mature tendons, and has one dermatan sulfate (DS) or chondroitin sulfate (CS) filament as a side chain which is associated with the surfaces of the collagen fibrils via its core protein. We identified a coordinated arrangement of decorin DS filaments in the equine SDFT. The sizes of the decorin DS filaments detected by Cupromeronic blue staining showed a unique regional variation; they were shortest in the MM region and longer in the MTJ and OTJ regions, and a considerable number of filaments were arranged obliquely to adjacent collagen fibrils in the MTJ region. This regional variation of the filaments may be an adaptation to lubricate the interfibrillar space in response to local mechanical requirements. The results of this study suggest that the MTJ region, which receives the muscular contractile force first, acts as a buffer for mechanical forces in the equine SDFT.
Keywords: collagen fibril, decorin, dermatan sulfate, horse, tendon
Introduction
Tendons transfer the forces generated by muscles to bones to generate smooth movement. They are primarily composed of collagen fibrils, and their mechanical properties are dependent on the organization and interactions of these fibrils. The relationships between the structure and mechanical behavior of tendons have been investigated with biomechanical experiments, which showed that variations in the mechanical properties of a tendon result from differences in the assembly of the component collagen fibrils. Generally, the stress–strain curves of tendons indicate the presence of viscoelastic properties (Puxkandl et al. 2002) which are affected by collagen fibril diameter; large-diameter fibrils bear high tensile strength and small-diameter fibrils contribute to elasticity (Parry et al. 1978a,b; Magnusson et al. 2003). Furthermore, differences in mechanical function in tendons are related to the substructures comprising collagen fibrils, non-collagenous proteins, and proteoglycans bearing glycosaminoglycan (GAG) chains (Puxkandl et al. 2002).
Decorin is the predominant proteoglycan in normal mature tendons. It belongs to the small leucine-rich proteoglycan family and consists of a core protein containing leucine repeats with a covalently attached GAG chain of either dermatan sulfate (DS) or chondroitin sulfate (CS) (Rees et al. 2000; Zhang et al. 2006). Several studies have indicated that each decorin core protein binds to the surface of a collagen fibril at 64–68-nm intervals by non-covalent bonding, whereas the decorin GAG chain covalently bonds with the core protein and extends from it to associate closely with a nearby decorin GAG chain through electrostatic interactions, which connects it to another decorin core protein on an adjacent fibril surface (Redaelli et al. 2003; Scott, 2003; Vesentini et al. 2005). The role of decorin in tendon is still controversial. Some studies have suggested that the GAGs of the decorin bridges guarantee the mechanical integrity of the interconnecting structures between collagen fibrils (Cribb & Scott, 1995; Puxkandl et al. 2002; Redaelli et al. 2003; Liu et al. 2005). Mechanical testing using tendons from decorin knockout mice showed complicated results; there were no changes in either tensile strength or elasticity of tail tendon fascicles compared with normal mice, but there were noted differences in patellar tendons (Robinson et al. 2005). Another experiment using human knee ligaments treated with chondroitinase B to remove DS demonstrated few differences in tensile and shear deformation under quasi-static loading conditions (Lujan et al. 2007). Recently, Fessel & Snedeker (2009) suggested that the decorin GAG chains were irrelevant to dynamic elastic behavior and to regulation of dynamic viscoelastic properties in tendon. Liao & Vesely (2007) proposed a mechanical contribution of GAG bridges among adjacent collagen fibrils to regulate slippage between collagen fibrils and reduce interfibrillar gaps during loading. The results of these studies support the theory that GAGs act to lubricate the interfibrillar space and support compressive loads via water retention, but at extremely low fibril deformation rates (Elliott et al. 2003; Scott, 2003; Basalo et al. 2007; Henninger et al. 2009).
The anatomical features of the mature equine superficial digital flexor tendon (SDFT) have been extensively studied as they form the physical basis for the athletic potential of horses. Recent reports describing the mature equine SDFT revealed graded structural differences along the longitudinal axis of the tendon which may have unique mechanical and functional characteristics. We previously identified regional differences in the distribution of collagen fibril diameters in the SDFT (Watanabe et al. 2005, 2007). We found predominantly small-diameter collagen fibrils (< 100 nm) in the region of the myotendinous junction (MTJ), mostly large-diameter fibrils (> 200 nm) in the region of the osteotendinous junction (OTJ), and a mixture of small- and large-diameter fibrils in the middle metacarpal (MM) region (Sese et al. 2007; Watanabe et al. 2007). This implies that the mechanical properties of the SDFT differ depending on location. Rigozzi et al. (2009) suggested that the mechanical properties of mouse Achilles tendon differ by region, and that this might be related to the distribution of GAGs. In the present study, we investigated the spatial arrangement of collagen fibrils among the regions with different distributions of collagen fibril diameters in the mature equine SDFT, and analyzed the relationship between the interaction of adjacent collagen fibrils and local decorin DS filaments. We aimed to clarify whether the adaptive alignment of these filaments fulfills the mechanical requirements of each region within the tendon. We discuss the possible functions of decorin in relation to the assembly of collagen fibrils and the regulation of collagen fibril diameters.
Materials and methods
Animals
Tissue samples were obtained from the normal SDFTs of four young thoroughbred horses (age, 2–3 years; mean ± SD, 2.75 ± 0.5 years). In each horse, a 2-cm-long sample was excised from each of the MTJ, MM, and OTJ regions. All samples were obtained from the central zone of each region, corresponding to the areas generally used for evaluation of collagen fibrils in the equine tendon (Birch et al. 1998; Watanabe et al. 2005, 2007). Each sample was hemisected; one section was prepared for morphological observations and the other was flash-frozen in liquid nitrogen and stored at −80 °C for morphological and biochemical analyses of GAGs.
Electron microscopy
One section from each sample was sliced under a dissecting microscope into 1 × 1 × 2 mm blocks, immersed in 3% glutaraldehyde in 0.1 m phosphate buffer for 24 h, and washed in phosphate buffer. Samples were postfixed in 1% (w/v) OsO4 for 30 min, dehydrated through a graded series of ethanols, and embedded in Quetol 812 (Nisshin EM, Tokyo, Japan). Ultrathin sections were obtained using an ultramicrotome (Reichert Supernova System; Leica, Austria) and mounted on copper grids. Sections were stained with 0.2% (w/v) tannic acid solution (Merck, Tokyo, Japan) for 15 min, 1% (w/v) uranyl acetate for 5 min, and 2% (w/v) lead citrate for 5 min. Tissue samples were examined under a transmission electron microscope (TEM: JEM-1220; JEOL, Tokyo, Japan) at an acceleration voltage of 80 kV.
Measurement of collagen fibril diameters and the spatial relationships between collagen fibrils
Four photomicrographs were randomly selected from each region of one tendon, and 1000 fibrils in total were measured on the four photomicrographs (250 on each micrograph) with image j version 1.30 software. Subsequently, the mass average diameter (MAD) of fibrils was calculated according to the method of Flint et al. (1984). Collagen fibril populations were characterized by MAD because it provides more representative and functional information than the arithmetic average of fibril diameters (Flint et al. 1984). The spatial relationships between?collagen fibrils were defined according to the method of Kuwaba et al. (2001) and are drawn schematically in Fig. 1. The center-to-center and surface-to-surface distances between collagen fibrils (n = 700 per micrograph) were measured with image j software.
Fig. 1.
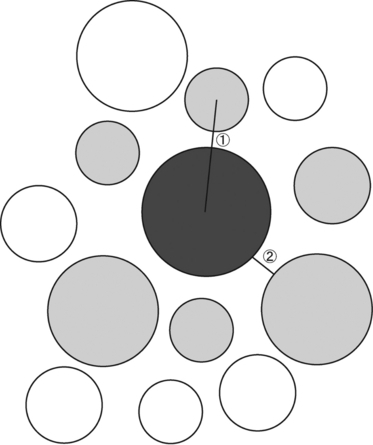
Schematic representation of the measurements of the center-to-center and surface-to-surface distances between collagen fibrils. The distances are defined according to Kuwaba et al. (2001). A collagen fibril (dark gray) surrounded by five or six adjacent fibrils (light gray) was randomly selected. The distance between the central fibril and each adjacent fibril was measured: 1, center-to-center distance; 2, surface-to-surface distance.
Cupromeronic blue stain
To examine DS filaments, samples from each region of each tendon were embedded in Tissue-Tek O.C.T. compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan) and frozen in liquid nitrogen. The frozen samples were sectioned longitudinally at a thickness of 10 μm and mounted on aqueous gelatin-coated (10% w/v at 40 °C) glass slides. Samples were stained with Cupromeronic blue in a critical electrolyte concentration technique to reveal sulfated GAGs (Scott et al. 1981; Haigh & Scott, 1986; Cribb & Scott, 1995). Sections were stained with 0.05% Cupromeronic blue (w/v) (Seikagaku Corp., Tokyo, Japan) in 0.025 sodium acetate buffer (pH 5.8) containing 3% glutaraldehyde (v/v) and 0.1 m MgCl2 for 6 h at room temperature, stained further with 0.034 m Na2WO4, embedded in Quetol 812, and cut into longitudinal ultrathin sections. The ultrathin sections were stained with uranyl acetate and examined under the TEM. Four photomicrographs from each region of each tendon were randomly selected. The lengths of the stained DS filaments and the angles between the longitudinal axes of the DS filaments and the collagen fibrils (n = 250 per photomicrograph) were measured with image j software. This angle was defined as the association angle and expressed as a value from 0 to 90 °C. To investigate whether decorin was also attached to CS chains, additional samples were pre-digested with 1 U of either chondroitinase ABC (Seikagaku Corp.), an enzyme that degrades DS and CS side-chains, or chondroitinase ACII (Seikagaku Corp.), an enzyme that specifically degrades CS chains, for 1 h at 37 °C prior to Cupromeronic blue staining.
Electrophoresis and western blotting
Frozen samples from each region of each tendon were dissected into 0.1 g blocks, which were homogenized and solubilized with CelLytic (Sigma, St. Louis, MO, USA) at 4 °C, according to the manufacturer's instructions. Protein concentrations were standardized by Lowry's method (Lowry et al. 1951). Samples containing the same amount of extracted protein were subjected to electrophoresis and Western blot analysis. To examine the molecular size of decorin DS, samples were subjected to SDS/polyacrylamide gel electrophoresis (SDS/PAGE) (10% gel) and Western blotting. Samples were boiled for 5 min with a sodium dodecyl sulfate (SDS) sample solution containing 1% SDS, 5% glycerol, and 0.1% bromophenol blue, and separated by SDS/PAGE. Samples in the gels were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore Corporation, Billerica, MA, USA) and analyzed by Western blotting. Membranes were blocked with skim milk powder (Wako, Tokyo, Japan) for 1 h to minimize nonspecific binding and washed four times with phosphate-buffered saline [PBS: 137 mm NaCl, 2.7 mm KCl, 8.1 mm Na2HPO4, 1.5 mm KH2PO4 (pH 7.4)] containing 0.2% Tween 20 (PBST).
To detect decorin, membranes were incubated with anti-human dermatan sulfate proteoglycan (1 : 2000 PBST; Seikagaku Corp.). The antibody for human decorin core protein cross-reacted with the equine counterpart, since exactly the same epitope amino acid sequence occurs in the equine decorin sequence (Sawada et al. 2002).
Following incubation with anti-human dermatan sulfate proteoglycan, blots were washed four times with PBST and incubated with peroxidase-labeled anti-mouse IgG (Amersham Biosciences, Little Chalfont, Bucks., UK) for 1 h at room temperature. Bound antibodies were visualized with an ECL plus Western Blotting Detection System (Amersham) according to the manufacturer's instructions. To examine the molecular size of the DS-depleted core protein of decorin, the proteoglycan fraction was pre-digested with chondroitinase ABC for 4 h at 37 °C prior to SDS/PAGE. The immunoreactivity of decorin was analyzed with nih image software, version 1.61.
Statistical analysis
Differences between representative values (MAD, center-to-center distance, surface-to-surface distance, DS filament length, and the absolute value of the association angle) of each region were tested with anova, and with a selected Scheffe's test for multiple comparisons. The significance level was set at 0.05.
Results
We used biochemical methods to analyze regional differences in decorin distribution within the equine SDFT. Broad bands with an apparent molecular weight of 70 kDa were identified in all samples (Fig. 2), indicating the presence of a proteoglycan with a heterogeneous GAG chain. Following pretreatment of the samples with chondroitinase ABC, a sharp band with an apparent molecular weight of 45 kD was revealed. This band represented the core protein of the proteoglycan molecule.
Fig. 2.
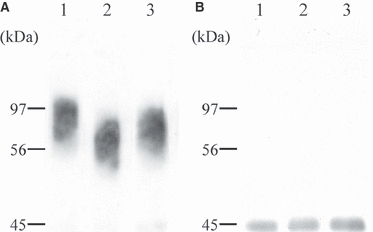
Western blot analysis of decorin extracted from three regions of the superficial digital flexor tendon. The same amounts of protein in the extract from each region were applied and immunostained with anti-human dermatan sulfate proteoglycan antibody before (A) and after (B) chondroitinase ABC treatment: lane 1, myotendinous region; lane 2, middle metacarpal region; lane 3, osteotendinous region.
Mean migration of the detected band representing the undigested proteoglycan molecule was greater in extracts from the MM region than in those from the other two regions, although migration of the core protein after chondroitinase treatment was the same in all samples. This implies that the decorin GAG chains from the MM region are shorter than those from the other regions.
Figure 3 shows cross-sectional TEM images of collagen fibrils in the three different regions. As the tendency of distribution of collagen fibrils was the same as previously reported (Watanabe et al. 2007), we emphasized the spatial relationships of the fibrils including the center-to-center and surface-to-surface distances between pairs of neighboring fibrils. No significant differences were observed in the mean surface-to-surface distances among the three regions. However, the mean center-to-center distance was significantly larger in the OTJ region than in the other regions, reflecting the presence of a greater number of large-diameter fibrils (Table 1).
Fig. 3.
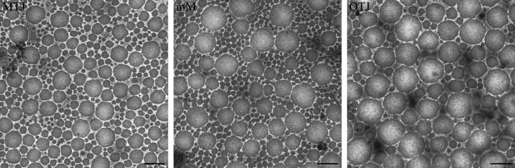
Electron micrographs showing cross-sections of collagen fibrils in the myotendinous junction (MTJ), middle metacarpal (MM) region, and osteotendinous junction (OTJ) in the superficial digital flexor tendon. Center-to-center and surface-to-surface distances between collagen fibrils were measured; means are shown in Table 1. Bar: 200 nm.
Table 1.
Center-to-center distances (nm), surface-to-surface distances (nm), length of dermatan sulfate (DS) filaments (nm), and association angles (°) in the different regions of the mature equine superficial digital flexor tendon
| Region | MAD | Center-to-center distance (nm) | Surface-to-surface distance (nm) | DS filament length (nm) | Association angle (º) |
|---|---|---|---|---|---|
| MTJ | 102.00 ± 5.6* | 82.14 ± 9.2 | 14.59 ± 0.9 | 41.46 ± 1.5 | 62.48 ± 0.8* |
| MM | 143.71 ± 8.9 | 84.18 ± 4.1 | 13.59 ± 1.7 | 30.53 ± 1.8* | 74.72 ± 2.0 |
| OTJ | 152.27 ± 7.5 | 119.79 ± 6.19* | 13.23 ± 0.7 | 38.74 ± 0.7 | 74.48 ± 1.0 |
MAD, mass average diameter; DS, dermatan sulfate; MTJ, myotendinous junction; MM, middle metacarpal; OTJ, osteotendinous junction.
The association angle is defined as the angle between the longitudinal axis of a DS filament and a collagen fibril. Data are mean ± standard error.
Value is significantly different from that of the other regions (P < 0.05).
Characteristic short filaments were identified along the collagen fibrils in longitudinal sections with Cupromeronic blue staining (Fig. 4A). These appeared periodically on almost every d-band of the collagen fibrils (Fig. 4B). Pretreatment of the samples with chondroitinase ACII had no effect on these filaments (data not shown). These data indicate that Cupromeronic blue stained only DS filaments, and that there were undetectable levels of CS in our specimens.
Fig. 4.
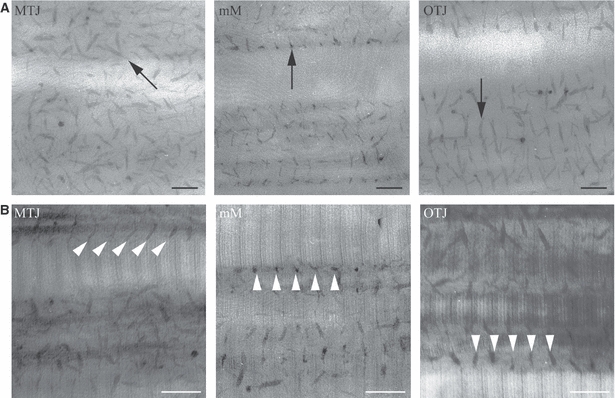
Electron micrographs showing longitudinal sections of collagen fibrils and dermatan sulfate (DS) filaments in the myotendinous junction (MTJ), middle metacarpal (MM) region, and osteotendinous junction (OTJ) in the superficial digital flexor tendon. (A) The samples were stained with cupromeronic blue to examine DS filaments. The lengths of the stained DS filaments were measured and the means are shown in Table 1. Arrows indicate DS filaments. Bar: 100 nm. (B) The samples were stained with cupromeronic blue, and then with uranyl acetate. The association angles between the longitudinal axes of the DS filaments and the collagen fibrils were measured and the means for each region are shown in Table 1. The angle was expressed as a value from 0 to 90 °C. Arrowheads indicate DS filaments. Bar: 100 nm.
We measured the length of the DS filaments and their association angles, defined as the angle between the longitudinal axis of a DS filament and a collagen fibril (Table 1). In the MM region, the filament lengths were significantly shorter and the molecular size of decorin DS significantly smaller than in the other regions. However, the molecular size of the core proteins after pretreatment with chondroitinase ABC was the same in all three regions (Fig. 2). These results confirm that DS filament lengths were significantly shorter in the MM region.
Cupromeronic blue staining showed that most DS filaments were arranged orthogonally to adjacent collagen fibrils. However, a considerable number of filaments in the MTJ region were arranged obliquely (Fig. 4), and the association angle was significantly smaller in this region than in the other regions.
Discussion
In a previous report, we described a graded arrangement of collagen fibrils with different diameters in the equine SDFT, with predominantly small-diameter fibrils in the region of the MTJ, and a gradual increase in large-diameter fibrils toward the OTJ (Watanabe et al. 2007). In this paper, we investigated the spatial arrangement of collagen fibrils and constructed a model showing their organization in the SDFT (Fig. 5). The surface-to-surface distances between neighboring fibrils were almost constant over the entire tendon, whereas the center-to-center distances varied according to fibril diameter. Small-diameter collagen fibrils predominate in the MTJ region, which is relatively rich in type V collagens. An increase in the relative ratio of type V collagen has been reported to decrease fibril diameter (Mizuno et al. 2001; Watanabe et al. 2007). These small-diameter fibrils serve as anchorages for the finger-like processes at the terminal ends of skeletal muscle fibers (Kvist et al. 1991) and they make firm contact with and directly fuse to the exterior of the fibro-reticular lamina of the basement membrane of the muscle fibers (Adachi & Hayashi, 1994). The small-diameter fibrils of the MTJ region have been observed by high-voltage electron microscopy to extend distally and have been found to fuse with each other to form thick fibrils in the MM region (Watanabe et al. 2007). In the OTJ region, thick fibrils predominate and fuse directly to bone. This organization is an example of a graded distribution of collagen supramolecular aggregates, and may be functionally significant, as graded interrelations are involved in the homeostasis of tissue structure and function (Hayashi et al. 1999).
Fig. 5.
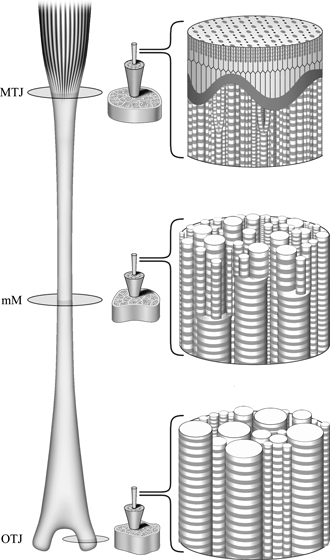
A model showing the graded organization of collagen fibrils in the superficial digital flexor tendon. In the myotendinous junction (MTJ) region (upper part of the tendon), collagen fibrils with a small diameter make firm contact with and fuse directly to the basement membrane of muscle fibers. Fine fibrils in the MTJ extend downwards and fuse with each other to form thick fibrils in the middle metacarpal (MM) region (middle part). In the osteotendinous junction (OTJ) region, thick fibrils are predominant and fuse directly to the bone (lower part).
Several studies have proposed models with short and/or discontinuous collagen fibrils, and with decorin-based DS GAGs on adjacent fibril surfaces working as functional cross-links which transfer forces and contribute to viscoelastic properties in tendons and ligaments (Cribb & Scott, 1995; Puxkandl et al. 2002; Raspanti et al. 2002; Redaelli et al. 2003; Vesentini et al. 2005). However, recent studies have shown contradictory results indicating that collagen is responsible for mechanical function but GAGs are not (Fessel & Snedeker; Lujan et al. 2007; Provenzano & Vanderby, 2006; Robinson et al. 2005; Screen et al. 2005). As shown in our previous report, there is frequent fusion between collagen fibrils in the MM region. Fibril fusion and continuity of fibrils in mature tendon has also been observed by scanning electron microscopy (Provenzano & Vanderby, 2006). These results support the idea that collagen fibrils, which are continuous from muscle to bone, are the basic structures responsible for the mechanical function of the tendon. It is plausible that the role of the GAGs is to lubricate the interfibrillar space and to support compressive loads via water retention.
The CS/DS proteoglycans decorin and biglycan are found in tendons (Scott, 1996; Iozzo, 1999). Decorin and biglycan show high homology, have a leucine-rich core protein with either one (decorin) or two (biglycan) covalently attached GAG side chains (Iozzo, 1999), and compete for collagen binding. The GAG filaments shown by Cupromeronic blue staining are mainly decorin and not biglycan, as evidence suggests that biglycan decreases rapidly to barely detectable levels during tendon development (Zhang et al. 2006) and that decorin becomes the predominant proteoglycan in the normal mature tendon (Scott, 1992, 1996; Rees et al. 2000; Redaelli et al. 2003; Zhang et al. 2006).
Chondroitinase digestion confirmed that DS is the major decorin GAG in the mature equine SDFT. We visualized the DS filaments (composed of decorin core proteins and DS side-chains) by Cupromeronic blue staining. These were shown to anchor at the d-band in the gap region on collagen fibrils and interconnect between the fibrils by non-covalent bonding; this is an example of coordinated interlinking by DS filaments (Redaelli et al. 2003; Vesentini et al. 2005). SDS-PAGE indicated that the molecular size of decorin DS was smaller in the MM region than in other regions. As there were no differences in the sizes of the core proteins among the regions, this was due to the presence of comparatively short-length DS side-chains in the MM region. Furthermore, the length of the DS filaments differed in a region-dependent manner; they were longer in the MTJ and OTJ regions than in the MM region. Although the mechanisms of DS filament formation by interconnecting decorin GAG chains remain controversial, it is important to note that the size differences demonstrated by our biochemical analyses corresponded with our morphological observations. Thus, decorin molecules with smaller DS side-chains form shorter DS filaments in the MM region.
Although the lengths of DS filaments differed among the three regions, the spacing between the collagen fibril surfaces did not change significantly, indicating that this was not directly affected by the DS filaments. In contrast, Kuwaba et al. (2002) reported that elongated dermatan sulfates were preferentially distributed among collagen fibrils separated by enlarged interfibrillar gaps. However, their observations were based on a dynamic aspect of tissue remodeling, the post-inflammatory healing process in the skin. We studied decorin in the mature equine tendon and suggest that decorin in mature tissues may exhibit different characteristics. The length of DS filaments was significantly greater in the MTJ region, and most DS filaments in this region ran obliquely from one fibril to another with a mean association angle of 62 degrees. This angle was significantly less than in the MM and OTJ regions. The oblique arrangement allows the accommodation of longer DS filaments with no change in the center-to-center or surface-to-surface distances between the fibrils.
Several DS filaments were well aligned at a fixed association angle on the same collagen fibril pair. Oblique DS filaments have also previously been observed in longitudinal sections of tendon and ligament (Cribb & Scott, 1995; Henninger et al. 2007, 2009). This alignment of filaments may occur as a result of reorganization by the sliding of fibrils against other fibrils to which the filaments are connected. The longer GAG chains of the DS filaments in the MTJ region could change the properties of fibrils to allow larger deformation. Alternatively, the oblique alignment may be involved in the formation of an interweaving or spiral structure of collagen fibrils. Redaelli et al. (2003) suggested that decorin altered the conformation of GAGs from a wavy arrangement to a linear structure with increasing levels of applied strain. In either case, the coordinated DS filament alignment would form the basis of the properties of that region of the tendon.
Obliquely running DS filaments would separate the force generated by muscle contraction into longitudinal and vertical elements. The longitudinal force causes the fibrils to slide against each other, whereas the vertical force either keeps fibrils separated or interconnects them via bridges of DS filaments. The MTJ region with collagen fibrils with long DS filament structure and added lubrication may act as a mechanical buffer which distributes contraction force from the muscle to the tendon.
Robinson et al. (2005) reported that different tendons in an animal had different mechanical properties. Tendons can enhance muscle performance, and this mechanism is frequently applied to limb tendons during running (Roberts, 2002). Superficial digital flexor tendonitis frequently occurs in the forelimbs of thoroughbred horses during racing (Gillis et al. 1997). Rupture of a tendon is most likely in a region with many small-diameter collagen fibrils. However, superficial digital flexor tendonitis almost always occurs in the MM region (Gillis et al. 1997). The MTJ region may avoid injury because the buffer action is superior to that of other regions. It is unclear why tendinitis occurs most frequently in the MM region, but the structure and composition of the collagen fibrils and decorin DS filaments in this region may be responsible. Furthermore, the OTJ region, which is rich in thick collagen fibrils, may be rather static, and a mechanically continuous transition is preferable to avoid an increase in stress at the junction between the tendon and bone.
This morphological and biochemical study revealed a graded organization of collagen fibrils and a coordinated arrangement of decorin DS filaments in the mature equine SDFT. We suggest that decorin is involved in the arrangement of collagen fibrils in the SDFT and that the region-specific morphological differences are functionally significant.
References
- Adachi E, Hayashi T. Anchoring of epithelia to underlying connective tissue: evidence of frayed ends of collagen fibrils directly merging with meshwork of lamina densa. J Electron Microsc (Tokyo) 1994;43:264–271. [PubMed] [Google Scholar]
- Basalo IM, Chahine NO, Kaplun M, et al. Chondroitin sulfate reduces the friction coefficient of articular cartilage. J Biomech. 2007;40:1847–1854. doi: 10.1016/j.jbiomech.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Birch HL, Bailey AJ, Goodship AE. Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular composition. Equine Vet J. 1998;30:534–539. doi: 10.1111/j.2042-3306.1998.tb04530.x. [DOI] [PubMed] [Google Scholar]
- Cribb AM, Scott JE. Tendon response to tensile stress: an ultrastructural investigation of collagen: proteoglycan interactions in stressed tendon. J Anat. 1995;187:423–428. [PMC free article] [PubMed] [Google Scholar]
- Elliott DM, Robinson PS, Gimbel JA, et al. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng. 2003;31:599–605. doi: 10.1114/1.1567282. [DOI] [PubMed] [Google Scholar]
- Fessel G, Snedeker JG. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol. 2009;28:503–510. doi: 10.1016/j.matbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Flint MH, Craig AS, Reilly HC, et al. Collagen fibril diameters and glycosaminoglycan content of skins – indices of tissue maturity and function. Connect Tissue Res. 1984;13:69–81. doi: 10.3109/03008208409152144. [DOI] [PubMed] [Google Scholar]
- Gillis C, Pool RR, Meagher DM, et al. Effect of maturation and aging on the histomorphometric and biochemical characteristics of equine superficial digital flexor tendon. Am J Vet Res. 1997;58:425–430. [PubMed] [Google Scholar]
- Haigh M, Scott JE. A method of processing tissue sections for staining with Cu-promeronic blue and other dyes, using CEC techniques, for light and electron microscopy. Basic Appl Histochem. 1986;30:479–486. [PubMed] [Google Scholar]
- Hayashi T, Hirose M, Yamato M. Reconstituted collagen assemblies as building-blocks for the construction of multicellular system in vitro. In: Ikeda Y, Okano T, et al., editors. Tissue Engineering for Therapeutic Use. 3rd edn. Amsterdam: Elsevier Science BV; 1999. pp. 109–118. [Google Scholar]
- Henninger HB, Mass SA, Underwood CJ, et al. Spatial distribution and orientation of dermatan sulfate in human medial collateral ligament. J Struct Biol. 2007;158:33–45. doi: 10.1016/j.jsb.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger HB, Maas SA, Shepherd JH, et al. Transversely isotropic distribution of sulfated glycosaminoglycans in human medial collateral ligament: a quantitative analysis. J Struct Biol. 2009;165:176–183. doi: 10.1016/j.jsb.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV. The biology of the small leucine-rich proteoglycans – functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- Kuwaba K, Kobayashi M, Nomura Y, et al. Elongated dermatan sulphate in post-inflammatory healing skin distributes among collagen fibrils separated by enlarged interfibrillar gaps. Biochem J. 2001;358:157–163. doi: 10.1042/0264-6021:3580157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaba K, Kobayashi M, Nomura Y, et al. Size control of decorin dermatan sulfate during remodeling of collagen fibrils in healing skin. J Dermatol Sci. 2002;29:185–194. doi: 10.1016/s0923-1811(02)00023-3. [DOI] [PubMed] [Google Scholar]
- Kvist M, Jozsa L, Kannus P, et al. Morphology and histochemistry of the myotendineal junction of the rat calf muscles. Histochemical, immunohistochemical and electron-microscopic study. Acta Anat (Basel) 1991;141:199–205. doi: 10.1159/000147122. [DOI] [PubMed] [Google Scholar]
- Liao J, Vesely I. Skewness angle of interfibrillar proteoglycans increase with applied load on mitral valve chordae tendinease. J Biomech. 2007;40:390–398. doi: 10.1016/j.jbiomech.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Liu X, Yeh ML, Lewis JL, et al. Direct measurement of the rupture force of single pair of decorin interactions. Biochem Biophys Res Commun. 2005;338:1342–1345. doi: 10.1016/j.bbrc.2005.10.096. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lujan TJ, Underwood CJ, Henninger HB, et al. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J Orthop Res. 2007;25:894–903. doi: 10.1002/jor.20351. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Kjaer M. Tendon properties in relation to muscular activity and physical training. Scand J Med Sci Sports. 2003;13:211–223. doi: 10.1034/j.1600-0838.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Adachi E, Imamura Y, et al. The fibril structure of type V collagen triple-helical domain. Micron. 2001;32:317–323. doi: 10.1016/s0968-4328(00)00036-6. [DOI] [PubMed] [Google Scholar]
- Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci. 1978a;203:305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- Parry DA, Craig AS, Barnes GR. Tendon and ligament from the horse: an ultrastructural study of collagen fibrils and elastic fibres as a function of age. Proc R Soc Lond B Biol Sci. 1978b;203:293–303. doi: 10.1098/rspb.1978.0106. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Vanderby R., Jr Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84. doi: 10.1016/j.matbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Puxkandl R, Zizak I, Paris O, et al. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc Lond B Biol Sci. 2002;357:191–197. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspanti M, Conqiu T, Guizzardi S. Structural aspects extracellular matrix of the tendon: an atomic force and scanning electron microscopy study. Arch Histol Cytol. 2002;65:37–43. doi: 10.1679/aohc.65.37. [DOI] [PubMed] [Google Scholar]
- Redaelli A, Vesentini S, Soncini M, et al. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons – a computational study from molecular to microstructural level. J Biomech. 2003;36:1555–1569. doi: 10.1016/s0021-9290(03)00133-7. [DOI] [PubMed] [Google Scholar]
- Rees SG, Flannery CR, Little CB, et al. Catabolism of aggrecan, decorin and biglycan in tendon. Biochem J. 2000;350:181–188. [PMC free article] [PubMed] [Google Scholar]
- Rigozzi S, Müller R, Snedeker JG. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J Biomech. 2009;42:1547–1552. doi: 10.1016/j.jbiomech.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Roberts TJ. The integrated function of muscles and tendons during locomotion. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1087–1099. doi: 10.1016/s1095-6433(02)00244-1. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Huang TF, Kazam E, et al. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;127:181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- Sawada H, Shimomura T, Kimata K, et al. Characterization of an anti-decorin monoclonal antibody, and its utility. J Biochem. 2002;132:997–1002. doi: 10.1093/oxfordjournals.jbchem.a003315. [DOI] [PubMed] [Google Scholar]
- Scott JE. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992;6:2639–2645. [PubMed] [Google Scholar]
- Scott JE. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry. 1996;35:8795–8799. doi: 10.1021/bi960773t. [DOI] [PubMed] [Google Scholar]
- Scott JE. Elasticity in extracellular matrix ‘shape modules’ of tendon, cartilage, etc. A sliding proteoglycan-filament model. J Physiol. 2003;553:335–343. doi: 10.1113/jphysiol.2003.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE, Orford CR, Hughes EW. Proteoglycan-collagen arrangements in developing rat tail tendon. An electron microscopicial and biochemical investigation. Biochem J. 1981;195:573–581. doi: 10.1042/bj1950573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screen HRC, Shelton JC, Chhaya VH, et al. The influence of noncollagenous matrix components on the micromechanical environment of tendon fascicles. Ann Biomed Eng. 2005;33:1090–1099. doi: 10.1007/s10439-005-5777-9. [DOI] [PubMed] [Google Scholar]
- Sese M, Ueda H, Watanabe T, et al. Characteristics of collagen fibrils in the entire equine superficial digital flexor tendon. Okajimas Folia Anat Jpn. 2007;84:111–114. doi: 10.2535/ofaj.84.111. [DOI] [PubMed] [Google Scholar]
- Vesentini S, Redaelli A, Montevecchi FM. Estimation of the binding force of the collagen molecule-decorin core protein complex in collagen fibril. J Biomech. 2005;38:433–443. doi: 10.1016/j.jbiomech.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hosaka Y, Yamamoto E, et al. Control of the collagen fibril diameter in the equine superficial digital?flexor tendon in horses by decorin. J Vet Med Sci. 2005;67:855–860. doi: 10.1292/jvms.67.855. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Imamura Y, Hosaka Y, et al. Graded arrangement of collagen fibrils in the equine superficial digital flexor tendon. Connect Tissue Res. 2007;48:332–337. doi: 10.1080/03008200701692800. [DOI] [PubMed] [Google Scholar]
- Zhang GY, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]


