Abstract
We investigated the distribution of fibrillin-2 and LTBP-2 (latent TGF-β binding protein-2) in the intervertebral disc of the adult bovine tail. The association of fibrillin-2 and of LTBP-2 with fibrillin-1 was examined by dual immunofluorescence staining. Both fibrillin-2 and LTBP-2 were found extensively distributed in all regions of the disc with the organisation of the network varying significantly region to region. In the outer annulus fibrosus (OAF) both fibrillin-2 and LTBP-2 co-localised with fibrillin-1 forming fibres running parallel to the collagen fibres of the lamellae with the microfibrillar network staining densely in between the adjacent lamellae and also at the boundaries of the collagen bundle compartments. In the inner annulus fibrosus (IAF) and nucleus pulposus (NP), co-localised fibrillin-1,2 and LTBP-2 formed a chondron-like structure around the cell. By contrast, the inter-territorial matrix of the IAF and NP contained a dense network of fibrillin-2 but only sparse/filamentous fibres of fibrillin-1 and LTBP-2. Dual immunostaining revealed that in this region, fibrillin-2 was highly colocalised with elastin. The LTBP-2 network co-localised well with that of fibrillin-1 in all regions and indeed is reported to bind strongly to fibrillin-1. However, interestingly LTBP-2 but not fibrillin-1 or fibrillin-2 was removed by hyaluronidase but not collagenase pre-digestion. Our results suggest that fibrillin-2 and LTBP-2 could play an important role in disc function.
Keywords: Fibrillin-2, LTBP-2, fibrillin-1, elastic fibres, disc
Introduction
The intervertebral disc plays an important biomechanical role, providing the spine with flexibility and carrying load. Morphologically, the disc is composed of a central region, the nucleus pulposus (NP), surrounded laterally by the annulus fibrosus (AF) and longitudinally by the cartilaginous endplates, which lie between the disc and the vertebral bodies (White & Panjabi, 1978). The biomechanical behaviour of the disc ultimately depends on the composition and organisation of its macromolecular constituents. The major constituents are fibrillar collagens and the large aggregating proteoglycan aggrecan; their role in the biomechanical responses of disc has long been of interest (Eyre et al. 2002; Roughley et al. 2002; Urban & Roberts, 2003). Minor constituents also play important roles in maintaining disc integrity and function; of these, the distribution and organisation of the network of elastic fibres has recently attracted attention (Yu et al. 2002, 2005; Smith & Fazzalari, 2006; Hayes et al. 2011).
Elastic fibres in general consist of a central core of elastin surrounded by microfibrils, of which fibrillins are the main constituent (Greenlee et al. 1966; Sakai et al. 1986; Montes, 1996). Three types exist in human tissues (fibrillin 1–3) with fibrillin-1 most widely distributed and extensively investigated (Ramirez et al. 2004; Hubmacher et al. 2006; Ramirez & Sakai, 2010). In the disc, the organisation of the elastic network, shown by immunostaining elastin and fibrillin-1, varies with region; in the annulus fibrosus, elastic fibres are aligned with collagen fibres within the lamellae and encircle collagen bundles (Yu et al. 2002), whereas in the NP, fibrillin-1 is concentrated around the cells with elastin fibres, seen mainly in the interterritorial matrix (Yu et al.2007). There is no information on the distribution of fibrillin-2 in the post-natal disc. Indeed, fibrillin-2 and fibrillin-3 are thought to be mainly involved in the early stage of microfibril formation during embryonic development, fibrillin-1 being the main component of microfibrils in a mature tissue (Zhang et al. 1994, 1995; Corson et al. 2004). However, fibrillin-2 has recently been reported to be present in post-natal tissues (Cain et al. 2006; Charbonneau et al. 2010).
Microfibrils are also associated with many other matrix proteins, including latent transforming growth factor binding proteins (LTBPs). There are four forms of LTBPs, LTBP1–4 (Annes et al. 2003; Hyytiainen et al. 2004), all of which, apart from LTBP-2, can bind covalently to transforming growth factor (TGF)-β (Rifkin, 2005). LTBP-2 is reported to co-localise with fibrillin-1 in human embryonic lung fibroblasts and aorta (Vehvilainen et al. 2009) and in human foetal disc (Hayes et al. 2011). Its distribution in adult disc is unknown.
The distribution of microfibrils is of interest as their role is not only biomechanical but biological in that they regulate the signaling of growth factors, particularly TGF-β (Ramirez et al. 2004). The main aim of the current study is to investigate the distribution of fibrillin-2 and LTBP-2 in adult disc tissue, with the ultimate aim to understand their roles in disc function.
Materials and Methods
Specimen preparation
Fresh bovine tails were obtained from three adult steers (18–24 months) from a local abattoir within 3 h of slaughter. Different regions of the disc were dissected out and immediately snap-frozen and stored at −80 °C until used. For the central region, the nucleus pulposus (NP), frozen sections were cut transversely at a thickness of 20 μm. The annulus fibrosus (AF) is formed from concentric collagen lamellae encircling the NP. The lamellae are formed from bundles of collagen fibres extending obliquely from one vertebral body to the next with their angle to the axis of the spine alternating between adjacent lamellae. To reflect the organisation of this structure adequately, frozen AF tissue sections at 20 μm thickness were cut obliquely at about 45º to the axis of the disc using a cryostat microtome; each section thus showed cross-sectional and ‘in-plane’ views of adjacent lamellae. Figure 1 illustrates specimen preparation. The sections were mounted on microscope slides (VWR International Ltd, UK) and stored at −20 °C for further use.
Fig. 1.
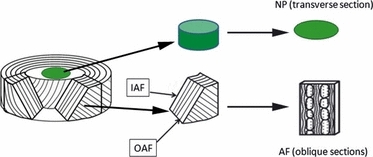
Schematic showing orientation of specimens from the different disc regions. NP, nucleus pulposus; AF, annulus fibrosus; IAF, inner annulus fibrosus; OAF, outer annulus fibrosus.
Dual immunofluorescence staining
Disc AF tissue is densely packed with matrix proteins, particularly collagen and proteoglycans, which can prevent antibody penetration. Therefore AF sections were pre-treated with hyaluronidase (Sigma H6254) or collagenase (Sigma C5138) as previously described (Yu et al. 2007).
Unfixed sections were dual immunostained either with fibrillin-2 together with fibrillin-1 or with LTBP-2 together with fibrillin-1 or with fibrillin-2 together with elastin. Table 1 lists the primary and secondary antibodies used.
Table 1.
List of antibodies used*
| Fibrillin-1 | Fibrillin-2 | LTBP-2 | Elastin | |
|---|---|---|---|---|
| 1st antibody | Mice anti-bovine fibrillin-1 from Abcam UK Ltd, UK, Cat. no: ab3090 | Rabbit anti-human fibrillin-2 antibody (cross-reacted with bovine) from Elastin Products Company, USA, Cat. no: PR225 | Rabbit anti-bovine LTBP-2 from Elastin Products Company, USA, Cat. no: PR410 | Mice anti-bovine elastin from Abcam UK Ltd, UK, Cat. No: ab9519 |
| Dilution: 1 : 50 Buffer: 50 mm Tris–HCl plus 10 mm calcium acetate | Dilution: 1 : 50 | Dilution: 1 : 50 | Dilution: 1 : 5 | |
| 2nd antibody | Cy3-conjugated donkey anti-mouse IgG | DyLight-488- conjugated donkey anti- rabbit IgG | DyLight-488- conjugated donkey anti- rabbit IgG | Cy3-conjugated donkey anti-mouse IgG |
| (Red) Stratech Scientific Ltd, UK, Product code: 715-165-151 Dilution: 1 : 100 | (Green) Stratech Scientific Ltd, Product code: 712-485-153 Dilution: 1 : 100 | (Green) Stratech Scientific Ltd, Product code: 712-485-153 Dilution: 1 : 100 | (Red) Stratech Scientific Ltd, UK, Product code: 715-165-151 Dilution: 1 : 100 |
Dilution with PBS unless otherwise indicated.
All the procedures were performed at room temperature. Phosphate-buffered saline (PBS) was used for washing (three times, 3 min each time) between each incubation unless otherwise indicated. After washing, 6–10% normal donkey serum (NDS) in Tris buffer (50 mm Tris–HCl plus 10 mm calcium acetate) was used to block sections for 30 min. The sections were then incubated with fibrillin-1 antibody (1 : 50 dilution with Tris buffer) or elastin antibody (1 : 50 dilution with PBS) overnight at 4 °C. After washing, the specimen was incubated with fluorescent Cy3-conjugated donkey anti-mouse IgG (Stratech Scientific Ltd, UK, Product code: 715-165-151, 1 : 100 dilution) for 30 min at room temperature. After washing, sections were blocked with NDS for 30 min and then incubated with fibrillin-2 antibody or LTBP-2 antibody (1 : 50 dilution with the PBS) overnight at 4 °C. After washing, the sections were incubated with donkey anti-rabbit IgG conjugated with fluorescent DyLight-488 (Stratech Scientific Ltd, UK: Product code: 712-485-153, 1 : 100 dilution). Finally, the specimens were washed again and mounted using hardset mounting medium containing 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratory, Cat. no: H-1500) to stain cell nuclei. Negative controls were incubated with normal mouse IgG and normal rabbit serum instead of the primary antibodies: no cross-reactivity was found.
Microscopy and image processing
A conventional fluorescence microscope (Leica DMRB) with three different filters was used to detect the three different dyes of fluorescent signals: blue fluorochrome filters for DAPI, green and violet fluorochrome filters (filter cubes from Chroma Technology Corp, USA, Cat. nos 31014 WB and 41001 HQ) for Cy-3 (orange) and DyLight-488 (green), respectively. Multiple images of the same site were taken manually without moving the section, either by changing the focus to track the fibres under same fluorescent filters or by switching the filters gently to investigate co-localisation.
Image processing and merges were performed by adobe photoshop. The colour balance control function of photoshop was used to change the colour of Cy3 to red to strengthen the contrast with green. Pictures taken from exactly the same specimen site were merged when appropriate.
Results
The outer annulus fibrosus (OAF)
Collagen is the principle structural component in the OAF region of the disc. Its network organisation has been described recently in detail in several studies (Pezowicz et al. 2005, 2006; Schollum et al. 2008), which described parallel collagen fibres organised in bundles constructed as individual compartments/subcompartments in the lamella. We found the organisation of fibrillin-1,2 and LTBP-2 is strongly related to the main structure of collagen organisation.
Fibrillin-2/fibrillin-1
Figure 2 shows the typical images of dual immunostained fibrillin-2 (Fig. 2A,B) and fibrillin-1 (Fig. 2C) in the OAF. Extensive fibrillin-2 staining of parallel fibres was seen within a lamella (Fig. 2A,B). In addition, fibrillin-2 stained densely in-between adjacent lamellae (white arrows in Fig. 2B) and at the boundaries of collagen bundles (yellow arrows in Fig. 2B). This organisation of fibrillin-2 resembles that of elastin and fibrillin-1 described previously (Yu et al. 2007). Indeed, our dual immunostaining confirmed that fibrillin-2 was highly co-localised with fibrillin-1 (Fig. 2D) within a lamella; there was relatively less co-localisation between adjacent lamellae (white arrows) and also at the boundaries (yellow arrows) of collagen bundles.
Fig. 2.
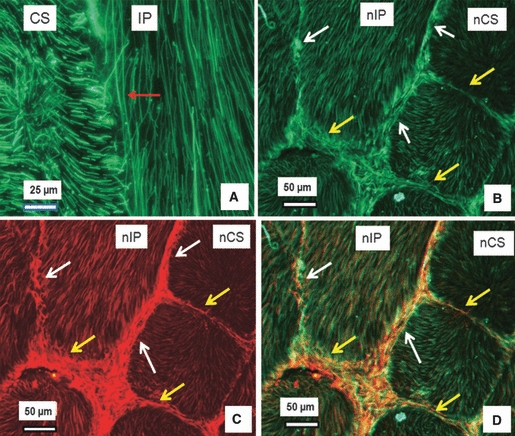
Dual immunostained fibrillin-2 and fibrillin-1 in the outer annulus fibrosus of the bovine disc. (A) Parallel fibrillin-2 fibres (green) in an inplane lamella (IP) with the red arrow highlighting a long fibrillin-2 fibre (longer than 170 μm). (B–D) Typical dual immunostaining of a section, with (B) showing organisation of fibrillin-2 (green), (C) fibrillin-1 organisation (red), and (D) the merged image of (B) and (C). The figures indicate that fibrillin-2 is highly co-localised with fibrillin-1; together, they are densely organised at the interlamellae (white arrows) and at the boundaries of collagen bundles (yellow arrows). CS, cross-sectioned lamella; nCS, nearly cross-sectioned lamella; IP, inplane lamella; nIP, nearly inplane lamella.
LTBP-2/fibrillin-1
Figure 3 shows the typical images of dual immunostained LTBP-2 (Fig. 3A,B) and fibrillin-1 (Fig. 3C). The organisation of LTBP-2 (Fig. 3A,B) appears very similar to that of both fibrillin-2 (Fig. 2A,B), and fibrillin-1 (Figs 2C and 3C), i.e. extensive, parallel fibres aligned in the direction of collagen fibres (Yu et al. 2007) in a lamella, and densely organised in-between adjacent lamellae (white arrows in Fig. 3B) and also at the boundaries (yellow arrows in Fig. 3B) of collagen bundles. LTBP-2 staining, unlike that of fibrillin-1 and fibrillin-2, was removed by hyaluronidase predigestion (Fig. 3E,F), but not by collagenase pre-digestion.
Fig. 3.
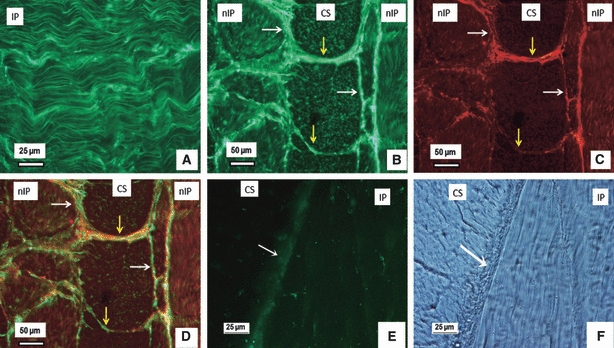
Dual immunostained LTBP-2 and fibrillin-1 in the outer annulus fibrosus. (A) Parallel LTBP-2 fibres (green) in the inplane (IP) lamella. (B–D) Typical dual immunostaining of a section with (B) showing LTBP-2 (green), (C) fibrillin-1(red) and (D) the merged image of (D) and (E). LTBP-2 appears highly co-localised with fibrillin-1; together they are densely organised in the interlamellae spaces (white arrows) and also at the boundaries of collagen bundles (yellow arrows). (E–F) Hyaluronidase overnight pre-digestion removed LTBP-2 (green in E), but not collagen structure (F), with white arrows indicating interlamellae. CS, cross-sectioned lamella; nCS, nearly cross-sectioned lamella; IP, inplane lamella; nIP, nearly inplane lamella.
The inner annulus fibrosus (IAF)
Fibrillin-2/Fibrillin-1
Figure 4 shows typical dual immunostained fibrillin-2 (Fig. 4A) and fibrillin-1 (Fig. 4B) in the IAF. In comparison with fibrillin-1, fibrillin-2 was organised not only around the cells (white arrow in Fig. 4A) but also in the inter-territorial matrix (yellow arrows in Fig. 4A,C), where little fibrillin-1 was present. These differences in distribution were more apparent in the NP region (data below). However, fibrillin-2 and fibrillin-1 were highly co-localised in the pericellular matrix, where they formed a chondron-like structure (Fig. 4D).
Fig. 4.
Dual immunostained fibrillin-2 (A) and fibrillin-1 (B) in the inner annulus fibrosus. The figure shows typical dual staining of a section, with (A) showing fibrillin-2 (green) distribution, (B) fibrillin-1 (red) distribution and (C) a merged image of (A) and (B) where the cells are stained with DAPI (blue). The figures show more fibrillin-2 in the inter-territorial matrix (A: yellow arrows) where little fibrillin-1 is present (B). White arrow in (A) shows that fibrillin-2 is also concentrated around cells. (D) A higher magnification of a merged image showing a network of fibrillin-1 and -2 forming a chondron-like structure encircling the cell and connecting with the fibres at the inter-territorial matrix (white arrows).
LTBP-2/fibrillin-1
The pattern of immunostaining of LTBP-2 was similar to that of fibrillin-1 (data not shown), i.e. filamentous in the inter-territorial matrix, but mostly distributed around cells. Details of LTBP-2 and fibrillin-1 organisation around the cells are shown in Fig. 5. These fibers appear well anchored into the cell membrane (white arrows) and connected with inter-territorial matrix (yellow arrow). In addition, LTBP-2 in IAF (and in NP) also appears to be removed by hyaluronidase predigestion (not shown).
Fig. 5.
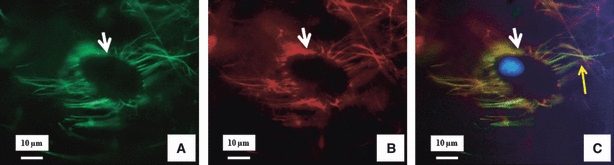
Dual immunostained LTBP-2 and fibrillin-1 around a cell of the inner annulus fibrosus region. The figures show a typical dual immunostained image of the same area: (A) LBPT-2 (green), (B) fibrillin-1 (red), (C) merged image of (A) and (B) with DAPI staining of the cell nucleus (blue). These images show the co-localised network anchored to the cell membrane (white arrows) and also connecting to the inter-territorial matrix (yellow arrow).
The nucleus pulposus
Fibrillin-2/fibrillin-1/elastin
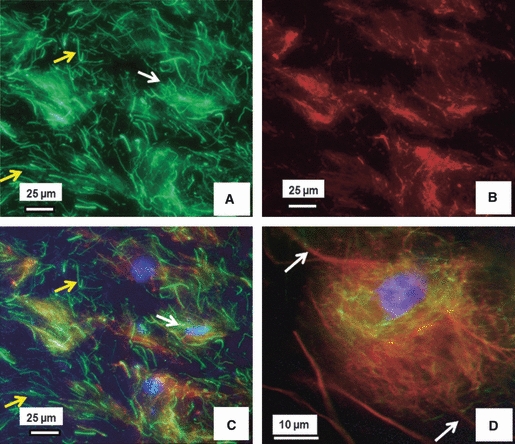
Figure 6 shows typical dual immunostained fibrillin-2 (Fig. 6A) and fibrillin-1 (Fig. 6B) in the NP. As seen also in the IAF (Fig. 4), long fibrillin-2 fibres were evident in the inter-territorial matrix (yellow arrows in Fig. 6A), where little fibrillin-1 was present (Fig. 6B). This distribution resembled findings on the distribution of elastin fibres and fibrillin-1 in this region (Yu et al. 2007). Dual immunostaining of fibrillin-2 and elastin confirm that fibrillin-2 is highly co-localised with elastin in the inter-territorial matrix (Fig. 7). In the pericellular matrix, co-localised fibrillin-1 and fibrillin-2 encircle the cell (Fig. 6D).
Fig. 6.
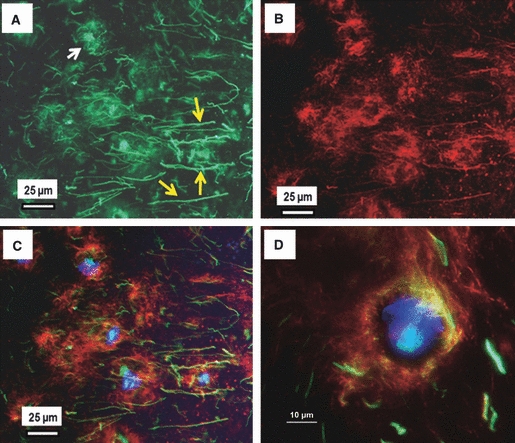
Dual immunostained fibrillin-2 and fibrillin-1 in the NP. Typical dual immunostained image of a section showing organisation of fibrillin-2 (green: A), fibrillin-1 (red: B), and the merged image (C) with DAPI staining of the nucleus (blue). The images show that fibrillin-2 is organised not only around the cells (white arrows in A) but also is extensively distributed in the inter-territorial matrix (yellow arrows in A) where little/filamentous fibrillin-1 is present. (D) Merged image of fibrillin-1 and -2 at a higher magnification, showing a network of fibrillin-1, and -2 forming a chondron-like structure around the cell.
Fig. 7.
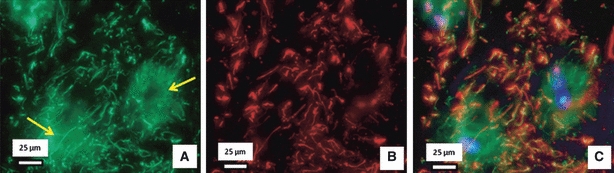
Typical dual immunostained fibrillin-2 and elastin in the NP. A typical dual immunostained section showing fibrillin-2 (green: A) and elastin (red: B). Fibrillin-2 appears organised not only around the cells (yellow arrows in A) but also in the inter-territorial matrix, whereas (B) elastin is distributed mainly in the inter-territorial matrix, (C) is a merged image of (A) and (B) plus DAPI stained cell nuclei, indicating that fibrillin-2 is highly co-localised with elastin in the inter-territorial matrix.
LTBP-2/fibrillin-1
Figure 8 shows typical dual immunostained LTBP-2 (Fig. 8A,D) and fibrillin-1 (Fig. 8B,E) in the NP. The organisation of LTBP-2 highly resembles that of fibrillin-1, i.e. densely encircling the cells with only sparse filamentous fibres seen in the inter-territorial matrix. At a higher magnification, co-localised LTBP-2 and fibrillin-1 forming a chondron-like structure encircling the cells (Fig. 8D-F).
Fig. 8.
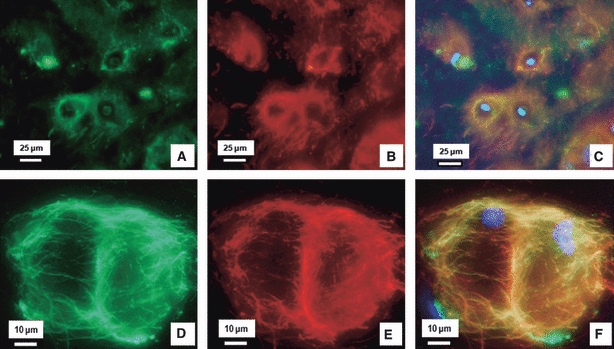
Dual immunostained LTBP-2 and fibrillin-1 in the NP. A typical dual stained image at low magnification (A–C) and higher magnification (D–F) showing LTBP-2 (green: A and D) and fibrillin-1 (red: B and E). (C, F) Merged images of (A) and (B), and (D) and (E), respectively, with cell nuclei (blue) stained with DAPI. (A–C) LTBP-2 appears highly co-localised with fibrillin-1 and together they are distributed densely around the cells. (D–F) At a higher magnification, co-localised LTBP-2 and fibrillin-1 clearly encircle cells.
Discussion
Here we describe the organisation of microfibrillar components of the elastic network in different regions of the intervertebral disc. We investigated the distribution of fibrillin-2 and LTBP-2 and their co-localisation with fibrillin-1 using dual immunostaining of fibrillin-2 with fibrillin-1 or LTBP-2 with fibrillin-1. Overall, both fibrillin-2 and LTBP-2 were evident in all disc regions. In terms of colocalisation with fibrillin-1, both fibrillin-2 and LTBP-2 were highly co-localised with fibrillin-1, especially in the lamellae of the OAF and in a chondron-like structure around the cell in the IAF and the NP. However, in the IAF and NP, surprisingly, fibrillin-2 was much more densely distributed in the inter-territorial matrix compared with fibrillin-1 and co-localised with elastin. LTBP-2 appeared highly co-localised with fibrillin-1 in all regions and, interestingly, was removed by hyaluronidase pretreatment.
Table 2 presents a summary of our findings.
Table 2.
Summary of the results*
| Fibrillin-2 | Fibrillin-1 | Association between fibrillin-2 and fibrillin-1 | ||||
|---|---|---|---|---|---|---|
| Disc region | Appearance | Staining density | Appearance | Staining density | ||
| OAF | Parallel fibres within lamellae, also dense in the interlamellar space and encircling collagen bundles | +++ | As fibrillin-2 | +++ | Highly co-localised within lamellae, Less co-localised in-between adjacent lamellae and boundaries of collagen bundles | |
| IAF | PM | Encircling cells | +++ | As fibrillin-2 | +++ | Co-localised |
| IM | Fibres | +++ | Filamentous | + | Few co-localised | |
| NP | PM | Encircling cells | +++ | As fibrillin-2 | +++ | Co-localised |
| IM | Fibres | ++ | Filamentous | +/− | Few co-localised | |
PM, pericellular matrix; IM, inter-territorial matrix.
LTBP-2 organisation resembles that of fibrillin-1 (not included in the Table).
+++ thick fibres, extensive and dense.
++ thick fibres, disperse.
+ thin fibres, less dense.
+/- thin fibres, very spare.
In the OAF, both fibrillin-2 and LTBP-2 appear as thick and long fibres aligned parallel to the collagen fibers in the in-plane lamellae (Figs 2A and 3A). In addition, they were both highly co-localised with fibrillin-1 (Figs 2 and 3), as shown previously for elastin (Yu et al. 2005, 2007). Furthermore, all fibrillins-1 and -2 and LTBP-2 were densely organised at the inter-lamellae junctions and at the boundaries of collagen bundle compartments. However, interestingly, they appear less co-localised in this region. Taken together, the results suggest a close functional relationship between the elastic fibre, microfibrils, LTBP-2 and collagen fibres in the OAF lamellae. Recent studies have demonstrated that disc inter-lamellae and adjacent collagen bundle compartment junctions play a complicated role in cohesion of the lamellae (Pezowicz et al. 2005, 2006; Schollum et al. 2008). Our data suggest that all these microfibrillar components could be involved in this cohesion.
In the IAF and NP, fibrillin-2 and LTBP-2 co-localised with fibrillin-1 in the pericellular region but rarely in the inter-territorial matrix. In the pericellular matrix, the co-localised network enclosed the cells (Figs 4D, 5C, 6D and 8C,F), forming a structure similar to the ‘chondron’ described around cells of articular cartilage (Poole et al. 1991, 1992) and also described around disc NP cells (Roberts et al. 1991; Cao et al. 2007). We found that the microfibrils appear anchored to the cell membrane and also connected to the fibres of the inter-territorial matrix (white arrows in Figs 4D and 5C), supporting the suggestion that the cell ‘chondron’ could play an important role in transducing signals between the cells and the inter-territorial matrix (Hing et al. 2002). Recently, several studies have reported that fibrillin microfibrils can regulate TGF-β activities (Ramirez et al. 2004; Choi et al. 2007). These chondron-like structures may thus also be involved in the biological regulation of cellular behaviour.
However, in the inter-territorial matrix of the IAF and NP, although there is an extensive fibrillin-2 fibre network (yellow arrows, Figs 4A,C and 6A), only a few fibrillin-1 (Figs 4B and 6B) and LTBP-2 (Fig. 8) fibres are present. Such differences in organisation between fibrillin-2 and fibrillin-1 resemble the differences between the organisation of elastin and fibrillin-1 fibres in the IAF and NP (Yu et al. 2007). Indeed, we have confirmed that fibrillin-2 rather than fibrillin-1 is co-localised with elastin in the inter-territorial matrix of the NP (Fig. 7). Our results are in agreement with those of a recent study on ligaments (Smith et al. 2011), which also found that fibrillin-2 but not fibrillin-1 co-localised with elastin in some regions of the tissue. It is possible that the regional differences in distribution between fibrillin-1 and fibrillin-2 arise because they play different roles in the disc matrix; the NP with its fibrillin-2/elastin network experiences mostly compressive loads, whereas the co-localised fibrillins, LTBP-2, elastin and collagen fibres in the OAF act to restrain the pressurised NP and therefore are mainly under tension (Nerurkar et al. 2010) Indeed, although they share some similarities, there are differences in skeletal manifestations of patients with Marfan syndrome (fibrillin-1 gene mutation; Lebreiro et al. 2010) and Beals syndrome (fibrillin-2 gene mutation; Tunçbilek & Alanay, 2006), with patients with Beals syndrome generally having more severe skeletal disorders. Our data, together the differences in clinical manifestations, and the results of Smith et al. (2011) suggest that fibrillin-2 plays a different role to fibrillin-1 in the skeletal system.
Unlike the region-specific association between fibrillin-2 and fibrillin-1, LTBP-2 consistently co-localised with fibrillin-1 in all regions. LTBP-2, which was firstly identified as an independent protein of LTBPs (Moren et al. 1994), has been shown to bind to fibrillin-1 but not to fibrillin-2 at the molecular level (Hirani et al. 2007), as confirmed in a study of different human cultured cell lines (Vehvilainen et al. 2009). Recently, a study of foetal human intervertebral disc also demonstrated the co-localisation of fibrillin-1 and LTBP-2 in the OAF (Hayes et al. 2011). Our data confirm these findings and provide additional information of LTBP-2 distribution in IAF and NP in adult disc. Surprisingly, we found that LTBP-2 is removed by hyaluronidase but not by collagenase pre-digestion. Of the elastic fibre proteins, such a characteristic appears unique to LTBP-2, as fibrillins-1 and -2, and elastin cannot be removed by hyaluronidase or collagenase pre-digestion. This result suggests that hyaluronidase cleaves the bonds between LTBP-2 and other matrix components. LTBP-2 is reported to bind strongly to heparin and has been reported to bind strongly to fibrillin-1 through its heparin-binding sites (Hirani et al. 2007) and also binds strongly to heparin-sulphate proteoglycans (HSPG; Parsi et al. 2010) such as perlecan, also found in the pericellular region of disc cells (Smith et al. 2009). If LTBP-2 bonds to fibrillin-1 or HSPG such as perlecan in disc tissue, hyaluronidase should not remove it, as it has been reported that both heparin and HSPG resist hyaluronidase digestion (Kanwar & Farquhar, 1979). However, hyaluronidase can digest hyaluronic acid, chondroitin and chondroitin-sulphate (Lindahl, 1970), and therefore the nature of binding of LTBP-2 to the disc matrix remains unclear.
In summary, we have demonstrated that fibrillin-2 and LTBP-2 are extensively organised in all regions of adult bovine disc. Their network organisation and degree of co-localisation with fibrillin-1 varies significantly in the different disc regions. Our results suggest that these proteins may play different but important functional roles which are as yet unidentified.
Acknowledgments
This work was supported financially by the Yves Cotrel Foundation and the British Scoliosis Foundation. B.L. was supported by the China Scholarship Council.
References
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Cain SA, Morgan A, Sherratt MJ, et al. Proteomic analysis of fibrillin-rich microfibrils. Proteomics. 2006;6:111–122. doi: 10.1002/pmic.200401340. [DOI] [PubMed] [Google Scholar]
- Cao L, Guilak F, Setton LA. Three-dimensional morphology of the pericellular matrix of intervertebral disc cells in the rat. J Anat. 2007;211:444–452. doi: 10.1111/j.1469-7580.2007.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau NL, Jordan CD, Keene DR, et al. Microfibril structure masks fibrillin-2 in postnatal tissues. J Biol Chem. 2010;285:20242–20251. doi: 10.1074/jbc.M109.087031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JB, Youn I, Cao L, et al. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech. 2007;40:2596–2603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson GM, Charbonneau NL, Keene DR, et al. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83:461–472. doi: 10.1016/j.ygeno.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Matsui Y, Wu JJ. Collagen polymorphisms of the intervertebral disc. Biochem Soc Trans. 2002;30:844–848. doi: 10.1042/bst0300844. [DOI] [PubMed] [Google Scholar]
- Greenlee TK, Ross R, Hartman JL. The fine structure of elastic fibers. J Cell Biol. 1966;30:59–71. doi: 10.1083/jcb.30.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, Smith SM, Gibson MA, et al. Comparative immunolocalization of the elastin fibre-associated proteins fibrillin-1, LTBP2, and MAGP-1 with components of the collagenous and proteoglycan matrix of the fetal human IVD. Spine (Phila Pa 1976) 2011;36(21):E1365–72. doi: 10.1097/BRS.0b013e31821fd23e. [DOI] [PubMed] [Google Scholar]
- Hing WA, Sherwin AF, Poole CA. The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge. Osteoarthritis Cartilage. 2002;10:297–307. doi: 10.1053/joca.2002.0517. [DOI] [PubMed] [Google Scholar]
- Hirani R, Hanssen E, Gibson MA. LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol. 2007;26:213–223. doi: 10.1016/j.matbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Hubmacher D, Tiedemann K, Reinhardt DP. Fibrillins: from biogenesis of microfibrils to signaling functions. Curr Top Dev Biol. 2006;75:93–123. doi: 10.1016/S0070-2153(06)75004-9. [DOI] [PubMed] [Google Scholar]
- Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41:233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- Kanwar YS, Farquhar MG. Isolation of glycosaminoglycans (heparan sulfate) from glomerular basement membranes. Proc Natl Acad Sci U S A. 1979;76:4493–4497. doi: 10.1073/pnas.76.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreiro A, Martins E, Cruz C, et al. Marfan syndrome: clinical manifestations, pathophysiology and new outlook on drug therapy. Rev Port Cardiol. 2010;29:1021–1036. [PubMed] [Google Scholar]
- Lindahl U. Attempted isolation of a heparin proteoglycan from bovine liver capsule. Biochem J. 1970;116:27–34. doi: 10.1042/bj1160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol Int. 1996;20:15–27. doi: 10.1006/cbir.1996.0004. [DOI] [PubMed] [Google Scholar]
- Moren A, Olofsson A, Stenman G, et al. Identification and characterization of LTBP-2, a novel latent transforming growth factor-beta-binding protein. J Biol Chem. 1994;269:32469–32478. [PubMed] [Google Scholar]
- Nerurkar NL, Elliott DM, Mauck RL. Mechanical design criteria for intervertebral disc tissue engineering. J Biomech. 2010;43:1017–1030. doi: 10.1016/j.jbiomech.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsi MK, Adams JR, Whitelock J, et al. LTBP-2 has multiple heparin/heparan sulfate binding sites. Matrix Biol. 2010;29:393–401. doi: 10.1016/j.matbio.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Pezowicz CA, Robertson PA, Broom ND. Intralamellar relationships within the collagenous architecture of the annulus fibrosus imaged in its fully hydrated state. J Anat. 2005;207:299–312. doi: 10.1111/j.1469-7580.2005.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezowicz CA, Robertson PA, Broom ND. The structural basis of interlamellar cohesion in the intervertebral disc wall. J Anat. 2006;208:317–330. doi: 10.1111/j.1469-7580.2006.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CA, Glant TT, Schofield JR. Chondrons from articular cartilage. (IV). Immunolocalization of proteoglycan epitopes in isolated canine tibial chondrons. J Histochem Cytochem. 1991;39:1175–1187. doi: 10.1177/39.9.1717545. [DOI] [PubMed] [Google Scholar]
- Poole CA, Ayad S, Gilbert RT. Chondrons from articular cartilage. V. Immunohistochemical evaluation of type VI collagen organisation in isolated chondrons by light, confocal and electron microscopy. J Cell Sci. 1992;103:1101–1110. doi: 10.1242/jcs.103.4.1101. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY. Biogenesis and function of fibrillin assemblies. Cell Tissue Res. 2010;339:71–82. doi: 10.1007/s00441-009-0822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY, Dietz HC, et al. Fibrillin microfibrils: multipurpose extracellular networks in organismal physiology. Physiol Genomics. 2004;19:151–154. doi: 10.1152/physiolgenomics.00092.2004. [DOI] [PubMed] [Google Scholar]
- Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- Roberts S, Menage J, Duance V, et al. Type III collagen in the intervertebral disc. Histochem J. 1991;23:503–508. doi: 10.1007/BF01041176. [DOI] [PubMed] [Google Scholar]
- Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869–874. doi: 10.1042/bst0300869. [DOI] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schollum ML, Robertson PA, Broom ND. ISSLS prize winner: Microstructure and mechanical disruption of the lumbar disc annulus: part I: a microscopic investigation of the translamellar bridging network. Spine (Phila Pa 1976) 2008;33:2702–2710. doi: 10.1097/BRS.0b013e31817bb92c. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Fazzalari NL. Regional variations in the density and arrangement of elastic fibres in the anulus fibrosus of the human lumbar disc. J Anat. 2006;209:359–367. doi: 10.1111/j.1469-7580.2006.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KD, Vaughan-Thomas A, Spiller DG, et al. The organisation of elastin and fibrillins 1 and 2 in the cruciate ligament complex. J Anat. 2011;218:600–607. doi: 10.1111/j.1469-7580.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Whitelock J, Iozzo R, et al. Topographical variation in the distributions of versican, aggrecan and perlecan in the foetal human spine reflects their diverse functional roles in spinal development. Histochemistry and Cell Biology. 2009;132:491–503. doi: 10.1007/s00418-009-0623-z. [DOI] [PubMed] [Google Scholar]
- Tunçbilek E, Alanay Y. Congenital contractural arachnodactyly (Beals syndrome) Orphanet J Rare Dis. 2006;1:20. doi: 10.1186/1750-1172-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehvilainen P, Hyytiainen M, Keski-Oja J. Matrix association of latent TGF-beta binding protein-2 (LTBP-2) is dependent on fibrillin-1. J Cell Physiol. 2009;221:586–593. doi: 10.1002/jcp.21888. [DOI] [PubMed] [Google Scholar]
- White AR, Panjabi MM. The clinical biomechanics of the occipitoatlantoaxial complex. Orthop Clin North Am. 1978;9:867–878. [PubMed] [Google Scholar]
- Yu J, Winlove PC, Roberts S, et al. Elastic fibre organization in the intervertebral discs of the bovine tail. J Anat. 2002;201:465–475. doi: 10.1046/j.1469-7580.2002.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Fairbank JC, Roberts S, et al. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine (Phila Pa 1976) 2005;30:1815–1820. doi: 10.1097/01.brs.0000173899.97415.5b. [DOI] [PubMed] [Google Scholar]
- Yu J, Tirlapur U, Fairbank J, et al. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J Anat. 2007;210:460–471. doi: 10.1111/j.1469-7580.2007.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Apfelroth SD, Hu W, et al. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J Cell Biol. 1994;124:855–863. doi: 10.1083/jcb.124.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hu W, Ramirez F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol. 1995;129:1165–1176. doi: 10.1083/jcb.129.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]


