Abstract
Changes in body weight due to changes in food intake are reflected by corresponding changes in the cardiac phenotype. Despite a growing body of literature on cardiac hypertrophy associated with obesity, little is known on the atrophic remodelling of the heart associated with calorie restriction. We hypothesized that, besides the cardiomyocyte compartment, capillaries and nerve fibres are involved in the atrophic process. C57Bl6 mice were kept on normal diet (control group) or at a calorie-restricted diet for 3 or 7 days (n = 5 each). At the end of the protocol, mice were killed and the hearts were processed for light and electron microscopic stereological analysis of cardiomyocytes, capillaries and nerve fibres. Body, heart and left ventricular weight were significantly reduced in the calorie-restricted animals at 7 days. Most morphological parameters were not significantly different at 3 days compared with the control group, but at 7 days most of them were significantly reduced. Specifically, the total length of capillaries, the volume of cardiomyocytes as well as their subcellular compartments and the interstitium were proportionally reduced during caloric restriction. No differences were observed in the total length or the mean diameter of axons between the cardiomyocytes. Our data indicate that diet-induced left ventricular atrophy leads to a proportional atrophic process of cardiomyocytes and capillaries. The innervation is not involved in the atrophic process.
Keywords: caloric restriction, electron microscopy, malnutrition, myocardial remodelling, stereology
Introduction
Cardiac atrophy occurs as a result of prolonged bed rest, mechanical unloading or during periods of catabolism, such as cancer cachexia or malnutrition (Vandewoude & Buyssens, 1992a; Hill & Olson, 2008; Brinks et al. 2009; Tian et al. 2010; Baskin & Taegtmeyer, 2011). During starvation, a decrease in the ratio of mitochondrial to myofibril volume as well as dilation of the cardiomyocyte T-system has been noted (Vandewoude & Buyssens, 1992a). Biochemical analysis of hearts from rabbits starved for 0, 3 or 7 days revealed that the diminished synthesis of new proteins, particularly those of myofibrils, and a decreased half-life of proteins significantly contribute to cardiac atrophy (Samarel et al. 1987). Interestingly, in mechanically unloaded hearts cardiac atrophy involves pathways of both protein synthesis and degradation, indicating an active atrophic remodelling process (Razeghi et al. 2003). In a recent study on the effects of cancer cachexia in the mouse, we had reported that myofibrillar volume decreases, although the total mass of the left ventricle and the total volume of the cardiomyocytes remains the same. In addition, we had reported that cancer cachexia is associated with a decrease in myocardial innervation to approximately 50% of control values (Mühlfeld et al. 2011). Whether this effect is related to the loss of body weight or other pathogenic factors such as increased cytokine levels is not clear.
Most of the studies on caloric restriction- or starvation-induced cardiac remodelling have focused on the atrophic response of the cardiomyocytes, whereas the remodelling of the cardiomyocyte microenvironment, namely capillaries and innervation, has not been investigated. In fact, only one study addressed the changes of the myocardial capillaries in malnutrition. Most of the parameters are relative values such as volume or surface density, and are therefore difficult to relate to the changes in the cardiomyocyte compartment (Vandewoude & Buyssens, 1992b). In particular, the relationship and time course of caloric restriction-induced changes in the different compartments of the myocardium is currently unknown. A disproportional remodelling between the compartments may be of clinical importance, particularly in patients who suffer from heart disease and start losing weight voluntarily or involuntarily.
In the light of these considerations and our previous work on cancer cachexia, we hypothesized that left ventricular remodelling induced by caloric restriction is a proportional atrophic process involving capillaries, nerve fibres and cardiomyocytes at the same time and to a similar degree. To test this hypothesis, we subjected mice to caloric restriction by 50% for 0, 3 and 7 days, and investigated the left ventricle using design-based stereological methods. Specifically, the total volume of cardiomyocytes and their organelles as well as the total length of capillaries and axons was estimated. In contrast to our hypothesis, remodelling of the left ventricle induced by caloric restriction only involves the cardiomyocyte and capillary compartment, not the innervation, and we propose that changes of the interplay of the different myocardial components need to be considered in future studies on atrophic remodelling.
Materials and methods
Animals
A total of 15 adult male 8-week-old C57BL/6J mice were purchased from Charles River Laboratories (France) and randomly assigned to a control, 3-day caloric restriction and 7-day caloric restriction group (n = 5 each). The mice were kept in custom-made metabolic cages (one mouse per cage) for 4 days to become accustomed to the new environment with ad libitum food and water access, controlled temperature and lighting (12 h light–dark cycle). During these 4 days, the average food intake per mouse was calculated, and then mice from the 3- and 7-day groups were subjected to caloric restriction by 50% of spontaneous intake. All experiments were performed according to the institutional guidelines that comply with national and international regulations, and were approved by the local authorities.
The animals were killed by isoflurane inhalation and subsequent exsanguination by cutting the abdominal caval vein. Afterwards, the thorax was opened, the heart was quickly excised and immersed in 4 °C cold fixative containing 4% paraformaldehyde in phosphate-buffered saline. The heart was kept in the fixative for at least 24 h at 4 °C. The heart was weighed, and the atria and right ventricle were removed from the left ventricles. The latter was weighed, cut longitudinally and three times transversely. The resulting eight pieces were systematically, uniformly, randomly sampled to obtain four tissue pieces for paraffin embedding. From the remaining four pieces, one was randomly chosen, weighed carefully and separately embedded in paraffin, whereas the other three specimens were embedded in epoxy resin. Paraffin embedding was performed according to standard protocols. For epoxy resin embedding, the samples were postfixed in 1% osmium tetroxide in aqua bidest, stained en bloc in half-saturated watery uranyl acetate, dehydrated in an ascending ethanol series and finally embedded in Epon.
Stereology
The stereological methods used in this study are well established and have been the subject of a recent review (Mühlfeld et al. 2010a). For light microscopic stereology, an Olympus BX51 microscope (Olympus, Hamburg, Germany), equipped with a digital camera (Olympus DP72) and the newCAST stereology software (Visiopharm, Horsholm, Denmark) was used. Transmission electron microscopy was performed using a LEO 902 electron microscope (Zeiss, Oberkochen, Germany). All fields of view (FOV) for stereological analysis were obtained by systematic uniform random sampling (Gundersen & Jensen, 1987).
For the stereological analysis of cardiomyocytes, semithin and ultrathin sections were cut from the Epon-embedded tissue blocks and stained with toluidine blue and lead citrate/uranyl acetate, respectively. Grids with test points were projected onto the FOV and the number of points hitting structures of interest was counted. At the light microscopic level (objective lens magnification: 40 ×), the number of points hitting cardiomyocytes and interstitium was counted and used to calculate the volume fraction of these compartments according to the equation VV(str/ref) = P(str)/P(ref), where VV(str/ref) is the volume fraction of a structure of interest in relation to a reference volume, P(str) is the number of points hitting the structure of interest and P(ref) is the total number of points hitting the reference volume. At the electron microscopic level (primary magnification: 7000 ×), the number of points hitting myofibrils, mitochondria, free sarcoplasm and the nucleus was counted to calculate the volume fraction of these compartments related to the cardiomyocyte as the reference volume as described above. The volume densities were multiplied by the respective reference volume to obtain the total volume of the compartments.
The total length of capillaries in the left ventricle was estimated by counting the number of capillary profiles within the area of an unbiased counting frame (Gundersen, 1977) using systematic uniform randomly sampled electron micrographs obtained at a primary magnification of 3000 ×. The length density was calculated according to the formula LV(cap/lv) = 2Q/A, where LV(cap/lv) is the length density of capillaries in relation to the left ventricle, Q is the number of transects of capillaries, and A is the total area used for counting. The total length was calculated by multiplication of the length density by the left ventricular volume. From the length density of capillaries, we estimated the mean cross-sectional area of tissue surrounding an average capillary by calculating the reciprocal of the length density. This parameter helps to evaluate the diffusion distance from the capillaries to the tissue, but it includes the capillary cross-sectional area of the capillary itself. Therefore, the volume of the capillaries (either including the capillary endothelium or not) was estimated using the point counting method on the same images used for length estimation. Assuming a cylindrical shape of the capillaries, the mean radius of the capillaries was calculated from the length and volume estimations.
The total length of axons ramifying between cardiomyocytes was estimated by a recently established method (Mühlfeld et al. 2010b). Nerve fibre profiles were visualized by immunohistochemistry for protein gene product 9.5 (PGP9.5; Gulbenkian et al. 1987). At an objective lens magnification of 40 ×, the number of PGP9.5-positive nerve fibre profiles was counted if they were lying inside the area of an unbiased counting frame projected onto randomly sampled FOV. Using the same equation as for the length density of capillaries, the length density of nerve fibres was calculated. At the electron microscopic level (primary magnification: 20 000 ×), systematic uniform random sampling was used to obtain FOV containing nerve fibres. From these pictures, the mean number of axon profiles per nerve fibre profile was counted and multiplied with the total length of nerve fibres to obtain the total length of axons in the ventricle. Additionally, the mean diameter of axons was measured as the largest diameter orthogonal to the longest axon profile diameter.
During paraffin embedding a high and unpredictable degree of tissue shrinkage occurs, and leads to false estimations if the densities obtained from the sections are simply multiplied by the reference volume before embedding (Dorph-Petersen et al. 2001). Therefore, from each animal, the one tissue block that had been embedded separately in paraffin was entirely cut into 7-μm-thick sections. Using the Cavalieri principle (Howard & Reed, 2005), the area of these sections was estimated, multiplied by the section thickness and the total number of sections. This microscopically determined volume after embedding and the volume measured before embedding were used to calculate the tissue shrinkage due to embedding. A factor correcting for tissue shrinkage was included to correct the length density of nerve fibres. No such correction was made for samples embedded in Epon because previous studies had shown that tissue shrinkage in epoxy resin is negligible (Dorph-Petersen et al. 2001; Eisele et al. 2008).
Statistics
All data are given as mean (standard deviation) in the tables or as individual values in the figures. Intergroup differences were tested by Kruskal–Wallis anova on Ranks and, where appropriate, subsequent non-parametric two-sided Mann–Whitney U-test for independent samples. Differences were considered statistically significant if P < 0.05.
Results
Animals
The initial body weight of the animals was not different among the three groups. At the time the animals were killed, they had a mean body weight of 24.9 ± 1.1 g in the control group, 19.5 ± 1.9 g in mice subjected to caloric restriction for 3 days (P < 0.05 vs. CG), and 16.8 ± 1.2 g after 7 days of calorie restriction (P < 0.05 vs. CG). The left ventricular weight and volume was significantly higher in controls than in calorie-restricted mice at 7 days. There was a tendency towards higher left ventricle-to-body weight ratios in the calorie-restricted animals, indicating a stronger decrease in body weight than in left ventricle weight, but this was not statistically significant (Table 1).
Table 1.
Body and heart weight
| Control group | 3 days CR | 7 days CR | |
|---|---|---|---|
| Initial body weight (g) | 22.82 (0.57) | 23.52 (2.01) | 22.18 (1.21) |
| Final body weight (g) | 24.88 (1.14) | 19.54 (1.92)** | 16.82 (1.25)** |
| Left ventricle weight (mg) | 96.64 (16.13) | 83.78 (8.91) | 72.26 (8.96)* |
| Left ventricle volume (mm3) | 91.17 (15.21) | 79.04 (8.41) | 68.17 (8.45)* |
| Left ventricle-to-body weight ratio | 3.87 (0.50) | 4.29 (0.17) | 4.32 (0.69) |
CR, caloric restriction.
P < 0.05 vs. control group
P < 0.01 vs. control group.
Cardiomyocytes
Figure 1A–C shows the morphology of the myocardium and the cardiomyocytes. The ratio of myocyte and interstitial volume was not affected by calorie restriction. The total volume of both compartments was significantly lowered after 7 days of caloric restriction. In addition, the volumes of myofibrils, mitochondria and sarcoplasm were significantly lower after 7 days of calorie restriction than in controls; however, there were no significant differences in the relationship of the different compartments, indicating a proportional atrophic remodelling of cardiomyocytes. Some of the parameters but not all were already significantly decreased after 3 days, indicating an early onset of atrophic remodelling (Table 2; Fig. 2).
Fig. 1.
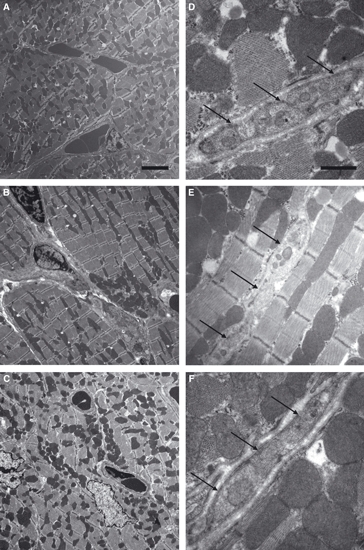
Transmission electron micrographs of cardiomyocyte and nerve fibre morphology. There were no qualitative changes in the myocardial ultrastructure during 7 days of caloric restriction. (A) Control group; (B) 3 days CR; (C) 7 days CR; scale bar: 4 μm. Nerve fibres were abundant between cardiomyocytes. They did not show any qualitative morphological differences between the groups. (D) Control group; (E) 3 days CR; (F) 7 days CR; scale bar: 2 μm. Arrows point at a nerve fibre between the cardiomyocytes.
Table 2.
Summary of stereological data
| Control group | 3 days CR | 7 days CR | |
|---|---|---|---|
| VV(myo/lv) (%) | 85.48 (2.00) | 84.09 (4.45) | 86.96 (2.98) |
| VV(int/lv) (%) | 14.52 (2.00) | 15.91 (4.45) | 13.04 (2.98) |
| V(myo, lv) (mm3) | 77.87 (12.50) | 66.35 (6.76)* | 59.37 (8.49)* |
| V(int, lv) (mm3) | 13.30 (3.27) | 12.68 (4.21) | 8.80 (1.77)* |
| VV(mf/myo) (%) | 52.77 (3.54) | 54.88 (1.35) | 54.66 (1.58) |
| VV(mi/myo) (%) | 36.25 (2.32) | 36.55 (1.54) | 35.70 (1.76) |
| VV(nuc/myo) (%) | 1.71 (0.58) | 1.56 (0.31) | 1.84 (0.45) |
| VV(sp/myo) (%) | 9.27 (2.30) | 6.98 (0.98) | 7.80 (0.70) |
| V(mf, myo) (mm3) | 40.98 (6.08) | 36.40 (3.64) | 32.43 (4.45)* |
| V(mi, myo) (mm3) | 28.40 (6.12) | 24.25 (2.54) | 21.19 (3.23)* |
| V(nuc, myo) (mm3) | 1.34 (0.56) | 1.04 (0.16) | 1.11 (0.39) |
| V(sp, myo) (mm3) | 7.14 (1.63) | 4.67 (1.04)* | 4.64 (0.82)* |
| L(ax, lv) (m) | 27.62 (10.06) | 24.36 (6.42) | 25.55 (6.14) |
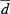 (ax) (ax) |
411 (32) | 415 (31) | 403 (18) |
| LV(cap/lv) (1/μm2) | 0.0049 (0.0004) | 0.0053 (0.0016) | 0.0050 (0.0004) |
| 1/LV(cap/lv) (μm2) | 204 (17) | 209 (83) | 199 (18) |
| L(cap, lv) (m) | 450 (94) | 414 (130) | 345 (64)* |
| VV(caplum/lv) (%) | 3.90 (0.81) | 4.22 (0.98) | 2.56 (0.17)** |
| V(caplum, lv) (mm3) | 3.65 (1.47) | 3.37 (1.08) | 1.76 (0.29)** |
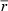 (caplum) (caplum) |
1.58 (0.14) | 1.63 (0.25) | 1.28 (0.04)* |
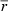 (cap) (cap) |
2.07 (0.17) | 2.09 (0.29) | 1.73 (0.05)* |
CR, caloric restriction; VV, volume density; V, total volume; LV, length density; L, total length; myo, cardiomyocytes; int, interstitium; lv, left ventricle; mf, myofibrils; mi, mitochondria; nuc, nuclei; sp, sarcoplasm; ax, axons; 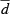 , mean diameter; cap, capillaries; caplum, capillary lumen;
, mean diameter; cap, capillaries; caplum, capillary lumen; 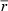 , mean radius.
, mean radius.
P < 0.05 vs. control group
P < 0.01 vs. control group.
Fig. 2.
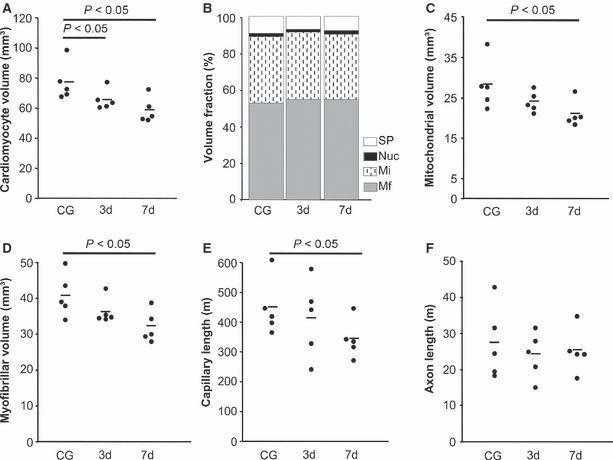
Main stereological results. Each data point represents one animal; the horizontal bar represents the group mean. Abbreviations: Mf, myofibrils; Mi, mitochondria; Nuc, nuclei; SP, sarcoplasm.
Capillaries
The total length and volume of capillaries was similar in controls and mice subjected to 3 days of caloric restriction. At 7 days, the total length and volume of capillaries was significantly smaller. Interestingly, the mean area of myocardium around each capillary showed a very narrow range among the groups. The mean radius of the capillary lumen was significantly smaller at 7 but not at 3 days of caloric restriction. Obviously, the mean radius of capillaries including the endothelium was larger than the lumen in all groups, but the additional contribution of the endothelium to the radius was similar in all groups (Table 2; Fig. 2).
Innervation
Neither the total length of axons in the left ventricle nor the mean diameter of the axons was affected by caloric restriction, indicating that the nervous system compartment does not take part in the structural remodelling of the left ventricle during the investigated time period (Table 2; Figs 1D–F, 2 and 3).
Fig. 3.
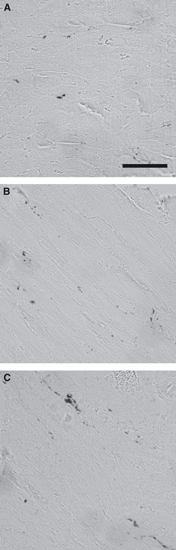
Light micrographs of nerve fibres marked with PGP9.5. There were no qualitative changes in the distribution of nerve fibres during 7 days of caloric restriction. (A) Control group; (B) 3 days CR; (C) 7 days CR; scale bar: 50 μm.
Discussion
The present study was designed to test whether three functionally and structurally different compartments of the left ventricle of the mouse heart undergo a proportional remodelling during a defined period of calorie restriction. The major result is that the cardiomyocyte and the capillary compartments show a proportional atrophic response to the calorie restriction. Most of the changes were significant at 7 days of caloric restriction only. The atrophic processes appeared to have already commenced at 3 days; however, only few parameters reached statistical significance between controls and mice calorie restricted for 3 days. This proportionality is nicely reflected by the small range of the mean area of myocardium surrounding each capillary, indicating that the diffusion distance is kept at a comparably small level in all three groups. Interestingly, the axon tree of the sympathetic neurons innervating the cardiomyocytes is not involved in this atrophic process.
The atrophic response of the cardiomyocytes we observed is characterized by a proportional decrease of various subcellular components. This is in contrast to our recent observation on the atrophic remodelling of the left ventricular myocardium of the mouse in cancer cachexia (Mühlfeld et al. 2011), where we observed a decrease of the myofibrillar compartment and an increase in the sarcoplasmic volume. The difference underlines the different pathogenic remodelling processes in calorie restriction and cachexia. The proportional atrophic process is in contrast to the observation that the ratio between mitochondrial and myofibrillar volume decreases in the ventricles of the starved female rat (Vandewoude & Buyssens, 1992a). Besides species and gender differences, a different calorie restriction protocol may account for these differences. In our study, the mice received 50% of their usual calorie intake, whereas in the Vandewoude and Buyssens study, the rats were subjected to a global caloric deprivation. In the opposite situation, i.e. cardiac hypertrophy due to diet-induced obesity, we have recently observed that the major subcellular compartments of the cardiomyocytes grow in proportion to one another, although the free sarcoplasmic compartment is intermingled with a higher number of larger lipid droplets (Gruber et al., in press). From these considerations we conclude that diet-induced left ventricular atrophy and hypertrophy are processes that lie within the normal range of cardiac plasticity, at least if the changes in diet are not as extreme as a zero-calorie nutrition.
When comparing the cardiomyocyte changes with the alterations in total capillary length, a clear relationship between the atrophic remodelling of both compartments becomes apparent, although the major changes of the capillaries occur between 3 and 7 days of calorie restriction. In particular, the reciprocal of the capillary length density, which is an unbiased parameter for the amount of tissue being supplied by one capillary, is not altered by calorie restriction. It should be noted that this parameter includes the capillaries itself. Therefore, the volume of the capillaries was estimated. Under the model-based assumption of the capillaries being cylindrical structures, the radius of the capillaries was calculated. The data show the luminal radius of the capillaries decreases at 7 days of caloric restriction, but the thickness of the endothelium is not altered. Taken together, our data support the conclusion that the diffusion distance for oxygen and other nutrients remains the same because of the proportionate atrophic response.
Again, in diet-induced hypertrophy we observed a similar growth response between cardiomyocytes and capillaries (Gruber et al., in press). The finding is also contrary to the observation that the maximal oxygen diffusion distance is reduced in starvation in the female rat heart (Vandewoude & Buyssens, 1992b). The same study differences mentioned above may account for this. One interesting aspect is that cardiac atrophy due to unloading also involves both the myocyte and capillary compartment (Rakusan et al. 1997), indicating that the regulation of the phenotypic plasticity of these two compartments is closely linked. Given the close correlation between these compartments with metabolic parameters (Stahl, 1967, Hoppeler et al. 1984), this observation is functionally reasonable and a misrelationship may be associated with pathological events.
In contrast to these findings, the innervation of the left ventricle was not altered structurally as assessed by the total length and the mean diameter of axons. Obviously, this does not imply that there are no changes in the autonomic regulation of the heart. The neurons could be changed in a different way, for example, their expression of neurotransmitters. On the other hand, it may also be possible that the combination of decreased myocyte volume and normal total axon length causes regional hyperinnervation of cardiomyocytes. Moreover, the presence of quantitative changes in myocardial innervation does not necessarily have functional consequences (Kiriazis et al. 2005). As such it is impossible to speculate about the functional significance of this finding. What is remarkable though is the discrepancy between the atrophic response of cardiomyocytes/capillaries and their innervation. A similarly unchanged myocardial innervation was observed in diet-induced obesity (Gruber et al., in press); however, in cancer cachexia cardiomyocyte remodelling was accompanied by a reduction of total axon length to 50% of control values (Mühlfeld et al. 2011). Thus, despite the ability of the cardiac innervation to undergo great quantitative changes, the axons are not quantitatively involved in cardiac atrophy during calorie restriction.
Concluding remarks
In summary, left ventricular atrophy due to calorie restriction leads to a significant reduction of the cardiomyocyte volume and total capillary length within 7 days. The atrophy of the cardiomyocytes involves myofibrils, mitochondria and free sarcoplasm to a similar degree. The axons within the microenvironment of the cardiomyocytes do not undergo atrophy during this period.
Author contributions
CG participated in the design of the study, the acquisition, analysis and interpretation of the data. NN participated in the acquisition, analysis and interpretation of the data. SN participated in the design of the study, the animal experiments, the analysis and interpretation of the data. GM and GK participated in the microscopic work, the acquisition and analysis of the data. RV participated in the design of the study, the animal experiments, the analysis and interpretation of the data. CM provided the concept of the study, planned and supervised the acquisition of the data, and participated in analysis and interpretation of the data. All authors have read and approved the manuscript.
References
- Baskin KK, Taegtmeyer H. Taking pressure off the heart: the ins and outs of atrophic remodelling. Cardiovasc Res. 2011;90:243–250. doi: 10.1093/cvr/cvr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinks H, Tevaearai H, Mühlfeld C, et al. Contractile function is preserved in unloaded hearts despite atrophic remodeling. J Thorac Cardiovasc Surg. 2009;137:742–746. doi: 10.1016/j.jtcvs.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Nyengaard JR, Gundersen HJ. Tissue shrinkage and unbiased stereological estimation of particle number and size. J Microsc. 2001;204:232–246. doi: 10.1046/j.1365-2818.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- Eisele JC, Schaefer IM, Nyengaard JR, et al. Effect of voluntary exercise on number and volume of cardiomyocytes and their mitochondria in the mouse left ventricle. Basic Res Cardiol. 2008;103:12–21. doi: 10.1007/s00395-007-0684-x. [DOI] [PubMed] [Google Scholar]
- Gruber C, Kohlstedt K, Loot AE, et al. Stereological characterization of left ventricular cardiomyocytes, capillaries and innervation in the non-diabetic, obese mouse. Cardiovasc Pathol. doi: 10.1016/j.carpath.2011.11.003. (in press) in press. [DOI] [PubMed] [Google Scholar]
- Gulbenkian S, Wharton J, Polak JM. The visualisation of cardiovascular innervation in the guinea pig using an antiserum to protein gene product 9.5 (PGP 9.5) J Auton Nerv Syst. 1987;18:235–247. doi: 10.1016/0165-1838(87)90122-6. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Notes on the estimation of the numerical density of arbitrary profiles. J Microsc. 1977;111:219–223. [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Lindstedt SL, Claassen H, et al. Scaling mitochondrial volume in heart to body mass. Respir Physiol. 1984;55:131–137. doi: 10.1016/0034-5687(84)90018-5. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology: Three-Dimensional Measurement in Microscopy. 2nd edn. Abingdon: Garland Science/BIOS Scientific; 2005. [Google Scholar]
- Kiriazis H, Du XJ, Feng X, et al. Preserved left ventricular structure and function in mice with cardiac sympathetic hyperinnervation. Am J Physiol Heart Circ Physiol. 2005;289:H1359–H1365. doi: 10.1152/ajpheart.01010.2004. [DOI] [PubMed] [Google Scholar]
- Mühlfeld C, Nyengaard JR, Mayhew TM. A review of state-of-the-art stereology for better quantitative 3D morphology in cardiac research. Cardiovasc Pathol. 2010a;19:65–82. doi: 10.1016/j.carpath.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Mühlfeld C, Papadakis R, Krasteva G, et al. An unbiased stereological method for efficiently quantifying the innervation of the heart and other organs based on total length estimations. J Appl Physiol. 2010b;108:1402–1409. doi: 10.1152/japplphysiol.01013.2009. [DOI] [PubMed] [Google Scholar]
- Mühlfeld C, Das SK, Heinzel FR, et al. Cancer induces cardiomyocyte remodeling and hypoinnervation in the left ventricle of the mouse heart. PLoS One. 2011;6:e20424. doi: 10.1371/journal.pone.0020424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusan K, Heron MI, Kolar F, et al. Transplantation-induced atrophy of normal and hypertrophic rat hearts: effect on cardiac myocytes and capillaries. J Mol Cell Cardiol. 1997;29:1045–1054. doi: 10.1006/jmcc.1996.0350. [DOI] [PubMed] [Google Scholar]
- Razeghi P, Sharma S, Ying J, et al. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation. 2003;108:2536–2541. doi: 10.1161/01.CIR.0000096481.45105.13. [DOI] [PubMed] [Google Scholar]
- Samarel AM, Parmacek MS, Magid NM, et al. Protein synthesis and degradation during starvation-induced cardiac atrophy in rabbits. Circ Res. 1987;60:933–941. doi: 10.1161/01.res.60.6.933. [DOI] [PubMed] [Google Scholar]
- Stahl WR. Scaling of respiratory variables in mammals. J Appl Physiol. 1967;22:453–460. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- Tian M, Nishijima Y, Asp ML, et al. Cardiac alterations in cancer-induced cachexia in mice. Int J Oncol. 2010;37:347–353. doi: 10.3892/ijo_00000683. [DOI] [PubMed] [Google Scholar]
- Vandewoude MF, Buyssens N. Effect of ageing and malnutrition on rat myocardium. I. The myocyte. Virchows Arch A Pathol Anat Histopathol. 1992a;421:179–188. doi: 10.1007/BF01611173. [DOI] [PubMed] [Google Scholar]
- Vandewoude MF, Buyssens N. Effect of ageing and malnutrition on rat myocardium. II. The microvasculature. Virchows Arch A Pathol Anat Histopathol. 1992b;421:189–192. doi: 10.1007/BF01611174. [DOI] [PubMed] [Google Scholar]


