Abstract
Amblyomma americanum (the lone star tick) is a broadly distributed tick that transmits multiple pathogens of humans and domestic animals. `Candidatus Rickettsia amblyommii' is a spotted-fever group rickettsial species that is potentially associated with human disease. In 2008 and 2009, we assayed over 500 unfed adult ticks from 19 Maryland populations for the presence of `Candidatus R. amblyommii'. Infection frequencies ranged from 33% to 100%, with an average infection rate of 60% in 2008 and 69% in 2009. Infection frequencies did not differ statistically between sexes. To develop a system in which to study `Candidatus R. amblyommii' in the laboratory, we used a cell line developed from Anopheles gambiae mosquitoes (Sua5B) to isolate and culture `Candidatus R. amblyommii' from field-collected A. americanum ticks from 2 localities in Maryland. After infection, Sua5B cells were infected for more than 40 passages. Infection was confirmed by Rickettsia-specific PCR, gene sequencing, and Rickettsia-specific fluorescence in situ hybridization (FISH). These data show that `Candidatus R. amblyommii' is widespread in Maryland A. americanum populations and that Sua5B cells are a useful tool for culturing Rickettsia infections from wild ticks.
Keywords: Cell culture, Vector-borne disease, Rickettsiosis, Intracellular
Introduction
Amblyomma americanum (the lone star tick) is broadly distributed across the eastern and central United States and transmits multiple pathogens of humans and domestic animals (Childs and Paddock, 2003; Goddard and Varela-Stokes, 2009). One bacterial species associated with this tick is `Candidatus Rickettsia amblyommii' (which we will refer to as R. amblyommii in the text), which was previously reported as a non-pathogenic Rickettsia species in several states (Burgdorfer et al., 1974, 1975, 1981; Loving et al., 1978). However, more recent studies have implicated this bacterium in human disease (Apperson et al., 2008; Sanchez et al., 1992). R. amblyommii infection is estimated to occur in in 40–60% of collected A. americanum (Clay et al., 2008; Castellaw et al., 2010; Smith et al., 2010).
R. amblyommii was previously cultured from A. cajennense in African green monkey kidney (Vero) cells (Labruna et al., 2004). Mosquito cells are often easier to culture than cells derived from mammals or ticks because they can be passaged rapidly (~1 week), can be grown at room temperature under ambient atmosphere, and potentially develop high bacterial titers (Rasgon et al., 2006; Sakamoto and Azad, 2007). Sakamoto and Azad (2007) identified several mosquito cell lines that seemed highly permissible to multiple Rickettsia species and suggested that these cell lines might be useful as screening tools to isolate and identify novel Rickettsia and other intracellular bacterial infections from arthropods. In particular, the Anopheles gambiae cell line Sua5B has been used to grow R. felis, R. montanensis, R. peacockii, and R. typhi, as well as several strains of the bacterial symbiont Wolbachia (Rasgon et al., 2006; Sakamoto and Azad, 2007; Hughes et al., 2011).
In this study, we examined the distribution and infection frequency of R. amblyommii in natural populations of A. americanum in Maryland. We also used Sua5B cells to isolate and culture R. amblyommii from wild A. americanum ticks collected from 2 Maryland populations.
Materials and methods
R. amblyommii infection survey
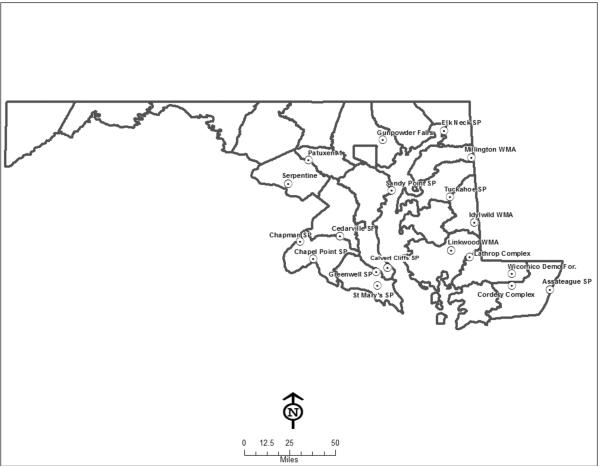
Unfed male and female adult A. americanum ticks were collected from 19 Maryland populations (Fig. 1) in 2008 and 2009 (Tables 1 and 2) by dragging. Ticks were brought live in collection vials to the Johns Hopkins School of Public Health (JHSPH) for processing. Ticks were bisected with a sterile razor blade and half stored at −20°C for archiving. Genomic DNA was extracted from the other half as previously described (Zhang et al., 2011).
Fig. 1.
Amblyomma americanum collection locations in 2008 and 2009.
Table 1.
2008 tick collection data.
| Location | County | Sex | N | # positive | % infected |
|---|---|---|---|---|---|
| Idylwild WMA | Caroline | Female | 19 | 12 | 63% |
| Male | 10 | 4 | 40% | ||
| Linkwood WMA | Dorchester | Female | 29 | 20 | 69% |
| Male | 19 | 12 | 63% | ||
| Assateague SP | Worchester | Female | 9 | 8 | 89% |
| Male | 7 | 7 | 100% | ||
| Cedarville SF | PG and Charles | Female | 2 | 1 | 50% |
| Male | 1 | 0 | 0 | ||
| Chapel Point SP | Charles | Female | 3 | 1 | 33% |
| Chapman SP | Charles | Female | 17 | 15 | 88% |
| Male | 9 | 3 | 33% | ||
| Serpentine | Montgomery | Female | 15 | 9 | 60% |
| Male | 17 | 8 | 47% | ||
| Patuxent 1 | Montgomery | Female | 1 | 1 | 100% |
| St Mary's SP | St Mary's | Female | 4 | 2 | 50% |
| Male | 1 | 1 | 100% | ||
| Greenwell SP | St Mary's | Female | 3 | 1 | 33% |
| Male | 1 | 0 | 0 | ||
| Sandy Point SP | Anne Arundel | Female | 8 | 4 | 50% |
| Male | 9 | 4 | 44% | ||
| Millington WMA | Kent | Female | 2 | 0 | 0 |
| Male | 3 | 1 | 33% | ||
| Calvert Cliffs SP | Calvert | Female | 7 | 4 | 57% |
| Elk Neck SP | Cecil | Female | 1 | 0 | 0 |
| Tuckahoe SP | Queen Anne's | Male | 1 | 1 | 100% |
|
| |||||
| Total | Female | 120 | 78 | 65.0% | |
| Male | 78 | 41 | 52.6% | ||
Table 2.
2009 tick collection data.
| Location | County | Sex | N | # positive | % infected |
|---|---|---|---|---|---|
| Idylwild WMA | Caroline | Female | 20 | 13 | 65% |
| Male | 17 | 15 | 88% | ||
| Linkwood WMA | Dorchester | Female | 2 | 0 | 0 |
| Male | 4 | 3 | 75% | ||
| Assateague SP | Worchester | Female | 4 | 2 | 50% |
| Male | 4 | 4 | 100% | ||
| Cordery Complex (WR10) | Worchester | Female | 30 | 26 | 87% |
| Male | 31 | 22 | 71% | ||
| Cedarville SF | PG and Charles | Female | 3 | 0 | 0 |
| Male | 3 | 1 | 33% | ||
| Serpentine | Montgomery | Female | 40 | 32 | 80% |
| Male | 25 | 21 | 84% | ||
| Calvert Cliffs SP | Calvert | Female | 24 | 9 | 38% |
| Male | 12 | 5 | 42% | ||
| Wicomico Demo Forest (W46) | Wicomico | Female | 16 | 11 | 69% |
| Male | 15 | 10 | 67% | ||
| Lathrop Complex (W06) | Wicomico | Female | 27 | 17 | 63% |
| Male | 25 | 18 | 72% | ||
| Gunpowder Falls | Baltimore | Male | 2 | 1 | 50% |
|
| |||||
| Total | Female | 166 | 110 | 66.3% | |
| Male | 138 | 100 | 72.5% | ||
PCR
DNA extractions were screened for spotted fever group (SFG) rickettsiae as described by Blair et al. (2004). rompA PCR was employed to further confirm Rickettsia infection by using primers Rr190.70p and Rr190.602n as described (Regnery et al., 1991). To further characterize the Rickettsia species, gltA (Regnery et al., 1991), rompB (Roux and Raoult, 2000), and GeneD genes (Sekeyova et al., 2001) were also amplified as described. PCR using template DNA from uninfected Sua5B cells was included as a negative control. All PCR products were separated on a 2.5% agarose tris-borate-EDTA (TBE) gel by electrophoresis and visualized using ethidium bromide staining under UV light. PCR products were directly sequenced.
Tick source material for culturing Rickettsia
Nine female ticks (collection #: 20090351-0359) were collected on June 12, 2009, at Calvert Cliffs SP, Calvert County, MD. Fifteen male ticks (collection #: 0100029-0043) were collected on May 13, 2010, at Serpentine SP, Montgomery County, MD. Ticks were brought alive to the JHSPH in collection vials and maintained until processing.
Cell line infection
Uninfected Anopheles gambiae Sua5B cells were cultured as previously described (Rasgon et al., 2006) in Falcon 24-well plates (Becton Dickinson). Ticks from each collection were pooled, surface-sterilized 3 times with 70% ethanol, then rinsed with sterile water for 5 min. Ticks were homogenized by using a TissueLyser II bead mill (Qiagen, Valencia, CA) with 5-mm stainless steel beads in 300 μl sterile Schneider's insect medium (Invitrogen Corporation, CA). Debris was separated by centrifugation at 200×G for 2 min, and the supernatant collected. The supernatant was passed through a sterile 2.7-μm Whatman filter and layered into cultured Sua5B cells from which the medium had been removed. The plate was centrifuged at 2500×G for one hour. Cells were incubated at room temperature with 100 U/ml penicillin for 2 days and transferred to 25-cm2 flasks for culturing in Schneider's medium supplemented with 10% fetal bovine serum (FBS). Cells were split and passaged once a week. Two independent cultures were attempted, one from ticks collected in 2009 and the other one from ticks collected in 2010.
Confirmation of Rickettsia infection of Sua5B cells
Genomic DNA from cells was isolated using the MasterPure DNA purification kit (Epicentre Biotechnologies, Madison, WI) and eluted in 100 μl sterile water. PCR to confirm and characterize Rickettsia infection of cells was carried out as described above. Rickettsia were also visualized in Sua5B cells using fluorescence in situ hybridization (FISH) as described (Sakamoto and Azad, 2007). Infected cells were grown overnight in Labtek chamber slides (8-well Permanox slides; Nalge Nunc International). Cells were fixed in 4% formalin phosphate-buffered saline at room temperature for 20 min, and the chamber walls and gasket removed. The Rickettsia-specific FISH probe was 5'-conjugated with rhodamine and is specific to all known Rickettsia 16S rRNA genes (5'-rhodamine-TCCACGTCGCCGTCTTGC, IDT, Coralville, IA) (Sakamoto and Azad, 2007). The probe was prepared to a final concentration of 10 pmol/ml hybridization solution as previously described (Rasgon et al., 2006). Probed slides were incubated in a humid chamber at 37°C overnight, then washed as described (Rasgon et al., 2006). Slides were mounted in 50 μl of ProLong (Invitrogen) with 1 ng/ml 4',6-diamidino-2-phenylindole (DAPI) and viewed by epifluorescent microscopy on an Olympus BX-41 compound microscope fitted with epifluorescent optics. Images were taken with a 0.5–0.75 s exposure in a two-by-two-pixel bin at gamma level one using a SPOT RT digital camera (Diagnostic Instruments, Inc., MI), and images merged with SPOT advanced imaging software (Universal Imaging, PA). Uninfected mosquito Sua5B cells were visualized as a negative control.
Results
Infection survey
In 2008, we assayed a total of 198 unfed adult ticks (120 females, 78 males) from 15 Maryland populations for R. amblyommii infection (Table 1). In 2009, we collected 304 ticks from 10 populations (166 females, 138 males) (Table 2). Infection frequencies in populations ranged from 33% to 100%. In total, 60% and 69% of ticks were positive for infection in 2008 and 2009, respectively. Infection frequencies were not statistically different between sexes (2008: female: 65%; male: 52.6%; P=0.1, Fisher's Exact Test; 2009: female: 66.3%; male: 72.5%; P=0.26, Fisher's Exact Test). BLAST search results of sequenced PCR amplicons indicated that rompA (452 bp) was 100% identical with R. amblyommii (EF689733), rompB (763 bp) was 100% identical with Candidatus R. amblyommii isolate 85–1084 (FJ455415), gltA was 100% identical with uncultured Rickettsia sp. clone from A. americanum (GQ302944), and GeneD (579 bp) was 99% identical with Candidatus R. amblyommii isolate 85–1084 (FJ358549).
Isolation and culture of R. amblyommii in Sua5B cells
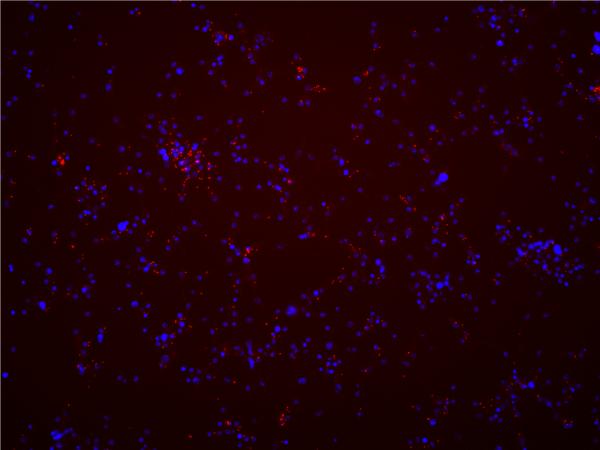
Rickettsia-specific PCR and sequencing was performed at passage number 3 post-infection to initially confirm Rickettsia presence in the cell line, and was repeated approximately every 10 passages thereafter. At the time of manuscript submission, cells were PCR-positive for 45 passages for the 2009 culture and for 20 passages for the 2010 culture. Obtained sequences from PCR amplicons were identical to those described above. Fluorescence in situ hybridization to visualize Rickettsia in Sua5B cells was performed at passage 43 (Fig. 2) for the 2009 infection and at passage 12 for the 2010 collection. Results from both were qualitatively similar. Rickettsiae were detectable as red fluorescent punctate dots in the cytoplasm of infected cells (Fig. 1) that were not visible in Rickettsia-negative cells (data not shown). The cell infection rate was greater than 95%, although interestingly the infection titer in individual cells was relatively low (<10 rickettsiae per cell).
Fig. 2.
Color-merged fluorescence in situ hybridization image of Rickettsia amblyommii-infected Sua5B cells (passage 43 post-infection). Individual Rickettsia bacteria are visible as punctate red dots. Cells were counterstained with DAPI to visualize mosquito cell nuclei (blue signal).
Discussion
In this study, we found that R. amblyommii infection is widespread in Maryland A. amblyommii populations, similar to previous studies in other areas of the United States. Although not confirmed, R. amblyommii has been implicated as a potential human pathogen (Apperson et al., 2008). Due to the extensive distribution and high frequency of infection of this bacterium in natural populations, the potential for R. amblyommii to cause human disease merits further study.
We were able to use a mosquito cell line to isolate R. amblyommii from wild specimens of A. americanum. Infection was stable in the cells for over 40 passages with no decrease in the cell infection rate, although the bacterial titer in individual cells remained relatively low. Our data indicate that cultured mosquito cells can be highly effective for isolating and cultivating Rickettsia infections from wild ticks. The Sua5B cell line, in particular, seems to be highly amenable to Rickettsia infection (Sakamoto and Azad, 2007; Hughes et al., 2011) as well as to infection by other obligate intracellular bacteria such as Wolbachia (Rasgon et al., 2006; Hughes et al., 2011). Mosquito cells have several advantages over other cell lines (tick- or mammal-derived) commonly used for Rickettsia culture – they can be cultured in standard commercially available media, can be grown at room temperature, and do not require additional carbon dioxide atmosphere. The permissiveness of this cell line to infection with intracellular bacteria makes it a useful tool for isolation of Rickettsia from wild-collected arthropods.
Acknowledgements
This research was funded by NIH/NIAID grants R21AI067386 and R03AI079297 to DEN and R21AI070178 to JLR. XZ was partially supported by the Ralph and Sylvia Barr fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. Tick-borne diseases in North Carolina: Is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- Blair PJ, Jiang J, Schoeler GB, Moron C, Anaya E, Cespedes M, Cruz C, Felices V, Guevara C, Mendoza L, Villaseca P, Sumner JW, Richards AL, Olson JG. Characterization of spotted fever group rickettsiae in flea and tick specimens from northern Peru. J. Clin. Microbiol. 2004;42:4961–4967. doi: 10.1128/JCM.42.11.4961-4967.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Adkins TR, Priester LE. Rocky Mountain spotted fever (tick-borne typhus) in South Carolina: an educational program and tick/rickettsial survey in 1973 and 1974. Am. J. Trop. Med. Hyg. 1975;24:866–872. doi: 10.4269/ajtmh.1975.24.866. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Cooney JC, Thomas LA. Zoonotic potential (Rocky Mountain spotted fever and tularemia) in the Tennessee Valley Region. Am. J. Trop. Med. Hyg. 1974;23:109–117. doi: 10.4269/ajtmh.1974.23.109. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Hayes SF, Thomas L, Lancaster JLJ. A new spotted fever group rickettsia from the lone star tick. In: Burgdorfer W, Anacker RW, editors. Rickettsiae and Rickettsial Diseases. Academic Press; New York: 1981. pp. 213–267. [Google Scholar]
- Castellaw AH, Showers J, Goddard J, Chenney EF, Varela-Stokes AS. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J. Med. Entomol. 2010;47:473–476. doi: 10.1603/me09263. [DOI] [PubMed] [Google Scholar]
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- Clay K, Klyachko O, Grindle N, Civitello D, Oleske D, Fuqua C. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Mol. Ecol. 2008;17:4371–4381. doi: 10.1111/j.1365-294x.2008.03914.x. [DOI] [PubMed] [Google Scholar]
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet. Parasitol. 2009;160:1–12. doi: 10.1016/j.vetpar.2008.10.089. [DOI] [PubMed] [Google Scholar]
- Hughes GL, Ren X, Ramirez JL, Sakamoto JM, Bailey JA, Jedlicka AE, Rasgon JL. Wolbachia infections in Anopheles gambiae cells: transcriptomic characterization of a novel host-symbiont interaction. PLoS Pathogens. 2011;7:e1001296. doi: 10.1371/journal.ppat.1001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna MB, Whitworth T, Bouyer DH, McBride J, Camargo LM, Camargo EP, Popov V, Walker DH. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the State of Rondônia, Western Amazon, Brazil. J. Med. Entomol. 2004;41:1073–1081. doi: 10.1603/0022-2585-41.6.1073. [DOI] [PubMed] [Google Scholar]
- Loving SM, Smith AB, DiSalvo AF, Burgdorfer W. Distribution and prevalence of spotted fever group rickettsiae in ticks from South Carolina, with an epidemiological survey of persons bitten by infected ticks. Am. J. Trop. Med. Hyg. 1978;27:1255–1260. doi: 10.4269/ajtmh.1978.27.1255. [DOI] [PubMed] [Google Scholar]
- Rasgon JL, Ren X, Petridis M. Can Anopheles gambiae be infected with Wolbachia pipientis? Insights from an in vitro system. Appl. Environ. Microbiol. 2006;72:7718–7722. doi: 10.1128/AEM.01578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux V, Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer membrane protein rOmpB (ompB) Int. J. Syst. Evol. Microbiol. 2000;50:1449–1455. doi: 10.1099/00207713-50-4-1449. [DOI] [PubMed] [Google Scholar]
- Sakamoto JM, Azad AF. Propagation of arthropod-borne Rickettsia spp. in two mosquito cell lines. Appl. Environ. Microbiol. 2007;73:6637–6643. doi: 10.1128/AEM.00923-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JL, Candler WH, Fishbein DB, Green CR, Cote TR, Kelly DJ, Driggers DP, Johnson BJB. A cluster of tick-borne infections: association with military training and asymptomatic infections due to Rickettsia rickettsii. Trans. R. Soc. Trop. Med. Hyg. 1992;86:321–325. doi: 10.1016/0035-9203(92)90330-f. [DOI] [PubMed] [Google Scholar]
- Sekeyova Z, Roux V, Raoult D. Phylogeny of Rickettsia spp. inferred by comparing sequences of `gene D', which encodes an intracytoplasmic protein. Int. J. Syst. Evol. Microbiol. 2001;51:1353–1360. doi: 10.1099/00207713-51-4-1353. [DOI] [PubMed] [Google Scholar]
- Smith MP, Ponnusamy L, Jiang J, Ayyash LA, Richards AL, Apperson CS. Bacterial pathogens in ixodid ticks from a Piedmont County in North Carolina: prevalence of rickettsial organisms. Vector-Borne Zoonotic Dis. 2010;10:939–952. doi: 10.1089/vbz.2009.0178. [DOI] [PubMed] [Google Scholar]
- Zhang X, Norris DE, Rasgon JL. Distribution and molecular characterization of Wolbachia endosymbionts and filarial nematodes in Maryland populations of the lone star tick (Amblyomma americanum) FEMS Microbiol. Ecol. 2011;77:50–56. doi: 10.1111/j.1574-6941.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]