Abstract
Ixodid ticks are vectors of human diseases such as Lyme disease, babesiosis, anaplasmosis, and tick-borne encephalitis. These diseases cause significant morbidity and mortality worldwide and are transmitted to humans during tick feeding. The tick-host-pathogen interface is a complex environment where host responses are modulated by the molecules in tick saliva to enable the acquisition of a blood meal. Disruption of host responses at the site of the tick bite may also provide an advantage for pathogens to survive and replicate. Thus, the molecules in tick saliva not only aid the tick in securing a nutrient-rich blood meal, but can also enhance the transmission and acquisition of pathogens. To investigate the effect of feeding and flavivirus infection on the salivary gland transcript expression profile in ticks, a first-generation microarray was developed using ESTs from a cDNA library derived from Ixodes scapularis salivary glands. When the salivary gland transcript profile in ticks feeding over the course of 3 days was compared to that in unfed ticks, a dramatic increase in transcripts related to metabolism was observed. Specifically, 578 transcripts were up-regulated compared to 151 down-regulated transcripts in fed ticks. When specific time points post attachment were analyzed, a temporal pattern of gene expression was observed. When Langat virus-infected ticks were compared to mock-infected ticks, transcript expression changes were observed at all 3 days of feeding. Differentially regulated transcripts include putative secreted proteins, lipocalins, Kunitz domain-containing proteins, anti-microbial peptides, and transcripts of unknown function. These studies identify salivary gland transcripts that are differentially regulated during feeding or in the context of flavivirus infection in Ixodes scapularis nymphs, a medically important disease vector. Further analysis of these transcripts may identify salivary factors that affect the transmission or replication of tick-borne flaviviruses.
Keywords: Tick vector, Ixodes scapularis, Nymph, Salivary gland, Gene expression, Feeding, Flavivirus
Introduction
Ixodid ticks carry a variety of pathogens including the bacteria that cause Lyme disease (Burgdorfer et al., 1982) and anaplasmosis (Pancholi et al., 1995), parasites that cause babesiosis (Spielman and Clifford, 1979), and flaviviruses that cause tick-borne encephalitis (TBE) (Zilber and Clifford, 1946), Omsk hemorrhagic fever (Kharitonova and Leoniv, 1985), Kyasanur forest disease (Work, 1958), and Alkurma hemorrhagic fever (Charrel et al., 2001). Transmission of these pathogens to humans generally occurs at the tick-host-pathogen interface during feeding. Ixodid ticks have a particularly slow feeding process and are associated with a host for days to weeks. They penetrate the host skin with long, barbed mouthparts and secrete a cement-like glue that anchors the tick to the host. Once established on the host, feeding begins slowly, and the tick takes in small amounts of blood while synthesizing cuticle to accommodate the increase in volume. The fast feeding stage is characterized by rapid uptake of the blood meal where the tick increases in volume until it becomes fully engorged and detaches from the host. The nutrients in the blood meal are concentrated by pumping water and ions via the saliva back into the host. Thus, tick feeding involves alternating between blood ingestion and salivation (Gregson, 1967). Ticks secrete a variety of bioactive molecules into their saliva to facilitate blood meal acquisition. These salivary molecules function to modulate host responses to the tick such as the hemostatic system, inflammation, and innate and adaptive immunity. Inhibitors of vasoconstriction, platelet aggregation, coagulation, inflammation, complement activation, NK cell activity, and T cell proliferation have been identified in tick saliva and play critical roles during feeding (Anguita et al., 2002; Gillespie et al., 2000; Ribeiro et al., 1985; Wikel, 1999).
The bioactive molecules present in tick saliva not only promote tick feeding, but also create an advantageous microenvironment at the tick bite site for pathogens to survive and replicate. Enhancement of tick-borne pathogen infection by salivary components is called saliva-assisted transmission (SAT) (Nuttall and Jones, 1991). The phenomenon of SAT emphasizes the complex evolution occurring at the tick-host-pathogen interface. Recently, tick salivary gland proteins that enhance the transmission or acquisition of Borrelia burgdorferi (Dai et al., 2009, 2010; Narasimhan et al., 2007b; Ramamoorthi et al., 2005), Anaplasma phagocytophilum (de la Fuente et al., 2006; Sukumaran et al., 2006), and Anaplasma marginale (de la Fuente et al., 2006) have been identified. One of these salivary proteins has been shown to interact directly with the pathogen (Ramamoorthi et al., 2005), while others appear to provide an advantage to the pathogen through inhibition of host responses (Narasimhan et al., 2007b; Tyson et al., 2007). Promotion of virus transmission by SAT has been demonstrated for only one tick-borne flavivirus, tick-borne encephalitis virus (TBEV) (Labuda et al., 1993). This study demonstrated that transmission of TBEV from infected guinea pigs to feeding ticks was significantly higher when salivary gland extract from fed ticks was combined with the virus inoculum. In addition, a greater number of guinea pigs had detectable viremia when compared with guinea pigs that received virus alone. More recently, tick saliva has been shown to enhance TBEV infection of dendritic cells and to modulate their maturation and release of inflammatory cytokines in response to infection in vitro (Fialova et al., 2010). Together, these studies suggest that tick saliva affects the transmission and infection kinetics of TBEV, but specific proteins responsible for these effects have not yet been characterized.
A detailed characterization of the salivary components in ixodid ticks would greatly enhance our understanding of the tick-host-pathogen interface and may provide strategies for controlling tick-borne infections. Identification of tick salivary gland components has significantly advanced with recent studies analyzing the sialotranscriptome of Ixodes scapularis (Ribeiro et al., 2006; Valenzuela et al., 2002) and with the I. scapularis genome project (Hill and Wikel, 2005; Pagel Van Zee et al., 2007). These undertakings were the first to demonstrate the complexity of tick saliva, the redundancy in protein function within gene families, and the abundance of molecules that could be targeted for vaccine research or exploited to develop novel pharmaceutical compounds.
To augment these studies, we developed a first generation microarray to observe salivary gland gene expression in I. scapularis nymphs. The first set of experiments compared salivary gland gene expression in unfed nymphs to that of nymphs that had fed on mice for up to 3 days. These studies demonstrated a temporal salivary gland gene expression profile during feeding including a dramatic up-regulation of transcription. The second set of experiments compared salivary gland gene expression of uninfected nymphs to those infected with Langat virus, a member of the TBEV serogroup, over a 3-day feeding period. Virus infection of nymphs resulted in distinct gene expression changes, providing insight into the response of the tick vector to flavivirus infection. These studies reveal potential targets for future development of novel vaccines against ticks and the pathogens they carry.
Materials and methods
Animals and ticks
I. scapularis ticks were obtained from the tick rearing facility at Oklahoma State University (Stillwater, OK). All ticks were housed in a relative humidity of 98% with a 14:10 h light:dark cycle. While ticks fed upon mice, the animals were housed in a wire-bottomed cage placed over a pan of water. For rearing, ticks were fed to engorgement on adult, white Rocky Mountain Laboratories (RML) mice. For microarray studies, nymphs were applied on the neck and shoulders of mice and allowed to attach. Partially-fed nymphs were collected at 24 h (day 1), 48 h (day 2), and 72 h (day 3) post placement on mice. All mouse work was performed in accordance with the RML Institutional Animal Care and Use Committee.
Synchronous infection of nymphs
Langat virus (LGTV) was kindly provided by Alexander Pletnev (NIAID) and propagated as previously described (Pletnev and Men, 1998). I. scapularis nymphs were infected with LGTV as previously described (Mitzel et al., 2007). Briefly, nymphs were collected in 1.5-ml sterile screw-cap centrifuge tubes and were placed at 65% relative humidity for dehydration. Ticks were then suspended in either 0.5 ml of complete DMEM containing 3×107 pfu/ml of LGTV, strain TP21, or 0.5 ml of PBS (mock) and incubated at 34°C for 45 min. Ticks were washed 2 times with PBS and were wicked free of excess moisture using Whatman paper. Ticks were placed at 98% relative humidity for 17 days to allow replication of LGTV, at which point they were fed on adult mice.
RNA isolation
Groups of 25 partially-fed nymphs from each experimental time point were flash frozen in liquid nitrogen and transferred to Lysing Matrix D tubes (MP Bio) containing 800 ul of RLT buffer (Qiagen). Whole ticks were homogenized using the FastPrep 24 (MP Bio) at speed level 6 m/s for 40 s, and the homogenate was cleared by centrifugation at 21,000 × g for 3 min. RNA was extracted using the RNeasy mini kit (Qiagen) following the manufacturer's instructions. RNA quality was analyzed on an Agilent 2100 Bioanalyzer.
cDNA library
The ESTs used to generate the microarray were derived from 6 cDNA libraries as previously described (Ribeiro et al., 2006). A detailed description of the annotated transcripts from the 6 salivary gland libraries can be found at Ribeiro et al. (2006). The microarray was developed using the 7476 ESTs generated from the 6 I. scapularis salivary gland cDNA libraries that were assembled to produce 3020 annotated transcripts.
Tick salivary gland gene expression microarray
Sure-print microarray slides, 4×44K format were purchased from Agilent Technologies. The microarray design was based on a sequencing library of 7476 EST sequences. 60-mer probes were selected using Agilent e-Array software http://earray.chem.agilent.com/earray. We searched for the 5 “best probes” of each EST by base composition. The array also included probes for 90 target sequences representing housekeeping gene transcripts and 67 probes to transcripts predicted to be related to immune functions.
RNA amplification and microarray hybridization
Total tick RNA samples were reverse transcribed and amplified using 50 ng following protocols from the Ovation RNA Amplification System V2 from Nugen Technologies (User Guide Cat. #D01014, Version 05.01.07, Part # 3100-12). The amplified cDNA samples were labeled with Cy3, and a pooled sample (comprised of equal portions of each of the test samples) was labeled with Cy5 following the protocol from the FL-Ovation cDNA Fluorescent Module from Nugen (User Guide Cat. #D01020, Version 02.20.08, Part # 4310-12). The samples were hybridized against the array slides at 65°C for 40 h as recommended in the FL-Ovation cDNA Fluorescent Module protocol. After hybridization, the slides were washed according to the Agilent Technologies Two-Color Microarray-Based Gene Expression Analysis (Quick Amp Labeling) protocol (Version 5.7, March 2008). The slides were scanned using the Agilent Microarray Scanner at 5 micron resolution using the extended dynamic range (XDR) mode at a 100:10 ratio. Image analysis was performed using Feature Extraction software from Agilent.
Statistical analysis
Test for expression differences was calculated using mixed effects ANOVA models in JMP Genomics (SAS Institute). Feeding time-dependent expression for each hybridization target was determined using exhaustive comparisons of each time point to other time points or groups of time points. Effect of LGTV infection was estimated using a 2-way ANOVA model and formal comparisons of infected versus mock condition at each time point. The microarray analysis comparing fed to unfed ticks was performed on 3 independent biological replicates. The microarray analysis comparing LGTV-infected and uninfected ticks was performed on 2 independent biological replicates.
Validation of array by quantitative real-time PCR
Quantitative real-time PCR (qPCR) reactions were performed in triplicate using Express Two-Step Superscript® qRT-PCR with Premixed ROX (Invitrogen). First strand cDNA synthesis used 750 ng of total RNA for each sample. The reactions were incubated at 25°C for 10 min, 42°C for 2 h, 85°C for 5 min. The custom primers and probes for PCR were designed using the Primer Express software (Applied Biosystems). Sequences for the primers and probes are shown in Supplemental Table 1. Reactions were run on the ABI PRISM 7900HT sequence detection system and analyzed using the SDS2.3 software (Applied Biosystems). The data were normalized to the I. scapularis 16s rRNA at each corresponding time point, and fold changes were determined using the standard curve method.
Results
Using ESTs derived from the cDNA libraries utilized in the sialotranscriptome analysis (Ribeiro et al., 2006), we developed a first-generation microarray to observe I. scapularis salivary gland transcript expression changes associated with tick feeding or with flavivirus infection.
Comparison of the salivary gland transcript expression profile between unfed and fed Ixodes scapularis nymphs
Blood feeding by ticks represents an enormous change from a conserving to a robust metabolism, which involves intense salivary biosynthetic capacity as well as increased water and ion transport. Microarray analysis comparing salivary transcripts from fed (days 1, 2, and 3) to unfed ticks confirmed this increase in metabolism showing 578 transcripts to be up-regulated and only 151 down-regulated gene products in feeding ticks (Table 1; specific contigs can be seen in Supplemental Table 2). Further analysis of Table 1 demonstrates 128 transcripts assigned to the secreted class are up-regulated, while only 3 secreted transcripts are down-regulated upon feeding. The down-regulated transcripts code for a threonine-rich protein (possibly a mucin or a protein needed for water vapor capture), for a Gly-rich peptide that is similar to antimicrobial peptides (AMP) of worms (Couillault et al., 2004) and for a member of the 8.9-kDa protein family in ticks, of unknown function, but that could be an AMP (Francischetti et al., 2009). Because transcripts containing a signal peptide encode proteins that are likely secreted into the saliva where they may function to modulate host responses during feeding, the secreted class of differentially regulated transcripts is of particular interest.
Table 1.
Differentially expressed family of transcripts when the comparison was made between fed (1, 2 and 3 days) or unfed ticks.
| Class | Down regulated | Up regulated |
|---|---|---|
| Unknown | 60 | 135 |
| Secreted | 3 | 128 |
| Metabolism, energy | 14 | 72 |
| Protein modification machinery | 8 | 45 |
| Protein synthesis machinery | 21 | 24 |
| Protein export machinery | 4 | 21 |
| Cytoskeletal | 0 | 15 |
| Transcription machinery | 14 | 14 |
| Proteasome machinery | 3 | 11 |
| Signal transduction | 2 | 11 |
| Oxidant metabolism/detoxication | 0 | 10 |
| Transporters/storage | 0 | 9 |
| Extracellular matrix/cell adhesion | 0 | 7 |
| Metabolism, nucleotide | 1 | 7 |
| Nuclear regulation | 4 | 6 |
| Metabolism, amino acid | 0 | 6 |
| Metabolism, carbohydrate | 0 | 6 |
| Metabolism, intermediate | 0 | 4 |
| Metabolism, lipid | 0 | 4 |
| Transcription factor | 4 | 2 |
| Lysosomal | 0 | 1 |
| Transposable element | 4 | 0 |
| Unknown, conserved | 9 | 40 |
| Total | 151 | 578 |
Nine transcripts coding for transporters or for storage proteins were up-regulated upon feeding, including 2 components of the V-ATPase complex, important for water and ion movement across membranes (Nelson, 2003), and 2 ion transporters.
Two transcripts coding for putative transcription factors (TF) were up-regulated while 4 were down-regulated upon feeding. Transcripts coding for the TF e(y)2, which is highly conserved in eukaryotes (Georgieva et al., 2001), were up-regulated in fed ticks. In contrast, the transcriptional repressor EED/ESC/FIE was down-regulated in fed ticks. Proteins of this family are important suppressors of homeotic genes (Ohad et al., 1999), possibly functioning to prevent differentiation of the salivary glands while the tick is in the unfed state. In the category of nuclear regulation, 6 transcripts were up-regulated during blood feeding including 4 histones, a product homologous to the Anapc13 protein, which is important for cell cycle control and a protein potentially involved in replication/repair.
In unfed ticks, a small protein associated with negative regulation of transcription from the Pol II promoter was up-regulated, as was another transcriptional regulator similar to human HCNGP, which is associated with proteins that may inhibit cell survival genes. Products coding for a CRE-binding protein as well as a homolog of the homeodomain only protein were up-regulated in unfed ticks. Up-regulation of these transcripts classified as TF is consistent with a state of transcriptional paralysis in unfed ticks. Also associated with transcriptional control, 4 transcripts involved with nuclear regulation were up-regulated in unfed ticks, including products associated with transcriptional control and histone acetylation, with histone acetyltransferase, and with the nucleosome assembly protein NAP-1, which in human and yeast facilitates the association of histones with DNA (Ishimi and Kikuchi, 1991). These transcripts suggest a role of histone modification in the DNA regulation of unfed ticks. Transcriptional up-regulation of a gene coding for the mitochondrial single-stranded DNA-binding protein was observed in unfed ticks and is important in mitochondrial genome maintenance and replication.
Somewhat puzzling was the up-regulation of 21 products associated with protein synthesis in unfed ticks, which consisted of elongation factors and ribosomal proteins, while 24 other products in the same category were found up-regulated in fed ticks, as expected. The same situation occurs with products associated with the transcription machinery. It is possible that some vital protein synthetic pathways are observed in unfed ticks (such as production of hygroscopic saliva), which use a unique set of proteins for transcriptional and protein synthesizing machinery, perhaps due to the vast biotic differences seen in unfed (dehydrated, metabolic conserving) and fed (overhydrated, high metabolism) ticks.
Many products classified as genes coding for metabolic enzymes, cytoskeletal and extracellular matrix components, as well as for protein modification and protein export machinery were up-regulated upon feeding, as expected.
Four transposable element-associated products were up-regulated in unfed ticks, all representing Class I elements. This finding is consistent with the idea that these elements transcribe more in organisms that are stressed, such as dehydrated, unfed ticks (Capy et al., 2000).
A number of unknown transcripts were found in this analysis, representing a large portion of the transcripts identified as significantly altered in their expression levels. Specifically, 175 unknown or unknown, but conserved transcripts were up-regulated, whereas 69 were down-regulated in fed ticks. Some of the transcripts of the unknown class could represent probes that aim solely to untranslated regions of genes as was shown to occur in an analysis of the sialotranscriptome of Anopheles gambiae (Arca et al., 2005). In addition, it is possible that some of the proteins encoded by transcripts with unknown function may be secreted into the saliva, but their secretory signal peptide was not present because the transcript lacked the 5’ end of the sequence. For example, a histamine release factor (HRF) detected in Dermacentor variabilis saliva did not contain a putative signal peptide (Mulenga et al., 2003). In addition, transcripts from Ixodes ricinus (Chmelar et al., 2008) and Ixodes scapularis (Dai et al., 2010) were shown to have homology to the HRF and also lack a secretion signal. For this reason and the obvious possibility that some of the unknown transcripts may have novel functions, the unknown transcripts that were differentially regulated in fed ticks are interesting targets for future study.
Temporal analysis of the salivary gland transcript expression profile in unfed and fed I. scapularis nymphs
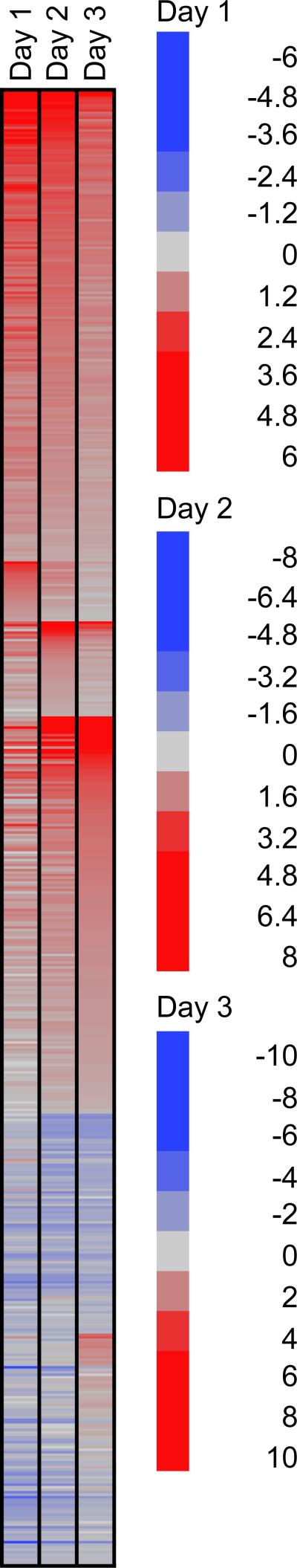
Because the acquisition of a blood meal by ticks is a highly dynamic process, we analyzed the transcript expression profile as ticks fed over a period of 3 days. To graphically depict expression patterns over the course of feeding, a heat map of the microarray data was generated showing relative expression of differentially regulated transcripts over the 3-day feeding period (Fig. 1). These data demonstrate the temporal pattern of salivary gland gene transcript expression over time.
Fig. 1.
Temporal pattern of salivary gland gene expression in nymphs. A heat map showing expression ratios (fed vs unfed) of 911 contigs that had statistically significant* changes during feeding or between any of the feeding timepoints. Red encodes positive values of the log2 ratio (up-regulation), while blue indicates down-regulation during feeding. Transcripts were sorted according to observed feeding time specificity of expression. *False Discovery Rate of 0.05.
The different times post attachment (24 h, 48 h, and 72 h) were compared to each other to ascertain if there were transcript expression changes that could be attributed to the different stages of feeding. The first day of feeding involves attachment to the host and transformation from a metabolically inactive to a metabolically active tick. Comparison of differentially expressed transcripts between day 1 and subsequent days post attachment may indicate genes that are associated with the attachment process, when up-regulated. Down-regulated transcripts in this comparison set may indicate genes that are significantly expressed in the mid and final feeding stages. Notably when comparing day 1 to days 2 and 3 post attachment, 56 transcripts coding for putative secreted proteins, as well as transcripts associated with energy metabolism, protein export machinery and cytoskeleton, which may represent the growth of the tissues as feeding progresses were found to be down-regulated during the earliest phase of blood meal acquisition (Table 2). Transcripts of the unknown class are the most abundant up-regulated gene products on day 1 (Table 2). Only 3 transcripts classified as putative secreted salivary products were up-regulated at 24 h post attachment, including a transcript coding for a Pro-Gly-rich peptide, which may be part of the attachment cement, and members of the 8.9-kDa and 18.7-kDa families, which have no known function (Table 2). Specific contigs that were differentially expressed on day 1 of feeding can be seen in Supplemental Table 3.
Table 2.
Differentially expressed family of transcripts when the comparison was made between day 1 of feeding with days 2 and 3.
| Class | Down regulated | Up regulated |
|---|---|---|
| Unknown | 69 | 20 |
| Secreted | 56 | 3 |
| Metabolism, energy | 38 | 1 |
| Protein modification machinery | 19 | 4 |
| Protein export machinery | 17 | 1 |
| Unknown, conserved | 13 | 0 |
| Cytoskeletal | 12 | 0 |
| Oxidant metabolism/detoxication | 7 | 1 |
| Extracellular matrix/cell adhesion | 7 | 0 |
| Signal transduction | 5 | 2 |
| Transcription machinery | 4 | 5 |
| Metabolism, carbohydrate | 4 | 0 |
| Metabolism, amino acid | 4 | 0 |
| Nuclear regulation | 3 | 2 |
| Metabolism, nucleotide | 3 | 1 |
| Metabolism, lipid | 3 | 1 |
| Transcription factor | 2 | 0 |
| Transporters/storage | 2 | 0 |
| Protein synthesis machinery | 1 | 1 |
| Metabolism, intermediate | 1 | 1 |
| Lysosomal | 1 | 0 |
| Proteasome machinery | 0 | 0 |
| Transposable element | 0 | 1 |
| Total | 271 | 44 |
The comparison in Table 3 shows differentially expressed transcripts unique to the second day of feeding (48 h post attachment) with relationship to both the previous and the following days. It provides for a relatively small number of differentially expressed transcripts, including very few related to metabolism, and protein synthesis, modification, and export as expected. Notably, 17 transcripts coding for secreted proteins are up-regulated, including several coding for Gly-Tyr-rich peptides that are similar to worm AMP, 2 members of the 9.4-kDa secreted protein family (164, 165) and a peptide containing a trypsin inhibitor like (TIL) domain that suggests it functions as a protease inhibitor and/or as an AMP (Otvos, 2000). None of these putative secreted proteins have been functionally characterized. Specific contigs that were differentially expressed on day 2 of feeding can be seen in Supplemental Table 4.
Table 3.
Differentially expressed family of transcripts when the comparison was made between day 2 of feeding with days 1 and 3.
| Class | Down regulated | Up regulated |
|---|---|---|
| Secreted | 8 | 17 |
| Extracellular matrix/cell adhesion | 0 | 5 |
| Unknown | 11 | 5 |
| Protein export machinery | 0 | 4 |
| Protein modification machinery | 1 | 4 |
| Protein synthesis machinery | 1 | 2 |
| Metabolism, energy | 0 | 2 |
| Nuclear regulation | 0 | 1 |
| Transcription machinery | 0 | 1 |
| Signal transduction | 1 | 1 |
| Cytoskeletal | 0 | 1 |
| Unknown, conserved | 0 | 1 |
| Transcription factor | 2 | 0 |
| Proteasome machinery | 1 | 0 |
| Transporters/storage | 0 | 0 |
| Oxidant metabolism/detoxication | 0 | 0 |
| Metabolism, carbohydrate | 0 | 0 |
| Metabolism, nucleotide | 0 | 0 |
| Metabolism, amino acid | 0 | 0 |
| Metabolism, lipid | 1 | 0 |
| Metabolism, intermediate | 0 | 0 |
| Transposable element | 0 | 0 |
| Lysosomal | 0 | 0 |
| Total | 26 | 44 |
The third day of nymphal feeding (72 h post attachment) is characterized by a relatively high number of up-regulated transcripts when compared to the previous 2 days of feeding (Table 4). Fifty-four transcripts coding for putative secreted proteins, possibly involved with salivary function were up-regulated on day 3 of feeding. This category includes members of TIL domain containing peptides as well as transcripts coding for putative protease inhibitors, including those coding for 2 different serpins (1070, 2940), 2 members of the Kunitz family (469, 470), and one cystatin. A transcript coding for a lipocalin was also differentially up-regulated on day 3. A member of the AMP defensin family as well as several members of the Gly-Tyr rich protein family, possibly an AMP as per their similarity to nematode peptides (Couillault et al., 2004), were up-regulated. Transcripts coding for a secreted ribonuclease were also up-regulated and could be related to AMP function (Deshpande and Shankar, 2002). Mucins, a Gly-rich peptide and a peritrophin were also up-regulated. Except for the cystatin, which has anti-inflammatory and immunosuppressive properties (Kotsyfakis et al., 2006, 2007, 2008), the remaining proteins have not been functionally characterized. Notably, many transcripts coding for proteins or enzymes associated with oxidant metabolism were up-regulated, including several coding for alkyl hydroperoxide reductases, selenoproteins, and superoxide dismutase. These proteins, if secreted, could help to counteract oxygen radicals released by activated leukocytes, or intracellularly to prevent oxidative damage that could result from the very active prostaglandin synthetic activity during feeding that leads to high amounts of PGE2 in tick saliva (Ribeiro et al., 1992; Sa-Nunes et al., 2007). Dopamine is a salivary secretagogue in ticks, and possibly associated with its catabolism is the increased expression of transcripts homologous to cytosolic sulfotransferases that are known detoxicants of catecholamines (Negishi et al., 2001). Enzymes associated with proline hydroxylation were also up-regulated, suggesting, as previously stated (Ribeiro et al., 2006), that many of the proline-rich salivary proteins could be modified to look more like collagen, which has many hydroxyproline and hydroxylysine post-translation modifications. Transcripts coding for homologs of the vertebrate cytokine Macrophage Inhibition Factor were also elevated (Orita et al., 2002), but it is not clear whether this ubiquitous protein is secreted in saliva, as it does not have a signal peptide. As in the previous comparisons, many transcripts of unknown function and putative secreted proteins were differentially transcribed. Specific contigs that were differentially expressed on day 3 of feeding can be seen in Supplemental Table 5.
Table 4.
Differentially expressed family of transcripts when the comparison was made between day 3 of feeding with days 1 and 2.
| Class | Down regulated | Up regulated |
|---|---|---|
| Unknown | 14 | 80 |
| Secreted | 3 | 54 |
| Metabolism, energy | 2 | 15 |
| Protein modification machinery | 5 | 14 |
| Cytoskeletal | 0 | 11 |
| Unknown, conserved | 2 | 9 |
| Oxidant metabolism/detoxication | 1 | 7 |
| Protein export machinery | 1 | 6 |
| Transcription factor | 1 | 5 |
| Transcription machinery | 9 | 4 |
| Signal transduction | 2 | 4 |
| Extracellular matrix/cell adhesion | 0 | 4 |
| Protein synthesis machinery | 4 | 3 |
| Metabolism, carbohydrate | 0 | 3 |
| Metabolism, amino acid | 0 | 3 |
| Metabolism, lipid | 0 | 3 |
| Nuclear regulation | 2 | 2 |
| Proteasome machinery | 0 | 2 |
| Transporters/storage | 2 | 2 |
| Metabolism, nucleotide | 1 | 1 |
| Transposable element | 0 | 1 |
| Lysosomal | 0 | 1 |
| Metabolism, intermediate | 0 | 0 |
| Total | 49 | 234 |
To confirm the validity of the microarray analysis, 15 transcripts shown to be differentially expressed in the microarray analysis were analyzed using quantitative real-time PCR (qPCR). I. scapularis 16s rRNA was used for normalization and fold changes were calculated using the standard curve method. Linear regression analysis was performed and correlation values calculated by plotting the fold changes observed in the microarray against the fold changes observed in qPCR. This analysis gave a good degree of correlation (R2=0.70, data not shown), typical for this kind of cross comparison, validating the results of the microarray analysis.
Comparison of the salivary gland transcription profile between Langat virus-infected and mock-infected I. scapularis nymphs
Validation of the microarray comparing fed to unfed ticks provided us with the background to observe the salivary gland transcript expression changes associated with flavivirus infection in ticks. LGTV-infected or mock-infected nymphs were fed on mice and subsequently harvested at 1, 2, and 3 days post attachment. Small, but significant groups of salivary gland transcript expression changes were observed at all 3 time points of feeding when LGTV-infected and mock-infected ticks were compared (Table 5). The small number of differentially expressed salivary gland transcripts observed in this study is similar to other studies involving infection of ticks with Borrelia burgdorferi and Theileria parva (Alarcon-Chaidez et al., 2006; Nene et al., 2004).
Table 5.
Differentially expressed transcripts in LGTV infected compared to mock infected nymphs over the average of the three feeding timepoints (A) or at 1 day (B), 2 days (C) or 3 days (D) post attachment.
| A. LGTV infected compared to Mock average of three time points | ||||
|---|---|---|---|---|
| contig | fold change | p value* | category | annotation |
| 112 | 3.038073 | 0.013 | s/hbp | 25 kDa salivary gland protein family |
| 216 | -1.07158 | 0.091 | uk | unknown |
| 2446 | 1.113103 | 0.031 | uk | unknown |
| B. Day 1 LGTV infected compared to mock infected | ||||
|---|---|---|---|---|
| contig | fold change | p value* | category | annotation |
| 1040 | 1.103896 | 0.057 | s | Thr rich protein |
| 1214 | -1.07742 | 0.010 | uk | unknown |
| 1294 | 1.028793 | 0.021 | s | unknown |
| 1368 | 1.414937 | 0.041 | s/hbp | putative 22.5 kDa secreted protein family |
| 141 | 1.669891 | 0.094 | s | putative secreted salivary gland peptide |
| 150 | -1.123 | 0.056 | h/ps | rRNA |
| 1541 | 1.770322 | 0.018 | s | putative 10.2 kDa secreted protein family |
| 156 | -1.78332 | 0.010 | s | putative secreted protein |
| 16 | 1.282444 | 0.021 | s | putative 5.3 kDa secreted protein family |
| 171 | -1.02389 | 0.086 | s | 16 kDa salivary gland proteinfamily |
| 2024 | 1.292348 | 0.013 | s | putative 8.4 kDa secreted protein family |
| 2446 | 1.202692 | 0.096 | uk | unknown |
| 2520 | -2.27403 | 0.075 | s | putative secreted protein |
| 2827 | 1.666958 | 0.027 | s/hbp | putative 22.5 kDa secreted protein family |
| 2850 | 1.066202 | 0.005 | h/cs | actin-5C - sperm individualization |
| 2940 | 1.042433 | 0.041 | s/serp | serpin |
| 379 | 1.168351 | 0.004 | h/pm | glutathione S-transferase Mu 1 |
| 380 | 1.350044 | 0.084 | s/hbp | lipocalin like protein |
| 429 | 1.118668 | 0.084 | h/cons | homolog of human multiple coagulation factor deficiency 2 |
| 498 | 1.808626 | 0.044 | s | unknown |
| 890 | 1.523475 | 0.019 | s | truncated secreted protein |
| 984 | 1.068396 | 0.058 | s/mp | metalloprotease |
| C. Day 2 LGTV infected compared to mock infected | ||||
|---|---|---|---|---|
| contig | fold change | p value* | category | annotation |
| IS07-1672 | -1.07540 | 0.065 | Immune | microplusin preprotein-like |
| IS07-20802 | -1.11664 | 0.056 | Immune | microplusin preprotein-like |
| 1117 | 1.346923 | 0.009 | uk | unknown |
| 112 | 3.930712 | 0.028 | s/hbp | 25 kDa salivary gland protein family; lipocalin |
| 1763 | -1.03699 | 0.083 | h/nr | DEAD-box protein |
| 1994 | -1.20701 | 0.038 | h/cs | tubulin beta-1 chain |
| 2159 | -1.06922 | 0.084 | h/tm | 67 kDa polymerase-associated factor PAF67 |
| 2607 | 1.069192 | 0.094 | uk | unknown |
| 2616 | -1.01096 | 0.086 | h/ps | translation initiation factor 3; kun |
| 2940 | -1.02945 | 0.042 | s/serp | serpin |
| 356 | -1.26118 | 0.024 | s/kun | Kunitz protease inhibitor |
| 468 | -1.06652 | 0.090 | s/hbp | lipocalin like protein |
| 469 | 1.648069 | 0.080 | s/kun | putative secreted protease inhibitor; kun |
| 476 | -1.18143 | 0.028 | s | unknown |
| 50 | -1.14065 | 0.043 | s | putative secreted protein |
| 576 | 1.023254 | 0.099 | h/meten | NADH dehydrogenase subunit 5 |
| D. Day 3 LGTV infected compared to mock infected | ||||
|---|---|---|---|---|
| contig | fold change | p value* | category | annotation |
| IS07-10903 | -1.11419 | 0.031 | Immune | defensin precursor |
| IS07-10904 | -1.23397 | 0.032 | Immune | antimicrobial peptide |
| 112 | 3.486134 | 0.038 | s/hbp | 25 kDa salivary gland protein family; lipocalin |
| 146 | 1.270404 | 0.011 | h/ps | rRNA intron-encoded homing endonuclease |
| 1573 | 1.240068 | 0.027 | h/meten | ATP synthase F0 subunit 6 |
| 1908 | -5.79522 | 0.078 | s | putative 18.7 kDa secreted protein family; lipocalin |
| 213 | -1.81942 | 0.095 | uk | unknown |
| 2446 | 1.229296 | 0.091 | uk | unknown |
| 411 | 2.330357 | 0.070 | s/hbp | 26 kDa salivary gland proteinfamily; lipocalin |
| 43 | 5.101603 | 0.062 | s | putative secreted protein |
| 498 | 1.421836 | 0.078 | s | unknown |
| 542 | -1.39584 | 0.017 | s | putative 8.9 kDa secreted protein family |
| 654 | 1.085064 | 0.080 | h/meten | NADH dehydrogenase subunit 3 |
Raw p values not adjusted for multiple comparisons, overall False Discovery Rate was 0.05.
Fold changes are shown in log2. Category abbreviations are as follows: s, secreted; uk, unknown; hbp, histamine binding protein; h, housekeeping; ps, protein synthesis; cs, cytoskeletal; serp, serpin; pm, post-translational modification machinery; cons, conserved; mp, metalloprotease; nr, nuclear regulation; tm, transcription machinery; kun, Kunitz domain containing protein; meten, energy metabolism.
When all 3 time points post attachment were averaged, 3 transcripts were statistically differentially regulated in LGTV-infected ticks compared to uninfected ticks (Table 5A). One of these is classified into the 25-kDa salivary gland protein family and has homology to the lipocalins. The other 2 transcripts (213, 2446) have no known function.
On day 1 of feeding (24 h post attachment), transcripts that were up-regulated in LGTV-infected nymphs include several putative secreted proteins, which fall into the 5.3-kDa, 8.4-kDa, 10.2-kDa, and 22.5-kDa (1368, 2827) protein families (Table 5B). Up-regulated transcripts also include a serpin, a glutathione S-transferase homolog, a metalloprotease, and a lipocalin-like protein. Only 5 transcripts were down-regulated on day 1 in LGTV-infected ticks and include rRNA, a 16-kDa salivary gland protein, 2 putative secreted proteins (156, 2520), and an unknown transcript.
Only 5 of 16 differentially regulated transcripts were up-regulated in LGTV-infected ticks 2 days post attachment (Table 5C). The up-regulated transcripts include a Kunitz domain-containing protein, an NADH dehydrogenase subunit, a member of the 25-kDa salivary gland protein family with homology to the lipocalins, and 2 unknown proteins (1117, 2607). The majority of differentially regulated transcripts on day 2 is down-regulated and includes 2 microplusin-related proteins, several housekeeping genes, a serpin, a lipocalin, and a Kunitz protease inhibitor.
The smallest number of differentially regulated transcripts in LGTV-infected ticks was observed 3 days post attachment (Table 5D). Up-regulated transcripts include rRNA, an ATP synthase subunit, an NADH dehydrogenase subunit, and a 26-kDa salivary gland protein family member with homology to lipocalins. One putative secreted protein had a fold change of 5.1 (log2), suggesting this transcript to be highly up-regulated in LGTV-infected ticks. Another interesting transcript is classified into the 25-kDa salivary gland protein family and also has homology to the lipocalins. This protein had a fold change of 3.5 (log2) compared to uninfected ticks on day 3. Down-regulated transcripts include a defensin precursor, an AMP, a putative 8.9 secreted protein family member, and a putative 18.7-kDa secreted protein family member with homology to the lipocalins. At all 3 time points of feeding, several differentially regulated transcripts were classified as unknown.
Sixteen transcripts were analyzed by qPCR to confirm the validity of the microarray results. All of the transcripts tested were validated in the direction of their change in the microarray analysis, but not necessarily in the magnitude of the change. For example, if the transcript was up-regulated in the microarray analysis, it was also found to be up-regulated by qPCR. However, the actual fold change values for each transcript varied leading to a low correlation when linear regression analysis was performed (R2=0.47, data not shown). The low correlation may be further explained because of differences in normalization methods or differences in the location of the primers for real-time PCR compared to hybridization location of the microarray probes.
Discussion
The microarray studies reported here demonstrate that acquisition of a blood meal over a 3-day period by I. scapularis nymphs is accompanied by a dramatic increase in salivary gland transcription, validating previous reports of tick salivary gland gene expression during feeding (Narasimhan et al., 2007a; Ribeiro et al., 2006; Valenzuela et al., 2002). In addition, these studies have shown for the first time that flavivirus infection alters salivary gland transcript expression in nymphs, the developmental stage most recognized for pathogen transmission (Nosek et al., 1972; Nuttall et al., 1994). The small size of the nymphs dictated that certain experimental procedures be performed such as using whole ticks as well as pooling ticks at each time point. Because tick attachment and initiation of feeding is not a synchronous process, pooling of ticks at each time point accounts for this potentially staggered transcription, although it limits our ability to determine gene expression changes in an individual tick. Moreover, because whole ticks were used, we cannot say with absolute certainty that the observed differentially regulated transcription is salivary gland-specific as some of the tick genes may also be expressed in other tissues, particularly the midgut tissue. Even with these limitations, this study identified numerous tick salivary molecules that are differentially regulated in response to tick feeding or flavivirus infection that may be exploited for the development of anti-tick or anti-flavivirus vaccines. At all 3 feeding times tested, the largest number of differentially regulated transcripts belongs to the unknown or secreted classes. Further analysis of these unknown and secreted molecules may reveal novel functions for tick proteins. Targeting tick salivary molecules that are required for the early stages of feeding will likely prove to be the most effective strategy for the development of anti-tick and possibly even anti-flavivirus vaccines, particularly because flaviviruses can be transmitted within 15 min of tick attachment (Ebel and Kramer, 2004). Thus, the differentially regulated transcripts observed on day 1 of feeding are currently being analyzed for this purpose. Among the 3 days of feeding that were compared, the third day showed the greatest variety of differentially regulated transcripts. Perhaps, this time point reflects the peak of metabolic activation and/or the point where the host mounts significant inflammation at the attachment site and requires more salivary products to disarm it.
Although I. scapularis is not a natural vector for LGTV (Smith, 1956), we have previously demonstrated the competence of I. scapularis for LGTV (Mitzel et al., 2007). LGTV is used to model the more pathogenic BSL3 and BSL4 tick-borne flaviviruses such as Powassan virus and TBEV, respectively, for which related ixodid ticks are the natural vectors. The commercial availability of I. scapularis ticks, their competence for LGTV, and their importance as a vector for several human pathogens makes them an ideal species for these studies.
One transcript that was up-regulated in LGTV-infected ticks belongs to the 5.3-kDa family. Transcripts in this family were also found to be up-regulated in Borrelia burgdorferi-infected I. scapularis nymphs (Ribeiro et al., 2006), suggesting a role for these proteins in immunity or host defense. Recently, one member of this family was shown to function as an AMP (Pichu et al., 2009). Interestingly, several transcripts classified as AMPs were consistently down-regulated in LGTV-infected ticks. The relevance of this finding is not known. Of particular interest is a transcript that is classified into the 25-kDa salivary gland protein family and has homology to lipocalins, which was significantly up-regulated in LGTV-infected ticks at day 2 and day 3 of feeding. Lipocalin proteins in ticks bind small molecules such as histamine and seratonin, suggesting a role for them as inflammatory modulators or anti-hemostatic proteins. However, the role of this protein family during LGTV infection remains to be determined.
The expression pattern of many of the gene products differentially regulated in response to LGTV infection is complex. Transcripts within a similar family were found to be both up-regulated and down-regulated in LGTV-infected ticks over the time course of feeding, such as that observed for Kunitz proteins, lipocalins, and secreted proteins. Moreover, similar gene families were differentially regulated in both feeding and LGTV-infected ticks. It is probable that evolution has resulted in gene families with redundancies such that different proteins with similar functions are needed depending on the stage of feeding or the pathogen present.
The results of these studies augment the growing knowledge of the components in tick saliva important for the acquisition of a blood meal, which furthers our understanding of the feeding process and may facilitate the development of novel methods to control tick populations and the pathogens they transmit. The feasibility of this idea has been demonstrated with the anti-vector vaccine, TRP64. This vaccine is derived from a Rhipicephalus appendiculatus cement protein and was shown to impair blood feeding of ticks on immunized hosts (Trimnell et al., 2002, 2005). Although produced as an anti-tick vaccine, the authors subsequently demonstrated that immunization with TRP64 protected mice against lethal infection with tick-transmitted TBEV (Labuda et al., 2006).
The transcript changes observed in LGTV-infected ticks may be specific to LGTV, but may also indicate proteins that function during tick-borne flavivirus infection in general. Transcripts up-regulated in LGTV-infected ticks may suggest roles for these proteins in enhancing LGTV replication or transmission as was observed for Salp15 and B. burgdorferi (Ramamoorthi et al., 2005) and Salp16 and Anaplasma phagocytophilum (Sukumaran et al., 2006). Alternatively, up-regulation of transcripts in LGTV-infected ticks may suggest a response of the tick vector to inhibit LGTV infection. These differentially expressed transcripts provide exciting targets for further analysis to determine if they have a functional role in flavivirus transmission or replication at the tick-host-pathogen interface.
Supplementary Material
Acknowledgements
We thank Anita Mora for technical assistance in the preparation of the tables and the figure. We also thank the Tick-Borne Flavivirus section and the Laboratory of Virology for helpful discussions. This research was supported by the Intramural Research Program at the National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcon-Chaidez F, Ryan R, Wikel S, Dardick K, Lawler C, Foppa IM, Tomas P, Cushman A, Hsieh A, Spielman A, Bouchard KR, Dias F, Aslanzadeh J, Krause PJ. Confirmation of tick bite by detection of antibody to Ixodes calreticulin salivary protein. Clin. Vaccine Immunol. 2006;13:1217–1222. doi: 10.1128/CVI.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E. Salp15, an Ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity. 2002;16:849–859. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- Arca B, Lombardo F, Valenzuela JG, Francischetti IM, Marinotti O, Coluzzi M, Ribeiro JM. An updated catalogue of salivary gland transcripts in the adult female mosquito, Anopheles gambiae. J. Exp. Biol. 2005;208:3971–3986. doi: 10.1242/jeb.01849. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease – a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Capy P, Gasperi G, Biemont C, Bazin C. Stress and transposable elements: co-evolution or useful parasites? Heredity. 2000;85(Pt 2):101–106. doi: 10.1046/j.1365-2540.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- Charrel RN, Zaki AM, Attoui H, Fakeeh M, Billoir F, Yousef AI, de Chesse R, De Micco P, Gould EA, de Lamballerie X. Complete coding sequence of the Alkhurma virus, a tick-borne flavivirus causing severe hemorrhagic fever in humans in Saudi Arabia. Biochem. Biophys. Res. Commun. 2001;287:455–461. doi: 10.1006/bbrc.2001.5610. [DOI] [PubMed] [Google Scholar]
- Chmelar J, Anderson JM, Mu J, Jochim RC, Valenzuela JG, Kopecky J. Insight into the sialome of the castor bean tick, Ixodes ricinus. BMC Genomics. 2008;9:233. doi: 10.1186/1471-2164-9-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, Ewbank JJ. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat. Immunol. 2004;5:488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- Dai J, Narasimhan S, Zhang L, Liu L, Wang P, Fikrig E. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the Lyme disease agent. PLoS Pathog. 2010;6:e1001205. doi: 10.1371/journal.ppat.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Wang P, Adusumilli S, Booth CJ, Narasimhan S, Anguita J, Fikrig E. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Microbe. 2009;6:482–492. doi: 10.1016/j.chom.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J, Almazan C, Blouin EF, Naranjo V, Kocan KM. Reduction of tick infections with Anaplasma marginale and A. phagocytophilum by targeting the tick protective antigen subolesin. Parasitol. Res. 2006;100:85–91. doi: 10.1007/s00436-006-0244-6. [DOI] [PubMed] [Google Scholar]
- Deshpande RA, Shankar V. Ribonucleases from T2 family. Crit. Rev. Microbiol. 2002;28:79–122. doi: 10.1080/1040-840291046704. [DOI] [PubMed] [Google Scholar]
- Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of powassan virus by deer ticks. Am. J. Trop. Med. Hyg. 2004;71:268–271. [PubMed] [Google Scholar]
- Fialova A, Cimburek Z, Iezzi G, Kopecky J. Ixodes ricinus tick saliva modulates tick-borne encephalitis virus infection of dendritic cells. Microbes Infect. 2010;12:580–585. doi: 10.1016/j.micinf.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci. 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva S, Nabirochkina E, Dilworth FJ, Eickhoff H, Becker P, Tora L, Georgiev P, Soldatov A. The novel transcription factor e(y)2 interacts with TAF(II)40 and potentiates transcription activation on chromatin templates. Mol. Cell Biol. 2001;21:5223–5231. doi: 10.1128/MCB.21.15.5223-5231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie RD, Mbow ML, Titus RG. The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite Immunol. 2000;22:319–331. doi: 10.1046/j.1365-3024.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- Gregson J. Observations on the movement of fluids in the vicinity of the mouthparts of naturally feeding Dermacentor andersoni Stiles. Parasitology. 1967;57:1–8. [Google Scholar]
- Hill CA, Wikel SK. The Ixodes scapularis Genome Project: an opportunity for advancing tick research. Trends Parasitol. 2005;21:151–153. doi: 10.1016/j.pt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Kikuchi A. Identification and molecular cloning of yeast homolog of nucleosome assembly protein I which facilitates nucleosome assembly in vitro. J. Biol. Chem. 1991;266:7025–7029. [PubMed] [Google Scholar]
- Kharitonova NN, Leoniv YA. Omsk Hemorrhagic Fever. 1985.
- Kotsyfakis M, Anderson JM, Andersen JF, Calvo E, Francischetti IM, Mather TN, Valenzuela JG, Ribeiro JM. Cutting edge: Immunity against a “silent” salivary antigen of the Lyme vector Ixodes scapularis impairs its ability to feed. J. Immunol. 2008;181:5209–5212. doi: 10.4049/jimmunol.181.8.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsyfakis M, Karim S, Andersen JF, Mather TN, Ribeiro JM. Selective cysteine protease inhibition contributes to blood-feeding success of the tick Ixodes scapularis. J. Biol. Chem. 2007;282:29256–29263. doi: 10.1074/jbc.M703143200. [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M, Sa-Nunes A, Francischetti IM, Mather TN, Andersen JF, Ribeiro JM. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J. Biol. Chem. 2006;281:26298–26307. doi: 10.1074/jbc.M513010200. [DOI] [PubMed] [Google Scholar]
- Labuda M, Jones LD, Williams T, Nuttall PA. Enhancement of tick-borne encephalitis virus transmission by tick salivary gland extracts. Med. Vet. Entomol. 1993;7:193–196. doi: 10.1111/j.1365-2915.1993.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Labuda M, Trimnell AR, Lickova M, Kazimirova M, Davies GM, Lissina O, Hails RS, Nuttall PA. An antivector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog. 2006;2:e27. doi: 10.1371/journal.ppat.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzel DN, Wolfinbarger JB, Long RD, Masnick M, Best SM, Bloom ME. Tick-borne flavivirus infection in Ixodes scapularis larvae: development of a novel method for synchronous viral infection of ticks. Virology. 2007;365:410–418. doi: 10.1016/j.virol.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Macaluso KR, Simser JA, Azad AF. The American dog tick, Dermacentor variabilis, encodes a functional histamine release factor homolog. Insect Biochem. Mol. Biol. 2003;33:911–919. doi: 10.1016/s0965-1748(03)00097-3. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Deponte K, Marcantonio N, Liang X, Royce TE, Nelson KF, Booth CJ, Koski B, Anderson JF, Kantor F, Fikrig E. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS One. 2007a;2:e451. doi: 10.1371/journal.pone.0000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Sukumaran B, Bozdogan U, Thomas V, Liang X, DePonte K, Marcantonio N, Koski RA, Anderson JF, Kantor F, Fikrig E. A tick antioxidant facilitates the Lyme disease agent's successful migration from the mammalian host to the arthropod vector. Cell Host Microbe. 2007b;2:7–18. doi: 10.1016/j.chom.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi M, Pedersen LG, Petrotchenko E, Shevtsov S, Gorokhov A, Kakuta Y, Pedersen LC. Structure and function of sulfotransferases. Arch. Biochem. Biophys. 2001;390:149–157. doi: 10.1006/abbi.2001.2368. [DOI] [PubMed] [Google Scholar]
- Nelson N. A journey from mammals to yeast with vacuolar H+-ATPase (V-ATPase). J. Bioenerg. Biomembr. 2003;35:281–289. doi: 10.1023/a:1025768529677. [DOI] [PubMed] [Google Scholar]
- Nene V, Lee D, Kang'a S, Skilton R, Shah T, de Villiers E, Mwaura S, Taylor D, Quackenbush J, Bishop R. Genes transcribed in the salivary glands of female Rhipicephalus appendiculatus ticks infected with Theileria parva. Insect. Biochem. Mol. Biol. 2004;34:1117–1128. doi: 10.1016/j.ibmb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Nosek J, Ciampor F, Kožuch O, Rajcani J. Localization of tick-borne encephalitis virus in alveolar cells of salivary glands of Dermacentor marginatus and Haemaphysalis inermis ticks. Acta Virol. 1972;16:493–497. [PubMed] [Google Scholar]
- Nuttall P, Jones L. Modern Acarology 2. Prague adn SPB academic publishing bv; Academia: 1991. Non-viraemic tick-borne transmission: mechanism and significance. [Google Scholar]
- Nuttall PA, Jones LD, Labuda M, Kaufman WR. Adaptations of arboviruses to ticks. J. Med. Entomol. 1994;31:1–9. doi: 10.1093/jmedent/31.1.1. [DOI] [PubMed] [Google Scholar]
- Ohad N, Yadegari R, Margossian L, Hannon M, Michaeli D, Harada JJ, Goldberg RB, Fischer RL. Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell. 1999;11:407–416. doi: 10.1105/tpc.11.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M, Yamamoto S, Katayama N, Fujita S. Macrophage migration inhibitory factor and the discovery of tautomerase inhibitors. Curr. Pharm. Des. 2002;8:1297–1317. doi: 10.2174/1381612023394674. [DOI] [PubMed] [Google Scholar]
- Otvos L., Jr. Antibacterial peptides isolated from insects. J. Pept. Sci. 2000;6:497–511. doi: 10.1002/1099-1387(200010)6:10<497::AID-PSC277>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Pagel Van Zee J, Geraci NS, Guerrero FD, Wikel SK, Stuart JJ, Nene VM, Hill CA. Tick genomics: the Ixodes genome project and beyond. Int. J. Parasitol. 2007;37:1297–1305. doi: 10.1016/j.ijpara.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Pancholi P, Kolbert CP, Mitchell PD, Reed KD, Jr., Dumler JS, Bakken JS, Telford SR, 3rd, Persing DH. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J. Infect. Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- Pichu S, Ribeiro JM, Mather TN. Purification and characterization of a novel salivary antimicrobial peptide from the tick, Ixodes scapularis. Biochem. Biophys. Res. Commun. 2009;390:511–515. doi: 10.1016/j.bbrc.2009.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnev AG, Men R. Attenuation of the Langat tick-borne flavivirus by chimerization with mosquito-borne flavivirus dengue type 4. Proc. Natl. Acad. Sci. USA. 1998;95:1746–1751. doi: 10.1073/pnas.95.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, Koski RA, Fikrig E. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem. Mol. Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Evans PM, MacSwain JL, Sauer J. Amblyomma americanum: characterization of salivary prostaglandins E2 and F2 alpha by RP-HPLC/bioassay and gas chromatography-mass spectrometry. Exp. Parasitol. 1992;74:112–116. doi: 10.1016/0014-4894(92)90145-z. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Makoul GT, Levine J, Robinson DR, Spielman A. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J. Exp. Med. 1985;161:332–344. doi: 10.1084/jem.161.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa-Nunes A, Bafica A, Lucas DA, Conrads TP, Veenstra TD, Andersen JF, Mather TN, Ribeiro JM, Francischetti IM. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J. Immunol. 2007;179:1497–1505. doi: 10.4049/jimmunol.179.3.1497. [DOI] [PubMed] [Google Scholar]
- Smith CE. A virus resembling Russian spring-summer encephalitis virus from an ixodid tick in malaya. Nature. 1956;178:581–582. doi: 10.1038/178581a0. [DOI] [PubMed] [Google Scholar]
- Spielman A, Clifford C. Human babesiosis on Natucket Island, USA: description of the vector, Ixodes (Ixodes) dammini, n. sp. (Acarina: Ixodidae). J. Med. Entomol. 1979;15:218–234. doi: 10.1093/jmedent/15.3.218. [DOI] [PubMed] [Google Scholar]
- Sukumaran B, Narasimhan S, Anderson JF, DePonte K, Marcantonio N, Krishnan MN, Fish D, Telford SR, Kantor FS, Fikrig E. An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. J. Exp. Med. 2006;203:1507–1517. doi: 10.1084/jem.20060208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimnell AR, Davies GM, Lissina O, Hails RS, Nuttall PA. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine. 2005;23:4329–4341. doi: 10.1016/j.vaccine.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Trimnell AR, Hails RS, Nuttall PA. Dual action ectoparasite vaccine targeting ‘exposed’ and ‘concealed’ antigens. Vaccine. 2002;20:3560–3568. doi: 10.1016/s0264-410x(02)00334-1. [DOI] [PubMed] [Google Scholar]
- Tyson K, Elkins C, Patterson H, Fikrig E, de Silva A. Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol. Biol. 2007;16:469–479. doi: 10.1111/j.1365-2583.2007.00742.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Francischetti IM, Pham VM, Garfield MK, Mather TN, Ribeiro JM. Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 2002;205:2843–2864. doi: 10.1242/jeb.205.18.2843. [DOI] [PubMed] [Google Scholar]
- Wikel SK. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 1999;29:851–859. doi: 10.1016/s0020-7519(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Work T. Russian spring-summer encephalitis virus in India. Kyasanur Forest disease. Prog. Med. Virol. 1958:1. [PubMed] [Google Scholar]
- Zilber A, Clifford C. Far Eastern tick-borne spring-summer encephalitis. American Reviews of Soviet Medicine. 1946:80. special supplement. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.