Abstract
Background.
We have recently documented the appearance of an anti-angiogenic peptide, endorepellin, in the urine of patients with chronic allograft dysfunction (CAD).
Methods.
Here, we analyzed using enzyme-linked immunosorbent assay the excretion of anti-angiogenic peptides endostatin, pigment epithelium-derived factor (PEDF) and Kruppel-like factor-2 (KLF-2), in healthy individuals, patients with stable graft function and patients with various degrees of CAD.
Results.
In healthy subjects and patients with CAD-0, endostatin, PEDF and KLF-2 excretions were at the level of detection. In contrast, there were significant differences between the patients with CAD-3 and CAD-0, CAD-1 and healthy controls for endostatin and CAD-0 versus CAD-3 for PEDF, but no differences in KLF-2 excretion. Receiver operating characteristic (ROC) curve analyses demonstrated a highly discriminative profile for all three biomarkers: the combination of these parameters offered 83% sensitivity and 90% specificity in distinguishing CAD-0 from CAD-1–3. The quality of these potential biomarkers of CAD was, however, highest in discriminating CAD status in biopsy-proven cases and dropped when CAD-0 was diagnosed based on clinical criteria.
Conclusions.
In conclusion, these findings indicate the diagnostic potential of urinary detection of endostatin, PEDF and to lesser degree KLF-2 and suggest a mechanistic role played by anti-angiogenic substances in the developing vasculopathy and vascular rarefaction in patients with CAD.
Keywords: endostatin, pigment epithelium-derived factor, Kruppel-like factor 2
Introduction
Chronic allograft dysfunction (CAD) is the most prevalent cause of late renal graft loss. This heterogeneous disease cannot be reliably diagnosed using non-invasive techniques and the histological diagnosis as a variable combination of fibrosis, vascular and glomerular damage is considered a gold standard [1–5].
Our previous mass spectroscopy studies demonstrated the de novo appearance in the urine of CAD patients of anti-angiogenic peptides [6]. Since vasculopathy is considered one of the leading factors in the development of graft disease [7], finding of anti-angiogenic proteins spurred the interest in examining these proteins in greater detail in individual patients. We have recently documented the appearance of one anti-angiogenic peptide, endorepellin, in the urine of patients with CAD occurring at the expense of the parent molecule, perlecan [6]. Here, we analyzed the excretion of three additional anti-angiogenic peptides endostatin [8, 9], pigment epithelium-derived factor (PEDF) [10–18] and Kruppel-like factor-2 (KLF-2) [19–22], in healthy individuals, patients with stable graft function and patients with various degrees of CAD.
Materials and methods
Patient characterization
Patient populations participating in this study have been described in detail in our previous publication [18]. Recruitment was performed at Westchester Medical Center (WMC) and New York Presbyterian Hospital (NYPH). Patients in NYPH underwent protocol biopsies, in WMC, CAD-0 diagnosis was made clinically. All patients completed a written consent form which permitted both urine and data collection in compliance with the Health Insurance Portability and Accountability Act following protocol approval by the Institutional Review Boards for clinical trials involving human subjects.
Urine specimens were centrifuged at 2500 r.p.m (700 g) for 10 min, aliquoted and stored at −80°C without protease inhibitors, as detailed in [6].
Clinical data, including blood pressure and serum creatinine, were abstracted from the patient records and presented previously [6]. All cases of CAD-1–3 were biopsy confirmed. The type and severity of allograft pathologies were classified according to the Banff’-97 criteria [23] and 2007 classification.
Enzyme-linked immunosorbent assay of endostatin, PEDF and KLF-2
Endostatin and PEDF levels were quantified using the commercial Quantikine Human Endostatin ELISA kit (R&D Systems, Minneapolis, MN) and ChemiKine PEDF ELISA kit (Millipore, Temecula, CA), according to the manufacturers’ instructions with minor modifications.
KLF-2 levels were measured using enzyme-linked immunosorbent assay (ELISA) developed in the laboratory, as detailed in Supplementary methods. Statistical analysis was performed as detailed in Supplementary methods.
Results
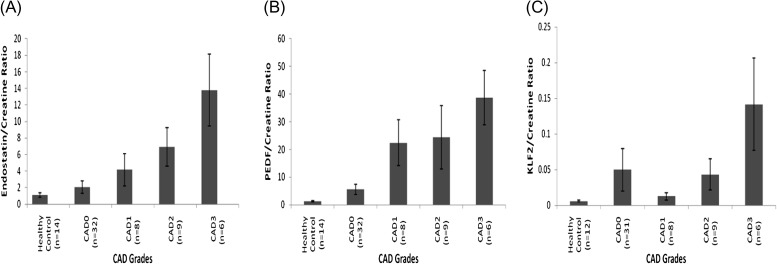
Results of ELISA detection of endostatin, PEDF and KLF-2 are summarized in Figures 1A–C and 2A–C. In healthy subjects and pooled patients with biopsy-confirmed IF/TA-0 combined with non-biopsied CAD-0, endostatin excretion was at the detection level. Kruskal–Wallis test (Table 1) showed that there were significant differences (P < 0.05) among the groups (CAD-0 versus CAD-2 and CAD-3 for endostatin). PEDF excretion in healthy controls and combined IF/TA-0 and CAD-0 patients was at the detection level. Kruskal–Wallis test showed that there were significant differences (P < 0.05) only between the groups CAD-0 versus CAD-3 (Table 1) KLF-2 excretion in healthy controls and combined IF/TA-0 and CAD-0 patients was also at the lower level of detection and Kruskal–Wallis test did not show difference among the groups. Regression analysis between endostatin, PEDF, KLF-2 and morphologic parameters is summarized in Table 2. Quartile analysis of data is presented in Supplementary figure 1.
Fig. 1.
(A) Endostatin, (B) PEDF and (C) KLF-2 urine concentrations, normalized using (urinary creatinine) in healthy non-transplant controls, in biopsy-confirmed and non-biopsy confirmed (CAD-0) and CAD-1, CAD-2 and CAD-3 patients. See statistical significance in Table 1.
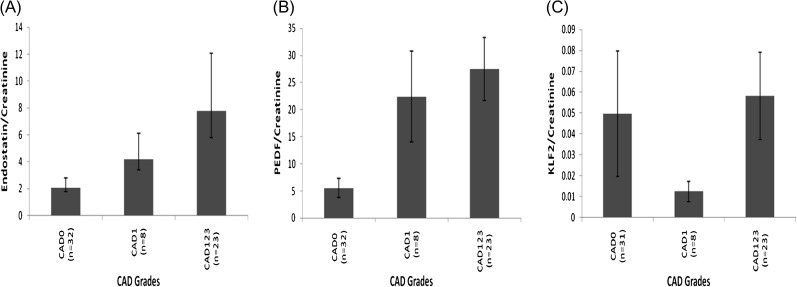
Fig. 2.
(A) Endostatin, (B) PEDF and (C) KLF-2 urine concentrations, normalized using (urinary creatinine), in CAD patients (1–3), in biopsy-confirmed and non-biopsy confirmed CAD-0 and in healthy non-transplant controls.
Table 1.
Kruskal–Wallis comparison followed by Dunn’s test for all the data obtaineda
| Endostatin | PEDF | KLF-2 | |
| CAD-0 versus CAD-1 | NS | NS | NS |
| CAD-0 versus CAD-2 | * | NS | NS |
| CAD-0 versus CAD-3 | * | * | NS |
| CAD-0 versus healthy | NS | NS | NS |
| CAD-1 versus CAD-2 | NS | NS | NS |
| CAD-1 versus CAD-3 | NS | NS | NS |
| CAD-1 versus healthy | NS | NS | NS |
| CAD-2 versus CAD-3 | NS | NS | NS |
| CAD-2 versus healthy | NS | NS | NS |
| CAD-3 versus healthy | NS | * | NS |
The total Type I error alpha is 0.05 and each comparison is adjusted by Dunn’s test rule. * means ‘significant difference’ and NS means ‘not significant’.
Table 2.
By two-group Wilcoxon test between endostatin, PEDF and KLF-2 levels and histological parameters of glomerular, tubulointerstitial and vascular disease (CG, CT, CV and CI) in CAD-1–3a
| Parameter | Endostatin | PEDF | KLF-2 |
| CG0:CG1 | No patients | No patients | No patients |
| CG0:CG2 | 0.4724 | 0.9253 | 0.8644 |
| CG0:CG3 | 0.2708 | 0.2405 | 0.5530 |
| CT0:CT1 | 0.5116 | 0.0099 | 0.8414 |
| CT0:CT2 | 0.0154 | 0.7330 | 0.8486 |
| CT0:CT3 | 0.5214 | 0.1317 | 0.4685 |
| CV0:CV1 | 0.8230 | 0.3523 | 0.7985 |
| CV0:CV2 | 0.4617 | 0.0556 | 0.2313 |
| CV0:CV3 | 0.3913 | 0.9644 | 0.8748 |
| CI0:CI1 | 0.7157 | 0.0409 | 0.7638 |
| CI0:CI2 | 0.0439 | 0.9279 | 0.8167 |
| CI0:CI3 | 0.6165 | 0.1559 | 0.4942 |
Regression analyses did not reveal any correlation between endostatin, PEDF or KLF-2 with the degree of renal dysfunction (as judged by serum creatinine concentration) or clinical parameters (data not shown). There was, however, significant correlation between endostatin, PEDF and KLF-2 levels in the urine. Analysis of correlation between endostatin, PEDF and KLF-2 levels and histological parameters of glomerular, tubulointerstitial and vascular disease in CAD-1–3 revealed a tight correlation between endostatin or PEDF with the severity of tubular and interstitial injury, but none showed correlation with the degree of glomerular involvement.
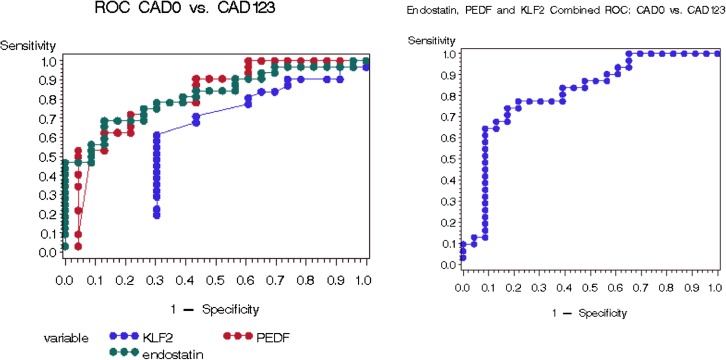
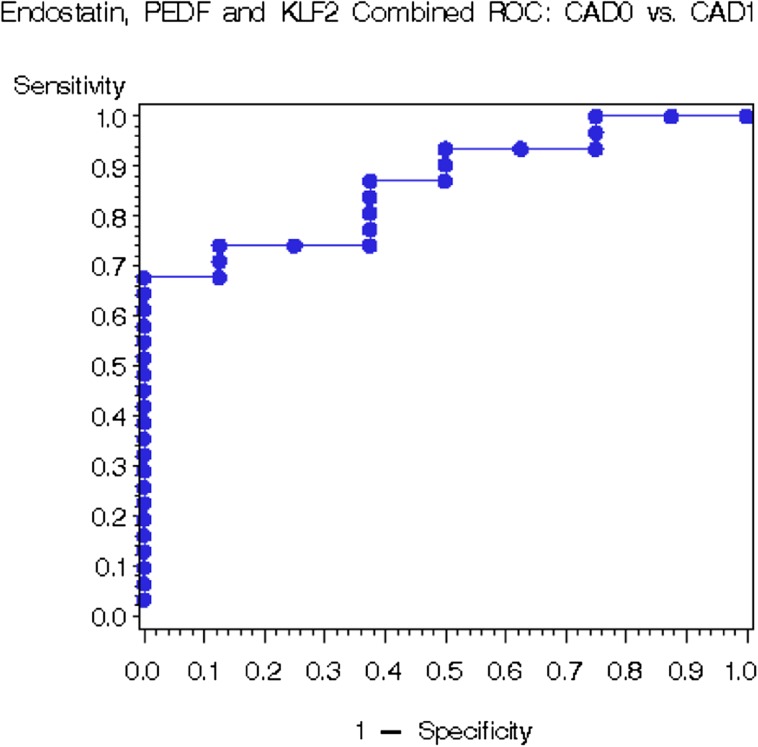
Receiver operating characteristic curve analyses demonstrated that PEDF has the highest potential as a biomarker, with the area under the curve (AUC) = 0.828, in distinguishing patients with IF/TA-0 + CAD-0 from groups CAD-1–3 (Figure 3). Moreover, endostatin, PEDF and KLF-2 allowed the detection of conversion from the biopsy-proven IF/TA-0 to CAD-1 with AUC of 0.861 (Figure 4). While these proteins poorly characterized clinically diagnosed CAD-0 (Supplementary figure 2 and Supplementary table 1), biopsy-confirmed IF/TA-0 showed a near perfect discrimination from CAD (Supplementary figure 3 and Supplementary table 2).
Fig. 3.
Receiver operating characteristic curve analysis for CAD as its discrimination threshold is varied, in CAD-0 (biopsy-confirmed and non-biopsy confirmed) versus CAD-1, -2 and -3 patients.
Fig. 4.
Receiver operating characteristic curve analysis for CAD as its discrimination threshold is varied, in CAD-0 (biopsy-confirmed and non-biopsy-confirmed) versus CAD-1 patients.
Discussion
Data presented herein demonstrated the validity of endostatin, PEDF and KLF-2 as potentially valuable candidate biomarkers of chronic allograft disease. The very fact that two of them are powerful anti-angiogenic substances (and the depletion of KLF-2 is also anti-angiogenic) nearly absent in transplant recipients with normal graft function but appearing in the urine in association with developing CAD may have diagnostic and pathogenetic significance.
It is of significant interest to compare the findings obtained in biopsy-proven IF/TA-0 patients and those which were categorized similarly, but based only on clinical criteria. The diagnostic power of tests was much reduced when similar analyses were performed using CAD-0 diagnosis made exclusively based on clinical presentations. One of the future diagnostic strategies may consist in a dynamic monitoring of urinary levels of PEDF and endostatin (KLF-2 appears to be of a lesser importance) in the post-transplant monitoring and consider a biopsy, if levels surge.
Heart and kidney transplants are the most vulnerable organs for development of graft vascular disease [7]. Identification of the surge in anti-angiogenic and anti-endothelial PEDF and endostatin in the urine of kidney allograft recipients may have pathogenetic significance.
Future studies should focus on the prospective consecutive screening of individual anti-angiogenic substances in the samples of urine obtained from transplant patients and validating their diagnostic and prognostic value in patients with chronic allograft disease as diagnosed using protocol biopsy.
Supplementary data
Supplementary data are available online at http://ndt.oxfordjournals.org.
Acknowledgments
These studies were supported in part by NIH grants DK54602 (M.S.G.), HL87062 (S.S.G.), AI51652 (M.S.) and Westchester Artificial Kidney Foundation. We express our gratitude to Dr E. O’Riordan, who has been instrumental in sample collection, and Dr C. Thompson for advice in the course of these studies.
Conflict of interest statement. None declared.
References
- 1.Chapman J, O’Connell P, Nankivell B. Chronic renal graft dysfunction. JASN. 2005;16:3015–3026. doi: 10.1681/ASN.2005050463. [DOI] [PubMed] [Google Scholar]
- 2.Cornell L, Colvin R. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens. 2005;14:229–234. doi: 10.1097/01.mnh.0000165888.83125.07. [DOI] [PubMed] [Google Scholar]
- 3.Nankivell B, Chapman J. Chronic allograft nephropathy: current concepts and future directions. Transplantation. 2006;81:643–654. doi: 10.1097/01.tp.0000190423.82154.01. [DOI] [PubMed] [Google Scholar]
- 4.Nankivell B, Borrows R, Fung C, et al. Delta analysis of posttransplantation tubulointerstitial damage. Transplant. 2004;78:434–441. doi: 10.1097/01.tp.0000128613.74683.d9. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz A, Mengel M, Gwinner W, et al. Risk factors for chronic allograft nephropathy after renal transplantation: a protocol biopsy study. Kidney Int. 2005;67:341–348. doi: 10.1111/j.1523-1755.2005.00087.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Riordan E, Orlova TN, Mendelev N, et al. Urinary proteomic analysis of chronic allograft nephropathy. Proteomics Clin Appl. 2008;2:1025–1035. doi: 10.1002/prca.200780137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell R. Graft vascular disease: immune response meets the vessel wall. Ann Rev Pathol. 2009;4:19–47. doi: 10.1146/annurev.pathol.3.121806.151449. [DOI] [PubMed] [Google Scholar]
- 8.O’Reilly M, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Olsen B. Increased angiogenic response in aortic explants of collagen XVIII/endostatin-null mice. Am J Pathol. 2004;165:415–424. doi: 10.1016/S0002-9440(10)63307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X, Zhang S, Barnstable C, et al. Molecular phylogeny of the antiangiogenic and neurotropic serpin, pigment epithelium-derived factor in vertebrates. BMC Genomics. 2006;7:248. doi: 10.1186/1471-2164-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Zhang S, Barnstable C, et al. PEDF induces apoptosis in human endothelial cells by activating p38 MAP kinase dependent cleavage of multiple caspases. Biochem Biophys Res Commun. 2006;348:1288–1295. doi: 10.1016/j.bbrc.2006.07.188. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Garcia N, Volpert O, Jimenez B. Pigment epithelium-derived factor as a multifunctional antitumor factor. J Mol Med. 2007;85:15–22. doi: 10.1007/s00109-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 13.Maik-Rachline G, Shaltiel S, Seger R. Extracellular phosphorylation converts PEDF from a neurotropic to an antiangiogenic factor. Blood. 2005;105:670–678. doi: 10.1182/blood-2004-04-1569. [DOI] [PubMed] [Google Scholar]
- 14.Filleur S, Volz K, Nelius T, et al. Two functional epitopes of PEDF block angiogenesis and induce differentiation in prostate cancer. Cancer Res. 2005;65:5144–5152. doi: 10.1158/0008-5472.CAN-04-3744. [DOI] [PubMed] [Google Scholar]
- 15.Cosgrove G, Brown K, Schiemann W, et al. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170:242–251. doi: 10.1164/rccm.200308-1151OC. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Zhang S, Lu K, et al. Decreased expression of PEDF is involved in the pathogenesis of diabetic nephropathy. Diabetes. 2005;54:243–250. doi: 10.2337/diabetes.54.1.243. [DOI] [PubMed] [Google Scholar]
- 17.Yamagishi S, Adachi H, Abe A, et al. Elevated serum levels of PEDF in the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:2447–2450. doi: 10.1210/jc.2005-2654. [DOI] [PubMed] [Google Scholar]
- 18.Motomiya Y, Yamagishi S, Adachi H, et al. Increased serum concentrations of pigment epithelium-derived factor in patients with end-stage renal disease. Clin Chem. 2006;52:1970–1971. doi: 10.1373/clinchem.2006.073171. [DOI] [PubMed] [Google Scholar]
- 19.Parmar K, Larman B, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkins G, Jain M. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 21.Das H, Kumar A, Lin Z, et al. Kruppel-like factor 2 regulates proinflammatory activation of monocytes. Proc Natl Acad Sci U S A. 2006;103:6653–6658. doi: 10.1073/pnas.0508235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins B, Wang Y, Mahabeleshwar G, et al. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racusen LC, Colvin R, Solez K, et al. Antibody-mediated rejection criteria—an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.