Abstract
Subtype-selective muscarinic antagonists effects on carbachol-induced and electric field-stimulated contractility of rat bladder were compared in vitro. Schild plot analysis of cumulative carbachol dose-response curves in the presence of antagonists was consistent with M3-mediated bladder contractions. However, nerveevoked contractions were inhibited 15% at 30 Hz (P < 0.01) by 10 nM pirenzepine (M1-selective antagonist), whereas 10 nM methoctramine (M2-selective antagonist) increased these contractions by 17% at 30 Hz (P < 0.01). Identical doses had no effect on carbachol-induced contractions, indicating prejunctional M1 facilitory and M2 inhibitory receptors. m1 Receptors could not be identified by subtype-selective antibodies, nor could the m1 transcript be identified by Northern hybridization. However, m1, m2, m3, and m4 transcripts were identified in rat bladder using the reverse transcriptase-polymerase chain reaction, providing support for the existence of the m1 subtype. In conclusion, strong evidence is provided for the existence of prejunctional M1 facilitory and M2 inhibitory and postjunctional M3 receptors modulating contractility in the rat urinary bladder.
Keywords: smooth muscle, reverse transcriptase-polymerase chain reaction, acetylcholine
Pharmacological data, based on the actions of subtypeselective antimuscarinic agents, can distinguish at least three distinct subtypes of muscarinic acetylcholine receptors: M1 receptors, which have a high affinity for pirenzepine (PZP), a low affinity for (11-(2-[(diethyl-amino)methyl]-1-piperidinyl acetyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepine-6-one (AFDX-116), and an intermediate affinity for p-fluoro hexahydrosilodifenidol (p-F-HHSiD); M2 receptors, which have a high affinity for AFDX-116 and methoctramine (Meth) and a low affinity for PZP and p-F-HHSiD; and M3 receptors, which have a high affinity for 4-diphenlacetoxy-N-methylpiperidine methiodide (4-DAMP) and p-F-HH-SiD and a low affinity for both PZP and AFDX-116 (3). Molecular techniques have identified five muscarinic receptor subtypes (m1-m5) arising from five separate genes (2). Immunological and molecular studies revealed that most tissues, including the urinary bladder, express a mixture of subtypes (7, 16, 26).
Acetylcholine acting via muscarinic receptors located on urinary bladder smooth muscle cells is the principal neurotransmitter inducing bladder muscle contraction during voiding (28, 29). Previous pharmacological studies have failed to reveal high-affinity PZP-binding sites, suggesting that no M1 receptors are present in the rat urinary bladder (18). Immunological studies using subtype-specific antibodies revealed the existence of m2 and m3 receptor subtypes (25, 26) but not the m1, m4, or m5 subtypes. Furthermore, Northern blot hybridization has identified mRNA encoding the m2 and m3 but not other subtypes of muscarinic receptors in the rat urinary bladder (16). Recent studies, on the other hand, show that low doses of PZP and oxotremorine inhibit electric field-stimulated release of [3H]acetylcholine from nerve terminals in the rat urinary bladder (20, 21). These prejunctional receptors inhibited by PZP appeared to be active only during high-frequency electrical stimulation, whereas the action of the receptors activated by oxotremorine predominated during low-frequency stimulation (21).
To delineate the function of the different muscarinic receptor subtypes in bladder contractility, we measured the effect of several subtype-selective muscarinic antagonists on both direct muscle stimulation by carbachol and on nerve-evoked contractions induced by electric field stimulation. In addition, we used reverse transcriptase-polymerase chain reaction (RT-PCR) to identify the muscarinic receptor subtype mRNAs (m1–m5) expressed in the rat urinary bladder. As such, unlike a previous report that characterized the muscarinic receptor subtypes involved in carbachol-induced contraction (26), this is the first report that combines both molecular and pharmacological methods to identify and functionally classify the muscarinic receptor subtypes involved in nerve-evoked contraction of the rat urinary bladder.
METHODS
Materials
The following drugs or chemicals were obtained from the sources indicated: carbachol and atropine from Sigma (St. Louis, MO) and methoctramine, 4-DAMP, 4-DAMP mustard, and p-F-HHSiD from Research Biochemi-cals International (Natick, MA).
Muscle strips
Urinary bladders were removed from 200- to 250-g male Sprague-Dawley rats (Ace Animals, Boyertown, PA) euthanized by decapitation. The urinary bladder body (tissue above the ureteral orifices) was dissected free of the serosa and surrounding fat. Muscarinic receptors are only associated with smooth muscle in the rat urinary bladder, as demonstrated by autoradiography (13), and no differences in contractility were seen when the mucosa was removed (unpublished observations); therefore, no further dissection was performed. The bladder was divided in the mid-sagittal plane and then cut into four longitudinal smooth muscle strips (~4 × 10 mm). The muscle strips were then stretched slowly to achieve a final isometric tension of 1 g in tissue baths containing 15 ml of modified Tyrode solution [(in mM) 125 NaCl, 2.7 KCl, 0.4 NaH2PO4, 1.8 CaCl2, 0.5 MgCl2, 23.8 NaHCO3, and 5.6 glucose] and equilibrated with 95% O2-5% CO2 at 37°C for 30 min.
Carbachol dose response
After equilibration to the bath solution, bladder strips were incubated for 30 min in the presence or absence of antagonist. Dose-response curves were derived from the peak tension developed after cumulative addition of carbachol (10 nM to 460 μM) added at 0.01 volume of the bathing solution. Cumulative dosing of carbachol as opposed to single doses was used based on the findings of Durant et al. (8), who showed no differences in tension when comparing these dosing regimens. Each strip was used for only one dose-response curve. Each concentration of antagonist was tested on 3-15 strips. Dose ratios were determined based on the average 50% effective concentration (EC50) values derived from dose-response curves of 16 antagonist-free strips performed in parallel with antagonist-treated strips. An EC50 value was determined for each strip via a Hill transformation of the data. The EC50 values determined in the presence of antagonist were used to generate Schild plots to calculate pA2 values for each antagonist.
Frequency response
Bladder strips as prepared above were desensitized to purinergic stimulation by the addition of two doses of 30 μM β,γ-methylene adenosine triphosphate separated by a 30-min rest period. The frequency-response curves shown in Fig. 2 were obtained with the continuous presence of β,γ-methylene adenosine triphosphate (final concentration 60 μM) in the bathing solution. To be sure that the purinergic response was continually desensitized, subsequent to some experiments, we measured the contractile response to electrical field stimulation after the addition of 10 μM atropine. This combination of purinergic desensitization and atropine inhibited >90% of the electric field-stimulated response, confirming the continued desensitization of the purinergic compo-nent of contraction throughout the experiment. Nerve-evoked contractions were induced by electric field stimulation generated by a solid-state square-wave stimulator (model S88, Grass Instruments, Quincy, MA) interfaced through a stimulus power booster (Stimu-Splitter II, Med-Lab Instruments, Loveland, CO) to maintain the amplitude, duration, and shape of the stimulus signal, which is transmitted simultaneously to 12 tissue baths in parallel. The 2.5-cm-long serpentine-shaped platinum electrodes are situated parallel to the long axis of the muscle strips ~1.25 cm apart in 15-ml organ baths (Radnoti Glass Technology, Monrovia, CA). The contractile response resulting from a submaximal (70% of maximum) field stimulation of 8 V at 1-ms duration at increasing frequencies (between 1 and 60 Hz) was recorded for each muscle strip, with a 4-min recovery period between each change in frequency. In preliminary experiments, no significant reduction in electric field-induced contractility was observed by multiple stimulations separated by a 4-min recovery period. Two stimulation paradigms were tested. In the first, stimulation at each frequency was continuous until peak tension was reached (5-10 s). In the second paradigm, 100 shocks were given at each frequency. The bladder strips were then incubated with or without antagonist for 30 min and then re-stimulated. In an attempt to use minimal quanti-ties of drugs, 3-ml tissue baths were constructed with platinum electrodes ~2 cm apart at the top and bottom of the long axis of the muscle strip. Identical stimulation paradigms were performed using this electrode configuration (except that 12 V at 1-ms pulse duration was used, because this gave a contraction of 70% of maximum).
Fig. 2.
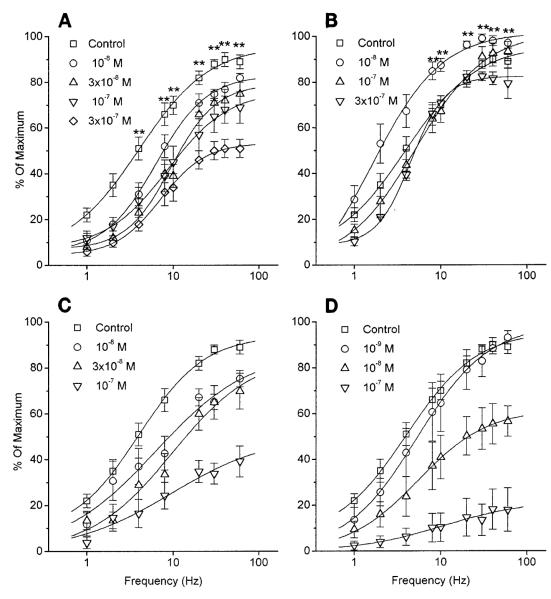
Muscarinic antagonists effect on electrically stimulated contraction of rat bladder strips in vitro. Average contractile response to electrical stimulation of 8 V, 1–60 Hz, 1-ms pulse duration, in presence of muscarinic antagonists. Data are expressed as percent of predrug maximum response for each strip. Pooled controls: (□) n = 12, max = 4.2 ± 0.7 g. PZP (A): 10 nM (○), n = 6, max = 5.7 ± 1.0 g; 30 nM (△), n = 9, max = 4.2 ± 0.4 g; 100 nM (▽), n = 3, max = 3.8 ± 0.9 g; 300 nM (◇), n = 3, max = 4.9 ± 1.0 g. Meth (B): 10 nM (○) n = 6, max = 4.0 ± 1.0 g; 100 nM (△), n = 7, max = 4.3 ± 0.7 g; 300 nM (▽), n = 3, max = 4.7 ± 0.7 g. ** Significant difference from control (P < 0.01). p-Fluoro hexahydrosilodifenidol (p-F-HHSiD, C): 10 nM (○), n = 6, max = 1.8 ± 0.5 g; 30 nM (△), n = 6, max = 2.7 ± 0.4 g; 100 nM (▽), n = 5, max = 2.0 ± 0.9 g. All p-F-HHSiD data points are significantly different from control (P < 0.01). 4-Diphenlacetoxy-N-methylpiperidine methiodide (4-DAMP, D): 1 nM (○), n = 2, max = 5.6 ± 1.6 g; 10 nM (△), n = 2, max = 6.6 ± 2.0 g; 100 nM (▽), n = 3, max = 4.2 ± 0.7 g. All 4-DAMP data points except 1 nM are significantly different from control (P < 0.01).
RT-PCR
Total RNA was isolated from rat bladder tissue using an RNA isolation kit (Stratagene, La Jolla, CA). Total RNA (10 μg) was reverse transcribed using oligo-dT primers. The reverse-transcribed products were screened for the presence of m1-m5 cDNA by PCR. PCR was carried out with pfu polymerase (Stratagene, La Jolla, CA) using two sets of oligonucleotide primers designed to be specific for the m1 receptor and a single set of oligonucleotide primers for m2-m5 receptors (Table 1). PCR reactions were performed on a DNA Pacer (Bellco Products, Vineland, NJ) and consisted of an initial cycle of 95°C for 5 min and 62°C for 5 min followed by 30 cycles of 95°C for 5 min, 62°C for 3 min, 72°C for 3 min, and a final cycle of 72°C for 10 min. The reaction products were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and photographed.
Table 1. PCR primers used for RT-PCR of m1–m5 mRNA.
| Primer | Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| ml-a-upper | CCCATCATCACTTTTGGCACA | |
| ml-a-lower | CTGCAGGGCGGCCAGCTCCCG | 123 |
| ml-b-upper | CCTCTGCTGCCGCTGTTG | |
| ml-b-lower | GGTGGGTGCCTGTGCTTCA | 175 |
| m2-upper* | CACGAAACCTCTGACCTACCC | |
| m2-lower* | TCTGACCY†GAMGAACCCAACTA | 686 |
| m3-upper* | GTCTGGCTTGGGTCATCTCTT | |
| m3-lower* | GCTGCTGCTGTGGTCTTGGTC | 433 |
| m4-upper* | TGGGTCTTGTCCTTTGTGCT | |
| m4-lower* | TTCATTGCCTGTCTGCTTTGTT | 587 |
| m5-upper* | CTGGTCTCCTTCATCCTCTGG | |
| m5-lower* | CCTGGGTTCTCTTTCCTGTTG | 394 |
PCR, polymerase chain reaction; RT, reverse transcriptase; bp, base pairs.
Sequences adapted from Wei et al. (27).
Mixed base code of CT; M, mixed base code of AC.
Statistical and data analysis
Results are reported as means ± SE. The contractility data curves displayed in Figs. 1 and 2 were generated by a curve-fitting program (Origin, MicroCal Software, Northampton, MA) based on a sigmoidal fit of the data. Statistical analysis was performed by analysis of variance with a post hoc Scheffé’s test (GB-STAT, Dynamic Microsystems, Silver Spring, MD). The slopes of Schild plots were analyzed for difference from unity using the 95% confidence interval.
Fig. 1.
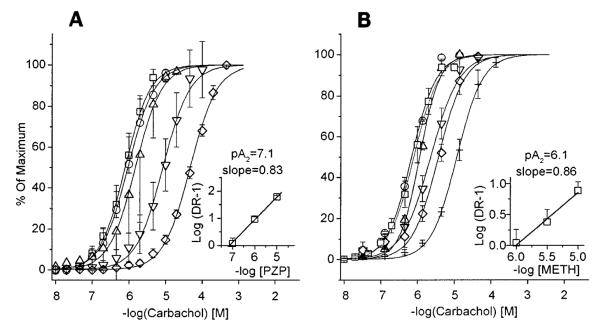
Carbachol dose-response displace-ment curves and Schild plots (insets) for pirenzepine (PZP, A) and methoctramine (Meth, B) effect on rat bladder strips in vitro. Data are expressed as percent of each individual strip’s maximal carbachol response. PZP: control (□), n = 16, maximum (max) = 4.8 ± 0.4 g; 10 nM (○), n = 4, max = 4.5 ± 1.4 g; 100 nM (△), n = 7, max = 4.2 ± 0.8 g; 1 μM (▽), n = 8, max = 4.0 ± 0.9 g; 10 μM (◇), n = 4, max = 4.3 ± 0.6 g. Meth: control (□), n = 16, max = 4.2 ± 0.8 g; 10 nM (○), n = 14, max = 4.3 ± 0.5 g; 100 nM (△), n = 6, max = 3.8 ± 0.4 g; 1 μM (▽) n = 5, max = 4.1 ± 1.0 g; 3 μM (◇), n = 3, max = 4.8 ± 1.2 g; 10 μM (+), n = 5, max = 3.0 ± 0.3 g. Slopes of Schild plots for both PZP and Meth are not significantly different from 1.0.
RESULTS
Carbachol response
Schild analysis of the shift in the carbachol dose-response curve for each of the muscarinic receptor antagonists revealed a dose-dependent competitive inhibition of bladder muscle contraction. PZP (1 nM-10 μM, pA2 = 7.1, slope = 0.83, not significantly different from unity) inhibited carbachol-stimulated muscle contractions at a concentration consistent with an M3 receptor directly mediating muscle contraction, as previously shown using Meth (pA2 = 6.1, slope = 0.86, not significantly different from unity), 4-DAMP, and p-F-HHSiD (26). Carbachol dose-response curves and Schild plots for PZP and Meth are shown in Fig. 1.
Electric field response
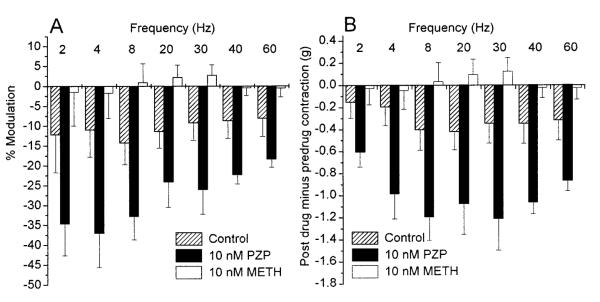
Two electric field-stimulation paradigms were used to characterize the effect of PZP and Meth on nerve-mediated contractions. Two electrode configurations were also tested. Both stimulation paradigms gave essentially identical results, and only the data obtained from the second paradigm, when strips were allowed to contract maximally to each frequency of stimulation, are presented. Electric field stimulation in the presence of various concentrations of PZP, Meth, 4-DAMP, and p-F-HHSiD revealed a dose-dependent inhibition of nerve-mediated contraction of bladder smooth muscle (Fig. 2). 4-DAMP and p-F-HHSiD inhibited both carbachol and electric field-stimulated muscle contractions at the same concentrations. However, 10 nM PZP, a dose that had no effect on carbachol-induced muscle contractions, significantly (P < 0.01) inhibited electric field-stimulated contractions at frequencies >2 Hz. Also, 10 nM Meth, a dose that also had no effect on carbachol-stimulated muscle contractions, significantly (P < 0.01) increased electric field-stimulated contractions over control at ≥8 Hz(Fig. 2). The M1 receptor-mediated facilitation as a percentage of the predrug contraction appeared to be greater at frequencies between 2 and 8 Hz. However, in terms of actual grams of tension difference, the M1-mediated facilitation was greater at frequencies >8 Hz. The M2 receptor-mediated inhibition appeared to be greater at frequencies between 8 and 20 Hz in terms of both percentage and actual grams of tension differences (Fig. 3).
Fig. 3.
Effect of frequency on M2 inhibition or M1 facilitation of rat urinary bladder contractility. A: average percent modulation of rat urinary bladder strip contractility to electrical stimulation of 8 V, 2-60 Hz, 1-ms pulse duration, produced by 10 nM PZP and 10 nM Meth, and, for time controls, run simultaneously without antagonist. Data are expressed as average percent modulation ± SE. %Modulation = 100 × (postdrug contraction in grams – predrug contraction in grams)/predrug contraction in grams for each frequency. B: actual difference in grams ± SE of postdrug contractions minus predrug base-line contractions for time control, 10 nM PZP, and 10 nM Meth.
Interestingly, when the stimulating electrodes were at the top and bottom of the long axis of the muscle strip, no significant M1 facilitation could be observed. At 20 Hz, both control and 10 nM PZP-treated strips reached 89.6% of the predrug maximum. It appears that the orientation of the electric field is also an important stimulation parameter.
RT-PCR
Oligo-dT primers were used in the RT reaction. This allowed direct amplification of RT products without further purification of the cDNA and potential loss of RT products before PCR. The use of oligo-dT primers also reduced the RT products expected to be produced from rRNA, and we felt that to identify low-abundance mRNAs, any method to selectively increase RT products from mRNA would be important. We were unable to amplify RT products directly from the RT reaction mix using random nine-nucleotide primers. With oligo-dT primers, we were able to identify transcripts for the m1-m4, but not the m5, muscarinic receptor in bladder RNA preparations (Fig. 4). Several groups, including ours, have previously been unable to detect m1 or m4 receptors in this tissue by Northern blot hybridization, radioligand binding, or immunoprecipitation (16, 18, 25, 26). Negative controls (no RT) yielded no products, confirming a lack of DNA contamination in the RNA samples.
Fig. 4.
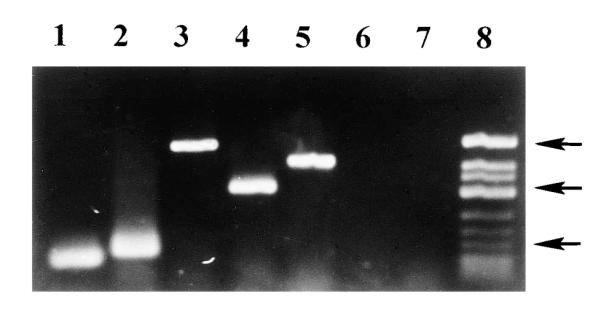
Ethidium bromide-stained agarose gel of reverse transcriptase-polymerase chain reaction (RT-PCR) products for the m1-m5 muscarinic receptors. Primers specific for muscarinic receptor subtypes were used to amplify DNA products, with the use of PCR, from a cDNA library prepared from rat urinary bladder RNA. Lane 1, m1-a primers, 123-base pair (bp) product; lane 2, m1-b primers, 175-bp product; lane 3, m2 primers, 686-bp product; lane 4, m3 primers, 433-bp product; lane 5, m4 primers, 587-bp product; lane 6, m5 primers, no product; lane 7, no RT control with m1-a primers, no product; lane 8, molecular weight marker. Top arrow, 725 bp; middle arrow, 417 bp; bottom arrow, 200 bp.
DISCUSSION
Previous studies have demonstrated that high-affinity PZP binding sites are not present in the rat urinary bladder (18). Other studies using subtype-specific antibodies failed to identify m1 receptors in this tissue by immunoprecipitation (25, 26) or by immuno-histochemical means (not shown). Immunoprecipitation analysis revealed that the m2 receptor accounts for between 80 and 90% of total rat bladder muscarinic receptors, with the m3 receptor accounting for ~10% (25, 26). Northern blot analysis has also failed to identify m1 receptor mRNA in the rat urinary bladder (16). These findings are consistent with a low density of m1 receptors in the rat bladder and may explain our inability to localize the m1 receptor to specific cell types.
Recent studies suggest that prejunctional M1 facilitory and M2 inhibitory receptors in the rat urinary bladder modulate acetylcholine release (20, 21). In these studies, M1-mediated facilitation of acetylcholine release was seen only during high-frequency continuous electrical stimulation. Acetylcholine release was reduced by 85% in the presence of 1 μM atropine (a nonselective muscarinic receptor antagonist) and by 70% in the presence of 50 nM PZP, implicating the M1 subtype in this response. Somogyi et al. (21) concluded that both M1 and M2 receptors are present prejunction-ally and that activation of M1 receptors leads to an increase in acetylcholine release, whereas activation of M2 receptors leads to a decrease in acetylcholine release from nerve terminals in the rat urinary bladder. A recent study by Tobin and Sjógren (23) also supports this localization and function of muscarinic receptor subtypes in the rabbit urinary bladder.
We have previously shown that the affinity of a series of muscarinic antagonists to inhibit direct muscarinic receptor stimulation by carbachol is most consistent with M3 receptors mediating rat bladder smooth muscle contraction (26). Different results were observed in the present study for inhibition of electric field-stimulated contractions. These contractions are considered to be primarily nerve evoked, because they are effectively (≥95%) blocked by 0.1 μM tetrodotoxin (19). They are also considered to be due to the release of acetylcholine by nerves, because in our experimental design, all postjunctional purinergic responses were desensitized. Although nerves releasing other neurotransmitters or peptides may be present in the rat urinary bladder, >90% of the contractile response to electric field stimulation is blocked with a combination of atropine and purinergic desensitization (data not shown); therefore, the sum net effect of these other transmitters is <10% of the maximum contraction.
Prejunctional receptors modulating acetylcholine release would not be expected to affect direct muscle stimulation due to carbachol. Although these prejunctional receptors may be activated by carbachol, in the absence of nerve stimulation, there is not enough acetylcholine released from nerve terminals to affect contraction, and, although prejunctional facilitory auto-receptors are activated, contractility is not enhanced. Therefore, the modulatory effects of prejunctional receptors will only be seen with nerve-evoked contractions.
Figure 2 shows that at a dose of 10 nM, the M1-selective antagonist PZP significantly decreased nerveevoked contractility. Conversely, 10 nM of the M2-selective antagonist Meth significantly increased nerveevoked contractions. Figure 1 shows that these doses of the two antagonists had no effect on carbachol-induced contractions, suggesting that these receptors are prejunctional. Unlike Somogyi et al. (20, 21), who reported facilitation only at high frequencies and inhibition at low frequencies, our results indicate both facilitation and inhibition at both low and high frequencies (Fig. 3). These differences could be due to differences in the electric stimulation parameters used in these studies. Whereas we stimulated submaximally at 8 V at a pulse duration of 1 ms (1-60 Hz), the results reported by Somogyi et al. (20, 21) were obtained with a stimulation of 100 V and a 0.25-ms duration (0.4 and 10 Hz). As an example, using the stimulation paradigm of Somogyi et al. (20, 21), 10 Hz resulted in a maximal contraction, whereas, using our conditions, 10 Hz resulted in a contraction of ~60% of maximum.
Our results correlate the changes in acetylcholine release seen by Somogyi et al. (21) with changes in smooth muscle contractility in the rat urinary bladder. In general agreement, Somogyi et al. (21) reported a 70% reduction in acetylcholine release with 50 nM PZP. Our results, a 15% reduction in rat bladder contractility at 30-Hz stimulation in the presence of 10 nM PZP, are similar to those of Tobin and Sjógren (23), who reported a 10% reduction in rabbit bladder contractility to the same dose of PZP. These findings provide functional evidence for prejunctional M1 facilitory and M2 inhibitory receptors (i.e., on nerve) and postjunctional M3 receptors mediating bladder muscle contraction. The prejunctional receptors may be localized to the prejunctional synaptosomal plasma membrane or may be located on the axon of the parasympathetic nerve, as is the case with α2-adrenoceptor on dog mesenteric nerve axons (5). The M1 receptors that enhance acetylcholine release may serve to ensure complete bladder emptying during micturition, whereas the M2 receptors may act in an autoinhibitory fashion to stop the release of acetylcholine from nerve endings and end the contraction.
Whereas a postjunctional muscle plasma membrane location of M1 and M2 receptors in bladder cannot be ruled out by these experiments, if they are in this location, antagonist affinities in our experimental paradigms indicate that they do not participate in transduc-ing the contraction. Other data obtained by selective alkylation of M3 receptors with 4-DAMP mustard suggest that the M2 receptor may be involved in blocking the increase in cAMP, and hence relaxation, induced by isoproterenol activation of β-adrenergic receptors, thereby participating in contraction (4, 8, 9).
The frequency-response curves seen in Fig. 2 appear to indicate that these antagonists are noncompetitive in nature. However, it is the concentration of acetylcholine in the vicinity of the postjunctional receptors that determines contractility. The acetylcholine in our experimental paradigm is released by parasympathetic nerves, is relatively short lived due to breakdown by acetylcho-linesterase, and cannot be increased without limit as is the case with carbachol concentration-effect curves. The stimulus used to elicit the release of acetylcholine is an electric field with varying frequency. It is possible that this stimulus is not able to evoke the release of enough acetylcholine to overcome the inhibitory affects of the antagonists; hence the antagonism would appear as noncompetitive. Another compounding factor is that these antagonists have both pre- and postjunctional effects at higher concentrations.
To reconcile our inability to identify bladder m1 receptors using subtype-selective antibodies or m1 receptor mRNA by Northern hybridization with the evidence presented by Somogyi et al. (21) and herein, we used a highly sensitive molecular means (RT-PCR) to identify m1, m2, m3, and m4 receptor mRNA in the rat urinary bladder (Fig. 4). Although this is a highly sensitive technique, we cannot localize the mRNA to prejunctional sites. Although these results do not prove the presence of prejunctional receptors, they are consistent with the premise that there are prejunctional m1 and m2 receptors. However, in the case of the rat urinary bladder, which does not contain intramural ganglion cells (11), the identified mRNA must originate from other cells in the bladder, be axonal in origin, or both. If the mRNA is derived solely from nonneuronal cells in the bladder, then the RT-PCR results would not support the proposed model. However, it is possible that the axons of the nerves innervating the bladder contain mRNA encoding the m1 and m2 receptor subtypes. A recent review (22) describes both mRNA localization and protein synthesis occurring within den-dritic processes of neurons and polyribosomes located in proximal axonal segments. Axonal localization of mRNA has been described for both invertebrates (6, 24) and vertebrates, specifically in rats (14, 17), along with protein synthesis occurring in the squid giant axons (12) and the axons of neurons regenerating in culture (15). Therefore, our demonstration of the presence of mRNA coding for prejunctional receptors in the rat urinary bladder may indicate that this mRNA is axonal in origin. In conjunction with the functional studies we describe, this constitutes strong evidence for prejunctional M1 and M2 receptor subtypes in rat bladder.
The function of the m4 receptor in the rat urinary bladder, if present, is unclear. In a recent study, Alberts (1) argued for the existence of prejunctional M4 inhibitory receptors in the guinea pig urinary bladder based on the correlation of the EC50 values of 20 muscarinic antagonists for increasing electrically stimulated acetylcholine release with published affinity values of these antagonists for M1-M4 receptors. The presence of M4 receptors, however, remains inconclusive because of the inability of the available antagonists to distinguish between M2 and M4 receptor subtypes. We were unable to detect the presence of m5 receptor mRNA using m5-specific primers and RT-PCR. The RT reaction used to produce the cDNA library was performed using oligo-dT primers to synthesize cDNA from mRNA. In contrast to the results reported here, when random primers were used to prime the RT reaction, no m1 PCR amplification product was observed from either rat or human (unpublished observation) cDNA prepared from urinary bladder RNA as previously described (30). The reason for the failure to identify m1 mRNA using the random primers is unclear, although it may be related to either a lower efficiency of RT using random primers or to random primers carried over from the RT reaction inhibiting the PCR reaction. Either way, care must be exercised in analyzing negative results obtained with RT-PCR if only one type of primer is used. This observation may help clarify some contradictory results obtained using RT-PCR when different types of oligonucleotide primers are used in the RT reaction.
In conclusion, the present study, based on both functional and molecular studies, indicates the presence of prejunctional M1 facilitory and M2 inhibitory receptors and postjunctional M3 receptors mediating rat bladder contraction. The physiological significance of prejunctional modulatory receptors in the bladder requires further investigation. It remains to be determined if these receptors are colocalized to the same nerves and under exactly what conditions in vivo these receptors come into play. Functional characterization of the muscarinic receptor subtypes in human bladder may allow for the clinical application of subtypeselective agents in the treatment of a variety of voiding dysfunctions while potentially minimizing the side effects of current cholinergic-based therapy.
Acknowledgments
We gratefully acknowledge the technical assistance of Sharon Filer-Maerten.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1-DK43333 (M. R. Ruggieri and G. R. Luthin) and RO1-DK39086 (M. R. Ruggieri).
REFERENCES
- 1.Alberts P. Classification of the presynaptic muscarinic receptor subtype that regulates 3H-acetylcholine secretion in the guinea pig urinary bladder in vitro. J. Pharmacol. Exp. Ther. 1995;274:458–568. [PubMed] [Google Scholar]
- 2.Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 3.Caulfield MP. Muscarinic receptors-characterization, coupling and function. Pharmacol. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 4.Choppin A, Eglen RM, Hedge SS. Muscarinic M2 receptors modulate relaxant responses to isoproterenol in rat urinary bladder (Abstract) Life Sci. 1997;60:1193. [Google Scholar]
- 5.Daniel EE, Gaspar V, Berzin I, Kwan C-Y. Characterization of α2-adrenoceptors and other adrenoceptors in mem-branes isolated from dog mesenteric nerve axons. J. Pharmacol. Exp. Ther. 1995;275:978–986. [PubMed] [Google Scholar]
- 6.Dirks RW, van Dorp AGM, van Minnen J, Fransen JAM, Van Der Ploeg M, Raap AK. Ultrastructural evidence for the axonal localization of caudodorsal cell hormone mRNA in the central nervous system of the mollusc Lymnaea stagnalis. Microsc. Res. Tech. 1993;25:12–18. doi: 10.1002/jemt.1070250104. [DOI] [PubMed] [Google Scholar]
- 7.Dórje F, Levey AI, Brann MR. Immunological detection of muscarinic receptor subtype proteins (m1-m5) in rabbit peripheral tissues. Mol. Pharmacol. 1991;40:459–462. [PubMed] [Google Scholar]
- 8.Durant PC, Shankley NP, Welsh NJ, Black JW. Pharmacological analysis of agonist-antagonist interactions at acetylcholine muscarinic receptors in a new urinary bladder assay. Br. J. Pharmacol. 1991;104:145–150. doi: 10.1111/j.1476-5381.1991.tb12399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eglen RM, Hedge SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- 10.Ehlert FJ, Thomas EA. Functional role of M2 muscarinic receptors in the guinea pig ileum. Life Sci. 1995;56:965–971. doi: 10.1016/0024-3205(94)00035-q. [DOI] [PubMed] [Google Scholar]
- 11.Gabella G, Uvelius B. Urinary bladder of the rat: fine structure of normal and hypertonic musculature. Cell Tissue Res. 1990;262:67–69. doi: 10.1007/BF00327747. [DOI] [PubMed] [Google Scholar]
- 12.Guiditta A, Menichini E, Perrone Capano C, Lan-gella M, Martin E, Castilgli E, Kaplan BB. Active polysomes in the axoplasm of the squid giant axon. J. Neurosci. Res. 1991;28:18–28. doi: 10.1002/jnr.490280103. [DOI] [PubMed] [Google Scholar]
- 13.Gunsena KT, Nimmo AJ, Morrison JFB, Whitaker EM. Effects of denervation on muscarinic receptors in the rat bladder. Br. J. Urol. 1995;76:291–296. doi: 10.1111/j.1464-410x.1995.tb07703.x. [DOI] [PubMed] [Google Scholar]
- 14.Jirkowski GG, Sanna PP, Bloom FE. mRNA coding for oxytocin is present in axons of the hypothalamo-neurohy-pophysial tract. Neurobiology (Bp.) 1990;87:7400–7404. doi: 10.1073/pnas.87.19.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenig E, Adams P. Local protein synthesizing activity in axonal fields regenerating in vitro. J. Neurochem. 1982;39:386–400. doi: 10.1111/j.1471-4159.1982.tb03960.x. [DOI] [PubMed] [Google Scholar]
- 16.Maeda A, Kubo Y, Mishina M, Numa S. Tissue distribution of mRNAs encoding muscarinic acetylcholine receptor subtypes. FEBS Lett. 1988;239:339–342. doi: 10.1016/0014-5793(88)80947-5. [DOI] [PubMed] [Google Scholar]
- 17.Mohr E, Fehr S, Richter D. Axonal transport of neuropeptide encoding mRNAs within the hypothalamo-hypophy-seal tract of rats. EMBO J. 1991;10:2419–2424. doi: 10.1002/j.1460-2075.1991.tb07781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monferini E, Firaldo E, Ladinski J. Characterization of muscarinic receptor subtypes in the rat urinary bladder. Eur. J. Pharmacol. 1988;147:453–485. doi: 10.1016/0014-2999(88)90180-x. [DOI] [PubMed] [Google Scholar]
- 19.Ruggieri MR, Whitmore KE, Levin RM. Bladder purinergic receptors. J. Urol. 1990;144:176–181. doi: 10.1016/s0022-5347(17)39405-3. [DOI] [PubMed] [Google Scholar]
- 20.Somogyi GT, de Groat WC. Evidence for inhibitory nicotinic and facilitory muscarinic receptors in cholinergic nerve terminals of the rat urinary bladder. J. Auton. Nerv. Syst. 1992;37:89–98. doi: 10.1016/0165-1838(92)90237-b. [DOI] [PubMed] [Google Scholar]
- 21.Somogyi GT, Tanowitz M, de Groat WC. M1 muscarinic receptor-mediated facilitation of acetylcholine release in the rat urinary bladder. J. Physiol. (Lond.) 1994;480:81–89. doi: 10.1113/jphysiol.1994.sp020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steward O, Banker GA. Getting the message from the gene to the synapse: sorting and intracellular transport of RNA in neurons. Trends Neurosci. 1992;15:180–186. doi: 10.1016/0166-2236(92)90170-d. [DOI] [PubMed] [Google Scholar]
- 23.Tobin G, Sjógren C. In vivo and vitro effects of muscarinic receptor antagonists on contractions and release of [3H] acetylcholine in the rabbit urinary bladder. Eur. J. Pharmacol. 1995;281:1–8. doi: 10.1016/0014-2999(95)00221-6. [DOI] [PubMed] [Google Scholar]
- 24.Van Minnen J. Axonal localization of neuropeptide-encoding mRNA in identified neurons of the snail Lymnaea stagnalis. Cell Tissue Res. 1994;276:155–161. doi: 10.1007/BF00354795. [DOI] [PubMed] [Google Scholar]
- 25.Wall SJ, Yasuda RP, Li M, Wolfe BB. Development of an antiserum against m3 muscarinic receptors: distribution of m3 receptors in rat tissues and cloned cell lines. Mol. Pharmacol. 1991;40:783–789. [PubMed] [Google Scholar]
- 26.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J. Pharmacol. Exp. Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- 27.Wei J, Walton EA, Milici A, Buccafusco JJ. m1-m5 muscarinic receptor distribution in rat CNS by RT-PCR and HPLC. J. Neurochem. 1994;63:815–821. doi: 10.1046/j.1471-4159.1994.63030815.x. [DOI] [PubMed] [Google Scholar]
- 28.Wein AJ, Levin RM, Barret DM. Voiding function: relevant anatomy, physiology, and pharmacology. In: Gillenwater JY, Grayhack JT, Howards SS, Duckett JD, editors. Adult and Pediatric Urology. Year Book Medical; Chicago, IL: 1987. [Google Scholar]
- 29.Yalla SV, Mcguire EJ, Elbadawi A, Blaivas JG. Neurourology and Urodynamics. Macmillan; New York: 1988. [Google Scholar]
- 30.Yamaguchi O, Sishudo K, Tamura K, Ogawa T, Fu-jimura T, Ohtsuka M. Evaluation of mRNAs encoding muscarinic receptor subtypes in human detrusor muscle. J. Urol. 1996;156:1208–1213. [PubMed] [Google Scholar]