Abstract
In vitro bladder contractions in response to cumulative carbachol doses were measured in the presence of selective muscarinic antagonists from rats that had their major pelvic ganglion bilaterally removed. Denervation induced both hypertrophy and a supersensitivity of the bladders to agonist. The affinities in control bladders for antagonism of carbachol-induced contractions were consistent with M3-mediated contractions. Affinities in denervated bladders for 4-diphenlacetoxy-N-methylpiperidine methiodide (8.5) and p-fluoro hexahydrosilodifenidol (6.6) were consistent with M2-mediated contractions, although the methoctramine affinity (6.5) was consistent with M3-mediated contractions. Subtype-selective immunoprecipitation of muscarinic receptors revealed a 50% increase in total and a 60% increase in M2 receptor density with no change in M3 receptor density in denervated bladders compared with normal or sham-operated controls. This increase in M2 receptor density is consistent with the change in affinity of the antagonists for inhibition of carbachol-induced contractions and may indicate that M2 receptors or a combination of M2 and M3 receptors directly mediates smooth muscle contraction in the denervated bladder.
Keywords: denervation, parasympathetic nerves, smooth muscle, muscarinic receptor subtypes
Pharmacological data, based on the actions of subtype-selective antimuscarinic agents, can distinguish at least three distinct subtypes of muscarinic acetylcholine receptors (M1, M2, M3). Molecular techniques have identified five muscarinic receptor subtypes (M1–M5) arising from five separate genes (2), and these can be quanti-fied using immunoprecipitation. The M1 receptors have a high affinity for pirenzepine (PZP), a low affinity for methoctramine, and an intermediate affinity for p-fluoro hexahydrosilodifenidol (p-F-HHSiD). The M2 receptors have a high affinity for (11-[2-(diethylamino)methyl]-1-piperidinyl acetyl)-5,11-dihydro-6H-pyrido[2,3-b][1,4]-benzodiazepine-6-one and methoctramine and a low affinity for PZP and p-F-HHSiD. The M3 receptors have a high affinity for 4-diphenlacetoxy-N-methylpiperidine methiodide (4-DAMP) and p-F-HHSiD and a low affinity for both PZP and methoctramine (6).
Acetylcholine acting via muscarinic receptors located on urinary bladder smooth muscle cells is the principal neurotransmitter inducing bladder muscle contraction during voiding (27). Immunological and molecular studies revealed that most tissues, including the urinary bladder, express a mixture of subtypes (8, 18, 20). Immunological studies using subtype-specific antibodies revealed the existence of M2 and M3 receptor subtypes (32, 33), but not the M1, M4, or M5 subtypes, in this tissue. The majority of the muscarinic receptors in the rat urinary bladder (70–90%) are of the M2 subtype, and the remaining receptors identifiable by this means are of the M3 subtype (33). The other subtypes may be present, but in such low amounts that they cannot be detected by this method. This is evidenced by our ability to detect mRNA coding for the M1, M2, M3, and M4 receptors in the rat urinary bladder using an extremely sensitive technique, RT-PCR (4).
Affinity values (pA2) derived from Schild plot analysis of the inhibition of carbachol-induced contractions of the rat urinary bladder are consistent with M3 receptors mediating contractility (4, 33). The presence of prejunctional M1 facilitory and M2 inhibitory receptors on parasympathetic nerves innervating the rat bladder has been demonstrated both by acetylcholine release (7, 29, 30) and functional studies (4). Other data obtained by selective alkylation of M3 receptors with 4-DAMP mustard suggest that the M2 receptor may be involved in inhibition of β-adrenergic receptor-induced relaxation (16). Rat bladder smooth muscle contracted with 90 mM KCl can be relaxed by isoproterenol stimulation of β-adrenergic receptors that act through elevation of cAMP. These strips will subsequently contract to carbachol. When the M3 receptor is selectively alkylated, a contractile function for the M2 receptor, which preferentially couples to the inhibition of adenylyl cyclase, can be observed. We have performed similar studies and obtained similar results, but in addition to the pharmacological analysis we used subtype-selective immunoprecipitation to determine the fraction of M2 and M3 receptors alkylated (5).
Unlike that of other mammalian species, the normal rat bladder does not contain intramural ganglia (12). After bilateral ablation of the major pelvic ganglion (denervation), degeneration of bladder nerves is expected to occur (10). The hypothesis of this study is that if all or a significant fraction of bladder M2 receptors are prejunctional, after denervation, the density of M2 receptors would decrease. To test this hypothesis we measured the density of M2 and M3 receptors by subtype-selective immunoprecipitation and calculated pA2 values for a panel of muscarinic antagonists for inhibition of carbachol-induced contractions 3 wk after bladder denervation.
METHODS
Materials
Frozen normal rat bladders were purchased from Pel Freeze Biologicals (Rogers, AR). The following drugs or chemicals were obtained from the sources indicated: carbachol, sodium cholate, protease inhibitors, atropine from Sigma Chemical, St. Louis, MO; methoctramine, 4-DAMP, 4-DAMP mustard, and p-F-HHSiD from Research Biochemicals International, Natick, MA; [3H]quinuclidinylbenzilate (QNB; 43 Ci/mM) from DuPont-New England Nuclear Research Products, Wilmington, DE; pansorbin from Calbiochem, La Jolla, CA; and digitonin from Gallard-Schlesinger Industries, Carle Place, NY.
Surgery
Rats (200- to 250-g female Sprague-Dawley rats from Ace Animals, Boyertown, PA) were anesthetized with 2% isoflurane in oxygen, and a midline incision was made in the lower abdomen. The pelvic plexus was exposed. For bilateral denervation, both the left and right major pelvic ganglia were cut with a Valleylab (Boulder, CO) hand stitching pencil attached to a model SSE 2 solid-state electrosurgery device (Valleylab). For sham-operated animals, the plexus was exposed but left intact. The subcutaneous tissue, muscle, and skin were sutured. After surgery, urine was expressed by the use of manual pressure on the lower abdomen of the denervated and sham-operated animals twice daily for 3 wk.
Muscle strips
Urinary bladders were removed from rats killed by decapitation. The urinary bladder body (tissue above the ureteral orifices) was dissected free of the serosa and surrounding fat. The bladder was divided in the midsagittal plane, then cut into longitudinal smooth muscle strips (~4 × 10 mm). The muscle strips were then suspended with 9.8 mN of isometric tension in tissue baths containing 15 ml of modified Tyrode solution (in mM: 125 NaCl, 2.7 KCl, 0.4 NaH2PO4, 1.8 CaCl2, 0.5 MgCl2, 23.8 NaHCO3, and 5.6 glucose) and equilibrated with 95% O2-5% CO2 at 37°C. The strips were tested for their ability to contract in response to electric field stimulation of 8 V, 30 Hz, 1-ms duration. The electric field stimulation was generated by a solid-state square-wave stimulator (model S88, Grass Instruments, Quincy, MA) interfaced through a stimulus power booster (Stimu-Splitter II; Med-Lab Instruments, Loveland, CO) to maintain the amplitude, duration, and shape of the stimulus signal, which is transmitted to 12 tissue baths in parallel simultaneously. The 2.5-cm long serpentine-shaped platinum electrodes are situated parallel to the long axis of the muscle strips ~1.25 cm apart in 15-ml organ baths (Radnoti Glass Technology, Monrovia, CA).
Carbachol dose response
After equilibration to the bath solution for 30 min, bladder strips were incubated for 30 min in the presence or absence of antagonist. Dose-response curves were derived from the peak tension developed following cumulative addition of carbachol (10 nM-300 μM final bath concentration). Each concentration of antagonist was tested on one strip from each of five denervated rats and on three strips from sham-operated controls. Dose ratios were determined on the basis of the average of the responses of five antagonist-free strips. An EC50 value was determined for each strip via a Hill transformation of the data spanning 10-90% of the maximal contraction. The EC50 values determined in the presence of antagonist were used to generate Schild plots to calculate pA2 values for each antagonist. If the slope of the Schild plot was not significantly different from unity, the slope of the Schild plot was constrained to unity to calculate the pKB value.
Immunoprecipitations
Immunoprecipitations were performed using antibodies as previously described (33), except that a two-step solubilization procedure was employed as described in Luthin et al. (17). Protein concentration in the solubilized receptor preparation was determined by a dye binding assay (Bio-Rad). Muscarinic receptor density is reported as femtomoles per milligram protein in this solubilized receptor preparation. Total muscarinic receptor levels were determined by desalting over Sephadex G-50 minicolumns as previously described (33). Precipitations on normal and sham-operated controls were done on pooled bladders (8-10 bladders per assay, 3 assays performed in triplicate for normal, 1 assay performed in quintuplet for sham), while precipitations were done on individual denervated bladders (n = 4, with at least duplicate determinations for each bladder).
Statistical and data analysis
Results are reported as means ± SE. The contractility data curves displayed in Fig. 2 were generated by a curve-fitting program (Origin, MicroCal Software, Northampton, MA) based on a sigmoidal fit of the data for the dose-response curves and a linear fit for the Hill and Schild plots. Statistical analysis of multiple-group comparison was performed by ANOVA with a post hoc Scheffé’s test (GB-STAT; Dynamic Microsystems, Silver Spring, MD) or Student’s t-test where appropriate. Statistically significant differences in the affinity values and departure from unity in the slopes derived from the Schild plots were determined using 95% confidence intervals.
Fig. 2.
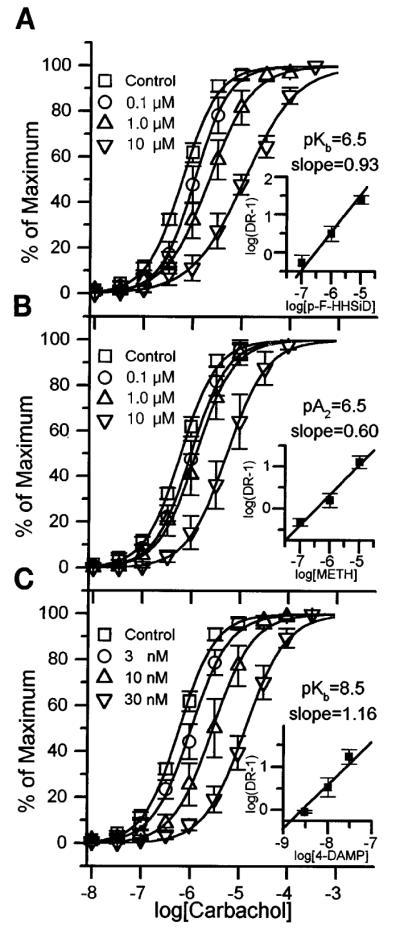
Carbachol dose-response (DR) displacement curves and Schild plot (insets) for p-fluoro hexahydrosilodifenidol (p-F-HHSiD; A), methoctramine (B), and 4-diphenlacetoxy-N-methylpiperidine methiodide (4-DAMP; C) effect on denervated rat bladder strips in vitro. Each antagonist curve represents average responses of 5 muscle strip preparations (1 strip from each of 5 denervated rats), whereas control curve represents the average of 14 muscle strips expressed as percent of each individual strip’s maximal carbachol response. These maximal responses (averages ± SE) were 32.3 ± 2.8 mN for control; 26.6 ± 2.2, 34.0 ± 5.4, and 27.2 ± 4.7 mN for 0.1, 1, and 10 μM p-F-HHSiD respectively; 26.2 ± 3.2, 34.2 ± 5.7, and 37.6 ± 5.9 mN for 1, 3, and 10 μM methoctramine respectively; and 37.8 ± 7.5, 36.7 ± 7.2, and 32.0 ± 3.7 mN for 3, 10, and 30 nM 4-DAMP, respectively. There was no significant difference in maximum between these groups.
RESULTS
Weight of bladders
Denervation induced hypertrophy of the rat urinary bladder. Denervated bladders weighed on average 505 ± 51 mg (range 368–848 mg, n = 9), which was significantly more (P < 0.01) than sham-operated bladders (98 ± 5 mg, range 76-120 mg, n = 10).
Agonist affinity
As can be seen in Fig. 1, the denervated bladder strips had an increased carbachol response while their response to electrical field stimulation was essentially zero and not significantly different from TTX-treated normal strips. There was no statistically significant difference between the EC50 values of carbachol for inducing contractions for control and sham-operated bladder strips (data not shown). As a consequence, these values were pooled for comparison with denervated bladders. The EC50 of carbachol for inducing contractions in denervated bladders (0.71 ± 0.09 μM) was significantly lower (P < 0.05) than pooled sham-operated and control bladders (1.26 ± 0.21 μM).
Fig. 1.
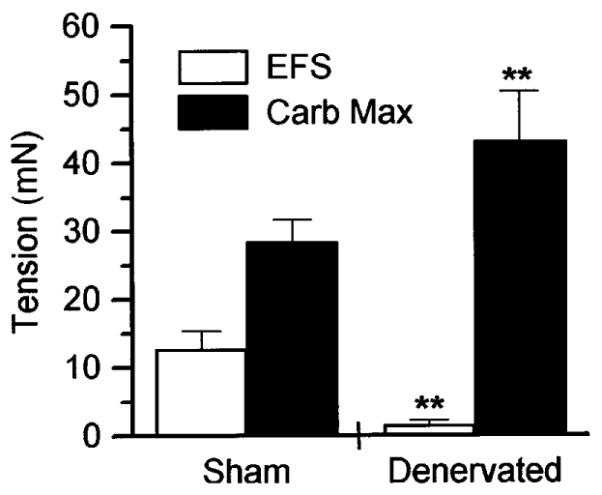
Tension developed by sham-operated control and denervated rat bladder muscle strips in vitro in response to electrical field stimulation (EFS) or carbachol-evoked maximum (Carb Max). Data shown are averages ± SE. In response to electric field stimulation (8 V, 1-ms duration at 30 Hz), actual tension developed was 12.7 ± 2.6 mN for sham-operated (n = 14 muscle strips) and 1.6 ± 0.7 mN for denervated rats (n = 60 muscle strips). Maximal carbachol responses were 28.4 ± 3.2 mN for sham-operated (n = 14 muscle strips) and 43.1 ± 7.3 mN for denervated rat bladder strips (n = 60 muscle strips). **Significantly different (ANOVA, P < 0.01) from sham-operated control.
Antagonist affinities
Schild analysis of the shift in the carbachol dose-response curves for a series of muscarinic receptor antagonists revealed a dosedependent competitive inhibition of bladder muscle contraction. As previously shown using PZP, methoctramine, 4-DAMP, and p-F-HHSiD, muscarinic receptor antagonists inhibited carbachol-stimulated muscle contractions in control bladders at a concentration consistent with M3 receptors directly mediating muscle contraction (4, 33). However, as shown in Fig. 2 and Table 1, in denervated bladders the affinities of 4-DAMP (pKB = 8.5 ± 0.2, slope not different from unity) and p-F-HHSiD (pKB = 6.5 ± 0.4, slope not different from unity) for inhibiting carbachol-induced contractions are consistent with M2 receptors directly mediating muscle contraction (6, 9). The affinity of methoctramine for inhibiting carbachol-induced contractions in the denervated rat bladders (pA2 = 6.5 ± 0.5, slope of the Schild plot was 0.60, significantly less than unity, not significantly different from control based on 95% confidence interval) is consistent with M3 receptors directly mediating muscle contraction (6, 9). In sham-operated controls, the affinities of methoctramine (pKB = 6.2 ± 0.4, slope not different from unity) and p-F-HHSiD (pKB = 7.7 ± 0.6, slope not different from unity) for inhibiting carbachol-induced contractions are consistent with M3 receptors directly mediating muscle contraction as is the case in normal bladders (Table 1).
Table 1. Affinities of carbachol and subtype-selective anticholinergics in unoperated control, sham-operated, and denervated rat urinary bladder.
| M1a | M2a | M3a | Control | Sham | Denervated | |
|---|---|---|---|---|---|---|
| PZP | 7.9–8.5 | 6.3–6.7 | 6.7–7.1 | 7.1b | ND | ND |
| p-F-HHSiD | 7.2–7.5 | 6.0–6.9 | 7.8–7.9 | 7.8b | 7.7 | 6.6* |
| Methoctramine | 7.1–7.6 | 7.8–8.3 | 6.3–6.9 | 6.1b | 6.2 | 6.5 |
| 4-DAMP | 8.9–9.2 | 8.0–8.4 | 8.9–9.3 | 9.1 | ND | 8.5* |
| Carbachol EC50 (μM) | 1.29±0.03 | 1.23±0.04 | 0.71±0.09* |
EC50 values are means ± SE. Values for denervated bladders are taken from Fig. 2. For control rat bladders, n = 6 bladder strips for each 4-diphenlacetoxy-N-methylpiperidine methiodide (4-DAMP) concentration (0, 1, 3, and 4 nM). For sham-operated animals, n = 3 bladder strips for each concentration of p-fluoro hexahydrosilodifenidol (p-F-HHSiD; 0, 0.1, 1, and 10 μM) or methoctramine (0, 1, and 10 μM). PZP, pirenzepine; ND, not determined.
From Caulfield (6) derived from both binding and functional studies.
From Wang et al. (33) derived from functional studies of rat bladder contractions.
Significantly different from control (P<0.05).
Immunoprecipitation
The total muscarinic receptor density (fmol/mg solubilized protein) in denervated bladders was significantly (P < 0.01) higher than in either sham-operated or unoperated control bladders (Fig. 3). Also as can be seen in Fig. 3, the density of M2 receptors was also significantly higher in denervated bladders than in either sham-operated (P < 0.05) or unoperated controls (P < 0.01). There was no difference in the density of M3 receptors. As a result of the selective increase in the density of M2 receptors, the M2-to-M3 ratio was significantly higher (P < 0.01) in the denervated bladders (9.6) than in either sham-operated (6.1) or unoperated controls (5.4). The sum of the M2 and M3 receptors precipitated accounted for 87, 92, and 87% of the total receptors solubilized for unoperated control, sham-operated control, and denervated bladders, respectively. The protein concentrations in the solubilized receptor preparations were 0.61 ± 0.03, 0.67, and 0.66 ± 0.03 mg/ml for unoperated control, sham-operated control, and denervated bladders, respectively; thus similar amounts of protein were solubilized for all groups studied.
Fig. 3.
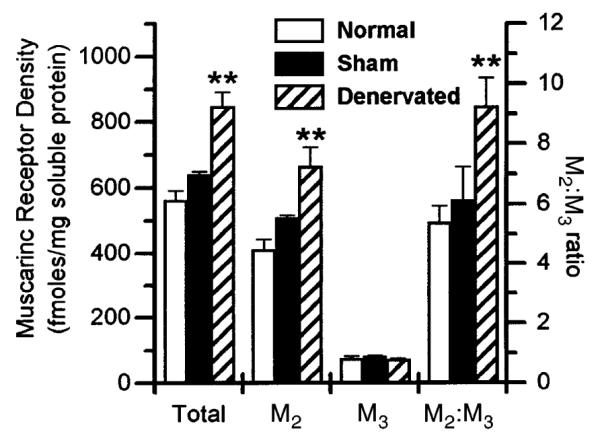
Precipitation of M2 and M3 muscarinic receptor subtypes from bladder of normal control, sham-operated control, and denervated rats. Receptors were labeled with [3H]quinuclidnylbenzilate and solubilized as described in Luthin et al. (17). Data shown are average femptomoles of receptor per milligram solubilized protein ± SE from individual denervated bladders (n = 4) or pooled normal (n = 3) or sham-operated controls (n = 1 performed in quintuplet) or ratio of M2 to M3 receptors. Protein concentration in solubilized receptor preparation was ~8% of protein concentration in crude homogenate. Compared with filtration binding, ~50% of muscarinic receptors were solubilized (data not shown). **Significant difference (P < 0.01) from control.
DISCUSSION
The denervation procedure used in this study was successful based on both the lack of contractile response of these bladders to electric field stimulation and also to the large increase in weight of the denervated bladders. The rats with denervated bladders were unable to void normally; therefore, the bladder was exposed to increased mechanical force, which resulted in an increase in bladder capacity and hypertrophy. In response to electric field stimulation, normal bladders contracted 60% while the denervated bladders contracted only 5% of their carbachol-evoked maximum (Fig. 1). Despite the predominance of M2 subtypes in rat bladder, pharmacological evidence based on the affinity of a panel of subtype-selective muscarinic antagonists is most consistent with M3 muscarinic receptors directly mediating smooth muscle contraction (Table 1; Ref. 33). On the basis of the pharmacological data obtained with denervated rat bladders (Fig. 2 and Table 1) compared with normal and sham-operated control rat bladders, it appears that in denervated rat bladders, M2 receptors can provide a contractile function that is mediated by M3 receptors in normal bladders. The reason that both 4-DAMP and p-F-HHSiD yielded affinities consistent with M2-mediated contraction whereas methoctramine yielded an affinity consistent with M3-mediated contraction is unknown. One possible explanation of this phenomenon is that in the denervated bladder either the M2 or M3 receptor subtype is sufficient to mediate contraction, perhaps independently of the other. Once the M3 receptors are blocked by an M3-selective antagonist, the M2 subtype is capable of mediating contraction. Until the antagonist concentration is high enough to block the M2 subtype, the contraction will not be antagonized, thus the appearance of an M2 affinity for M3-selective antagonists (4-DAMP and p-F-HHSiD). The reverse would be true for an M2-selective antagonist such as methoctramine and would yield an affinity consistent with M3-mediated contraction.
The slope of the Schild plot for the M2-selective antagonist methoctramine is significantly less than 1, unlike the slope of the Schild plots for the M3-selective antagonists 4-DAMP and p-F-HHSiD, which are not different from unity. One possible explanation for a Schild plot having a slope of less than unity is the involvement of more than one receptor subtype mediating the response. The reason that the Schild plot for methoctramine has a slope of less than 1 while the slope of the Schild plots for 4-DAMP and p-F-HHSiD are not less than 1 is not clear. On the basis of the affinity of muscarinic antagonists for inhibiting carbachol-induced contractions in denervated rat bladder and on the shallow slope of the Schild plot for methoctramine, a combination of M2 and M3 receptors could be mediating contraction in this tissue. Future experiments using antagonists with greater subtype selectivity (not currently commercially available) may help clarify the contribution of the M2 and M3 receptor subtypes to contraction in this tissue. This apparent change in function or “unmasking” of a function not previously identifiable by this means could be the direct result of the selective increase in M2 receptor density in response to denervation (Fig. 3). If this change in function of receptor subtypes seen in denervated bladders is a common phenomenon seen with other receptors and under pathophysiological conditions, then therapeutics developed for the treatment of pathological conditions based on their effectiveness in normal tissue may prove clinically ineffective.
In normal bladders, the M2 receptor appears to provide a contractile function by inhibition of β-adrenergic receptor-induced relaxation, which is only displayed in the presence of an inactivated M3 receptor population (16). Hegde et al. (16) also reported that in urethan-anesthetized rats the rank order of potency of intravenously injected muscarinic antagonists for antagonizing volume-induced bladder contractions correlated most favorably with M2 receptor involvement. It is possible that these antagonists influence volume-induced bladder contractions at the neuronal level and not by acting directly on the smooth muscle of the bladder. We hope through this study to help to clarify the pre- and postjunctional localization of these receptor targets. Our results support the localization of a major proportion of M2 receptors to smooth muscle cells in the denervated rat bladder.
Consistent with other reports, in denervated bladders, we observed a shift to the left in the carbachol concentration-effect curve termed “increased responsiveness” or “denervation-induced supersensitivity” (11, 21). Does the density of the receptor determine the responsiveness of the tissue? On the basis of the work of Nilvebrant et al. (21), this does not appear to be the case. This group showed that the density of muscarinic receptors declined with urinary diversion and that these bladders were supersensitive to the muscarinic receptor agonist methacholine. Apparently, the sensitivity of the bladder to muscarinic agonists can increase without increases in the density of muscarinic receptors. On the basis of this evidence, they suggested that supersensitivity may be induced by a lower level of receptor stimulation and that regulation of muscarinic receptor density may be influenced by bladder function. Muscarinic receptor desensitization, as a result of phosphorylation and internalization, induced by receptor activation has been described (14, 31). In normal bladders, some fraction of the muscarinic receptors may not be responsive to agonist (i.e., desensitized) because of tonic nervous stimulation. If this is the case, a hypo- or submaximal responsiveness may be the norm. With no nervous activity as in denervated bladders or no reflex-induced contractions as in diverted bladders, a greater fraction of the muscarinic receptors may be responsive to agonist. Consequently, these bladders would appear as either hyper-responsive or supersensitive. If this is the case, supersensitivity may be a misnomer and really refer to maximum responsiveness, because the normal physiological responsiveness is already desensitized to some degree.
In general agreement with others, we show that denervation of the rat urinary bladder induced a 48% increase in the density of total muscarinic receptors (13, 21). Gunasena et al. (13) reported a 37% increase in total [3H]QNB binding 2 wk after ganglionic denervation in the whole bladder, with a twofold increase in smooth muscle. Nilvebrant et al. (21) showed a 98 and 137% increase in [3H]QNB binding 1 and 3 wk postdenervation, respectively. The greater increase in density seen by Nilvebrant et al. (21) may be due to differences in experimental procedure, such as the fact that the urine from the bladders of the denervated animals used in our study was expressed twice daily whereas the urine from the denervated rats in the Nilvebrant et al. (21) study was expressed once per day. Unlike other reports, which measured total muscarinic receptor density and not the density of the individual subtypes, we show that the increase in muscarinic receptor density in denervated bladders is due to a selective increase in density of the M2 receptor subtype. The increase in total receptor density was the result of an ~60% increase in the density of M2 receptors with no change in the density of M3 receptors (Fig. 3).
This selective increase in the density of M2 receptors results in a significant increase in the M2-to-M3 ratio from ~5.4 in normal to ~9.5 in denervated bladders. We have previously published data indicating that the M2-to-M3 ratio is 9 in normal rat bladders (33). The bladders used in that study were obtained frozen from the supplier, and we subsequently determined that some of the bladders were likely contaminated with prostate tissue. In the current report, a different precipitation procedure (two-step solubilization) was used and extreme care was taken to remove all prostate tissue from the bladders. The rat prostate contains many proteases, and the M3 receptor appears to be selectively susceptible to proteolysis (most likely due to its large i3 loop), thus rendering it unable to be precipitated (23). As a result of these changes in procedure, we have been able to precipitate a greater density of M3 receptors, yielding an M2-to-M3 ratio of 5.4 in normal bladders. The selective increase in M2 receptor density is unlike that seen in the fetal, virgin, and gravid rabbit urinary bladders, where although there are differences in total muscarinic receptor density, the M2-to-M3 ratio remains constant (3). Our results using human bladder tissue indicate that patients in acute urinary retention also have an upregulation of total muscarinic receptor density, with no change in the M2-to-M3 ratio (M. R.Ruggieri, S. B. Brandes, G. J. Wise, and V. Krichevsky, unpublished observations).
Differences in the solubility of muscarinic receptor subtypes transfected into insect salivary cells with the M3 receptor being the least soluble have been reported by Rinken and colleagues (24, 25). Several differences, including the use of a different ligand (N-[3H]methylscopolamine), the use of transfected insect cells, and radiolabeling of the receptor after solubilization, which significantly decreases yield (24), negate direct comparison with our solubilization efficiency. Our experience to date, whether using parotid, bladder, or brain tissue, is solubilization of 40-50% of total muscarinic receptors (23). We precipitate nearly 90% of the solubilized receptors by subtype-selective immunoprecipitation. In the present study, nearly identical amounts of protein were solubilized from the three different groups of bladders, and between 87 and 92% of the solubilized receptors were precipitated. In conjunction with the data obtained by others (13, 21) and the pharmacological data presented in this study, we believe that it is unlikely that the increase in density of the M2 receptor was due to differences in solubility of the different subtypes. These immunoprecipitation results are consistent with the pharmacological data and also support the localization of M2 muscarinic receptors to smooth muscle cells in the denervated rat urinary bladder.
It is unlikely that denervation induced the expression of the M1, M4, or M5 receptor subtypes. In our previous study on normal rat bladders, the M2 and M3 receptor subtypes accounted for all measurable precipitated receptors, because no M1, M4, or M5 receptors could be immunoprecipitated (33). In the present study 87% of total receptors can be accounted for by a combination of the M2 and M3 subtypes in both control and denervated bladders. Therefore, if induction of other subtypes occurred in response to denervation, they do not contribute significantly to the total. Furthermore, the pharmacological data are consistent with this finding. The apparent change in function of the M2 receptor in denervated bladders, which may be an unmasking of a function not measurable in normal bladders, could be the result of its increased density regardless of any change in the density of M3 receptors or due to the increased ratio of M2 to M3 receptors. If the former were correct, any bladder that had an increased density of M2 receptors would be expected to contract due to M2 receptor activation. If the latter were correct, any condition that resulted in an elevated M2-to-M3 ratio regardless of the absolute density of receptor subtypes would be expected to result in M2 receptor-mediated contraction. On the basis of other results (5) in which pharmacological data are consistent with M3 receptor-mediated direct contraction even after an increase in the M2-to-M3 ratio due to selective alkylation, it appears that the absolute level of M2 receptors is more important than the M2-to-M3 ratio in determining which subtype mediates contraction. However, other differences resulting from adaptation of the bladder induced by either hypertrophy or the increased mechanical stretch imposed on the denervated bladder may not allow for straightforward comparison of the results from these two paradigms.
Alternately, the change in function of the M2 muscarinic receptor in denervated bladders may be a result of changes in the coupling of this subtype with the signal transduction mechanisms that result in contraction. The change in coupling may have been induced by one of the above-mentioned factors. The M1, M3, and M5 receptors are thought to preferentially couple to phosphoinositide (PI) hydrolysis through activation of phospholipase C by members of the Gq family of heterotrimeric guanine nucleotide binding regulatory proteins (G proteins), whereas the M2 and M4 receptor subtypes are thought to couple preferentially to the inhibition of adenylyl cyclase through Gi (5). Agonist stimulation of bladder muscarinic receptors modestly inhibits adenylyl cyclase in the rabbit (26) and guinea pig (22), but does not significantly stimulate PI hydrolysis in the rabbit bladder (26). However, muscarinic receptor-induced PI hydrolysis has been described in the rat (19), guinea pig (22), and human bladder (1). In cultured human bladder smooth muscle cells (15) and guinea pig bladder (22), activation of the M3 receptor increases PI hydrolysis. In a previous report we confirmed coupling of rat bladder M2 and M3 receptor subtypes to members of both the Gi and Gq/11 subfamilies of G proteins (33). On the basis of the promiscuous coupling of muscarinic receptors to different families of G proteins in the rat bladder, it is possible that M2 receptors as a result of their increased density may couple to PI hydrolysis in the denervated rat bladder and induce contraction independent of cAMP. Confirmation of this prediction awaits further analysis.
In conclusion, denervation induces an increase in M2 muscarinic receptor density with no change in M3 receptor density in the rat urinary bladder. Concomitant with these changes, the affinity of a series of muscarinic receptor antagonists for inhibition of carbachol-induced smooth muscle contraction switches from being consistent with M3 receptor-mediated contraction in control bladders to M2-mediated or a combination of M2 and M3 receptor-mediated contraction in denervated bladders.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1-DK-43333 (to M. R. Ruggieri and G. R. Luthin) and RO1-DK-39086 (to M. R. Ruggieri).Address for reprint requests: M. R. Ruggieri, Temple Univ. School of Medicine, Urology Research Laboratory, 3400 North Broad St., Philadelphia, PA 19140.We gratefully acknowledge the technical assistance of Sharon Filer-Maerten and the surgical assistance of Dr. Steven Brandes.
REFERENCES
- 1.Anderson KE, Holmquist F, Fovaeus M, Hedlund H, Sundler R. Muscarinic receptor stimulation of phosphoinositide hydrolysis in the human isolated urinary bladder. J. Urol. 1991;146:1156–1159. doi: 10.1016/s0022-5347(17)38030-8. [DOI] [PubMed] [Google Scholar]
- 2.Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 3.Brandes SB, Ruggieri MR. Muscarinic receptor subtypes in normal, fetal and gravid rabbit bladder, heart and uterus. Adv. Exp. Med. Biol. 1995;385:241–249. doi: 10.1007/978-1-4899-1585-6_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braverman AS, Luthin GR, Ruggieri MR. Prejunctional M1 facilitory and M2 inhibitory muscarinic receptors mediate rat bladder contractility. Am. J. Physiol. 1998;274:R517–R523. doi: 10.1152/ajpregu.1998.274.2.r517. Regulatory Integrative Comp. Physiol. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braverman AS, Luthin GR, Ruggieri MR. The M2 muscarinic acetylcholine receptor contributes to rat urinary bladder contraction. Part 2. Soc. Neurosci. Abstr. 1997;23:2022. [Google Scholar]
- 6.Caulfield MP. Muscarinic receptors-characterization, coupling and function. Pharmacol. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 7.D’Agostine G, Kilbinger H, Chiari MC, Garna E. Presynaptic inhibitory muscarinic receptors modulating [3H]acetylcholine release in the rat urinary bladder. J. Pharmacol. Exp. Ther. 1986;239:522–526. [PubMed] [Google Scholar]
- 8.Dórje F, Levey AI, Brann MR. Immunological detection of muscarinic receptor subtype proteins (M1-M5) in rabbit peripheral tissues. Mol. Pharmacol. 1991;40:459–462. [PubMed] [Google Scholar]
- 9.Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- 10.Ekström J, Elme M. Choline acetyltransferase activity in the denervated urinary bladder of the rat. Acta Physiol. Scand. 1977;111:81–86. doi: 10.1111/j.1748-1716.1977.tb05983.x. [DOI] [PubMed] [Google Scholar]
- 11.Ekström J, Malmberg L. Development of supersensitivity to methacholine in the rat detrusor following either parasympathetic denervation or decentralization. Acta Physiol. Scand. 1984;122:175–179. doi: 10.1111/j.1748-1716.1984.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 12.Gabella G, Uvelius B. Urinary bladder of the rat: fine structure of normal and hypertonic musculature. Cell Tissue Res. 1990;262:67–69. doi: 10.1007/BF00327747. [DOI] [PubMed] [Google Scholar]
- 13.Gunasena KT, Nimmo AJ, Morrison JFB, Whitaker EM. Effects of denervation on muscarinic receptors in the rat bladder. Br. J. Urol. 1995;76:291–296. doi: 10.1111/j.1464-410x.1995.tb07703.x. [DOI] [PubMed] [Google Scholar]
- 14.Haga T, Haga K, Kameyama K, Nakata H. Phosphorylation of muscarinic receptors: regulation by G proteins. Life Sci. 1993;52:421–428. doi: 10.1016/0024-3205(93)90297-g. [DOI] [PubMed] [Google Scholar]
- 15.Harriss DR, Marsh KA, Birmingham AT, Hill SJ. Expression of muscarinic M3-receptors coupled to inositol phospholipid hydrolysis in human detrusor cultured smooth muscle cells. J. Urol. 1995;154:1241–1245. [PubMed] [Google Scholar]
- 16.Hegde SS, Choppin A, Bonhas D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luthin GR, Harkness J, Artymyshyn RP, Wolfe BB. Antibodies to a synthetic peptide can be used to distinguish between muscarinic acetylcholine receptor binding sites in brain and heart. Mol. Pharmacol. 1988;34:327–333. [PubMed] [Google Scholar]
- 18.Maeda A, Kubo Y, Mishina M, Numa S. Tissue distribution of mRNAs encoding muscarinic acetylcholine receptor subtypes. FEBS Lett. 1988;239:339–342. doi: 10.1016/0014-5793(88)80947-5. [DOI] [PubMed] [Google Scholar]
- 19.Mimata H, Wheeler MA, Fukumoto Y, Takigawa H, Nishimoto T, Weiss RM, Latifpour J. Enhancement of muscarinic receptor-coupled phosphatidyl inositol hydrolysis in diabetic bladder. Mol. Cell. Biochem. 1995;152:71–76. doi: 10.1007/BF01076465. [DOI] [PubMed] [Google Scholar]
- 20.Monferini E, Firaldo E, Ladinski J. Characterization of muscarinic receptor subtypes in the rat urinary bladder. Eur. J. Pharmacol. 1988;147:453–485. doi: 10.1016/0014-2999(88)90180-x. [DOI] [PubMed] [Google Scholar]
- 21.Nilvebrant L, Ekström J, Malmberg L. Muscarinic receptor density in the rat urinary bladder after denervation, hypertrophy and urinary diversion. Acta Pharmacol. Toxicol. 1986;59:306–314. doi: 10.1111/j.1600-0773.1986.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 22.Noronha-Blob L, Lowe V, Patton A, Canning B, Costello D, Kinnier WJ. Muscarinic receptors: relationships among phosphoinositide breakdown, adenylate cyclase inhibition, in vitro detrusor muscle contractions and in vivo cystometrogram studies in guinea pig bladder. J. Pharmacol. Exp. Ther. 1989;249:843–851. [PubMed] [Google Scholar]
- 23.Pontari MA, Luthin GR, Braverman AS, Ruggieri MR. Characterization of muscarinic cholinergic receptor subtypes in rat prostate. J. Recept. Signal Transduct. Res. 1998;18:151–166. doi: 10.3109/10799899809047742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinken A, Kameyama K, Haga T, Engstrom L. Solubilization of muscarinic receptor subtypes from baculovirus infected Sf9 insect cells. Biochem. Pharmacol. 1994;48:1245–1251. doi: 10.1016/0006-2952(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 25.Rinken A. Subtype-specific changes in ligand binding properties after solubilization of muscarinic receptors from baculovirusinfected Sf9 insect cell membranes. J. Pharmacol. Exp. Ther. 1995;272:8–14. [PubMed] [Google Scholar]
- 26.Ruggieri MR, Bode DC, Levin RM, Wein AJ. Muscarinic receptor subtypes in human and rabbit bladder. Neurourol. Urodyn. 1987;6:119–128. [Google Scholar]
- 27.Ruggieri MR, Whitmore KE, Levin RM. Bladder purinergic receptors. J. Urol. 1990;144:176–181. doi: 10.1016/s0022-5347(17)39405-3. [DOI] [PubMed] [Google Scholar]
- 29.Somogyi GT, de Groat WC. Evidence for inhibitory nicotinic and facilitory muscarinic receptors in cholinergic nerve terminals of the rat urinary bladder. J. Auton. Nerv. Syst. 1992;37:89–98. doi: 10.1016/0165-1838(92)90237-b. [DOI] [PubMed] [Google Scholar]
- 30.Somogyi GT, Tanowitz M, de Groat WC. M1 muscarinic receptor-mediated facilitation of acetylcholine release in the rat urinary bladder. J. Physiol. (Lond.) 1994;480:81–89. doi: 10.1113/jphysiol.1994.sp020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobin AB, Keys B, Nahorski SR. Identification of a novel receptor kinase that phosphorylates a phospholipase Clinked muscarinic receptor. J. Biol. Chem. 1996;271:3907–3916. doi: 10.1074/jbc.271.7.3907. [DOI] [PubMed] [Google Scholar]
- 32.Wall SJ, Yasuda RP, Li M, Wolfe BB. Development of an antiserum against m3 muscarinic receptors: distribution of M3 receptors in rat tissues and cloned cell lines. Mol. Pharmacol. 1991;40:783–789. [PubMed] [Google Scholar]
- 33.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J. Pharmacol. Exp. Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]


