Abstract
Introduction
Gleason 4+3 prostate cancer is associated with worse clinicopathologic outcomes than Gleason 3+4. Whether the increased risk associated with Gleason 4+3 is equivalent to ≥4+4 is unclear.
Methods
We reviewed data from two separate cohorts pulled from the SEARCH Database. The first consisted of 374 men with biopsy Gleason 3+4 or greater, and the second consisted of 636 men with RP Gleason 3+4 or greater. We estimated the odds ratio of unfavorable surgical pathology for biopsy Gleason categories using logistic regression analysis. Using a Cox proportional hazards regression model, we estimated the relative risk of biochemical progression associated with each biopsy and RP Gleason category.
Results
In the biopsy Gleason cohort, Gleason 4+3 was associated with increased odds of extracapsular extension (p=0.01) and seminal vesicle invasion (p<0.001) relative to biopsy Gleason 3+4. Biopsy Gleason 4+3 was associated with similar odds of adverse pathology relative to biopsy Gleason ≥4+4 (all p values >0.10), except higher-grade pathological tumors among men with biopsy Gleason ≥4+4 (p=0.001). After adjusting for multiple clinical characteristics, biopsy Gleason 4+3 was associated with increased recurrence risk relative to 3+4 (p=0.001), but similar progression risk as biopsy Gleason ≥4+4 (p=0.53). In the RP Gleason cohort and after adjustment for multiple clinicopathologic features, RP Gleason 4+3 was associated with increased progression risk relative to RP Gleason 3+4 (p=0.03), but similar progression risk as RP Gleason ≥4+4 (p= 0.24).
Conclusions
In a multicenter database using pooled data from multiple pathologists, Gleason scores 4+3 and ≥4+4 exhibited similar clinicopathologic outcomes.
Keywords: Prostate cancer, radical prostatectomy, Gleason, prostate biopsy, progression
INTRODUCTION
Biopsy Gleason score helps predict pathological stage1 and risk of progression following therapy.2 While initial nomograms did not distinguish between Gleason 3+4 and 4+3,3–4 recent studies suggest that Gleason 4+3 is associated with more advanced disease1, 5–6 and greater risk of progression.6–7 Whether the greater risk associated with Gleason 4+3 is equivalent to that of ≥4+4 is unknown.
Though updated nomograms1 suggest that biopsy Gleason ≥4+4 is associated with a slightly increased risk of adverse pathology relative to 4+3, these differences are small and of unclear clinical relevance. We hypothesized the risk of adverse pathology and biochemical progression associated with biopsy and radical prostatectomy (RP) Gleason 4+3 would be higher than 3+4 but equivalent to ≥4+4. To address this issue, we examined the risk of adverse pathology and biochemical progression after RP as a function of Gleason score in the multicenter Shared Equal Access Regional Cancer Hospital (SEARCH) RP Database.8 Of note, we used two distinct cohorts, both pulled from the SEARCH database: men with a biopsy Gleason 3+4 or greater (biopsy cohort) and men with a RP Gleason 3+4 or greater (RP cohort).
MATERIALS AND METHODS
Study population
After obtaining Institutional Review Board approval from each institution, data from patients treated with RP between 1988 and 2005 at the Veterans Affairs Medical Centers in West Los Angeles, Palo Alto, San Francisco, California and Augusta, Georgia were combined into the SEARCH database. This database includes information on patient age at the time of surgery, race, clinical stage, grade of cancer on diagnostic biopsy, preoperative PSA, surgical specimen pathology (tumor grade, stage, and margin status), and follow-up PSA data for a mean and median of 55 and 46 months (range: 1–192 months). Patients treated with preoperative androgen deprivation or radiation therapy were excluded. Of the 1,419 patients in the SEARCH Database, 29 men diagnosed from transurethral resection were excluded. An additional 112 men with missing biopsy Gleason score, PSA, clinical stage, or ethnicity were excluded, resulting in a potential study population of 1,278 men. Biochemical progression was defined as a single PSA >0.2 ng/ml, two consecutive values at 0.2 ng/ml, or secondary treatment for an elevated PSA following RP. Prostatectomy specimens were sectioned per each institution’s protocol.8 Patients with missing follow-up information were excluded from analyses involving progression but were included in those evaluating risk of adverse pathology.
Statistical analysis
Gleason score was categorized as 3+4, 4+3, or ≥4+4. Group characteristics were compared using the rank-sum test for continuous variables and Chi-square test for categorical variables. The odds ratio of the outcome variables of positive surgical margins (PSM), extracapsular extension (ECE), and seminal vesicle invasion (SVI) was estimated for the predictor variables of biopsy Gleason 3+4 vs. 4+3 and 4+3 vs. ≥4+4 using logistic regression analysis: there were fifteen men with lymph node metastases (LNM). Analyses were mutually adjusted for PSA (continuous after logarithmic transformation), number of biopsy cores obtained (continuous), age at RP (continuous), year of RP (continuous), clinical stage (T1 vs. T2/T3), race (black, white, other), and center (categorical term).
Time to biochemical progression was compared between Gleason score categories using Kaplan-Meier plots and the log-rank test. The relative risk of biochemical progression for Gleason score was estimated using Cox proportional hazards regression models. Analyses of biopsy Gleason score were mutually adjusted for PSA, age, year of surgery, clinical stage, race, number of biopsy cores obtained, and center. Analyses of RP Gleason score were adjusted for PSA, age, year of surgery, clinical stage, race, center, PSM, ECE, SVI, and LNM. Given that crude, age-adjusted, and multivariable results were similar, only multivariable results are shown.
The distribution of all clinicopathological variables was similar among the four centers within the SEARCH database. Therefore, data from all centers were combined for analyses. All statistical analyses were performed using SPSS 12.0 and STATA 9.0.
RESULTS
Biopsy Gleason cohort demographics
Of 1,278 men, 456 (36%) had a biopsy Gleason of 3+4 or higher. Of these, 82 men with <6 biopsy cores obtained or missing biopsy data were excluded, resulting in a biopsy Gleason cohort of 374 men. In general, biopsy Gleason 4+3 was associated with higher-risk disease than 3+4 as evidenced by higher pathological grade, and more ECE and SVI (Table 1). There were no significant differences between biopsy Gleason 4+3 and ≥4+4 in any clinicopathological characteristic except for higher-grade pathological tumors in the Gleason ≥4+4 category.
Table 1.
Clinical and pathological characteristics of men with biopsy Gleason sum 7–10 tumors in the SEARCH Database
| Biopsy Gleason score | p value* (3+4 vs. 4+3) |
p value* (4+3 vs. ≥4+4) |
|||
|---|---|---|---|---|---|
| 3+4 | 4+3 | ≥4+4 | |||
| Number of patients | 201 | 78 | 95 | ||
| Age (years) – mean | 61.8 ± 6.7 | 62.2 ± 7.1 | 62.0 ± 5.9 | 0.59† | 0.67† |
| Median | |||||
| PSA (ng/ml) – mean | 10.1±7.4 | 11.8 ± 10.9 | 11.6 ± 8.1 | 0.47† | 0.57† |
| Median | 8.2 | 8.9 | 9.2 | ||
| Race: | 0.24 | 0.44 | |||
| White (%) | 112 (56) | 48 (62) | 56 (59) | ||
| Black (%) | 76 (38) | 22 (28) | 33 (24) | ||
| Other (%) | 13 (6) | 8 (10) | 16 (17) | ||
| Clinical Stage: | 0.20 | 0.75 | |||
| Tl (%) | 97 (48) | 31 (40) | 40 (42) | ||
| T2/T3 (%) | 104 (52) | 47 (60) | 55 (58) | ||
| Pathological Gleason score: | 0.001 | 0.001 | |||
| ≤3+3 (%) | 66 (33) | 8 (10) | 13 (14) | ||
| 3+4 (%) | 77 (38) | 32 (41) | 16 (17) | ||
| 4+3 (%) | 35(17) | 21 (27) | 21 (22) | ||
| ≥4+4 (%) | 23 (11) | 17 (22) | 45 (47) | ||
| Extracapsular extension (%) | 64 (32) | 39(51) | 44 (46) | 0.003 | 0.52 |
| Positive margins (%) | 76 (39) | 37 (47) | 41 (43) | 0.18 | 0.57 |
| Seminal vesicle invasion (%) | 16 (8) | 23 (30) | 21 (22) | <0.001 | 0.25 |
| Positive nodes (%) | 3 (1) | 4 (5) | 4 (4) | 0.11 | 0.76 |
p value by Chi-square test except where noted
p value by ranksum
Biopsy Gleason and pathological findings
After adjusting for multiple clinical characteristics, biopsy Gleason 4+3 was associated with increased odds of ECE (p=0.01) and SVI (p<0.001) compared to 3+4 (Table 2). There were no significant differences in odds of PSM, ECE, or SVI between men with biopsy Gleason 4+3 and ≥4+4.
Table 2.
Multivariable logistic regression analysis of association between biopsy Gleason score and adverse pathology in patients with biopsy Gleason sum 7–10 tumors in the SEARCH Database
| Odds Ratio | 95% CI | p value | |
|---|---|---|---|
| 4+3 vs. 3+4 | |||
| Positive surgical margins | 1.63 | 0.92 – 2.89 | 0.09 |
| Extracapsular extension | 2.16 | 1.18 – 3.94 | 0.01 |
| Seminal vesicle invasion | 6.43 | 2.76 – 14.98 | <0.001 |
| ≥4+4 vs. 4+3 | |||
| Positive surgical margins | 0.87 | 0.45 – 1.69 | 0.69 |
| Extracapsular extension | 0.78 | 0.40 – 1.54 | 0.48 |
| Seminal vesicle invasion | 0.61 | 0.28 – 1.29 | 0.19 |
Biopsy Gleason and biochemical progression
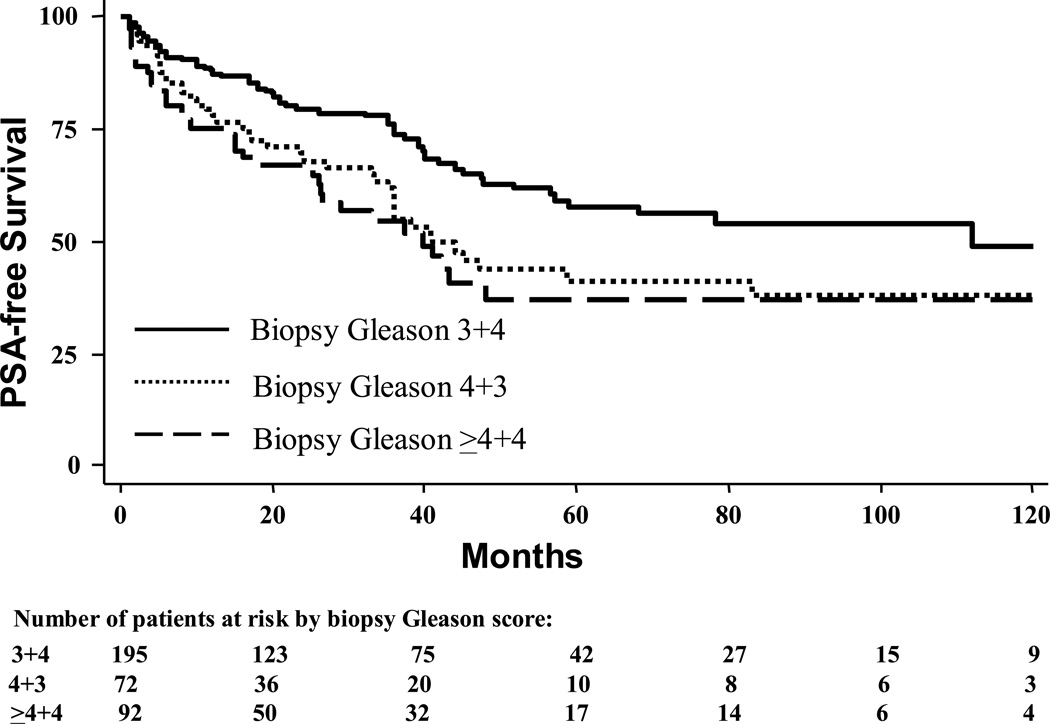
Among men in the biopsy Gleason cohort who did not recur, mean and median follow-up was 43 and 33 months, respectively (range: 1–164). During this time, 140 (37%) men experienced biochemical progression. Biopsy Gleason 4+3 was associated with an increased progression risk relative to 3+4 (log-rank, p=0.002), but similar progression risk as ≥4+4 (log-rank, p=0.54, Figure 1). After adjusting for multiple clinical characteristics, biopsy Gleason 4+3 was associated with increased biochemical progression compared to 3+4 (HR 2.08, 95% CI 1.34–3.21, p=0.001), but similar progression risk as biopsy Gleason ≥4+4 (HR 0.86, 95% CI 0.54–1.37, p=0.53).
Figure 1.
Actuarial 10-year biochemical recurrence rates of patients with biopsy Gleason sum 7–10 tumors treated with radical prostatectomy segregated by biopsy Gleason score. 2-way log-rank values: 3+4 vs. 4+3, p=0.002; 4+3 vs. ≥4+4, p=0.54.
RP Gleason cohort demographics and biochemical progression
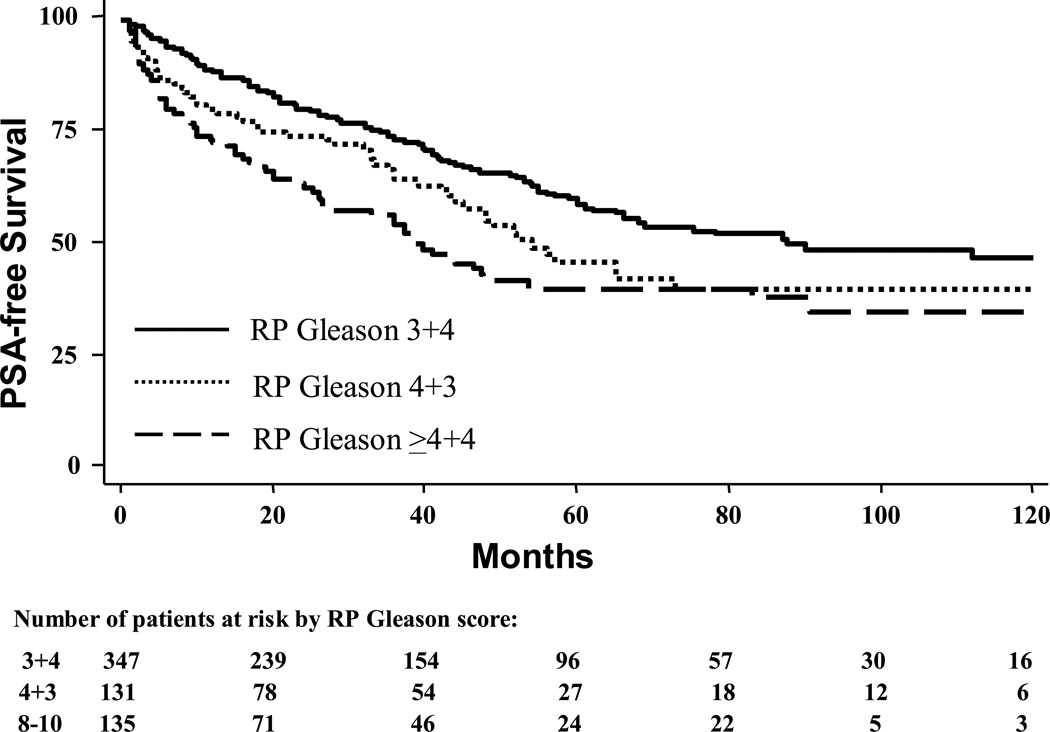
Of 1,278 men, 636 (50%) had a RP Gleason of 3+4 or higher (Table 3) and these men formed the RP Gleason cohort. Men with a RP Gleason score of 4+3 had an increased progression risk relative to 3+4 (log-rank, p=0.03), though similar risk as ≥4+4 (log-rank, p=0.14, Figure 2). After adjusting for multiple clinicopathological characteristics, RP Gleason 4+3 remained associated with a significantly increased progression risk relative to 3+4 (HR 1.44, 95% CI 1.04–2.01, p=0.03). After multivariable adjustment, the weak association between RP Gleason ≥4+4 and increased recurrence risk relative to 4+3 was attenuated and not statistically significant (HR 1.29, 95% CI 0.84–1.97, p=0.24).
Table 3.
Clinical and pathological characteristics of men with RP Gleason sum 7–10 tumors in the SEARCH Database
| RP Gleason score | p value* (3+4 vs. 4+3) |
p value* (4+3 vs. ≥4+4) |
|||
|---|---|---|---|---|---|
| 3+4 | 4+3 | ≥4+4 | |||
| Number of patients | 359 | 135 | 142 | ||
| Age (years) – mean | 62.2 ± 6.9 | 62.7 ± 6.5 | 62.3 ± 6.8 | 0.59† | 0.67† |
| Median | |||||
| PSA (ng/ml) – mean | 11.4 ± 12.8 | 12.5 ± 8.9 | 14.5 ± 18.4 | 0.02† | 0.95† |
| Median | 8.4 | 9.6 | 9.8 | ||
| Race: | 0.85 | 0.18 | |||
| White (%) | 198 (55) | 78 (58) | 86 (61) | ||
| Black (%) | 135 (38) | 47 (35) | 38 (27) | ||
| Other (%) | 26 (7) | 10 (7) | 18 (13) | ||
| Clinical Stage: | 0.01 | 0.55 | |||
| Tl (%) | 162 (45) | 43 (32) | 50 (35) | ||
| T2/T3 (%) | 197 (55) | 92 (68) | 92 (65) | ||
| Biopsy Gleason score: | <0.001 | 0.02 | |||
| ≤3+3 (%) | 208 (58) | 45 (33) | 34 (24) | ||
| 3±4 (%) | 89 (25) | 40 (30) | 34 (24) | ||
| 4+3 (%) | 38 (11) | 23 (17) | 21 (15) | ||
| ≥4+4 (%) | 24 (7) | 27 (20) | 53 (37) | ||
| Extracapsular extension (%) | 114 (32) | 47 (36) | 81 (60) | 0.40 | <0.001 |
| Positive margins (%) | 161 (45) | 54 (41) | 80 (58) | 0.42 | 0.01 |
| Seminal vesicle invasion (%) | 32 (9) | 24 (18) | 36 (26) | 0.004 | 0.12 |
| Positive nodes (%) | 9 (3) | 3 (2) | 8 (6) | 0.76 | 0.17 |
p value by Chi-square test except where noted
p value by ranksum
Figure 2.
Actuarial 10-year biochemical recurrence rates of patients with radical prostatectomy Gleason sum 7–10 tumors treated with radical prostatectomy segregated by radical prostatectomy Gleason score. 2-way log-rank values: 3+4 vs. 4+3, p=0.03; 4+3 vs. ≥4+4, p=0.14.
DISCUSSION
Multiple studies have demonstrated that Gleason score is important in predicting pathological stage and biochemical progression following therapy.1, 2, 9 While Gleason 7 can be stratified into 3+4 versus 4+3,1, 5, 7, 10–13 whether the greater risk associated with Gleason 4+3 is equivalent to that of ≥4+4 is unclear. In a large multicenter study examining biopsy and RP Gleason scores, we found that while clinicopathologic outcomes and progression risk among men with Gleason 4+3 tumors were worse than 3+4, there were no significant differences in outcomes between 4+3 and ≥4+4. If confirmed in other studies, these data suggest men with Gleason 4+3 and ≥4+4 should be counseled similarly with regard to treatment options and prognosis of these high-grade and potentially aggressive tumors.
The presence of Gleason pattern 4 tumor in the biopsy1–2 or RP specimen14–15 is a poor prognostic factor. Moreover, the exact percentage of pattern 4 in the biopsy16 or RP17 specimen is one of the strongest prognostic factors for progression following treatment. Yet, most pathology reports do not include exact percentage of pattern 4. Rather, they include a rough approximation in the form of a primary and secondary Gleason score. A specimen with 5–50% pattern 4 is Gleason 3+4, 50–95% pattern 4 is Gleason 4+3, and >95% pattern 4 is Gleason 4+4. Given the importance of percent Gleason pattern 4, it is not surprising that multiple studies have found that men with Gleason 4+3 (i.e. 50–95% pattern 4) have worse outcomes than men with Gleason 3+4 (i.e. 5–50% pattern 4).1, 5, 7, 10–13 The current study supports the continued risk stratification of men with Gleason 7 based on primary Gleason score.
To our knowledge, the current study is the first to investigate whether the increased risk of poor outcome associated with Gleason 4+3 approaches that of Gleason ≥4+4. Circumstantial evidence suggests that outcomes may be similar, as prognostic models predicting either pathological stage1 or prostate cancer-specific death18 show limited or no separation between Gleason 4+3 and ≥4+4. While pre-19 and post-operative20 nomograms predicting progression after RP do portend differences between Gleason 4+3 vs. ≥4+4, these nomograms did not examine Gleason score as a composite sum, but rather as separate primary and secondary Gleason scores. Since these nomograms did not directly compare outcomes between Gleason 4+3 and ≥4+4, it is unclear whether they can be used to imply that men with Gleason ≥4+4 exhibit worse outcomes than those with Gleason 4+3. In the current multicenter study, with the exception of higher-grade pathological disease among Gleason ≥4+4, we found no significant differences in adverse pathological outcomes or biochemical progression between Gleason 4+3 and ≥4+4. This implies that the small component of pattern 3 (i.e. <50%) in Gleason 4+3 disease does not mitigate the high-risk associated with a majority pattern 4 tumor in the specimen.
The findings of our study were based upon Gleason grading. Over time there has been a shift toward assigning higher Gleason grades.21 While we adjusted for year of surgery in multivariable analyses, it remains possible that differing pathological Gleason score interpretations influenced our results. Unfortunately, due to the small sample size, we were unable to evaluate whether the prognostic value of Gleason grading has changed over time. Thus, our results must be viewed as preliminary and require validation in external datasets. Moreover, Gleason grading is semi-subjective in nature with a moderate degree of intra- and inter-observer variability.22 In addition, our study used non-centralized pathologic evaluation from multiple pathologists. Whether single-center or single-pathologist analyses would reveal similar associations is unknown. The SEARCH Database does not contain data on the presence of high-grade tertiary Gleason patterns. Only a single Gleason score for each diagnostic biopsy was assigned, even when biopsy cores contained disparate Gleason scores. In these few cases, the highest Gleason score was utilized as the overall Gleason score. Whether this is the most appropriate method to assign overall Gleason score is unknown and requires further study. Moreover, our analysis did not examine the impact of quantitative pathology, such as number of positive cores or surface area of cancer involvement. It remains unknown whether such analyses would have influenced our results. Finally, our primary end-point was PSA recurrence. While prostate cancer mortality would be a better end-point, we currently do not have sufficient follow-up to measure this outcome, and early PSA recurrence has been linked with increased risk of prostate cancer death.23
CONCLUSIONS
In a multicenter database using pooled data from multiple pathologists, Gleason scores 4+3 and ≥4+4 exhibited similar clinicopathologic outcomes, regardless of whether the biopsy or RP specimen was examined.
Acknowledgments
Supported by the Department of Veterans Affairs, National Institute of Health R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA), the Georgia Cancer Coalition (MKT), the Department of Defense, Prostate Cancer Research Program, (SJF), and the American Urological Association Foundation Astellas Rising Star in Urology Award (SJF). Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Partin AW, Mangold LA, Lamm DM, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 2.Diblasio CJ, Kattan MW. Use of nomograms to predict the risk of disease recurrence after definitive local therapy for prostate cancer. Urology. 2003;62 Suppl 1:9. doi: 10.1016/j.urology.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico AV, Whittington R, Malkowicz BM, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 4.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer: a multi-institutional update. JAMA. 1997;277:1445. [PubMed] [Google Scholar]
- 5.Makarov DV, Sanderson H, Partin AW, et al. Gleason score 7 prostate cancer on needle biopsy: is the prognostic difference in Gleason scores 4 + 3 and 3 + 4 independent of the number of involved cores? J Urol. 2002;167:2440. [PubMed] [Google Scholar]
- 6.Rasiah KK, Stricker PD, Haynes AM, et al. Prognostic significance of Gleason pattern in patients with Gleason score 7 prostate carcinoma. Cancer. 2003;98:2560. doi: 10.1002/cncr.11850. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalgo ML, Bastian PJ, Mangold LA, et al. Relationship between primary Gleason pattern on needle biopsy and clinicopathologic outcomes among men with Gleason score 7 adenocarcinoma of the prostate. Urology. 2006;67:115. doi: 10.1016/j.urology.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Freedland SJ, Amling CL, Dorey F, et al. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology. 2002;60:670. doi: 10.1016/s0090-4295(02)01847-2. [DOI] [PubMed] [Google Scholar]
- 9.Lerner SE, Blute ML, Bergstralh EJ, et al. Analysis of risk factors of progression in patients with pathologically confined prostate cancer after radical retropubic prostatectomy. J Urol. 1996;156:137. [PubMed] [Google Scholar]
- 10.Tollefson MK, Leibovich BC, Slezak JM, et al. Long-term prognostic significance of primary Gleason pattern in patients with Gleason score 7 prostate cancer: impact on prostate cancer specific survival. J Urol. 2006;175:547. doi: 10.1016/S0022-5347(05)00152-7. [DOI] [PubMed] [Google Scholar]
- 11.Han M, Snow PB, Epstein JI, et al. A neural network predicts progression for men with Gleason score 3+4 versus 4+3 tumors after radical prostatectomy. Urology. 2000;56:994. doi: 10.1016/s0090-4295(00)00815-3. [DOI] [PubMed] [Google Scholar]
- 12.Sakr WA, Tefilli MV, Grignon DJ, et al. Gleason score 7 prostate cancer: a heterogeneous entity? Correlation with pathologic parameters and disease-free survival. Urology. 2000;56:730. doi: 10.1016/s0090-4295(00)00791-3. [DOI] [PubMed] [Google Scholar]
- 13.Chan TY, Partin AW, Walsh PC, et al. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology. 2000;56:823. doi: 10.1016/s0090-4295(00)00753-6. [DOI] [PubMed] [Google Scholar]
- 14.Stamey TA, Yemoto CM, McNeal JE, et al. Prostate cancer is highly predictable: a prognostic equation based on all morphological variables in radical prostatectomy specimens. J Urol. 2000;163:1155. doi: 10.1016/s0022-5347(05)67713-0. [DOI] [PubMed] [Google Scholar]
- 15.Epstein JI, Pound CR, Partin AW, et al. Disease progression following radical prostatectomy in men with Gleason score 7 tumor. J Urol. 1998;160:97. [PubMed] [Google Scholar]
- 16.Freedland SJ, Csathy GS, Dorey F, et al. Percent prostate needle biopsy tissue with cancer is more predictive of biochemical failure or adverse pathology after radical prostatectomy than prostate specific antigen or Gleason score. J Urol. 2002;167:516. doi: 10.1016/S0022-5347(01)69076-1. [DOI] [PubMed] [Google Scholar]
- 17.Stamey TA, McNeal JE, Yemoto CM, et al. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 18.D’Amico AV, Cote K, Loffredo M, et al. Pretreatment predictors of time to cancer specific death after prostate specific antigen failure. J Urol. 2003;169:1320. doi: 10.1097/01.ju.0000049200.30192.d1. [DOI] [PubMed] [Google Scholar]
- 19.Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephenson AJ, Scardino PT, Eastham JA, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 22.Carlson GD, Calvanese CB, Kahane H, et al. Accuracy of biopsy Gleason scores from a large uropathology laboratory: use of a diagnostic protocol to minimize observer variability. Urology. 1998;51:525. doi: 10.1016/s0090-4295(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 23.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]