Abstract
We have constructed stable virus-like particles displaying the HIV-1 Gag(p17) protein as an N-terminal fusion with an engineered protein domain from the Geobacillus stearothermophilus pyruvate dehydrogenase subunit E2. Mice immunized with the Gag(p17)-E2 60-mer scaffold particles mounted a strong and sustained antibody response. Antibodies directed to Gag(p17) were boosted significantly with additional immunizations, while anti-E2 responses reached a plateau. The isotype of the induced antibodies was biased towards IgG1, and the E2-primed CD4+ T cells did not secrete IFNγ. Using transgenic mouse model systems, we demonstrated that CD8+ T cells primed with E2 particles were able to exert lytic activity and produce IFNγ. These results show that the E2 scaffold represents a powerful vaccine delivery system for whole antigenic proteins or polyepitope engineered proteins, evoking antibody production and antigen specific CTL activity even in the absence of IFNγ-producing CD4+ T cells.
Keywords: Virus-like particles, HIV, vaccine, E2 scaffold, Gag(p17)
Introduction
After twenty-five years of HIV research and several failed vaccine trials (Buchbinder et al., 2008), despite some evidence for modest vaccine protection in humans (Rerks-Ngarm et al., 2009), new vaccine modalities are still needed to elicit the high-titer and durable immune responses. These responses include, but may not be limited to, cytotoxic T cells (CTL) directed to multiple viral proteins, strong T helper responses, and neutralizing antibodies (NAbs) that are effective against a broad range of primary HIV-1 isolates. Proof-of-principle experiments in nonhuman primate challenge models have been useful in understanding the role of antibodies in blocking infection, when they are present at sufficient titers in advance of exposure (Baba et al., 2000; Hessell et al., 2009; Hessell et al.; Mascola et al., 2000; Shibata et al., 1999). Similarly, the presence of T cell responses is correlated with viral control, as shown by T cell depletion studies in nonhuman primates (Lifson et al., 2001; Schmitz et al., 2005) and protection from disease when strong T cell responses are induced by vaccination with recombinant viral vectors (Hansen et al., 2009; Santra et al., 2005). Efforts have thus been focused on designing vaccines and delivery systems that can elicit strong, durable immunity that is directed against multiple viral antigens, to prevent ready escape. Current methods for eliciting T-cell responses for HIV, in particular, have presented limitations due to poor immunogenicity that is limited in seropositive individuals (O’Brien et al., 2009). However, in the case of some vectors, such as adenovirus, strategies have been proposed to circumvent anti vector immunity (Roberts et al., 2006). Moreover, combining two or more vaccine modalities in “prime-boost” regimens can elicit stronger immune responses than either vaccine alone (Kibuuka et al., 2010; Koup et al., 2010; Rerks-Ngarm et al., 2009).
The inactivated HIV virion itself has long been considered as a vaccine candidate and this approach, especially for therapeutic vaccines, was championed by polio vaccine luminary Jonas Salk (Salk, 1987). Protective efficacy with formalin-inactivated SIV in nonhuman primates was demonstrated (Johnson et al., 1992; Murphey-Corb et al., 1990) but the vaccine was complicated by the presence of host HLA, which was shown to be the mechanism for protection-unrelated to the SIV antigens (Arthur et al., 1995; Stott, 1991). Recent advances in understanding of virus assembly have led to the development of methods that chemically and irreversibly inactivate whole virions and that maintain the receptor binding activity of Envelope (Arthur et al., 1998; Lifson et al., 2004). The attractiveness of VLPs that can mimic the natural HIV virion without any risk of HIV infection led to the early development of recombinant Gag-Env particles produced in mammalian cells by recombinant vaccinia virus (Haffar et al., 1990; Haffar et al., 1992; Haffar et al., 1991; Luo, Li, and Yong Kang, 2003), or rhinovirus chimeras (Arnold et al., 1994; Lapelosa et al., 2010), yeast (Tsunetsugu-Yokota et al., 2003), or in other systems, reviewed in (Deml et al., 2005). While clearly immunogenic in eliciting Gag-specific CTL (Paliard et al., 2000) and antibodies, including some neutralizing antibodies (Ding et al., 2002), none of these approaches has elicited strong protective immunity. Explanations for low level immunogenicity may include dose, stochiometry of key immunogens such as Envelope (when present), or delivery or adjuvant systems employed to date.
We have previously designed and investigated a new delivery vehicle in which antigenic determinants are inserted on the surface of an icosahedral scaffold formed by the acyltransferase component (E2 protein) of the multienzyme pyruvate dehydrogenase complex (PDH) from Geobacillus stearothermophilus, and reported the ability of this scaffold to display peptides in a high immunogenic form (De Berardinis et al., 2003; Domingo et al., 2003). The core C-terminal catalytic domain of E2 self-assembles into trimers, which in turn aggregate to generate a 60-chain core with icosahedral symmetry (Domingo, Orru, and Perham, 2001; Henderson, Perham, and Finch, 1979; Perham, 2000). Moreover this 60-meric icosahderal structure can be regenerated with high efficiency from denaturing conditions in vitro, without the need of chaperonins (Allen and Perham, 1997; Lessard et al., 1998). The robustness of this peptide based virus-like particle has rendered it an attractive macromolecular scaffold for presentation of exogenous molecules on its surface (De Berardinis et al., 2003; Domingo et al., 2003; Domingo, Orru, and Perham, 2001) and for molecular encapsulation in its cavity (Dalmau et al., 2008; Dalmau, Lim, and Wang, 2009a; Dalmau, Lim, and Wang, 2009b). Efficient refolding of E2 to the 60-mer is also possible with foreign peptides replacing the natural peripheral domains, as N-terminal fusions to the core domain. Thus, a suitably engineered E2 core (E2DISP) can display 60 copies of heterologous peptides on the surface of a high molecular mass scaffold (De Berardinis et al., 2003; Domingo et al., 2003; Domingo, Orru, and Perham, 2001). This property can be exploited for vaccine design. Here we further expanded the delivery properties of the E2 scaffold by displaying a whole protein at the surface of the scaffold, and analysed the in vivo induction of B and T cell responses.
Results
Construction of E2 particles
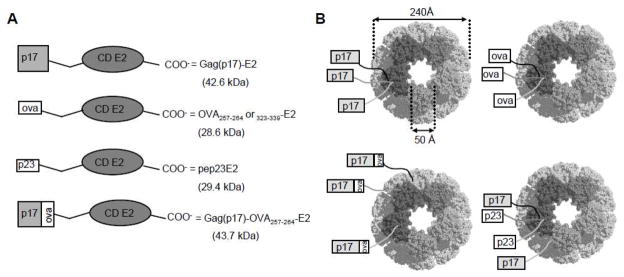
We constructed OVA257-264 E2 and OVA323-339 E2 particles expressing the OVA peptides at the N-terminus of E2. We also constructed Gag(p17)-E2, and Gag(p17)-OVA257-264-E2 particles respectively expressing the HIV-1 Gag-p17 protein at the N-terminus of E2 or, in addition, the OVA257-264 peptide between the E2 carrier protein and the HIV-1 Gag(p17) protein. Both the Gag(p17)-E2 and Gag(p17)-OVA257-264-E2 fusion proteins also assembled into 24nm virus-like E2 particles expressing up to 60 copies of the HIV-1 Gag(p17) or Gag(p17)-OVA257-264 antigens on their surface (see Fig. 1). Hybrid particles displaying equimolar amounts of pep23E2 and Gag(p17)-E2 particles which co-express a strong T helper epitope from HIV-1 reverse transcriptase were also generated (Fig. 1) according to previously described methodologies (Domingo et al., 2003). Correct folding of these particles was demonstrated by gel filtration chromatography to confirm size and electron microscopy analysis (data not shown).
Figure 1. Schematic representation of E2 constructs.
(A) p17-Gag protein or OVA323-339, OVA257-264 and pep23 peptides, fused at the N terminus of E2 catalytic domain (CD). In Gag(p17)-OVA257-264-E2 fusion protein the OVA257-264 epitope is inserted between the Gag(p17) protein and E2 CD. Molecular weight of single chains is reported. (B) Representation of 60-mer E2 complexes. pep23E2/Gag(p17)-E2 hybrid particles express simultaneously Gag(p17) protein and pep23 peptide on the same scaffold.
Generation of Gag(p17) specific CTLs
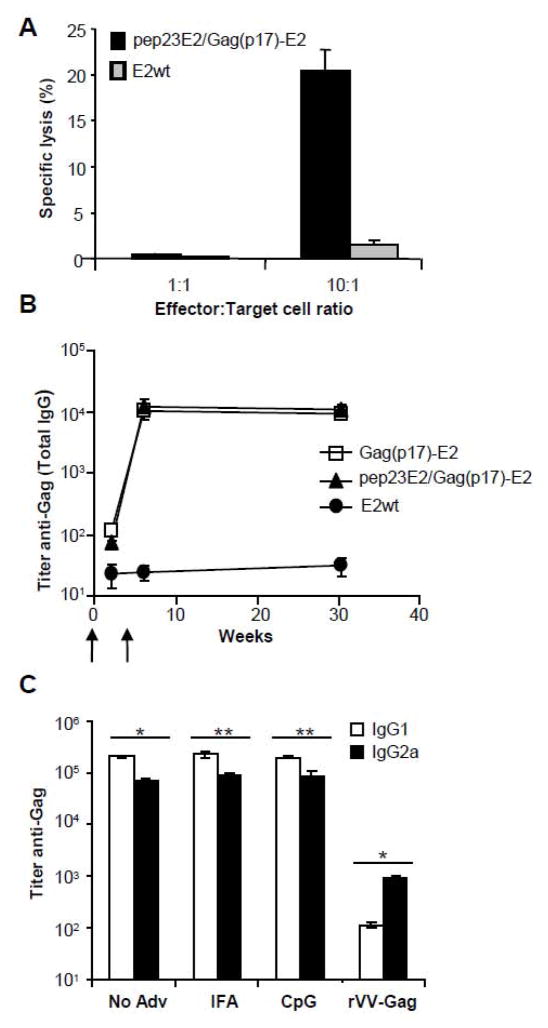
To investigate the ability of Gag(p17)-E2 particles to elicit peptide-specific CTL responses in vivo, HHD (HLA-A2.1/H2-Db) transgenic mice were immunized with double-display pep23E2/Gag(p17)-E2 particles and the generation of CTLs specific for the HLA-A2 restricted Gag(p17) epitope SLYNTVATL was examined. Since we previously reported that in the HHD mouse model the immunization with E2 particles co-expressing helper and cytotoxic T cell epitopes is a requirement for inducing a CTL response, we used the pep23E2/Gag(p17)-E2 particles co-expressing the pep23 helper epitope to ensure strong T cell help in these mice (Domingo et al., 2003). Splenocytes were isolated from mice immunized twice and restimulated in vitro for 5 days with syngeneic LPS-induced blast cells pulsed with the same E2 particles used for immunization. Effector cell-mediated cytotoxic activities were tested in 51Cr release assays towards RMA-S-HHD (Tap−, HLA-A2.1+) target cells loaded with the Gag(p17) SLYNTVATL synthetic peptide. As shown in figure 2A, specific cytotoxic activities were generated in splenocytes isolated from mice immunized (in the absence of adjuvants) with pep23E2/Gag(p17)-E2 particles. This response was similar in mice immunised in the presence of adjuvant (data not shown). In contrast, no cytotoxic activity was found in splenocytes isolated from mice immunized with E2 wt particles (Figure 2A). Splenocytes isolated from unimmunized mice and restimulated in vitro with pep23E2/Gag(p17)-E2 particles-pulsed LPS-blasts did not exert cytotoxic activity (data not shown).
Figure 2. Humoral and cytotoxic response induced by Gag(p17)-E2 particles.
(A) Cytotoxic response of splenocytes from HHD mice immunized twice in the absence of adjuvants with pep23E2/Gag(p17)-E2 (black bars) or E2wt (gray bars), and challenged after in vitro restimulation against RMA-S HHD target cells prepulsed with SLYNTVATL peptide. The percentage of antigen specific killing is shown on y-axis after subtraction of background killing of unpulsed targets and represent the mean ± SD of cytotoxic activity of 5 mice in each group. Effector/target ratios are shown on the x-axis. (B) BALB/cxC57BL/6 F1 mice were immunized with Gag(p17)-E2 (squares), pep23E2/Gag(p17)-E2 (triangles) or E2wt (circles) particles at weeks 0 and 4 (arrows). On week 2, 6 and 30, mice were bled and sera were analyzed by ELISA for Gag-specific total IgG endpoint titers. Figure represents the media ± SD of total IgG titer of 9 mice in each group. (C) Production of anti Gag isotypes in mice immunized twice with Gag(p17)-E2 in presence of IFA, CpG, or without adjuvant. Immunization with vaccinia virus Gag (rVV-Gag) was also performed as control of the isotype IgG2a induction. The values represent the mean ±SD of 9 mice in each group; *=P<0.01, **=P<0.05.
Induction of anti-Gag(p17) antibodies
We measured total IgG titers against Gag and E2 proteins in the sera of mice (three groups of three mice each) bled after one or two doses of Gag(p17)-E2, pep23E2/Gag(p17)-E2 or E2 wt particles, which were administered s.c. either in the presence or in the absence of adjuvants. The IgG antibodies against Gag were scarcely detectable after one dose but were strongly evident after two doses (Fig. 2B). No differences were observed between groups of mice immunized with particles displaying Gag(p17) alone or hybrid particles displaying pep23E2/Gag(p17)-E2 particles (Fig. 2B). Antibodies directed against E2 were detectable after one dose and boosted by the second dose (not shown). The high titer of antibodies persisted for 30 weeks (Fig. 2B). IgG isotype was significantly biased in favor of IgG1 as compared to the response obtained in mice immunized with vaccinia Gag used as a control (Fig. 2C). The antibody production was similar in the sera of mice immunized in the absence or in the presence of IFA or CpG adjuvants (Fig. 2C). In separate experiments, we immunized C57BL6xBALB/c mice three times (weeks 0, 4, and 31) with Gag(p17)-E2 alone; E2-specific responses were not further enhanced by the third immunization, while the Gag-specific responses increased significantly with the third dose (data not shown).
We conclude that: 1) the HIV-1 Gag(p17) protein displayed on Gag(p17)-E2 particles is strongly immunogenic in the absence of adjuvant; 2) responses to Gag are primed by one dose and boosted with a second or third dose; 3) antibodies level persisted for many weeks, at least 27 weeks; 4) although responses to E2 increase with a boosting dose, this does not abrogate the boosting effect for Gag responses; and 5) co-expression on E2 of exogenous helper epitopes is not needed for antibody production in the immunized mice.
Role of CD4+ T cells in IgG1 induction
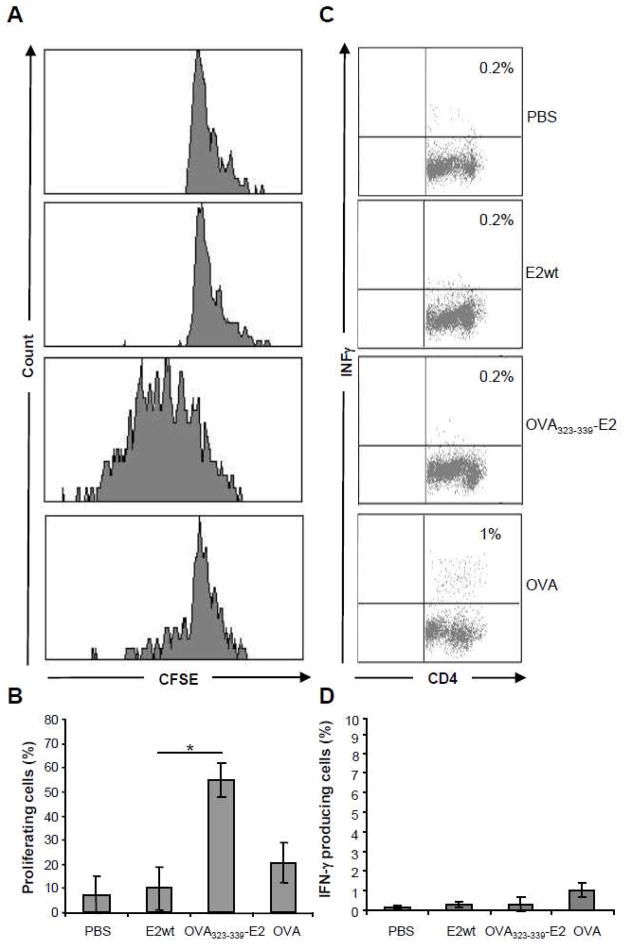
In order to analyse the role of CD4+ T cell help in the induction of an antibody response characterized by the IgG1 isotype, we assessed the production of IFNγ in CD4+ T cells isolated from C57BL/6xBALB/c (MHC H-2bd) F1 mice immunized with Gag(p17)-E2, and stimulated with overlapping peptides (15 amino acid residue length) encompassing the Gag(p17) sequence. The CD4+ T cells from mice immunized with one or two doses of Gag(p17)-E2 particles did not produce IFNγ upon restimulation with 15-mer Gag peptides (data not shown). This result may be consistent with the lack of T helper epitopes restricted by mouse MHC H-2bd in the Gag(p17) sequence, as these epitopes are not reported in the NIH database. Thus, in order to assess the role of CD4+ T cell help in the induction of antibodies with the IgG1 isotype, we chose to study the response to a well-established epitope such as the MHC H-2b-restricted chicken ovalbumin 323–339 (OVA323-339) epitope. For this study we used OVA323-339E2 particles and the OT-II mice which have CD4+ T cells expressing a transgenic TCR specific for the OVA323-339 epitope. According to established protocols (Parish CR, 2009) we thus transferred 3×106 CFSE-labeled, OVA323-339-specific, CD4+ OT-II T cells into C57BL/6 (MHC IAb) recipient mice. The day after, mice were immunized either s.c. with 100μg of E2 particles displaying the OVA323-339 peptide, or i.p. with 500μg of soluble ovalbumin plus poly(I:C). As control, immunization with E2 wt particles was also performed. After 3 days, isolated splenocytes were evaluated for OT-II proliferation as assayed by CFSE dilution, and the results illustrated in figure 3A–B show a strong proliferative response of OT-II CD4+ T cells in OVA323-339E2 treated mice as compared to the proliferative response observed in mice which received soluble OVA plus poly(I:C). We also measured IFNγ production by intracellular staining of splenocytes. As illustrated in figure 3C–D, CD4+ OT-II cells did not produce IFNγ upon restimulation with the OVA323-339 synthetic peptide. Nonspecific stimulation with PMA plus ionomycin gave rise to high levels of IFNγ (data not shown).
Figure 3. Priming of OT-II cells with E2 particles.
C57BL/6 recipient mice (5 per group) of CFSE-labelled OT-II T cells were immunized with E2wt particles, OVA323-339 E2 particles or soluble ovalbumin (OVA) plus poly (I:C) or PBS. 3 days after immunization, mice were sacrificed and the spleen cells analysed by flow cytometry for CFSE content (A–B), or restimulated in vitro with OVA323-339 synthetic peptide for IFNγ production analysis (C–D).
(A) Figure shows data from a single representative mouse for each group. Profiles are gated on Vα2+ CD4+ cells. (B) Mean values of all mice in each group. Bars represent mean ± SD of percentage values of CFSE-labelled proliferating cells obtained in 3 independent experiments; *=P<0.01. (C) IFNγ production of CD4+ gated cells. The percentages of IFNγ positive cells are indicated in the upper right corners. Figure shows data from a single representative mouse for each group. (D) Data of IFNγ production by CD4+ T cells from all immunised mice. Bars represent mean ± SD of values obtained in 3 independent experiments.
Priming with E2 particles induces antigen specific CD8+ T cells able to produce IFNγ
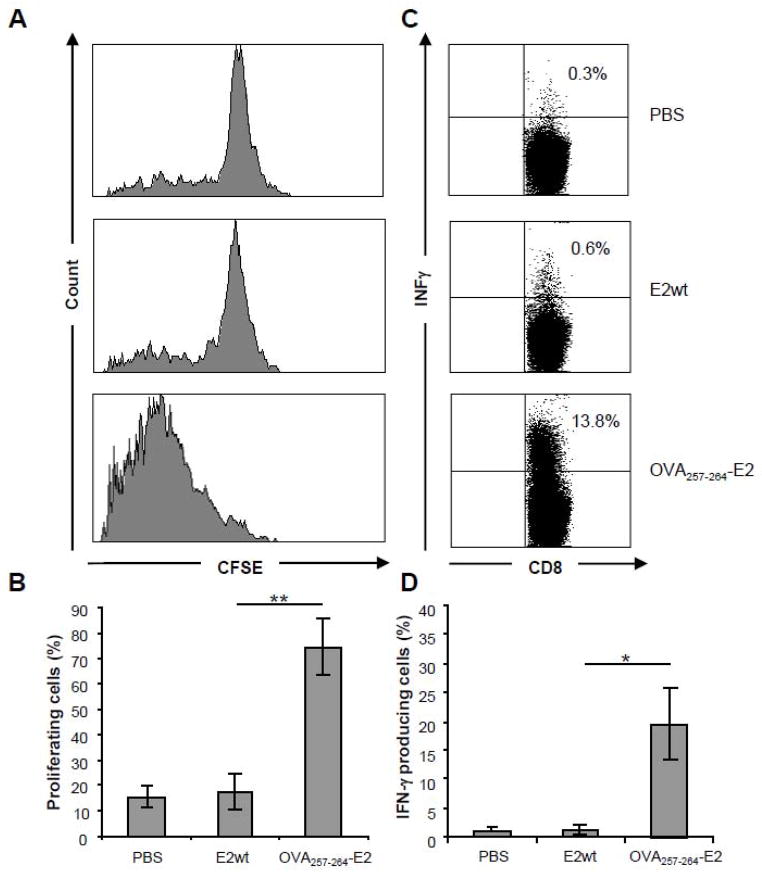
We also performed a study using the OT-I model characterized by CD8+ T cells expressing a transgenic TCR able to recognize the CTL epitope SIINFEKL from chicken ovalbumin residues 257–264 (OVA257-264). In this case we did not use E2 particles co-expressing the helper epitope pep23 because we previously reported (Del Pozzo et al.; Mascolo et al., 2007) that co-expression of a helper epitope in the immunizing particles is not necessary to obtain a CTL response specific towards the OVA257-264 peptide in C57BL/6 wild type mice. Thus, after the adoptive transfer of CSFE-labelled OT-I/CD8+ T cells, C57BL/6 recipient mice were immunized with E2 particles expressing the OVA257-264 peptide. After 3 days splenocytes were isolated from the immunized mice and respectively evaluated for OT-I proliferation and IFNγ production. As illustrated in figure 4A-B, a specific proliferation, as assessed by CSFE dilution, was observed on CD8+ T cells isolated from mice immunized with OVA257-264E2 particles. In contrast to OT-II CD4+ T cells, the OT-I CD8+ T cells were able to produce IFNγ upon restimulation with the specific OVA257-264 synthetic peptide (Fig. 4C–D).
Figure 4. Priming of OT-I CD8+ cells and INFγ production.
C57BL/6 recipient mice (5 per group) of CFSE-labelled OT-I T cells were immunized with E2wt particles, OVA257-264 E2 particles or PBS. 3 days after immunization, mice were sacrificed and the spleen cells analysed by flow cytometry for CFSE content (A–B), or restimulated in vitro with OVA257-264 synthetic peptide for IFNγ production analysis (C–D).
(A) Figure shows data from a single representative mouse for each group. Profiles are gated on Vα2+ CD8+ cells. (B) Mean values of all mice in each group. Bars represent mean ± SD of percentage values of CFSE-labelled proliferating cells obtained in 3 independent experiments; **=P<0.05. (C) IFNγ production of CD8+ gated cells. The percentages of IFNγ positive cells are indicated in the upper right corners. Figure shows data from a single representative mouse for each group. (D) Data of IFNγ production by CD8+ T cells from all immunised mice. Bars represent mean ± SD of values obtained in 3 independent experiments; *=P<0.01.
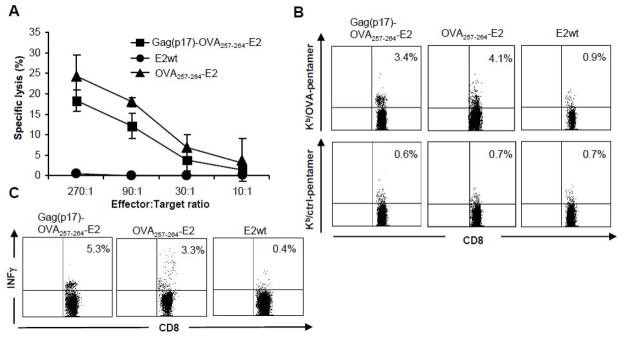
Induction of cytotoxic T cells by Gag(p17)-OVA257-264-E2 particles
We thus constructed a fusion protein expressing the OVA257-264 CTL epitope between the E2 carrier and the Gag(p17) protein, and assessed the production of OVA specific CTL in C57BL/6 wild type mice. Aim of this analysis was to demonstrate the ability of E2 particles to be processed and to cross-present the OVA257-264 CTL epitope even if it was located in an internal position in comparison with E2 particles expressing the OVA257-264 peptide as N-terminal epitope.
C57BL/6 mice (MHC H-2b) were immunized with two subcutaneous injections of the Gag(p17)-OVA257-264-E2, OVA257-264-E2 or E2 wild type particles. Two weeks after the second immunization, the mice were sacrificed and the splenocytes were isolated, and restimulated in vitro with syngeneic LPS-induced blast cells pulsed with the OVA257-264 peptide. After seven days, effector cells were tested for OVA257-264 peptide specific responses by cytotoxic assay, pentamer staining and intracellular staining. Figure 5A shows the cytotoxic activity of splenocytes isolated from mice immunized with Gag(p17)-OVA257-264-E2 particles, OVA257-264-E2 or with E2 particles. The results indicate that Gag(p17)-OVA257-264-E2 and OVA257-264-E2 particles elicited the induction of OVA257-264 specific cytotoxic T cells. Using pentamer staining we further characterized the effector cell population. As illustrated in figure 5B, CD8+ T cells specifically stained by the OVA-pentamers-PE (carrying the OVA peptide SIINFEKL), were present in splenocytes isolated from mice immunized with Gag(p17)-OVA257-264-E2 or OVA257-264-E2 particles. Furthermore, we also visualized, by intracellular staining, the production of IFNγ by CD8+ T cells isolated from the immunized mice and restimulated with the OVA257-264 synthetic peptide (Fig. 5C).
Figure 5. Antigen specific cytotoxic T cells raised in mice immunized with Gag(p17)- OVA257-264-E2 particles.
C57BL/6 mice (5 per group) were immunized twice with Gag(p17)-OVA257-264-E2, OVA257-264 -E2 or E2wt particles. 14 days after the second immunization splenocytes were isolated and cultured in vitro for 7 days in the presence of synthetic OVA257-264 peptide. (A) The cultured cells were used as effector cells in a lytic assay towards EL4 target cells prepulsed with OVA257-264 peptide. The percentages of antigen specific killing is shown on y-axis after subtraction of background killing of unpulsed targets and represent the mean ± SD of cytotoxic activities of all mice in each group. Data of three different experiments are reported. Effector/target ratios are shown on the x-axis.
(B) Pentamer staining of cultured cells. The dot plots represent flow cytometric analysis of spleen cells stained with anti-CD8 FITC mAb and either OVA-pentamers-PE carrying the OVA peptide (SIINFEKL) or ctrl-pentamers-PE carrying the control ctrl (SSYSYSSL) peptide. Figure shows data from a single representative mouse for each group and are representative of at least three different experiments.
(C) The percentage of CD8+ cells producing INFγ upon restimulation with OVA257-264 synthetic peptide is shown in the upper right corner of each plot after intracellular staining and FACS analysis. Figure shows data from a single representative mouse for each group and are representative of at least three different experiments.
Discussion
We previously described the immunogenicity of small epitopes (9–15 aa) displayed on the E2 scaffold (De Berardinis et al., 2003; Domingo et al., 2003). However, larger protein antigens may be more useful, as they contain multiple T cell epitopes as well as antibody determinants in their native context. Each E2 chain in the Geobacillus stearothermophilus PDH complex naturally displays, at the N-terminus of the acyltransferase core domain, 187 amino acid residues in the form of the two folded protein domains (lipoyl and peripheral subunit binding) and two flexible linkers. Moreover, the E2 system is naturally designed to present up to 60 copies of the E1 (~150 kDa) or E3 (~100 kDa) enzymes noncovalently attached on its surface. Thus, in theory, it is possible that large polypeptides can be attached to the E2 scaffold, which is formed by the acetyltransferase core domain.
In this context, we have expressed on E2 the HIV-1 Gag(p17) protein. Expression of this protein allowed the correct folding of the 60-mer scaffold. Moreover, Gag(p17) was expressed in a native form being recognised by a monoclonal antibody specific for a conformational epitope of the Gag(p17) protein (De Berardinis and Haigwood, 2004). Here we show the ability of Gag(p17) to induce in vivo a sustained humoral immune response. In fact, high titers of antibodies were detected after immunization in the absence of added adjuvants and the specific antibodies were still present in mice sera 30 weeks after the immunizations. The presence of an exogenous T cell help co-displayed by the E2 particles was not necessary for mice to mount a sustained antibody response. This result is in agreement with previously reported data on antibody production by immunizing with E2 particles not co-expressing an exogenous helper peptide (Domingo et al., 2003; Domingo, Orru, and Perham, 2001). However, we cannot exclude that the primed mice may recognize MHC H2bd restricted T helper epitope/s present in the E2 scaffold.
The analysis of IgG subclasses raised in mice sera indicates a bias towards IgG1 response, suggesting that a Th2-type response (Reiner and Locksley, 1995) is preferentially induced by E2 antigen delivery. However, we do not have a direct evidence for the role of E2-mediated T cell help in the induction of the anti-Gag IgG1 response. Immune responses in humans against most infections are of mixed pattern or balanced between Th1 and Th2. The balance needed between the induction of an effective antibody response versus a T cell response in protecting against live infection, is not well understood. However, we can hypothesize that in order to combat specific pathogens, it may be advantageous to preferentially induce a Th1 or Th2-type of response. Moreover, it is known that addition of a different kind of adjuvant may shift the immune response towards alternative pathways. In this context, it is important to assess which type of response is induced by a particular delivery vehicle in order to use it for eliciting a preferred pattern of cytokines response. Here we have analysed the CD4+ T cell response induced by E2 delivery using the OT-II mouse model. We observed that the proliferating OT-II CD4+ T cells, primed with E2 particles carrying the OVA323-339 peptide, did not produce IFNγ. Future work will be addressed to identify the cytokines produced by the CD4+ T cells upon priming via E2 antigen delivery.
We also studied the CD8+ T cell response in HHD transgenic mice, in the OT-I model and in C57BL/6 mice. In order to study the response in HHD mice we used E2 particles co-displaying the helper peptide pep23 because we previously observed that a CTL response can be induced in these mice only by E2 particles co-displaying an exogenous strong helper epitope (Domingo et al., 2003). On the other hand, according to previous findings in another system (Del Pozzo et al.; Mascolo et al., 2007) we found that that co-expression of the pep23 helper epitope was not necessary to induce a CD8+ T cell response against the OVA257-264 antigenic peptide SIINKFEL in OT-I and in C57BL/6 wild type mice. The data obtained, using the OT-I model system, show the production of IFNγ by antigen specific CD8+ T cells. These results are in contrast to data obtained with CD4+ T cells in the OT-II model. Moreover, in wild type C57BL/6 mice immunized with E2 particles carrying the OVA257-264 peptide as a fusion with the Gag(p17) we also induced antigen-specific CD8+ cytotoxic T cells able to produce IFNγ. CTL specific for the HLA-A2 restricted Gag(p17) epitope SLYNTVATL were also induced in HHD transgenic mice immunized with pep23E2/Gag(p17)-E2 particles. It should be emphasized that in both of these latter constructs the CTL epitopes were located in an internal position, requiring processing for class I presentation. This suggests that E2 delivery may be employed to obtain response towards whole immunogenic proteins or to display engineered constructs encompassing multiple epitopes. In this context we have previously observed that E2 particles can reach both the MHC class I and class II compartments (De Berardinis et al., 2003).
Vaccines designed to limit or prevent HIV infection are generally thought to require broad sustained humoral and cellular immune responses to be effective (Fauci et al., 2008). These types of responses have been difficult to elicit with existing approaches, even by combining modalities. Thus, both novel immunogens and more effective antigen presentation strategies are needed to complement the existing array of options or to be used in prime-boosting immunization strategies (Barnett et al., 1997; Doria-Rose et al., 2003; Law et al., 2007). From these data we can conclude that the Gag(p17) HIV-1 protein is effectively presented to the immune system by the E2 scaffold. In immunized mice this scaffold elicits, not only the production of a sustained humoral response, but also the induction of cytotoxic T cells. The ability of virus-like particles similar in size to the E2 scaffold to elicit both types of immunity represents an advantage over recombinant proteins, which primarily target antibody generation and viral vectors that are most effective in eliciting cell mediated responses. In theory, multiple HIV antigens and immune effectors/adjuvants can be presented on the same scaffold, limited only by steric constraints and solubility. In this context, the E2 antigen delivery system, which affords simplicity and safety along with the capability of inducing sustained humoral and cellular antigen-specific immune responses, represents a promising new tool that can be combined with other approaches to advance the field of vaccinology.
Materials and methods
Construction of E2 vectors
The OVA257-264-E2, OVA323-339-E2, Gag(p17)-E2 and Gag(p17)-OVA257-264-E2 vectors were constructed from the previously described pETE2DISP (De Berardinis et al., 2003; Domingo et al., 2003; Domingo, Orru, and Perham, 2001), to allow the expression, as N-terminal fusion to the E2 core scaffold, of the ovalbumin (OVA) peptides 257-264 and 323-339 (OVA257-264 and OVA323-339), the HIV-1 Gag(p17) protein and the OVA257-264 peptide fused to the HIV-1 Gag(p17) protein. Oligonucleotide sequences encoding the OVA peptides sequence SIINFEKL and ISQAVHAAHAEINEAGR with the protruding ends for Nco I and Xma I restriction enzymes (New England Biolabs, MA, USA) were purchased from Primm srl (Naples, Italy). OVA oligonucleotide sequences were inserted into the Nco I and Xma I digested pETE2DISP by ligation with T4 DNA ligase (New England Biolabs) to generate OVA257-264-E2 and OVA323-339-E2 vectors.
The segment of DNA encoding the HIV-1 Gag(p17) protein was amplified by polymerase chain reaction (PCR) from pNL4-3Gag vector (gift of Dr. Ned Landau) using the two oligonucleotides SEQ3: 5′CATGCCATGGCCGGTGCGA3′ and SEQ4: 5′CATGCCATGGCGTAATTTTGGCTGACC3′. Gag(p17) PCR product digested with Nco I and Xma I restriction enzymes was ligated into the previously digested pETE2DISP to generate Gag(p17)-E2 vector. Oligonucleotide sequences SEQ3 and SEQ5: 5′CATGCCCGGGCGTAATTTTGGCTGACC3′ were employed to amplify Gag(p17) cDNA from Gag(p17)-E2 vector and to introduce Nco I restriction sites at the 5′ and 3′ ends. The Gag(p17) amplified cDNA was thus digested and inserted into the Nco I digested OVA257-264-E2 vector by ligation with T4 DNA ligase to generate Gag(p17)-OVA257-264-E2 vector.
The BL21 (DE3) E. coli bacteria cells containing circular plasmids were selected on LB medium plates containing ampicillin (Sigma, Milan, Italy) and successful costruction of the plasmids was confirmed by DNA sequence analysis (Primm srl.). The construction of pep23E2 vector, expressing the KDSWTVNDIQKLVGK T helper epitope from reverse transcriptase of HIV-1 was described previously (Domingo et al., 2003).
Expression and purification of E2 proteins
1 liter of LB medium containing 100μg/ml ampicillin and 30μg/ml kanamycin (Sigma) was inoculated with 10 ml of an overnight culture of E. coli BL21 (DE3) co-transformed with OVA257-264-E2, OVA323-339-E2, Gag(p17)-E2 or Gag(p17)-OVA257-264-E2 and with pGroEL/ES vectors (De Berardinis et al., 2003; Domingo et al., 2003; Domingo, Orru, and Perham, 2001). Cultures were grown at 37°C until an optical density of 0.6 (600nm) was obtained. The cells were then heat-induced overnight at 30°C with 2mM Isopropyl β-D-1-thiogalactopyranoside (IPTG). The Gag(p17)-E2 cell pellet was resuspended in buffer A: 30mM potassium phosphate pH 6.4, 1mM phenylmethanesulphonylfluoride (PMSF, Sigma) containing 0.1mg/ml lysozyme (Sigma), and lysed by sonication. The supernatant containing Gag(p17)-E2 protein was recovered by centrifugation (10000 × g for 1h). The proteins that were precipitated between 35% and 40% saturation of ammonium sulphate were redissolved in buffer A, dialysed against two changes of the same buffer and applied on a Pharmacia Mono S HR10/10 HP cation exchange column previously equilibrated with buffer (20mM Tris-HCl pH 8.5, 10mM EDTA, 1mM PMSF). The Gag(p17)-E2 protein was eluted with a linear gradient of 0-1 M NaCl in buffer A at a flow rate of 1ml/min over 200ml. The Gag(p17)-OVA257-264-E2 cell pellet was resuspended in 20 mM potassium phosphate pH 7.4, 1mM PMSF (buffer B) containing 0.1mg/ml lysozyme and lysed by sonication. The supernatant containing Gag(p17)-OVA257-264-E2 protein recovered by centrifugation (10000 x g for 1h) was applied on a Biorad UNOS12 cation exchange column previously equilibrated with buffer B. The Gag(p17)-OVA257-264-E2 protein was eluted with a linear gradient of 0-1 M NaCl in buffer B at a flow rate of 1 ml/min over 200 ml. The fractions containing the E2 proteins were concentrated using a Centriprep30 (Millipore, MA) and loaded into a Pharmacia Superose-6 gel filtration column previously equilibrated with 50 mM potassium phosphate. The concentration of all the E2 constructs was determined by Coomassie dye binding method (Bradford assay). OVA257-264-E2, OVA323-339-E2 and pep23E2 complexes were purified using the same condition of E2 wild type (E2 wt) complex purification that was previously described (De Berardinis et al., 2003; Domingo et al., 2003; Domingo, Orru, and Perham, 2001). The hybrid assembly, pep23E2/Gag(p17)-E2, displaying both Gag(p17) and pep23, was generated by in vitro denaturation and refolding of mixtures of E2pep23 and Gag(p17)-E2 according to previously described protocol (Domingo et al., 2003). Briefly, equimolar quantities of purified pep23E2 and Gag(p17)-E2 were mixed and dialyzed against buffer B containing 5M GuHCl and 1mM DTT, followed by dialysis against buffer B without 5M GuHCl. Reconstitution of soluble E2 60-mer core structures in high yield (>90%) was confirmed by gel filtration and electron microscopy as previously described (Domingo et al., 2003). The purified proteins samples were stored at −80°C.
Mice
Female C57BL/6xBALB/c F1 hybrid mice (Jackson Laboratory, Bar Harbor, ME, USA) were housed at the University of Washington, USA. HHD transgenic mice (Pascolo et al., 1997) expressing a chimeric HLA-A2.1/H-2-Db MHC class I molecule on a C57BL/6 genetic background, were kindly provided by Dr. F. A. Lemonnier (Pasteur Institute, Paris, France). C57BL/6 mice, C57BL/6 OT-II Thy1.1+ or OT-I transgenic mice were purchased from Charles River Laboratory (Lecco, Italy). Mice were maintained at the animal facility of CNR, Naples, Italy. All animals were housed in specific pathogen free condition in accordance with the standards outlined by the National Institutes of Health Guide for the Care and Use of Laboratory animals. All experiments with mice were performed in accordance with European Union Laws and guidelines, and were approved by our institutional review board. The animal procedures (i.e. immunization, sacrifice) were performed according to rules approved by the ethical committee. In all case, 8-12 weeks old age-matched male mice were used for comparative studies.
Peptides
Overlapping 15-mer peptides encompassing the HIV Gag(p17) sequence (HIV-1 Con B Gag(p17) 15-mer) were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS (NIAID, NIH, USA). The OVA257-264 and OVA323-339 peptides from chicken ovalbumin were purchased from Primm srl.
Antibody response and ELISA assay
C57BL/6xBALB/c F1 hybrid mice were immunized subcutaneously (s.c.) with 100μg of one of the following constructs: E2wt, Gag(p17)-E2, pep23E2/Gag(p17)-E2. Proteins were administered alone, or in combination with IFA (Sigma) or CpG-ODN (Cell Sciences, MA). 107 plaque forming units (PFU) of recombinant vaccinia virus expressing HIV Gag (rVV-Gag) in 1x PBS were delivered intraperitoneally. Mice were immunized at week 0 and 4 and bled by retro-orbital bleed two weeks after each immunization and after 30 weeks from the first immunization. Sera were collected from each mouse and analysed by ELISA.
Briefly, 2μg/ml p55 Gag (Chiron, Emeryville, CA) or E2 protein were diluted in 1x carbonate/bicarbonate buffer (Sigma) and coated onto 96-well plates (MaxiSorp™, NUNC, NY) overnight at 4°C. Plates were blocked in Blotto (1x PBS/5% dry milk/1% normal goat sera) for one hour at room temperature then washed five times and incubated for five minutes in Wash Buffer (1x PBS/0.1% Triton X-100). Three-fold serial dilutions of sera were performed with Disruption Buffer (1x PBS/5% FBS/2% BSA/1% Triton X-100) and incubated in plates for one hour at room temperature. After washing, biotin-conjugated goat anti-mouse total IgG (1:1200) was diluted in Disruption Buffer and applied at 100μl/well. After washing, a 1:800 dilution of ExtrAvidin-conjugated horse-radish peroxidase (HRP, Sigma) was added.
For isotype-specific ELISAs, HRP-conjugated goat anti-mouse IgG1 (1:4000, Southern Biotech, Birmingham, AL), IgG2a (1:4000, Southern Biotech) were used, diluted in Disruption Buffer. After a final wash, plates were developed with 100μl/well TMB liquid substrate (Sigma). Reactions were stopped with 1N H2SO4 (Fisher). Plates were read on a Molecular Device luminometer at A450-650 using SoftMax pro (Sunnyvale, CA, USA). The endpoint titer was calculated as the lowest positive value for each sample that was three-times the average background of naïve mouse sera included on each plate.
Adoptive transfer and in vivo proliferation assay
CD4+ or CD8+ OVA-specific T cells were obtained from spleen of OT-II or OT-I mice by negative selection using the CD4+ and CD8+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Enrichment for T cells was confirmed by flow cytometry and was typically 95–99% pure. The cells were labelled with 5μM of Carboxyfluorescein Succinimidyl ester (CFSE, Sigma) in PBS for 10 minutes at 37°C, washed two times with PBS/0.1% BSA and twice with PBS.
3×106 CFSE labeled OT-I CD8+ or OT-II CD4+ T cells were injected i.v. into C57BL/6 recipients. 24 hours later, mice were immunized subcutaneously (s.c.) with 100μg of each of the following constructs: OVA257-264-E2, OVA323-339-E2, E2wt or PBS. 5 C57BL/6 mice received 500μg soluble ovalbumin protein plus 50 μg of poly(I:C) in PBS intra peritoneum. After 3 days, mice were sacrificed and cell suspensions of spleen were isolated and stained with PE conjugated anti-Vα2 and PE-Cy5-conjugated anti-CD8 or PE-Cy7-conjugated anti CD4 mAbs (BD Pharmingen, Milan, Italy). The CFSE fluorescence intensity was then evaluated by multicolor flow cytometry analysis using a FACS Canto cytometer (Becton Dickinson, CA, USA).
Intracellular staining
The analysis was performed on 3×106 splenocytes coltured with 10μg/ml of synthetic peptides (OVA257-264 or OVA323-339) for 5 hours in presence of brefeldin A (Sigma) (10mg/ml) in a U-bottom 96 well plate. Cells were cultured in absence of peptide (negative control) or with 30ng/ml of Phorbol 12-myristate 13-acetate (PMA), 1μg/ml of ionomycin (Sigma) as positive control. The cells were washed and stained with PE-Cy5 anti-CD8 or PE-Cy7 anti-CD4 mAb on ice for 30 minutes The cells were treated with Leucoperm™ kit (AbD Serotec, Kidlington, UK) at room temperature for 30 minutes, stained with PE conjugated anti-INFγ mAb (eBioscience, Hatfield, UK) according to manufacture instructions and analyzed by a FACS Canto cytometer (Becton Dickinson).
Cytotoxicity tests
Cytotoxic activity was assayed in HHD transgenic mice or C57BL/6 mice.
HHD transgenic mice were immunized at days 0 and 14 by injecting s.c. at the base of the tail 140μg of double displaying pep23E2/Gag(p17)-E2 particles in the absence of adjuvants. A control group of mice received the same amount of E2 wild type particles. After 7 days mice were sacrificed and isolated splenocytes (5×106) were co-cultured with antigen-pulsed γ-irradiated (10,000 rad) lipopolysaccaride-blasts (LPS-blasts) (2.5×106) produced from syngeneic unimmunized animals. LPS-blast cells consisted of splenocytes that have been cultured in 25μg/ml of LPS in RPMI-1640, supplemented with 10% FCS, 5×10−5 M 2-ME, 1mM glutamine, 1mM sodium pyruvate, and 7μg/ml dextran sulphate for 3 days and pulsed for 3 hours with 50μg/ml of the same E2 preparations used for the immunization procedure. After 5 days of co-culture, effector cells were harvested and assayed for cytotoxic activity by the standard 51Cr release assay, using as target RMA-S HHD cells prepulsed or not with 10μg/ml synthetic Gag peptide SLYNTVATL. The percentage of specific lysis was calculated as described (Domingo et al., 2003).
C57BL/6 mice were immunized twice at day 0 and 14 with 100μg of Gag(p17)-OVA257-264-E 2 or OVA257-264-E2 particles both displaying 5μg of OVA257-264. A control group of mice received two administrations of E2 particles in the same conditions as above. All mice were sacrificed and analysed on day 28. Splenocytes (4×106 cells/well) were stimulated in 24 well plates with LPS-blasts (2×106) from syngeneic unimmunized mice, which were prepulsed with OVA257-264 (10μg/ml) and γ-irradiated, in presence of interleukin 2 (IL-2) at 20U/ml. As positive control, spleen cells from each mouse were stimulated in parallel cultures with irradiated BALB/c female spleen cells bearing the H-2d alloantigen. After 7 days, the specific CTL activity of effector cell cultures was determined using the JAM assay (Matzinger, 1991) with EL4 cells (H-2b) prepulsed or not with 10μg/ml of OVA257-264 peptide as target cells. The percentage of specific lysis was calculated as described (Matzinger, 1991).
Pentamer staining
Splenocytes were isolated from C57BL/6 mice 14 days after the second immunization with Gag(p17)-OVA257-264-E2, OVA257-264-E2 or E2 particles and restimulated in vitro with syngeneic LPS-induced blast cells pulsed with specific OVA257-264 peptide. After 7 days, the effector cells (1×106 cells/100μl) were incubated with mouse anti-Fc receptor mAb (2.4G2 mAb, BD Pharmingen) in ice for 15 min to block Fc receptors and stained with PE-labeled SIINFEKL Kb pentamer or PE-labeled SSYSYSSL Kb pentamer (ProImmune, Oxford, UK) in ice for 45 minutes. FITC-conjugated anti-CD8 mAb (ProImmune) was added for additional 15 minutes. The cells were washed, fixed using PBS solution with 30% methanol and 0.4% parafolmaldehyde. A minimum of 50,000 live, CD8-positive, gated events were acquired and analysed by flow cytometry on a FACS Canto (Becton Dickinson).
Statistical Analysis
Statistical analyses were performed using the unpaired, two-tailed Student’s t-test. Differences were considered statistically significant when P < 0.05.
Acknowledgments
We thank Pasquale Barba for technical assistance. This work was supported by grants from the US PHS: NIH R21 AI062418 (N.D.R. and N.L.H.) and NIH R01 AI074379 (N.L.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen MD, Perham RN. The catalytic domain of dihydrolipoyl acetyltransferase from the pyruvate dehydrogenase multienzyme complex of Bacillus stearothermophilus. Expression, purification and reversible denaturation. FEBS Lett. 1997;413(2):339–43. doi: 10.1016/s0014-5793(97)00932-0. [DOI] [PubMed] [Google Scholar]
- Arnold GF, Resnick DA, Li Y, Zhang A, Smith AD, Geisler SC, Jacobo-Molina A, Lee W, Webster RG, Arnold E. Design and construction of rhinovirus chimeras incorporating immunogens from polio, influenza, and human immunodeficiency viruses. Virology. 1994;198(2):703–8. doi: 10.1006/viro.1994.1082. [DOI] [PubMed] [Google Scholar]
- Arthur LO, Bess JW, Jr, Chertova EN, Rossio JL, Esser MT, Benveniste RE, Henderson LE, Lifson JD. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res Hum Retroviruses. 1998;14(Suppl 3):S311–9. [PubMed] [Google Scholar]
- Arthur LO, Bess JW, Jr, Urban RG, Strominger JL, Morton WR, Mann DL, Henderson LE, Benveniste RE. Macaques immunized with HLA-DR are protected from challenge with simian immunodeficiency virus. J Virol. 1995;69(5):3117–24. doi: 10.1128/jvi.69.5.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6(2):200–6. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- Barnett SW, Rajasekar S, Legg H, Doe B, Fuller DH, Haynes JR, Walker CM, Steimer KS. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997;15(8):869–73. doi: 10.1016/s0264-410x(96)00264-2. [DOI] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau M, Lim S, Chen HC, Ruiz C, Wang SW. Thermostability and molecular encapsulation within an engineered caged protein scaffold. Biotechnol Bioeng. 2008;101(4):654–64. doi: 10.1002/bit.21988. [DOI] [PubMed] [Google Scholar]
- Dalmau M, Lim S, Wang SW. Design of a pH-dependent molecular switch in a caged protein platform. Nano Lett. 2009a;9(1):160–6. doi: 10.1021/nl8027069. [DOI] [PubMed] [Google Scholar]
- Dalmau M, Lim S, Wang SW. pH-triggered disassembly in a caged protein complex. Biomacromolecules. 2009b;10(12):3199–206. doi: 10.1021/bm900674v. [DOI] [PubMed] [Google Scholar]
- De Berardinis P, Haigwood NL. New recombinant vaccines based on the use of prokaryotic antigen-display systems. Expert Rev Vaccines. 2004;3(6):673–9. doi: 10.1586/14760584.3.6.673. [DOI] [PubMed] [Google Scholar]
- De Berardinis P, Sartorius R, Caivano A, Mascolo D, Domingo GJ, Del Pozzo G, Gaubin M, Perham RN, Piatier-Tonneau D, Guardiola J. Use of fusion proteins and procaryotic display systems for delivery of HIV-1 antigens: development of novel vaccines for HIV-1 infection. Curr HIV Res. 2003;1(4):441–6. doi: 10.2174/1570162033485168. [DOI] [PubMed] [Google Scholar]
- Del Pozzo G, Mascolo D, Sartorius R, Citro A, Barba P, D’Apice L, De Berardinis P. Triggering DTH and CTL activity by fd filamentous bacteriophages: role of CD4+ T cells in memory responses. J Biomed Biotechnol. 2010:894971. doi: 10.1155/2010/894971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deml L, Speth C, Dierich MP, Wolf H, Wagner R. Recombinant HIV-1 Pr55gag virus-like particles: potent stimulators of innate and acquired immune responses. Mol Immunol. 2005;42(2):259–77. doi: 10.1016/j.molimm.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Ding J, Smith AD, Geisler SC, Ma X, Arnold GF, Arnold E. Crystal structure of a human rhinovirus that displays part of the HIV-1 V3 loop and induces neutralizing antibodies against HIV-1. Structure. 2002;10(7):999–1011. doi: 10.1016/s0969-2126(02)00793-1. [DOI] [PubMed] [Google Scholar]
- Domingo GJ, Caivano A, Sartorius R, Barba P, Backstrom M, Piatier-Tonneau D, Guardiola J, De Berardinis P, Perham RN. Induction of specific T-helper and cytolytic responses to epitopes displayed on a virus-like protein scaffold derived from the pyruvate dehydrogenase multienzyme complex. Vaccine. 2003;21(13–14):1502–9. doi: 10.1016/s0264-410x(02)00664-3. [DOI] [PubMed] [Google Scholar]
- Domingo GJ, Orru S, Perham RN. Multiple display of peptides and proteins on a macromolecular scaffold derived from a multienzyme complex. J Mol Biol. 2001;305(2):259–67. doi: 10.1006/jmbi.2000.4311. [DOI] [PubMed] [Google Scholar]
- Doria-Rose NA, Ohlen C, Polacino P, Pierce CC, Hensel MT, Kuller L, Mulvania T, Anderson D, Greenberg PD, Hu SL, Haigwood NL. Multigene DNA Priming-Boosting Vaccines Protect Macaques from Acute CD4(+)-T-Cell Depletion after Simian-Human Immunodeficiency Virus SHIV89.6P Mucosal Challenge. J Virol. 2003;77(21):11563–77. doi: 10.1128/JVI.77.21.11563-11577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Johnston MI, Dieffenbach CW, Burton DR, Hammer SM, Hoxie JA, Martin M, Overbaugh J, Watkins DI, Mahmoud A, Greene WC. HIV vaccine research: the way forward. Science. 2008;321(5888):530–2. doi: 10.1126/science.1161000. [DOI] [PubMed] [Google Scholar]
- Haffar O, Garrigues J, Travis B, Moran P, Zarling J, Hu SL. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J Virol. 1990;64(6):2653–9. doi: 10.1128/jvi.64.6.2653-2659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar OK, Moran PA, Smithgall MD, Diegel ML, Sridhar P, Ledbetter JA, Zarling JM, Hu SL. Inhibition of virus production in peripheral blood mononuclear cells from human immunodeficiency virus (HIV) type 1-seropositive donors by treatment with recombinant HIV-like particles. J Virol. 1992;66(7):4279–87. doi: 10.1128/jvi.66.7.4279-4287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar OK, Smithgall MD, Moran PA, Travis BM, Zarling JM, Hu SL. HIV-specific humoral and cellular immunity in rabbits vaccinated with recombinant human immunodeficiency virus-like gag-env particles. Virology. 1991;183(2):487–95. doi: 10.1016/0042-6822(91)90978-k. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15(3):293–9. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CE, Perham RN, Finch JT. Structure and symmetry of B. stearothermophilus pyruvate dehydrogenase multienzyme complex and implications for eucaryote evolution. Cell. 1979;17(1):85–93. doi: 10.1016/0092-8674(79)90297-6. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5(5):e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 84(3):1302–13. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Montefiori DC, Goldstein S, Hamm TE, Zhou J, Kitov S, Haigwood NL, Misher L, London WT, Gerin JL, et al. Inactivated whole-virus vaccine derived from a proviral DNA clone of simian immunodeficiency virus induces high levels of neutralizing antibodies and confers protection against heterologous challenge. Proc Natl Acad Sci U S A. 1992;89(6):2175–9. doi: 10.1073/pnas.89.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibuuka H, Kimutai R, Maboko L, Sawe F, Schunk MS, Kroidl A, Shaffer D, Eller LA, Kibaya R, Eller MA, Schindler KB, Schuetz A, Millard M, Kroll J, Dally L, Hoelscher M, Bailer R, Cox JH, Marovich M, Birx DL, Graham BS, Michael NL, de Souza MS, Robb ML. A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-Uninfected East Africans (RV 172) J Infect Dis. 2010;201(4):600–7. doi: 10.1086/650299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup RA, Roederer M, Lamoreaux L, Fischer J, Novik L, Nason MC, Larkin BD, Enama ME, Ledgerwood JE, Bailer RT, Mascola JR, Nabel GJ, Graham BS. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS One. 2010;5(2):e9015. doi: 10.1371/journal.pone.0009015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapelosa M, Arnold GF, Gallicchio E, Arnold E, Levy RM. Antigenic characteristics of rhinovirus chimeras designed in silico for en5hanced presentation of HIV-1 gp41 epitopes. J Mol Biol. 2010;397(3):752–66. doi: 10.1016/j.jmb.2010.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M, Cardoso RM, Wilson IA, Burton DR. Antigenic and immunogenic study of membrane-proximal external region-grafted gp120 antigens by a DNA prime-protein boost immunization strategy. J Virol. 2007;81(8):4272–85. doi: 10.1128/JVI.02536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard IA, Domingo GJ, Borges A, Perham RN. Expression of genes encoding the E2 and E3 components of the Bacillus stearothermophilus pyruvate dehydrogenase complex and the stoichiometry of subunit interaction in assembly in vitro. Eur J Biochem. 1998;258(2):491–501. doi: 10.1046/j.1432-1327.1998.2580491.x. [DOI] [PubMed] [Google Scholar]
- Lifson JD, Rossio JL, Piatak M, Jr, Bess J, Jr, Chertova E, Schneider DK, Coalter VJ, Poore B, Kiser RF, Imming RJ, Scarzello AJ, Henderson LE, Alvord WG, Hirsch VM, Benveniste RE, Arthur LO. Evaluation of the safety, immunogenicity, and protective efficacy of whole inactivated simian immunodeficiency virus (SIV) vaccines with conformationally and functionally intact envelope glycoproteins. AIDS Res Hum Retroviruses. 2004;20(7):772–87. doi: 10.1089/0889222041524661. [DOI] [PubMed] [Google Scholar]
- Lifson JD, Rossio JL, Piatak M, Jr, Parks T, Li L, Kiser R, Coalter V, Fisher B, Flynn BM, Czajak S, Hirsch VM, Reimann KA, Schmitz JE, Ghrayeb J, Bischofberger N, Nowak MA, Desrosiers RC, Wodarz D. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001;75(21):10187–99. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Li Y, Yong Kang C. Budding and secretion of HIV Gag-Env virus-like particles from recombinant human adenovirus infected cells. Virus Res. 2003;92(1):75–82. doi: 10.1016/s0168-1702(02)00316-7. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6(2):207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- Mascolo D, Barba P, De Berardinis P, Di Rosa F, Del Pozzo G. Phage display of a CTL epitope elicits a long-term in vivo cytotoxic response. FEMS Immunol Med Microbiol. 2007;50 (1):59–66. doi: 10.1111/j.1574-695X.2007.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145(1–2):185–92. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M, Martin L, Davison-Fairburn B, Montelaro RC, Miller M, West M, Ohkawa S, Baskin GTB, Zhang J-Y, Putney SD, Allison AC, Eppstein DA. A formalin inactivated whole SIV vaccine and a glycoprotein-enriched subunit vaccine confer protection against experimental challenge with pathogenic live SIV in rhesus monkeys. Develop Biol Standard. 1990;72:273–285. [PubMed] [Google Scholar]
- O’Brien KL, Liu J, King SL, Sun YH, Schmitz JE, Lifton MA, Hutnick NA, Betts MR, Dubey SA, Goudsmit J, Shiver JW, Robertson MN, Casimiro DR, Barouch DH. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat Med. 2009;15(8):873–5. doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X, Liu Y, Wagner R, Wolf H, Baenziger J, Walker CM. Priming of strong, broad, and long-lived HIV type 1 p55gag-specific CD8+ cytotoxic T cells after administration of a virus-like particle vaccine in rhesus macaques. AIDS Res Hum Retroviruses. 2000;16(3):273–82. doi: 10.1089/088922200309368. [DOI] [PubMed] [Google Scholar]
- Parish CR, GM, Quah BJ, Warren HS. Use of the intracellular fluorescent dye CSFE to monitor lymphocyte migration and proliferation. Curr Protoc Immunol. 2009;Chapter4(Unit4):9. doi: 10.1002/0471142735.im0409s84. [DOI] [PubMed] [Google Scholar]
- Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med. 1997;185(12):2043–51. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perham RN. Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions. Annu Rev Biochem. 2000;69:961–1004. doi: 10.1146/annurev.biochem.69.1.961. [DOI] [PubMed] [Google Scholar]
- Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, Lifton MA, Lemckert AA, Holterman L, Chen B, Dilraj A, Carville A, Mansfield KG, Goudsmit J, Barouch DH. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441(7090):239–43. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- Salk J. Prospects for the control of AIDS by immunizing seropositive individuals. Nature. 1987;327(6122):473–6. doi: 10.1038/327473a0. [DOI] [PubMed] [Google Scholar]
- Santra S, Seaman MS, Xu L, Barouch DH, Lord CI, Lifton MA, Gorgone DA, Beaudry KR, Svehla K, Welcher B, Chakrabarti BK, Huang Y, Yang ZY, Mascola JR, Nabel GJ, Letvin NL. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J Virol. 2005;79(10):6516–22. doi: 10.1128/JVI.79.10.6516-6522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Johnson RP, McClure HM, Manson KH, Wyand MS, Kuroda MJ, Lifton MA, Khunkhun RS, McEvers KJ, Gillis J, Piatak M, Lifson JD, Grosschupff G, Racz P, Tenner-Racz K, Rieber EP, Kuus-Reichel K, Gelman RS, Letvin NL, Montefiori DC, Ruprecht RM, Desrosiers RC, Reimann KA. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated rhesus macaques. J Virol. 2005;79(13):8131–41. doi: 10.1128/JVI.79.13.8131-8141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5(2):204–10. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- Stott EJ. Anti-cell antibody in macaques. Nature. 1991;353(6343):393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- Tsunetsugu-Yokota Y, Morikawa Y, Isogai M, Kawana-Tachikawa A, Odawara T, Nakamura T, Grassi F, Autran B, Iwamoto A. Yeast-derived human immunodeficiency virus type 1 p55(gag) virus-like particles activate dendritic cells (DCs) and induce perforin expression in Gag-specific CD8(+) T cells by cross-presentation of DCs. J Virol. 2003;77(19):10250–9. doi: 10.1128/JVI.77.19.10250-10259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]