Abstract
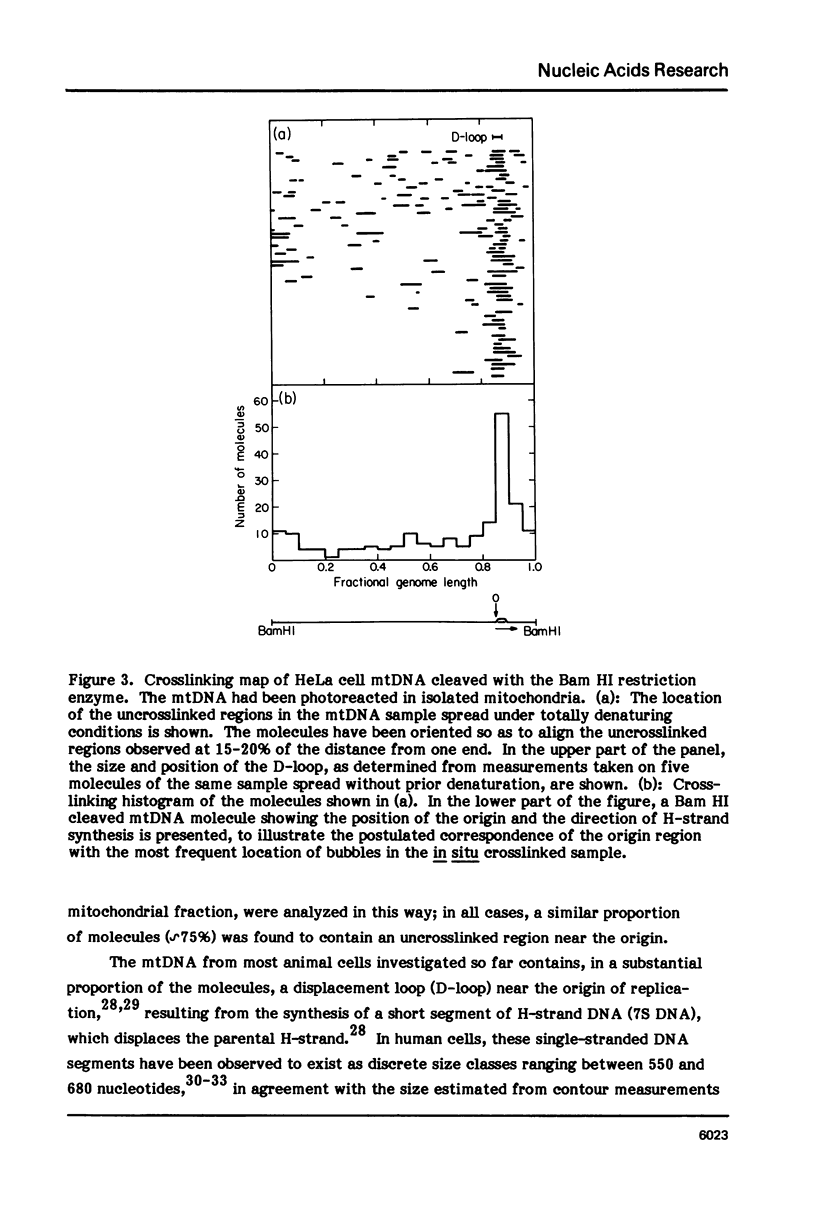
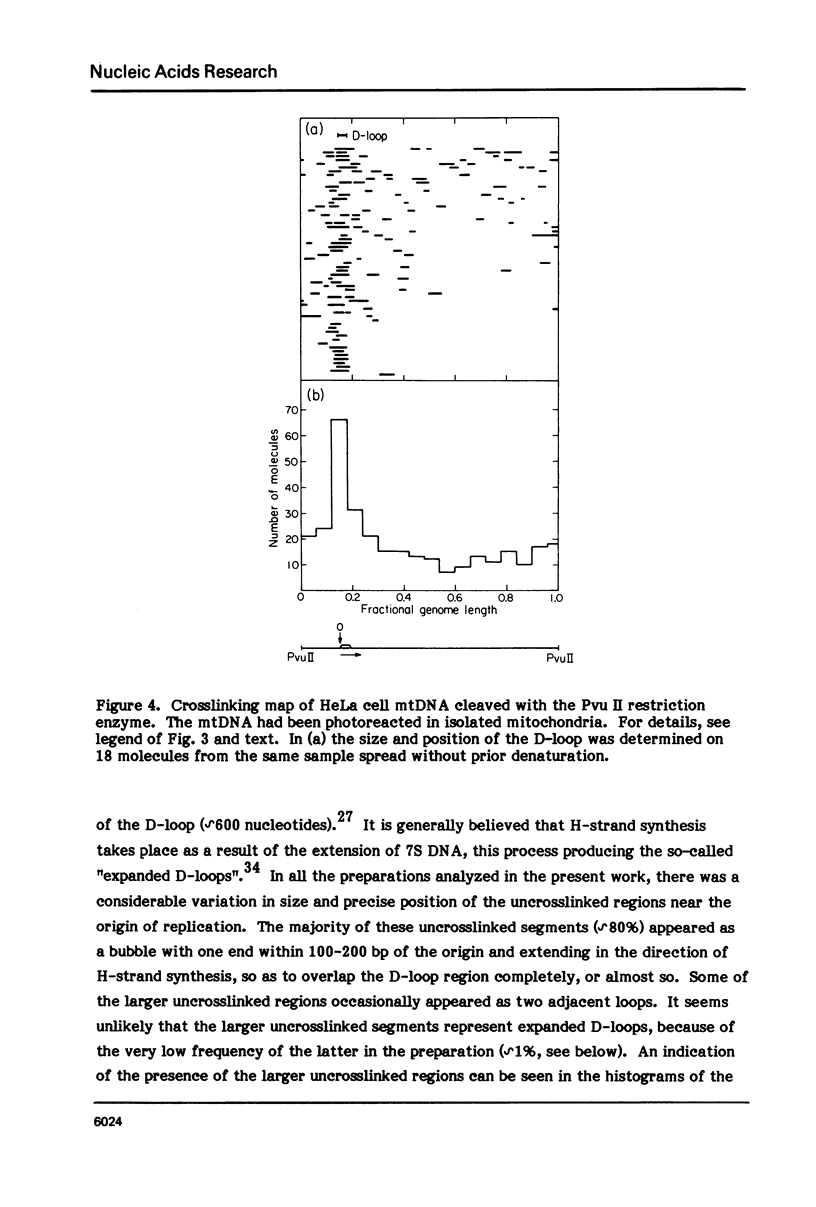
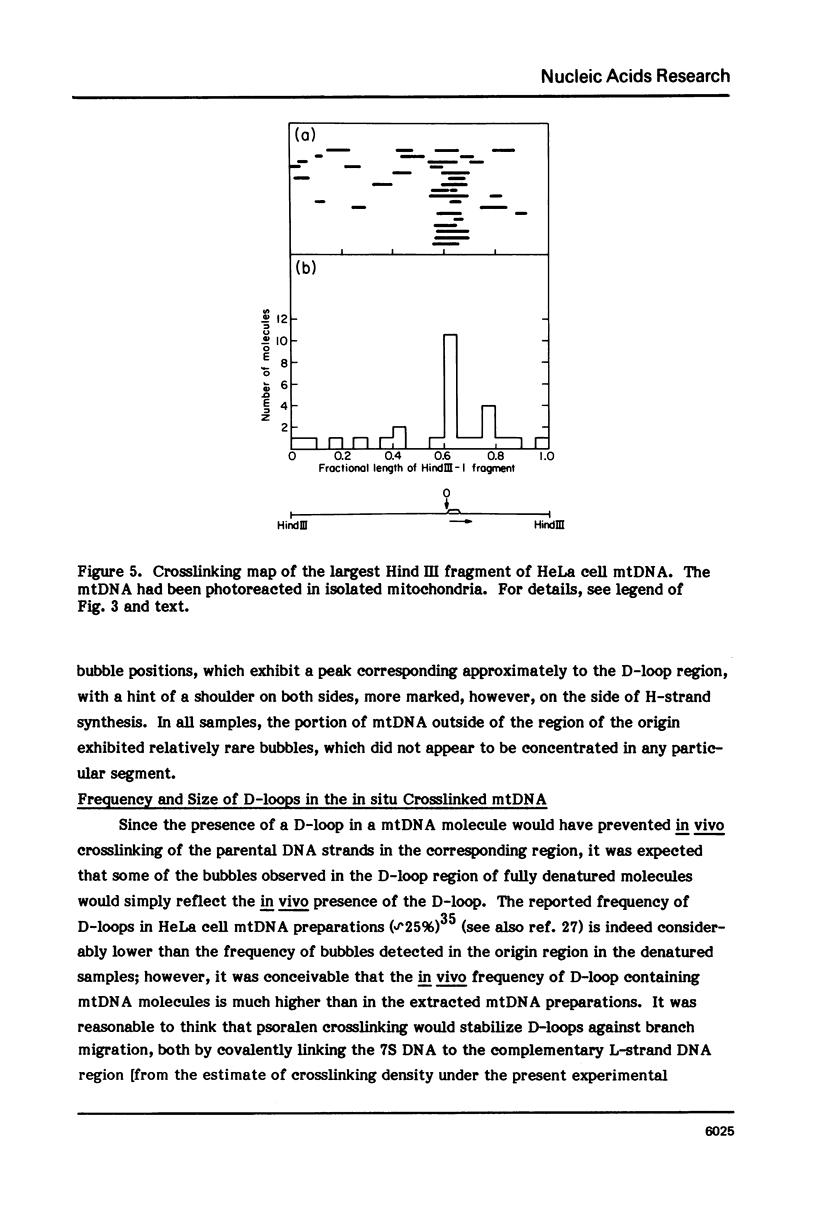
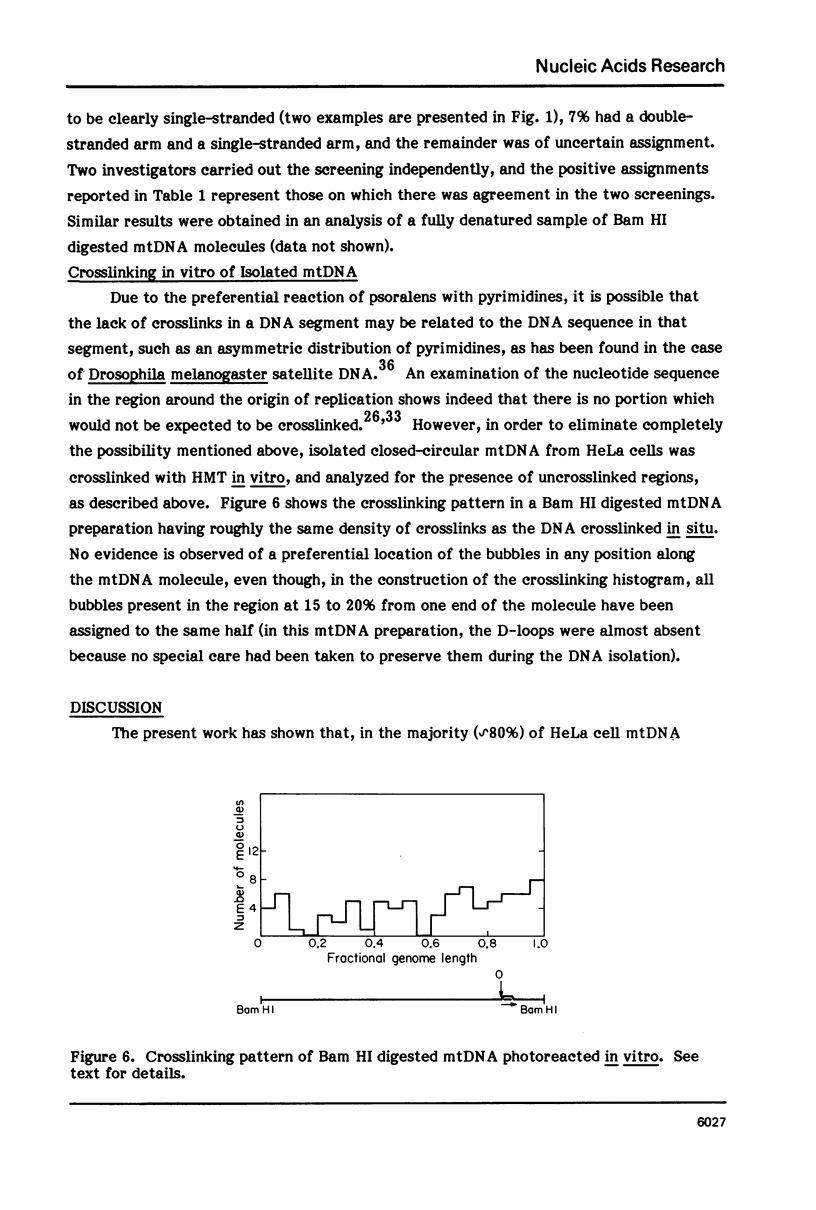
The in vivo association with proteins of HeLa cell mitochondrial DNA (mtDNA) has been investigated by analyzing the pattern of in situ crosslinking of the DNA by 4'-hydroxymethyl-4, 5',8-trimethylpsoralen (HMT). Either isolated mitochondria or whole cells were irradiated with long wavelength UV light in the presence of ths psoralen derivative, and the mtDNA was then isolated and analyzed in the electron microscope under totally denaturing conditions. No evidence of nucleosomal structure was found. The great majority of the molecules (approximately 90%) had a double-stranded DNA appearance over most of their contour length, with one to several bubbles occupying the rest of the contour, while the remaining 10% of the molecules appeared to be double-stranded over their entire length. Analysis of restriction fragments indicated the presence, in approximately 80% of the molecules, of a protected segment (300 to 1500 bp long) in a region which was centered asymmetrically around the origin of replication so as to overlap extensively the D-loop. Control experiments showed that at most 30% of the bubbles found near the origin could represent D-loops or expanded D-loops: furthermore, it could be excluded that some sequence peculiarity would account for the preferential location of bubbles near the origin of replication. The data have been interpreted to indicate that, in at least 55% of HeLa cell mtDNA molecules, the region around the origin is protected from in situ psoralen crosslinking by proteins or protein complexes which are associated in vivo with the DNA.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albring M., Griffith J., Attardi G. Association of a protein structure of probable membrane derivation with HeLa cell mitochondrial DNA near its origin of replication. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1348–1352. doi: 10.1073/pnas.74.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaldi F., Attardi G. Partial sequence analysis of ribosomal RNA from HeLa cells. I. Oligonucleotide pattern of 28 s and 18 s RNA after pancreatic ribonuclease digestion. J Mol Biol. 1968 May 14;33(3):737–755. doi: 10.1016/0022-2836(68)90317-3. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Attardi B., Cravioto B., Attardi G. Membrane-bound ribosomes in HeLa cells. I. Their proportion to total cell ribosomes and their association with messenger RNA. J Mol Biol. 1969 Aug 28;44(1):47–70. doi: 10.1016/0022-2836(69)90404-5. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Broude N. E., Budowsky E. I. The reaction of glyoxal with nucleic acid components. 3. Kinetics of the reaction with monomers. Biochim Biophys Acta. 1971 Dec 30;254(3):380–388. doi: 10.1016/0005-2787(71)90868-9. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Shine J., Goodman H. M. Human mitochondrial DNA: analysis of 7S DNA from the origin of replication. Proc Natl Acad Sci U S A. 1978 Feb;75(2):735–739. doi: 10.1073/pnas.75.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Vinograd J. Restriction endonuclease cleavage maps of animal mitochondrial DNAs. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4617–4621. doi: 10.1073/pnas.71.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron F., Jacq C., Rouvière-Yaniv J. Characterization of a histone-like protein extracted from yeast mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4265–4269. doi: 10.1073/pnas.76.9.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré D., Attardi G. Biochemical and electron microscopic characterization of DNA-RNA complexes from HeLa cell mitochondria. Biochemistry. 1978 Aug 8;17(16):3263–3273. doi: 10.1021/bi00609a014. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Karrer K. M. Chromatin structure of the ribosomal RNA genes of Tetrahymena thermophila as analyzed by trimethylpsoralen crosslinking in vivo. J Mol Biol. 1980 Feb 5;136(4):395–416. doi: 10.1016/0022-2836(80)90397-6. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Pardue M. L. Electron microscopy of DNA crosslinked with trimethylpsoralen: test of the secondary structure of eukaryotic inverted repeat sequences. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2644–2648. doi: 10.1073/pnas.73.8.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T., Potter D., Pardue M. L. Electron microscopy of DNA cross-linked with trimethylpsoralen: a probe for chromatin structure. Biochemistry. 1977 Nov 29;16(24):5313–5321. doi: 10.1021/bi00643a024. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Crews S., Ojala D., Posakony J., Nishiguchi J., Attardi G. Nucleotide sequence of a region of human mitochondrial DNA containing the precisely identified origin of replication. Nature. 1979 Jan 18;277(5693):192–198. doi: 10.1038/277192a0. [DOI] [PubMed] [Google Scholar]
- Fukuhara H. Relative proportions of mitochondrial and nuclear DNA in yeast under various conditions of growth. Eur J Biochem. 1969 Nov;11(1):135–139. doi: 10.1111/j.1432-1033.1969.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Clayton D. A. Displacement-loop replication initiation sequence in animal mitochondrial DNA exists as a family of discrete lengths. Proc Natl Acad Sci U S A. 1978 Feb;75(2):677–681. doi: 10.1073/pnas.75.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa T., Kawano S., Hizume M. A method of isolation of mitochondrial nucleoid of Physarum polycephalum and evidence for the presence of a basic protein. Exp Cell Res. 1976 Feb;97(2):435–440. doi: 10.1016/0014-4827(76)90638-8. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA. I. Intramitochondrial distribution and structural relations of single- and double-length circular DNA. J Mol Biol. 1969 Jun 28;42(3):521–528. doi: 10.1016/0022-2836(69)90240-x. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. A detailed physical map of HeLa cell mitochondria DNA and its alignment with the positions of known genetic markers. Plasmid. 1977 Nov;1(1):78–105. doi: 10.1016/0147-619x(77)90010-5. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Precise localization of the origin of replication in a physical map of HeLa cell mitochondrial DNA and isolation of a small fragment that contains it. J Mol Biol. 1978 Jul 5;122(3):301–319. doi: 10.1016/0022-2836(78)90192-4. [DOI] [PubMed] [Google Scholar]
- Posakony J. W., England J. M., Attardi G. Mitochondrial growth and division during the cell cycle in HeLa cells. J Cell Biol. 1977 Aug;74(2):468–491. doi: 10.1083/jcb.74.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter D. A., Fostel J. M., Berninger M., Pardue M. L., Cech T. R. DNA-protein interactions in the Drosophila melanogaster mitochondrial genome as deduced from trimethylpsoralen crosslinking patterns. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4118–4122. doi: 10.1073/pnas.77.7.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickwood D., Jurd R. D. A general method for the isolation and partial characterization of mitochondrial nucleoids by centrifugation in metrizamide gradients [proceedings]. Biochem Soc Trans. 1978;6(1):266–268. doi: 10.1042/bst0060266a. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Replication of mitochondrial DNA in mouse L cells and their thymidine kinase - derivatives: displacement replication on a covalently-closed circular template. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3810–3814. doi: 10.1073/pnas.69.12.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevaljević L., Petrović L. S., Rickwood D. Isolation and partial characterization of a mitochondrial deoxyribonucleic acid-protein complex from sea urchin embryos. Mol Cell Biochem. 1978 Nov 16;21(3):139–143. doi: 10.1007/BF00240132. [DOI] [PubMed] [Google Scholar]
- Shen C. J., Hearst J. E. Detection of long-range sequence order in Drosophila melanogaster satellite DNA IV by a photochemical crosslinking reaction and denaturation microscopy. J Mol Biol. 1977 May 25;112(3):495–507. doi: 10.1016/s0022-2836(77)80195-2. [DOI] [PubMed] [Google Scholar]
- Storrie B., Attardi G. Expression of the mitochondrial genome in HeLa cells. 13. Effect of selective inhibition of cytoplasmic or mitochondrial protein synthesis on mitochondrial nucleic acid synthesis. J Mol Biol. 1972 Nov 14;71(2):177–199. doi: 10.1016/0022-2836(72)90345-2. [DOI] [PubMed] [Google Scholar]
- Van Tuyle G. C., McPherson M. L. A compact form of rat liver mitochondrial DNA stabilized by bound proteins. J Biol Chem. 1979 Jul 10;254(13):6044–6053. [PubMed] [Google Scholar]
- Wieshahn G. P., Hyde J. E., Hearst J. E. The photoaddition of trimethylpsoralen to Drosophila melanogaster nuclei: a probe for chromatin substructure. Biochemistry. 1977 Mar 8;16(5):925–932. doi: 10.1021/bi00624a018. [DOI] [PubMed] [Google Scholar]
- Wiseman A., Attardi G. Reversible tenfod reduction in mitochondria DNA content of human cells treated with ethidium bromide. Mol Gen Genet. 1978 Nov 16;167(1):51–63. doi: 10.1007/BF00270321. [DOI] [PubMed] [Google Scholar]




