Abstract
Apoptosis is an important morphogenetic event in embryogenesis as well as during postnatal life. In the last 2 decades, apoptosis in tooth development (odontogenesis) has been investigated with gradually increasing focus on the mechanisms and signaling pathways involved. The molecular machinery responsible for apoptosis exhibits a high degree of conservation but also organ and tissue specific patterns. This review aims to discuss recent knowledge about apoptotic signaling networks during odontogenesis, concentrating on the mouse, which is often used as a model organism for human dentistry. Apoptosis accompanies the entire development of the tooth and corresponding remodeling of the surrounding bony tissue. It is most evident in its role in the elimination of signaling centers within developing teeth, removal of vestigal tooth germs, and in odontoblast and ameloblast organization during tooth mineralization. Dental apoptosis is caspase dependent and proceeds via mitochondrial mediated cell death with possible amplification by Fas-FasL signaling modulated by Bcl-2 family members.
Introduction
The phenomenon of apoptosis was defined almost 40 years ago (Kerr et al., 1972) and the process is well known as a sculptor of development (Doseff, 2004). Dysfunction in the regulation or execution of apoptotic cell death is implicated in a wide spectrum of abnormalities and diseases (Meier et al., 2000). Teeth are a great example of a shaped organ, and apoptosis has been reported at many stages of tooth development (odontogenesis) (Matalova et al., 2004). However, detailed investigations and functional experiments revealing the role of apoptotic signaling, and the molecular networks involved in dental apoptosis, have only been performed in the last decade. The mouse is a common model for cell death studies (Ranger et al., 2001) and also in odontogenesis research (Fleischmannova et al., 2008), and therefore we have concentrated on knowledge from the mouse.
The characteristic morphological features of apoptosis include blebbing, chromatin condensation, nuclear fragmentation, loss of adhesion, and cell shrinkage. These observable changes are accompanied by internucleosomal cleavage of DNA (Cohen et al., 1994); phosphatidylserine externalization (Martin et al., 1995); and proteolytic cleavage of a number of intracellular substrates (Martin and Green, 1995). The entire process of apoptosis takes from 30 min (Bronckers et al., 2000; Savill et al., 1993) to 1 h (Satchell et al., 2003). The consequent clearance of apoptotic cell and bodies is essential for tissue homeostasis (Savill and Fadok, 2000).
Caspases are the key components involved in apoptosis. Caspases are cysteine proteases that cleave a set of proteins to initiate and promote apoptotic signaling. At least 15 mammalian caspases have been indentified so far and play roles in apoptosis, inflammation, and also in nonapoptotic pathways (Kuranaga, 2011; Lamkanfi et al., 2007). The best known caspases are the initiators (activators), which includes caspase-2, -8, -9, and −10, and the effectors (executors), to which belong the trio of caspase-3, −6, and −7 (Nicholson, 1999; Shi, 2002). The complex caspase networks allow for compensation between different caspases, which has been reported particularly in vitro (Zheng et al., 2000).
Targeted disruption of caspase genes in mice has revealed different requirements for individual caspases during mammalian development (Zheng et al., 1999). For example, caspase-8−/− embryos die around E11 days with impaired heart development (Varfolomeev et al., 1998), whereas caspase-9−/− mice die before or shortly after birth and exhibit severe brain abnormalities (Kuida et al., 1998). Caspase-3−/− knock-out is usually perinatally lethal (Kuida et al., 1996). Cells contain natural inhibitors of caspases, such as IAPs (inhibitory apoptosis proteins), and XIPA or cytokine response modifier A (CrmA) (Ray et al., 1992; Riedl et al., 2001; Sun et al., 1999; Takahashi et al., 1998). Mammalian IAPs are able to inhibit large groups of ligands and transducers of the tumor necrosis factor (TNF) family of receptors, pro-apoptotic members of the ced-9/Bcl-2 family, cytochrome c, and chemotherapeutic agents (Deveraux and Reed, 1999; LaCasse et al., 1998). Fluoromethylketones (z-VAD-fmk) are widely used as artificial caspase inhibitors (Lavrik et al., 2005). A generalized caspase inhibition by z-VAD-fmk has been shown to disturb early mammalian development (Zakeri et al., 2005).
The caspase cascade can be organized into two distinct pathways: either initiated from outside the cell (extrinsic or death receptor pathway), or from inside the cell (intrinsic or mitochondrial pathway). The mitochondrial pathway includes activation of pro-apoptotic factors, such as Bax, which antagonizes the antiapoptotic effect of other Bcl-2 family members, such as Bcl-2, by the formation of heterodimers (Zimmermann et al., 2001). Translocation of Bax, Bid, or Bad to the mitochondria induces cytochrome c release. Cytochrome c forms a complex with the pro-apoptotic factor Apaf-1 (apoptotic protease activating factor-1) and procaspase-9, resulting in the formation of an apoptosome and leading to activation of caspase-9 in an energy-dependent process (Zou et al., 1997). Caspase-9 further cleaves procaspase-3 and caspase-3 activation is followed by the completion of cell destruction. Pro-apoptotic factor Smac/DIABLO (Du et al., 2000; Verhagen et al., 2000) is released from the mitochondria along with cytochrome c during apoptosis. This protein functions to promote caspase activation by associating with the apoptosome and by inhibiting IAPs. It has been shown that cytochrome c and Smac/DIABLO are released in a coordinated manner (Ekert et al., 2001). Caspase-3 is considered to be the most prevalent, and central caspase and is activated by both the receptor-mediated and mitochondrial pathway (Hengartner, 2000). Death receptor mediated caspase-3 activation occurs after ligand binding and receptor oligomerization causing activation of caspase-12 (or 10) via the corresponding death domain (DD). The best known example is TNF–TNFR coupling, such as Fas (CD95/APO-1)–FasL (CD178). This coupling is common in the immune system but also in kidney, testis, lung, intestine, and eye apoptosis (Watanabe-Fukunaga et al., 1992). Fas-mediated apoptosis can be modulated by cFLIP, which resembles procaspase-8 but without the active proteinase site (Irmler et al., 1997).
To complete the apoptotic process in a physiological way, apoptotic cells/bodies must be removed before breakdown of the membrane and secondary necrosis. This mission is commonly performed by professional phagocytes belonging to the monocyte–macrophage system. In the embryonic tooth germs, however, many stages of apoptosis occur before vascularisation has become established (Lechguer et al., 2008), and so the apoptotic cells must be removed by other means. Clearance of apoptotic cells by neighboring cells within the tissue is likely to occur. Moreover, phagocytosis of apoptotic cells may contribute to the promotion of proliferation within the tissue by supported release of growth and survival factors (Golpon et al., 2004; Kuranaga, 2011).
Apoptosis in Odontogenesis—Where, When, and Why?
Morphogenesis of teeth is based on reciprocal interactions between epithelial cells and cranial neural–crest-derived mesenchyme (Cobourne and Sharpe, 2003; Tucker and Sharpe, 1999). The initiation of the molar tooth germ in the mouse begins at embryonic day (E) 11. In all mammals, tooth development passes through well-defined stages of epithelial thickening: dental lamina, tooth bud, cap and tooth bell, followed by extracellular matrix secretion and mineralization (Caton and Tucker, 2009). Tooth development is mediated by the spatiotemporal expression of tooth-related genes and heterogenous factors employed in cell proliferation, adhesion, migration, cell cycle regulation, and also apoptotic inhibition and stimulation (Tucker and Sharpe, 2004). The importance of factors produced during epithelial–mesenchymal communication was shown in vitro in an elegant experiment performed by Vaahtokari et al. (1996). When E13 dental mesenchyme or epithelium, respectively, are cultured alone, the tissue undergoes severe apoptosis but when the tissues are cultured in combination, apoptosis is prevented in the cells near the interface of the tissues. Importantly, FGF-4-releasing beads rescued the surrounding cells from apoptosis when dental mesenchyme or epithelium was cultured alone (Vaahtokari et al., 1996). Thus, disruption of signaling factors in vivo, impacting on epithelial–mesenchymal interactions, may consequently lead to apoptosis. Apoptosis may therefore play a role in dental diseases and dismorphology as a consequence of defective tissue interactions. Apoptosis is likely to be involved particularly in oligodontia, hypodontia, agenesis, and periodontal disease (reviewed in Fleischmannova et al., 2008). Defects in apoptosis and/or in removal of ectopic tooth germs, may also play a role in determining the number of teeth, leading to the formation of supernumerary tooth formation, or influencing the ultimate size of a tooth. As such, apoptosis seems an attractive candidate factor for macrodontia (Kim et al., 2006).
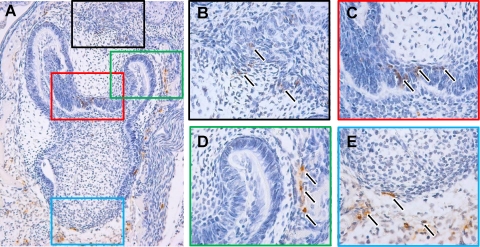
Apoptosis has multiple roles in dental development from the beginning of tooth formation to the completion of root development. Apoptosis has been observed in early morphogenesis, as well as during hard tissue formation and during tooth eruption (Matalova et al., 2004). A comprehensive study of the temporospatial distribution of apoptosis in prenatal mouse molar development was provided by Lesot et al. (1996), followed by a prenatal comparison in the field vole by Setkova et al. (2006). Apoptosis during postnatal stages of tooth–bone interactions in the mouse first molar was recently reported by Lungova et al. (2011) and in the mouse third molar by Chlastakova et al. (2011). The most important functional aspects of dental apoptosis are related to the elimination of the signaling centers of the enamel knots, elimination of vestigal tooth germs, and odontoblasts and ameloblasts structural organization, whereas apoptosis in surrounding tissues is related to tooth growth and eruption (Fig. 1).
FIG. 1.
Distribution of apoptosis in mouse odontogenesis. (A) First molar tooth germ, perinatal stage, TUNEL assay, hematoxylin counterstain, magnification 100×. (B) Detail of the stalk connecting tooth germ with the dental lamina, 400×, arrows point to apoptotic cells. (C) Detail of odontoblast and ameloblast layers, 400×; arrows point to apoptotic cells. (D) Detail of the growing molar cusp with apoptosis in the surrounding bone, 400×; arrows point to apoptotic cells. (E) Detail of the tips of the growing cervical loop enclosing dental mesenchyme and apoptosis in the surrounding tissue, 400×; arrows point to apoptotic cells. am, ameloblasts; ep, dental epithelium; me, dental mesenchyme; od, odontoblasts; ls, lamina stalk.
Signaling centers
In the mouse molars, the primary enamel knot (PEK) is involved in the bud–cap transition (Cho et al., 2007; Jernvall et al., 1998). The secondary enamel knots (SEK) are present at the bell stage and are considered to determine the cusp position and promote their growth by creating folds in the dental epithelium (Jernvall and Thesleff, 2000). The primary and secondary enamel knots express a range of signaling molecules, such as Fgf4 (Jernvall and Thesleff, 2000). The tertiary enamel knots (TEK) appear next to the enamel free areas at the cusp tips, and are thought to play a role in controlling enamel deposition (Luukko et al., 2003). Molar enamel knot cells are the most common populations for studying the molecular mechanisms of apoptosis during tooth development (Matalova et al., 2010).
In the mouse incisors, the localization of enamel knots differs as just a single enamel knot forms. (Kieffer et al., 1996). This observation indirectly supports a role for apoptosis in tooth crown formation.
More than 50 genes, such as Shh, BMP-2, -4, -7, and Fgf-4 have been identified as actively transcribed in the enamel knots (Jernvall et al., 2000). After fulfilling their signaling mission, most of the PEK cells undergo apoptosis, which has been postulated as a mechanism by which this signaling center can be silenced.
Vestigal dental primordial
Apoptosis is also believed to contribute to the elimination of the diastemal dental lamina, together with diastemal and antemolar vestigal tooth buds (Tureckova et al., 1996). These are believed to represent vestiges of ancestral teeth suppressed during evolution (Peterkova et al., 2000). Vestigial structures that appear in the diastema over a particular developmental period disappear during later development and do not give rise to functional teeth (Peterkova et al., 2002; Prochazka et al., 2010). Two prominent rudiments (MS, R2) can be observed in each lower jaw quadrants, of which R2 becomes later incorporated into the first molar (Prochazka et al., 2010). These primordial tooth germs are arrested at the bud stage and undergo apoptosis correlated with the expression of Bmps (Peterkova et al., 1998). However, the arrest of rudimentary buds is prevented in a number of mouse mutants, where apoptosis is inhibited. In these mice, the vestigal buds carry on proliferating and form premolar-like teeth in front of the first molar (Cobourne and Sharpe, 2010). In Sprouty mutants, Fgf signaling is elevated and apoptosis is inhibited in the R2, which continues proliferating to form a functional tooth (Peterkova et al., 2009). A similar reduction in apoptosis, as indicated by loss of p21, is observed when Shh signaling is elevated, again leading to formation of a premolar-like tooth (Ohazama et al., 2009). In contrast, in Wise (also known as Ectodin, Sostdc1, and Usag1) mutants, with elevated Wnt signaling, no difference was observed in apoptosis between control and mutant molar tooth germs, despite the formation of an R2 tooth, indicating that in these mice the rudimentary tooth can be revitalized without changes in apoptosis (Ahn et al., 2010). Apoptosis can also be inhibited by the ectopic application of antiapoptotic factors such as Fgfs, which can rescue tooth development in these normally vestigal buds in vitro (Li et al., 2011).
In the incisor region, vestigal tooth germs have also been reported (Peterkova et al., 1993, 2002), and apoptosis may also have a role in regulating the fate of these tooth germs, and therefore the number of incisors that form. In wild-type mice a patch of apoptosis is observed in the incisor tooth epithelium near to the oral surface, which is associated with Wnt activity (Munne et al., 2009). In the Wise mutant this patch of apoptosis is lost and an additional incisor tooth germ develops from this region, forming an ectopic incisor (Munne et al., 2009). There is thus a balance between proliferation and apoptosis, controlled by signaling molecules that defines how many teeth form. Such a balance of signaling molecules has also been shown to be necessary for apoptosis regulation in other shaped organs, such as limbs (Montero et al., 2001). Apoptosis modulation can be therefore considered as an important step in rudimentary tooth revitalization (Peterkova et al., 2009).
Odontoblasts and ameloblasts
Throughout the lifetime of a tooth, dentin is constantly formed, starting with active primary dentinogenesis, occurring until closure of the root apex, followed by physiological secondary dentin formation, which continues at a much slower rate (Ten Cate, 1998). As a result of dentin matrix production, odontoblasts become crowded by the reduction in pulp space. Therefore, the number of odontoblast must be reduced by apoptosis, occurring particularly at the beginning of secondary dentin formation. Nerve growth factor (NGF) is likely to be an important factor for either the survival or apoptosis of odontoblasts during pulp reduction (Mitsiadis et al., 1993). In ameloblasts, apoptosis can be detected at a range of stages of enamel development: in differentiated, transitional, as well as maturing ameloblasts (Bronckers et al., 2000). During enamel formation, about half of the ameloblast population between late secretory and late maturation stages undergo cell death (Smith, 1998). Apoptosis is likely to regulate the ameloblast cell number by elimination of daughter cells during preameloblast mitosis. During the transition stage ameloblasts shorten from tall secretory cells to short maturation-stage cells and start to undergo apoptosis. Later on, the ameloblasts completely regress, leaving behind the firm enamel.
Interdental bone and periodontal ligament
As the growing tooth must interact with the surrounding tissues in order to establish a functional tooth–bone complex (Diep et al., 2008; Fleischmannova et al., 2010), apoptosis is involved also in the formation of this interface. The exact distribution of apoptotic cells is related to tooth growth and bone remodeling (Chlastakova et al., 2011; Lungova et al., 2011). In the postnatal molar, apoptosis participates in removal of cells, particularly osteoblasts in the mandibular region, working together with osteoclasts to remodel the bone around the developing tooth. Apoptosis of osteoclast precursors may be a way of controlling the osteoclast cell population, effectively reducing bone resorption (Hughes and Boyce, 1997). At more advanced developmental stages, apoptotic cells and bodies accumulate in the cell layers above the tooth cusps, in the path of eruption.
Apoptosis has also been observed in the fibroblast-like cells of the periodontal ligament (Cerri et al., 2000; Pycroft et al., 2002). The mechanism of cell death in the periodontal ligament of teeth during eruption may be different from that in the periodontium of erupted teeth of limited growth, such as the molars of adult rodents (Bronckers et al., 2000). Gingival tissue has a high cell turnover, and apoptosis has been demonstrated to occur in humans, perhaps playing a role in preventing over proliferation (Yoshioka et al., 1996).
Other cell populations
Apoptosis is observed particularly in the subodontoblastic layer and pulp fibroblasts (Mitsiadis et al., 2008), indicating a role in pulp homeostasis (Nishikawa and Sasaki, 1999). The pulp is also eliminated by apoptosis during the process of physiological root resorption in primary teeth. However, the sequence of events and the definitive reason for the physiological death of pulp cells remain unclear (Monteiro et al., 2009).
Apoptosis has also been detected in the stratum intermedium (Bronckers et al., 1996; Vaahtokari et al., 1996) and stellate reticulum in mammalian (Baratella et al., 1999; Vaahtokari et al., 1996) and reptile teeth (Buchtova et al., 2008). In reptiles the extent of cell death in the stellate reticulum has been linked to the level of Shh signaling in this tissue, and may play a role in modulating the size of the resulting tooth (Handrigan and Richman, 2010). Apoptosis may also play a key role in removing cells from the center of the tooth germ in developing fangs, creating the central canal along which venom can travel (Zahradnicek et al., 2008).
Apoptosis in Odontogenesis—How?
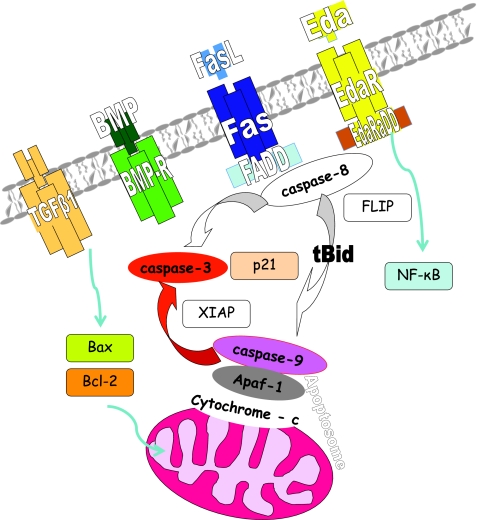
Several apoptosis related molecules have been already identified to be essential in dental apoptosis, and some others are likely to participate in apoptosis amplification and modulation (Fig. 2).
FIG. 2.
Schematic display of the molecular interactions of apoptosis related molecules in dental apoptosis indicating the central position of the mitochondria pathway, which has been shown to be essential. Ligand binding to the corresponding membrane receptor and consequent conformation changes starts activation of the initiator caspases, such as caspase-8, which in turn, cleaves effector caspases, such as caspase-3. However, effector caspases can be activated also by the mitochondria pathways starting with release of cytochrome c and formation of the apoptosome (cytochrome c+Apaf-1+caspase-9). Both pathways are interconnected by tBid and can be modulated by Bcl-2 family members (Bax, Bcl-2) and apoptosis inhibitors, such as FLIP or XIAP. Increased levels of the cyclin dependent kinase inhibitor p21 and decreased level of NF-kappa B supports commitment of the cell to apoptosis.
Caspases
The appearance of apoptotic cells detected by TUNEL and the presence of apoptotic bodies in the EK correlates with caspase-3 activation (Shigemura et al., 2001). The functional impact of caspase-3 deficiency on dental apoptosis was investigated in caspase-3 knock-out mice (Matalova et al., 2006). In these mice, apoptosis in the PEK was inhibited and first molar tooth morphogenesis displayed temporary alteration. However, these initial morphological changes were compensated during later development, and the adult showed no dental phenotype. In caspase-9 and Apaf-1 mutant mice apoptosis in the PEK was not observed (Setkova et al., 2007). The mitochondrial pathway, mediated by apoptosome formation, was therefore proposed as the major signaling in enamel knot apoptosis. However, unlike in caspase-3 mutants, no morphological changes were found at the bell stage in caspase-9 and Apaf-1 mutant mice, and despite the lack of apoptosis in the primary enamel knot, the cap-to-bell transition corresponded to the wild-type phenotype (Setkova et al., 2007). It is possible that amplification mechanisms, particularly via death receptors, may work synergistically with mitochondrial caspase cleavage to initiate cell death (Matalova et al., 2005).
General caspase inhibition has been tested ex vivo using molar culture; the results, however, have been divergent. For example, no histological changes were observed in molar explant cultures after pharmaceutical inhibition of caspases for 2 days at E13.5 in the mouse, despite lack of apoptosis leading to the persistence of the enamel knots (Coin et al., 2000). In these experiments the expression of Shh, Msx-2, Bmp-2, and Bmp-4 decreased in the persistent enamel knots, thus indicating that loss of enamel knot signaling could occur in the absence of elimination of the enamel knot itself. Loss of apoptosis, therefore, did not impinge on the resultant tooth shape. According to these results, the role of apoptosis here would be just a mechanism to scavenge the terminated cells rather than terminating the signaling role of the enamel knots. In contrast, Kim et al. (2006) demonstrated that the tooth shape was altered when they treated explants cultures with the same inhibitor at the early cap stage. The control teeth showed proper crown formation, with six cusps; however, treated teeth displayed a shorter crown height and a longer mesiodistal diameter; moreover, in dose-dependent manner (25M, 50M, and 25M, and 100M inhibitor). These results indicate that presence of a persistent enamel knot can alter the morphology of the resultant tooth, agreeing with the caspase-3 knockout data. Further work is needed to be able to dissect out the exact role of apoptosis in the enamel knots.
Nonapoptotic functions of caspases have also been reported, involving osteoclast differentiation (Szymczyk et al., 2006). Such nonapoptotic functions appear to be related to formation of the tooth–bone complex.
Bcl-2 family
Bcl-2 has been found in the enamel organ (Slootweg and de Weger, 1994), ameloblasts (Kondo et al., 2001), odontoblasts, and subodontoblastic layers (Piattelli et al., 2000). Bax is an antagonist of Bcl-2, and the Bcl-2/Bax ratio determines cell fate (Kitamura et al., 2001).
Govorko et al. (2010) observed that in most cells contributing to the tooth germ both Bcl-2 and Bax proteins are simultaneously expressed. Although Bax expression prevails in the cells of the outer enamel epithelium, Bcl-2 expression dominate in the areas of inner enamel epithelium. In the enamel reticulum, some cells express exclusively Bcl-2 or Bax, whereas others coexpress both proteins. The condensed ectomesenchymal cells of the tooth, mostly expressed only Bax protein.
Overexpression of human Bcl-2 in odontoblasts in transgenic mice prevented in vivo and in vitro odontoblast development, leading to a defect in differentiation and maturation (Zhang et al., 2010). Odontoblast apoptosis was inhibited by overexpression of Bcl-2; however, surprisingly no significant difference was revealed in the density of odontoblasts between the transgenic and wild-type animals. Moreover, neither the proliferation nor senescence rate of odontoblasts was affected. The seemingly unaffected odontoblast density may result from a proportional alteration in the volume of the pulp chamber (transgenics have larger pulp chambers than the wild type) or other unidentified mechanisms.
p21, p53, p63, and p73
It was reported that apoptosis in the EK is linked to the expression of the cyclin-dependent kinase inhibitor p21 in mouse molars (Jernvall et al., 1998). p21 expression is induced by BMP-4 in isolated dental epithelium, which is expressed in the underlying dental mesenchyme at the onset of EK formation (Jernvall et al., 1998). The general caspase inhibitor, Z-VAD-fmk, was able to completely inhibit p21-induced apoptosis, indicating that p21 acts through the caspase pathway (Wood and Newcomb, 2000). However, mice deficient in p21 have no tooth defects, indicating that it does not play an essential role in tooth development (Deng et al., 1995), or that other molecules, such as p27 or p57, might compensate for its function (Mills et al., 1999).
Strong expression of p53 in tooth germs suggests its engagement in dental apoptosis. Mice lacking p53 are susceptible to spontaneous tumours but develop normally (Donehower et al., 1992). In contrast, p63 is essential for tooth development. p63 deficiency results in disturbed skin appendage development, including anodontia (Mikkola, 2007). Possible tissue specific compensation by p63 or p73 have been described in other systems (Cui et al., 2005; Suliman et al., 2001) and may be also involved in odontogenesis.
Death receptors
Despite the evidence that dental apoptosis occurs via the mitochondrial pathway (Setkova et al., 2007), death receptors may be involved at least at the amplification level. Immunohistochemical data from developing rodent and human teeth suggest possible involvement of Fas (CD95)–FasL (CD178) and TNF pathways (Hatakeymata et al., 2000; Matalova et al., 2005). High expression of Fas receptor was found in the primary enamel knot area of the field vole, whereas the proliferating cervical loop was almost negative. Fas ligand expression correlated with Fas but it was also present in the protruding cervical loop (Matalova et al., 2005). Expression of FasL in Fas-negative cells may play roles in establishing the space for growing tooth germs, or immune protected areas, as described in other tissues (French et al., 1996). Fas knock-outs (lpr mice) have no apparent changes in the adult tooth phenotype (Matalova et al., 2005), whereas the impact of FasL deficiency (glc mice) has not been investigated yet.
A role for TNF/NF-kappa B has been reported in molar tooth cusp morphogenesis (Ohazama and Sharpe, 2004). Alterations in signaling through the death receptor Edar, which acts upstream of NFkappa B, leads to defects in the formation of the enamel knot, with cusp defects in the resultant teeth (Tucker et al., 2000). Cusp defects are also observed in mice with mutations in other parts of this signalling pathway, the ligand Eda, and intracellular adapter protein Edaradd (Headon et al., 2001; Kere et al., 1996).
Transforming growth factor beta (TGFβ) signaling
Apoptosis in ameloblasts has been connected to upregulation of TGF-β1 in the maturation-stage enamel organ. In mature tissues, and especially in tissues of epithelial origin, TGF-β1 is generally considered to be a growth inhibitor that may also promote apoptosis. TGF-β1 induces immediate–early stress–response genes that lead to a reduction in Bcl-2 expression and an induction of Bax expression. In keeping with this, a doubling of TGF-β1 transcription was observed in the maturation-stage enamel organ (P11), compared to the secretory-stage enamel organ (P4) (Tsuchiya et al., 2009). TGFβ and induction of apoptotic cascade have also been demonstrated in epithelial cells during rat molar tooth eruption (Moriguchi et al., 2010).
Other molecular players
Dental apoptosis has been associated with the expression of several other molecules, in particular, transcription factor. Egr-1, N-myc, c-fos, and Msx-2. These genes are all localized in developing teeth; however, with the exception of Msx-2, no confirmed correlation has been shown with cell death in odontogenesis (Jernvall and Thesleff, 2000).
BMPs interplay with FGFs and homeobox genes such as Msx-1 and Msx-2 (Cobourne and Sharpe, 2005) as well as other signalling pathways, such as Shh and Wnt (Cobourne and Sharpe, 2005), to modulate survival and apoptosis (Cobourne et al., 2001; Jernvall et al., 1998; Peterková et al., 2003). In particular, Bmp-4 and Bmp-2 appear to be involved in apoptosis stimulation in the dental epithelium (Tureckova et al., 1996). The growth inhibitory effect of this pathway seems to involve activation of p21 (Pouliot and Labrie, 2002).
Basic overview of apoptosis related molecules and their distribution within developing tooth germ is shown in Figure 3.
FIG. 3.
Schematic overview of temporospatial distribution of apoptosis related molecules during odontogenesis showing expression (mRNA in italics) in the epithelium and mesenchyme at the initiation (epithelial thickening), bud, cap, and bell stages and at the secretory phase. pek, primary enamel knot; sek, secondary enamel knots.
Nondevelopmentally induced apoptosis
Several studies have observed that apoptosis is more evident in odontoblasts after injury to odontoblastic processes, as seen after cavity preparation (Bronckers et al.,1996; Goldberg et al., 1994; Kitamura et al., 2001). Compressive forces can also induce apoptosis (Goga et al., 2006; Kobayashi et al., 2000). Apoptosis and expression of propapoptotic factors has also been associated with periodontitis and cyst formation (Martins et al., 2011).
Recently, a spectrum of different substances, such as implanted dental material, food and drugs compounds, and bacterial molecules have been tested to detect whether these substances are able to provoke apoptosis in respective parts of the tooth or in the dental environment. Sodium fluoride (NaF) is used as a prophylactic for caries; however, extreme acute or chronic exposure may result in enamel and skeletal fluorosis and other failures (Robinson et al., 2004). Toxic effects of NaF consist of inhibition of protein synthesis, alteration in cellular metabolism, induction of inflammatory cytokines, and apoptosis (Elliot et al., 2001). In ameloblasts treated with NaF an increased level of casapase-8 was observed, whereas in odontoblasts, an increased level of Bax was detected. An increased level of Bid was observed in both cell populations (Jacinto-Aleman et al., 2010). NaF may influence both the intrinsic and extrinsic apoptotic signaling pathways.
Conclusion
Apoptosis in tooth development has been observed at many stages and is undoubtedly involved in the balance between proliferation and cell elimination. The location of apoptotic cells indicates key roles in controlling a number of processes; however, the essential function of apoptosis has not yet been explicitly demonstrated. Removal of cell death pathway components results in a variety of phenotypes, with initial defects often corrected during later development. Compensatory mechanisms therefore appear to kick in to result in the eventual death of the cells. Thus, although it appears likely that apoptosis has a role in creating the final tooth morphology or controlling the number of teeth, this has proved difficult to show. Such compensatory mechanisms and alternative pathways are not restricted to the tooth but have been previously demonstrated for structures like the digits. High apoptosis is observed between the digits during development, leading to their eventual separation. When the apoptotic machinery is disrupted, such as in Apaf-1 mutants (Cecconi et al., 1998), apoptosis between the digits is initially prevented and webbing occurs. These interdigital cells are not normal, however, and eventually die by nonapoptotic (necrotic) mechanisms, producing a normal final digit pattern (Montero and Hurle, 2010).
Despite the fact that the primary role of apoptosis in tooth development and related disorders (such as cleft lip and palate) has not been clearly shown, the secondary effects accompany several disorders. Understanding of the molecular signaling thus can be beneficial not only for our understanding of basic mechanisms but also for diagnosis and treatment of disorders.
Acknowledgments
Research in apoptosis in the Brno lab was supported by the Grant Agency of the Czech Academy of Sciences IAA600450904 (embryonic apoptosis) and the Grant Agency of the Czech Republic 203/08/1680 (functional diagnostics of apoptotic cells). Collaboration between the CR and UK if funded by a Royal Society International joint grant (JP080875).
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Ahn Y. Sanderson B.W. Klein O.D. Krumlauf R. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development. 2010;127:3221–3231. doi: 10.1242/dev.054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratella L. Arana-Chavez V.E. Katchburian E. Apoptosis in the early involuting stellate reticulum of rat molar tooth germs. Anat. Embryol. 1999;200:49–54. doi: 10.1007/s004290050258. [DOI] [PubMed] [Google Scholar]
- Bronckers A.L. Lyaruu D.M. Goei W. Litz M. Luo G. Karsenty G., et al. Nuclear DNA fragmentation during postnatal tooth development of mouse and hamster and during dentin repair in the rat. Eur. J. Oral. Sci. 1996;104:102–111. doi: 10.1111/j.1600-0722.1996.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Bronckers A.L. Goei S.W. Dumont E. Lyaruu D.M. Wöltgens J.H. Van Heerde W.L., et al. In situ detection of apoptosis in dental and periodontal tissues of the adult mouse using annexin-V-biotin. Histochem. Cell Biol. 2000;113:293–301. doi: 10.1007/s004180000137. [DOI] [PubMed] [Google Scholar]
- Buchtova M. Handrigan G.R. Tucker A.S. Scott L. Town L. Fu K., et al. Initiation and patterning of the snake dental lamina are dependent on Sonic hedgehog signaling. Dev. Biol. 2008;319:132–145. doi: 10.1016/j.ydbio.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Catón J. Tucker A.S. Current knowledge of tooth development: patterning and mineralization of the murine dentition. J. Anat. 2009;214:502–515. doi: 10.1111/j.1469-7580.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi F. Alvarez-Bolado G. Meyer B.I. Roth K.A. Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- Cerri P.S. Freymuller E. Katchburian E. Apoptosis in the early developing periodontium of rat molars. Anat. Rec. 2000;258:136–144. doi: 10.1002/(SICI)1097-0185(20000201)258:2<136::AID-AR3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chlastakova I. Lungova V. Wells K. Tucker A.S. Radlanski R.J. Misek I., et al. Morphogenesis and bone integration of the mouse mandibular third molar. Eur. J. Oral Sci. 2011;119:265–273. doi: 10.1111/j.1600-0722.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- Cho S.W. Lee H.A. Cai J.L. Lee M.J. Kim J.Y. Ohshima H., et al. The primary enamel knot determines the position of the first buccal cusp in developing mice molars. Differentiation. 2007;75:441–451. doi: 10.1111/j.1432-0436.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- Cobourne M.T. Sharpe P.T. Tooth and jaw: molecular mechanisms of patterning in the first branchial arch. Arch. Oral Biol. 2003;48:1–14. doi: 10.1016/s0003-9969(02)00208-x. [DOI] [PubMed] [Google Scholar]
- Cobourne M.T. Sharpe P.T. Sonic hedgehog signaling and the developing tooth. Curr. Top. Dev. Biol. 2005;65:255–287. doi: 10.1016/S0070-2153(04)65010-1. [DOI] [PubMed] [Google Scholar]
- Cobourne M.T. Sharpe P.T. Making up the numbers: the molecular control of mammalian dental formula. Semin. Cell. Dev. Biol. 2010;21:314–324. doi: 10.1016/j.semcdb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Cobourne M.T. Hardcastle Z. Sharpe P.T. Sonic hedgehog regulates epithelial proliferation and cell survival in the developing tooth germ. J. Dent. Res. 2001;80:1974–1979. doi: 10.1177/00220345010800110501. [DOI] [PubMed] [Google Scholar]
- Cohen G.M. Sun X.M. Fearnhead H. Macfarlane M. Brown D.G. Snowden R.T., et al. Formation of large molecular weight fragments of DNA is a key committed step of apoptosis in thymocytes. J. Immunol. 1994;153:507–516. [PubMed] [Google Scholar]
- Coin R. Kieffer S. Lesot H. Vonesch J.L. Ruch J.V. Inhibition of apoptosis in the primary enamel knot does not affect specific tooth crown morphogenesis in the mouse. Int. J. Dev. Biol. 2000;44:389–396. [PubMed] [Google Scholar]
- Cui R.T. Nguyen T.T. Taube J.H. Stratton S.A. Feuerman M.H. Barton M.C. Family members p53 and p73 act together in chromatin modification and direct repression of alpha-fetoprotein transcription. J. Biol. Chem. 2005;280:39152–39160. doi: 10.1074/jbc.M504655200. [DOI] [PubMed] [Google Scholar]
- Deng C. Zhang P. Harper J.W. Elledge S.J. Leder P. Mice lacking p21 cIP11/WAf undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L. Reed J.C. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- Diep L. Matalova E. Mitsiadis T.A. Tucker A.S. Contribution of the tooth bud mesenchyme to alveolar bone. J. Exp. Zool. Part B Mol. Dev. Evol. 2009;312B:510–517. doi: 10.1002/jez.b.21269. [DOI] [PubMed] [Google Scholar]
- Donehower L.A. Harvey M. Slagle B.L. McArthur M.J. Montgomery C.A. Butel J.S., et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Doseff A.I. Apoptosis: the sculptor of development. Stem Cells Dev. 2004;13:473–483. doi: 10.1089/scd.2004.13.473. [DOI] [PubMed] [Google Scholar]
- Du C. Fang M. Li Y. Li L. Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Ekert P.G. Silke J. Hawkins C.J. Verhagen A.M. Vaux D.L. DIABLO promotes apoptosis by removing MIHA/XIAP from processed caspase 9. J. Cell Biol. 2001;152:483–490. doi: 10.1083/jcb.152.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot J. Scarpello J.H. Morgan N.G. Effects of tyrosine kinase inhibitors on cell death induced by sodium fluoride and pertusis toxin in the pancreatic beta-cell line, R1Nm5F. Br. J. Pharmacol. 2001;132:119–126. doi: 10.1038/sj.bjp.0703783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmannova J. Matalova E. Tucker A.S. Sharpe P.T. Mouse models of tooth abnormalities. Eur. J. Oral Sci. 2008;116:1–10. doi: 10.1111/j.1600-0722.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- Fleischmannova J. Matalova E. Sharpe P.T. Misek I. Radlanski R.J. Formation of the tooth–bone interface. J. Dent. Res. 2010;89:108–115. doi: 10.1177/0022034509355440. [DOI] [PubMed] [Google Scholar]
- French L.E. Hahne M. Viard I. Fas and Fas Ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and co-expression in adult tissues characterized by apoptotic cell turnover. J. Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goga Y. Chiba M. Shimizu Y. Mitani H. Compressive force induces osteoblast apoptosis via caspase-8. J. Dent. Res. 2006;85:240–244. doi: 10.1177/154405910608500307. [DOI] [PubMed] [Google Scholar]
- Goldberg M. Lasfargues J.J. Legrand J.M. Clinical testing of dental materials—histological considerations. J. Dent. 1994;22:25–28. doi: 10.1016/0300-5712(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Golpon H.A. Fadok V.A. Taraseviciene-Stewart L. Scerbavicius R. Sauer C. Welte, et al. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 2004;18:1716–1718. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- Govorko D.K. Becic T. Vukojevic K. Mardesic-Brakus S. Biocina-Lukenda D. Saraga-Babic M. Spatial and temporal distribution of Ki-67 proliferation marker, Bcl-2 and Bax proteins in the developing human tooth. Arch. Oral Biol. 2010;55:1007–1016. doi: 10.1016/j.archoralbio.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Handrigan G.R. Richman J.M. Autocrine and paracrine Shh signaling are necessary for tooth morphogenesis, but not tooth replacement in snake and lizards (Squamata) Dev. Biol. 2010;337:171–186. doi: 10.1016/j.ydbio.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S. Tomichi N. Ohara-Nemoto Y. Satoh M. The immunohistochemical localistaion of Fas and Fas ligand in jaw bone and tooth germ of human fetuses. Calcif. Tissue Int. 2000;66:330–337. doi: 10.1007/s002230010069. [DOI] [PubMed] [Google Scholar]
- Headon D.J. Emmal S.A. Ferguson B.M. Tucker A.S. Justice M.J. Sharpe P.T., et al. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature. 2001;414:913–916. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- Hengartner M.O. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hughes D.E. Boyce B.F. Apoptosis in bone physiology and disease. Mol. Pathol. 1997;50:132–137. doi: 10.1136/mp.50.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmler M. Thome M. Hahne M. Schneider P. Hofmann K. Steiner V., et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Jacinto-Alema L.F. Hernandez-Guerrero J.C. Trejo-Solıs C. Jimenez-Farfan M.D. Fernández-Presas A.M. In vitro effect of sodium fluoride on antioxidative enzymes and apoptosis during murine odontogenesis. J. Oral Pathol. Med. 2010;39:709–714. doi: 10.1111/j.1600-0714.2010.00918.x. [DOI] [PubMed] [Google Scholar]
- Jernvall J. Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- Jernvall J. Åberg T. Kettunen P. Keranen S. Thesleff I. The life history of an embryonic signalling center: BMP-4 induces p-21 and is associated with apoptosis in the mouse tooth enamel knot. Development. 1998;125:161–169. doi: 10.1242/dev.125.2.161. [DOI] [PubMed] [Google Scholar]
- Jernvall J. Keranen S.V.E. Thesleff I. Evolutionary modification of development in mammalian teeth: quantifying gene expression patterns and topography. Proc. Natl. Acad. Sci. USA. 2000;97:14444–14448. doi: 10.1073/pnas.97.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kere J. Srivasta A.K. Montonen O. Zonana J. Thomas N. Ferguson B., et al. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by a mutation in a novel transmembrane protein. Nat. Genet. 1996;13:409–416. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- Kerr J.F. Wyllie A.H. Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer S. Peterková R. Vonesch J.L. Ruch J.V. Peterka M. Lesot H. Morphogenesis of the lower incisor in the mouse from the bud to early bell stage. Int. J. Dev. Biol. 1999;43:531–539. [PubMed] [Google Scholar]
- Kim J.Y. Cha Y.G. Cho S.W. Kim E.J. Lee M.J. Lee J.M., et al. Inhibition of apoptosis in early tooth development alters tooth shape and size. J. Dent. Res. 2006;85:530–535. doi: 10.1177/154405910608500610. [DOI] [PubMed] [Google Scholar]
- Kitamura C. Kimura K. Nakayama T. Toyoshima K. Terashita M. Primary and secondary induction of apoptosis in odontoblasts after cavity preparation of rat molars. J. Dent. Res. 2001;80:1530–1534. doi: 10.1177/00220345010800061001. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. Hashimoto F. Miyamoto H. Kanaoka K. Miyazaki-Kawashita Y. Nakashima T., et al. Force-induced osteoclast apoptosis in vivo is accompanied by elevation in transforming growth factor beta and osteoprotegerin expression. J. Bone Miner. Res. 2000;15:1924–1934. doi: 10.1359/jbmr.2000.15.10.1924. [DOI] [PubMed] [Google Scholar]
- Kondo S. Tamura Y. Bawden J.W. Tanase S. The immunohistochemical localization of Bax and Bcl-2 and their relation to apoptosis during amelogenesis in developing rat molars. Arch. Oral Biol. 2001;46:557–568. doi: 10.1016/s0003-9969(00)00139-4. [DOI] [PubMed] [Google Scholar]
- Kuida K. Zheng T.S. Na S. Kuan C. Yang C.D. Karasumaya H., et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Kuida K. Haydar T.F. Kuan C.Y. Gu Y. Taya C. Karasuyama H., et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- Kuranaga E. Caspase signaling in animal development. Dev. Growth Diff. 2011;53:137–148. doi: 10.1111/j.1440-169X.2010.01237.x. [DOI] [PubMed] [Google Scholar]
- Lacasse E.C. Baird S. Korneluk R.G. Mackenzie A.E. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M. Kanneganti T.D. Franchi L. Núńez G. Caspase-1 inflammasomes in infection and inflammation. J. Leukoc. Biol. 2007;82:1–6. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- Lavrik I.N. Golks A. Krammer P.H. Caspases: pharmacological manipulation of cell death. J. Clin. Invest. 2005;115:2665–2672. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechguer N.A. Kuchler-Bopp S. Hu B. Haïkel Y. Lesot H. Vascularization of engineered teeth. J. Dent. Res. 2008;87:1138–1143. doi: 10.1177/154405910808701216. [DOI] [PubMed] [Google Scholar]
- Lesot H. Vonesch J.L. Peterka M. Turecková J. Peterková R. Ruch J.V. Mouse molar morphogenesis revisited by threedimensional reconstruction. II. Spatial distribution of mitoses and apoptosis in cap to bell staged first and second upper molar teeth. Int. J. Dev. Biol. 1996;40:1017–1031. [PubMed] [Google Scholar]
- Li L. Yuan G. Liu C. Zhang L. Zhang Y. Chen Y., et al. Exogenous fibroblast growth factor 8 rescues development of mouse diastemal vestigal tooth ex vivo. Dev. Dyn. online March. 2011:15. doi: 10.1002/dvdy.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungová V. Radlanski R.J. Tucker A.S. Renz H. Míšek I. Matalová E. Tooth-bone morphogenesis during postnatal stages of mouse first molar development. J. Anat. 2011;218:699–715. doi: 10.1111/j.1469-7580.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukko K. Loes S. Furmanek T. Fjeld K. Kvinnsland I.H. Kettunen P. Identification of a novel putative signaling center, the tertiary enamel knot in the postnatal mouse molar tooth. Mech. Dev. 2003;120:270–276. doi: 10.1016/s0925-4773(02)00458-6. [DOI] [PubMed] [Google Scholar]
- Martin S.J. Green D.R. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Martin S.J. Reutelingsperger C.P. McGahon A J. Rader J.A. Van Schie R.C. Laface D.M., et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins C.A. Rivero E.R.C. Dufloth R.M. Figueiredo C.P. Vieira D.S.C. Immunohistochemical detection of factors related to cellular proliferation and apoptosis in radicular and dentigerous cysts. J. Endod. 2011;37:36–39. doi: 10.1016/j.joen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Matalova E. Tucker Sharpe P.T. Death in the life of a tooth. J. Dent. Res. 2004;83:11–16. doi: 10.1177/154405910408300103. [DOI] [PubMed] [Google Scholar]
- Matalova E. Tucker A.S. Misek I. Apoptosis-related factors (Fas receptor, Fas ligand, FADD) in early tooth development of the field vole (Microtus agrestis) Arch. Oral Biol. 2005;50:165–169. doi: 10.1016/j.archoralbio.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Matalova E. Sharpe P.T. Lakhani S.A. Roth K.A. Flavell R.A. Setkova J., et al. Molar tooth development in caspase-3 deficient mice. Int. J. Dev. Biol. 2006;50:491–497. doi: 10.1387/ijdb.052117em. [DOI] [PubMed] [Google Scholar]
- Matalova E. Dubska L. Fleischmannova J. Chlastakova I. Janeckova E. Tucker A.S. Cell proliferation and apoptosis in the primary enamel knot measured by flow cytometry of laser microdissected symplex. Arch. Oral Biol. 2010;55:570–575. doi: 10.1016/j.archoralbio.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Meier P. Finch A. Evan G. Apoptosis in development. Nature. 2000;407:796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- Mikkola M.L. p63 in skin appendage development. Cell Cycle. 2007;6:285–290. doi: 10.4161/cc.6.3.3798. [DOI] [PubMed] [Google Scholar]
- Mills A.A. Zheng B. Wang X.J. Vogel H. Roop D.R. Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Mitsiadis T.A. Couble P. Dicou E. Rudkin B.B. Magloire H. Patterns of nerve growth factor (NGF) proNGF, and p75, NGF receptor expression in the rat incisor: comparison with expression in the molar. Differentiation. 1993;54:161–175. doi: 10.1111/j.1432-0436.1993.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Mitsiadis T.A. De Bari C. About I. Apoptosis in developmental and repair-related human tooth remodeling: a view from the inside. Exp. Cell Res. 2008;314:869–877. doi: 10.1016/j.yexcr.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Monteiro J. Day P. Duggal M. Morgan C. Pulpal status of human primary teeth with physiological root resorption. Int. J. Paediatr. Dent. 2009;19:16–25. doi: 10.1111/j.1365-263X.2008.00963.x. [DOI] [PubMed] [Google Scholar]
- Montero J.A. Hurle J.M. Sculpturing digit shape by cell death. Apoptosis. 2010;15:365–375. doi: 10.1007/s10495-009-0444-5. [DOI] [PubMed] [Google Scholar]
- Montero J.A. Macias D. Ganan Y. Merino R. Chimal-Monroy J. Hurle J.M. Interactions between FGFs and BMPs in the control of programmed cell death in the developing limb. Int. J. Dev. Biol. 2001;45:S113–S114. [Google Scholar]
- Moriguchi M. Yamada M. Miake Y. Yanagisawa T. Transforming growth factor beta inducible apoptotic cascade in epithelial cells during rat molar tooth eruptions. Anat. Sci. Int. 2010;85:92–101. doi: 10.1007/s12565-009-0061-y. [DOI] [PubMed] [Google Scholar]
- Munne P.M. Tummers M. Jarvinen E. Thesleff I. Jernvall J. Tinkering with the inductive mesenchyme: Sostdc1 uncovers the role of dental mesecnhyme in limiting tooth number. Development. 2009;136:393–402. doi: 10.1242/dev.025064. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Diff. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- Nishikawa S. Sasaki F. Apoptosis of dental pulp cells and their elimination by macrophages and MHC class II-expressing dendritic cells. J. Histochem. Cytochem. 1999;47:303–312. doi: 10.1177/002215549904700304. [DOI] [PubMed] [Google Scholar]
- Ohazama A. Sharpe P.T. TNF signalling in tooth development. Curr. Opin. Genet. Dev. 2004;14:513–519. doi: 10.1016/j.gde.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Ohazama A. Haycraft C.J. Seppala M. Blackburn J. Ghafoor S. Cobourne M., et al. Primary cilia regulate Shh activity in the control of molar tooth number. Development. 2009;136:897–903. doi: 10.1242/dev.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkova R. Peterka M. Vonesch J.L. Ruch J.V. Multiple developmental origin of the upper incisor in mouse: histological and computer assisted 3D reconstruction. Int. J. Dev. Biol. 1993;37:581–588. [PubMed] [Google Scholar]
- Peterkova R. Peterka M. Vonesch J.L. Tureckova J. Viriot L. Ruch J.V., et al. Correlation between apoptosis distribution and BMP-2 and BMP-4 expression in vestigial tooth primordia in mice. Eur. J. Oral Sc. 1998;106:667–670. doi: 10.1046/j.0909-8836..t01-5-.x. [DOI] [PubMed] [Google Scholar]
- Peterkova R. Peterka M. Viriot L. Lesot H. Dentition development and budding morphogenesis. J. Craniofac. Genet. Dev. Biol. 2000;20:158–172. [PubMed] [Google Scholar]
- Peterková R. Peterka L. Viriot L. Lessot H. Development of the vestigial tooth primordia as a part of mouse odontogenesis. Connect. Tissue Res. 2002;43:120–128. doi: 10.1080/03008200290000745. [DOI] [PubMed] [Google Scholar]
- Peterková R. Peterka M. Lesot H. The developing mouse dentition: a new tool for apoptosis study. Ann. N. Y. Acad. Sci. 2003;1010:453–466. doi: 10.1196/annals.1299.083. [DOI] [PubMed] [Google Scholar]
- Peterkova R. Churava S. Lesot H. Rothova M. Prochazka J. Peterka M., et al. Revitalization of a diastemal tooth primordium in Spry2 null mice results from increased proliferation and decreased apoptosis. J. Exp. Zool. Part B Mol. Dev. Evol. 2009;312B:292–308. doi: 10.1002/jez.b.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piattelli A. Rubini C. Fioroni M. Ciavarelli L. De Fazio P. Bcl-2, p53, and MIB-1 in human adult dental pulp. J. Endod. 2000;26:225–227. doi: 10.1097/00004770-200004000-00007. [DOI] [PubMed] [Google Scholar]
- Pouliot F. Labrie C. Role of Smad1 and Smad4 in the induction of p21WAF1, Cip1 during bone morphogenetic protein-induced growth arrest in human breast cancer cells. J. Endocrinol. 2002;72:187–198. doi: 10.1677/joe.0.1720187. [DOI] [PubMed] [Google Scholar]
- Prochazka J. Pantalacci S. Churava S. Rothova M. Lambert A. Lesot H., et al. Patterning by heritage in mouse molar row development. Proc. Natl. Acad. Sci. USA. 2010;107:15497–15502. doi: 10.1073/pnas.1002784107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycroft J.M. Hann A. Moxham B.J. Apoptosis in the connective tissues of the periodontal ligament and gingivae of rat incisor and molar teeth at various stages of development. Connect. Tissue Res. 2002;43:265–279. doi: 10.1080/03008200290000763. [DOI] [PubMed] [Google Scholar]
- Ranger A.M. Malynn B.A. Kosmeyer S.J. Mouse model of cell death. Nat. Genet. 2001;28:113–118. doi: 10.1038/88815. [DOI] [PubMed] [Google Scholar]
- Ray C.A. Black R.A. Kronheim S.R. Greenstreet T.A. Sleath P.R. Salvesen G.S., et al. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Riedl S.J. Renatus M. Schwarzenbacher R. Zhou Q. Sun C. Fesik S.W., et al. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- Robinson C. Connell S. Kirkham J. Brookes S.J. Shore R.C. Smith A.M. The effect of fluoride on the developing tooth. Caries Res. 2004;38:268–276. doi: 10.1159/000077766. [DOI] [PubMed] [Google Scholar]
- Satchell P.G. Gutmann J.L. Witherspoon D.E. Apoptosis: an introduction for the endodontist. Int. Endod. J. 2003;36:237–245. doi: 10.1046/j.1365-2591.2003.00657.x. [DOI] [PubMed] [Google Scholar]
- Savill J. Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- Savill J. Fadok V. Henson P. Haslety C. Phagocytic recognition of cells undergoing apoptosis. Immunol. Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Setkova J. Lesot H. Matalova E. Witter K. Matulova P. Misek I. Proliferation and apoptosis in early molar morphogenesis—voles as models in odontogenesis. Int. J. Dev. Biol. 2006;50:481–489. doi: 10.1387/ijdb.052067js. [DOI] [PubMed] [Google Scholar]
- Setkova J. Matalova E. Sharpe P.T. Misek I. Tucker A. Primary enamel knot cell death in Apaf-1 and caspase-9 deficient mice. Arch. Oral Biol. 2007;52:15–19. doi: 10.1016/j.archoralbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- Shigemura N. Kiyoshima T. Sakai T. Matsuo K. Momoi T. Yamaza H., et al. Localization of activated caspase-3-positive and apoptotic cells in the developing tooth germ of the mouse lower forst molar. Histochem. J. 2001;33:253–258. doi: 10.1023/a:1017900305661. [DOI] [PubMed] [Google Scholar]
- Slootweg P.J. de Weger R.A. Immunohistochemical demonstration of bcl-2 protein in human tooth germs. Arch. Oral Biol. 1994;39:545–550. doi: 10.1016/0003-9969(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Smith C.E. Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- Suliman A. Lam A. Datta R. Srivastava R.K. Intracellular mechanism of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001;20:2122–2133. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- Sun C. Cai M. Gunasekera A.H. Meadows R.P. Wang H. Chen J., et al. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature. 1999;401:818–821. doi: 10.1038/44617. [DOI] [PubMed] [Google Scholar]
- Szymczyk K.H. Freeman T.A. Adams C.S. Srinivas V. Steinbeck M.J. Active caspase-3 is required for osteoclast differentiation. J. Cell. Physiol. 2006;209:836–844. doi: 10.1002/jcp.20770. [DOI] [PubMed] [Google Scholar]
- Takahashi R. Deveraux Q. Tamm I. Welsh K. Assamunt N. Salvesen G.S., et al. A single bir domain of xiap sufficient for inhibiting caspases. J. Biol. Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- Ten Cate A.R. Dentinogenesis. In: Ten Cate A.R., editor. Oral Histology: Development, Structure, and Function. 5th. CV Mosby; St. Louis, MO: 1998. pp. 128–149. [Google Scholar]
- Tsuchiya M. Sharma R. Tye C.E. Sugiyama T. Bartlett J.D. Transforming growth factor-b1 expression is up-regulated in maturation-stage enamel organ and may induce ameloblast apoptosis. Eur. J. Oral Sci. 2009;117:105–112. doi: 10.1111/j.1600-0722.2009.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A.S. Sharpe P.T. Molecular genetics of tooth morphogenesis and patterning, the right shape in the right place. J. Dent. Res. 1999;78:826–834. doi: 10.1177/00220345990780040201. [DOI] [PubMed] [Google Scholar]
- Tucker A. Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat. Rev. Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- Tucker A.S. Headon D.J. Schneider P. Ferguson B.M. Overbeek P. Tschopp J., et al. Edar/Eda interactions regulate enamel knot formation in tooth morphogenesis. Development. 2000;127:4691–4700. doi: 10.1242/dev.127.21.4691. [DOI] [PubMed] [Google Scholar]
- Tureckova J. Lesot H. Vonesh J.L. Peterka M. Peterková R. Ruch J.V. Apoptosis is involved in disapearance of the diastemal dental primordia in mouse embryo. Int. J. Dev. Biol. 1996;40:483–489. [PubMed] [Google Scholar]
- Vaahtokari A. Åberg T. Thesleff I. Apoptosis in developing tooth: association with an embryonic signalling center and suppression of EGF and FGF-4. Development. 1996;122:121–129. doi: 10.1242/dev.122.1.121. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E.E. Schuchmann M. Luria V. Chiannilkulchai N. Beckmann J.S. Mett I.L., et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1 and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Verhagen A.M. Ekert P.G. Pakusch M. Silke J. Connolly L.M. Reid G.E., et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R. Brannan C.I. Itoh N. Yonehara S. Copeland N.G. Jenkins N. A., et al. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J. Immunol. 1992;148:1274–1279. [PubMed] [Google Scholar]
- Wood D.E. Newcomb E.W. Cleavage of Bax enhances its cell death function. Exp. Cell Res. 2000;256:375–382. doi: 10.1006/excr.2000.4859. [DOI] [PubMed] [Google Scholar]
- Yoshioka C. Muraki Y. Fukuda J. Haneji T. Kobayashi N. Identification of the Fas antigen in human gingiva. J. Dent. Res. 1996;75:1353–1357. doi: 10.1177/00220345960750060501. [DOI] [PubMed] [Google Scholar]
- Zahradnicek O. Horacek I. Tucker A.S. Viperous fangs: molecular evidence for the infolding theory of venom-canal development. Mech. Dev. 2008;125:786–796. doi: 10.1016/j.mod.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Zakeri Z. Lockshin R.A. Criado-Rodrigues L.M. Martinez A.C. A generalized caspase inhibitor disrupts early mammalian development. Int J. Dev. Biol. 2005;49:43–47. doi: 10.1387/ijdb.041920zz. [DOI] [PubMed] [Google Scholar]
- Zhang W. Ju J. Gronowicz G. Odontoblast-targeted Bcl-2 overexpression impairs dentin formation. J. Cell. Biochem. 2010;111:425–432. doi: 10.1002/jcb.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T.S. Hunot S. Kuida K. Flavell R.A. Caspase knockouts: matters of life and death. Cell Death Diff. 1999;6:1043–1053. doi: 10.1038/sj.cdd.4400593. [DOI] [PubMed] [Google Scholar]
- Zheng X.M. Resnick R.J. Shalloway D. A phosphotyrosine displacement mechanism for activation of Src by PTP. EMBO J. 2000;19:964–978. doi: 10.1093/emboj/19.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K.C. Bonzon C. Green D.R. The machinery of programmed cell death. Pharmacol. Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- Zou H. Henzel W.J. Liu X. Lutschg A. Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]