Abstract
The purpose of this study was to characterize the muscarinic receptor subtypes in the individual lobes of the rat prostate. Immunoprecipitation was performed on homogenates of these 3 lobes using antibodies to the m1-m4 muscarinic receptor subtypes. Reverse transcriptase polymerase chain reaction assays (RT-PCR) were also performed using primers specific for each of the five muscarinic receptor subtypes (m1-m5). The susceptibility of the receptors to degradation by endogenous prostate proteases was assessed by mixing rat ventral prostate with rat heart (m2) and rat parotid (m3) prior to immunoprecipitation. In the ventral lobe, transcripts for the m1-m4 subtypes were amplified whereas in the dorsal and lateral lobes only the m2 and m3 sets of primers amplified PCR products of the predicted size. Immunoprecipitation of the ventral lobe resulted in predominantly m3 receptors, while the majority of receptors immunoprecipitated from lateral and dorsal lobes were the m2 subtype. The m3 muscarinic subtype was apparently susceptible to degradation by prostate proteases whereas the m2 subtype was not. These results demonstrate a regional distribution in the subtypes of muscarinic receptors in the rat prostate, and a greater susceptibility of the m3 receptor to degradation during immunoprecipitation than the m2 subtype.
INTRODUCTION
The rat prostate is innervated by branches of the autonomic nervous system originating in the pelvic ganglion (1). This ganglion receives innervation from the pelvic nerve, carrying motor and sensory information from the sympathetic and parasympathetic fibers, and the hypogastric nerve which carries post ganglionic sympathetic fibers (2–4). These nerves mediate their effects on the prostate through adrenergic and cholinergic receptors and are necessary to maintain the functional and structural integrity of the rat prostate (5,6).
The presence of muscarinic cholinergic receptors has been documented in the rat prostate by radioligand binding studies (7–9). Muscarinic receptors have been shown to be involved in prostatic secretion in animals (10,11), and may also play a role in prostatic growth and hypertrophy (12, 13). The rat prostate is composed of three lobes, ventral, dorsal and lateral (14). There are regional differences between the individual lobes in the secretion and/or accumulation of many substances including zinc, acid phosphatase, prostate binding protein (prostatein), and fructose (15–17). The individual lobes of the rat prostate also demonstrate different response in growth following unilateral parasympathectomy (12). Our hypothesis is that differences in the regional composition of muscarinic receptors may give a structural basis for these observed regional differences in physiology.
At least three types of muscarinic receptors, M1, M2 and M3, are pharmacologically distinguishable using presently available antagonists (18). Using the binding of relatively subtype selective drugs, the pharmacologic profile of the muscarinic receptors in the whole rat prostate is consistent with the M3 subtype (7). Five genes coding for muscarinic receptor subtypes (m1-m5) have been cloned that share the same proposed overall structure and a large degree of protein sequence homology (19). The purpose of this study is to determine the composition and distribution of the molecular subtypes of muscarinic receptors (m1-m5) in the individual lobes of the rat prostate using subtype specific antibodies, and RT-PCR for the mRNA.
MATERIALS AND METHODS
Materials
Carbachol, goat anti-mouse IgG1-agarose, sodium cholate, GTP and atropine were purchased from Sigma Chemical Company (St. Louis MO). [3H]Quinuclidinyl benzilate (39 Ci/mmol) was purchased from DuPont-New England Nuclear Research Products (Wilmington DE). Pansorbin was purchased from Calbiochem Inc. (LaJolla CA). Digitonin was purchased from Gallard-Schlesinger Industries Inc. (Carle Place NY).
Dissection of Rat Prostate
Rats were sacrificed by cervical dislocation. A midline incision was made through the peritoneum. After identification of the bladder, the rat prostate was separated into three respective lobes, ventral, lateral and dorsal, as described previously (14). The names of the lobes of the rat prostate correspond to their position relative to the rat urethra. The ventral prostate is easily visible as two large lobes inferior to the bladder and was removed first. The small lateral lobes are found on either side of the urethra, and are visible only after removing the ventral prostate. The dorsal prostate was approached by separating the seminal vesicles at their base and incising the tissue overlying the posterior aspect of the urethra. The bladder was left in place to facilitate dissection and identification of the urethra. After removal, the prostate tissue was immediately frozen on dry ice.
Immunoprecipitation
Immunoprecipitation was performed using antibodies to the m1-m4 muscarinic receptor subtypes as previously described (19, 20). Membranes were prepared following homogenization in 10 volumes of 10mM Tris (pH 7.4) with 1mM EDTA (TE) containing the following protease inhibitors at 100 µg/ml each (maximum soluble concentration) in a protease inhibitor cocktail (PIC): pepstatin, leupeptin, aprotinin, soybean and lima bean trypsin inhibitors, and α2-macroglobulin. Membranes were incubated with 3H-QNB (1.5 nM) in TE/PIC buffer at 25 C for 30 minutes followed by centrifugation at 20,000 X G for 12 minutes at 4C. The supernatant was discarded, the pellet was solubilized, and solubilized receptors were immunoprecipitated as we have previously described (19–21). Total receptor concentration was determined by desalting over Sephadex G-50 minicolumns. A separate group of rat ventral prostate tissue was processed as above with the exception of labeling the muscarinic receptors with 3H-QNB after solubilization of receptors.
Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was isolated from rat prostate tissue using an RNA isolation kit (Stratagene, La Jolla, CA). 10 µg of total RNA was reverse transcribed using oligo(dT) primers. The reverse transcribed products were screened for the presence of m1-m5 cDNA by PCR. PCR was carried out using oligonucleotide primers designed to be specific for the m1-m5 receptors (Table 1). PCR reactions (initial cycle 95 C for 5 minutes, 62 C for 5 minutes, 30 cycles of 95 C for 5 minutes, 62 C for 3 minutes, 72 C for 3 minutes; extension cycle of 72 C for 10 minutes.) were performed on a DNA Pacer (Bellco Products, Inc., Vineland, NJ).
TABLE 1.
Muscarinic Receptor Oligonucleotide PCR Primers Used for RT-PCR
| Primer Name | Sequence (5'–3') | Product Size |
|---|---|---|
| m1-upper | CCTCTGCTGCCGCTGTTG | |
| m1-lower | GGTGGGTGCCTGTGCTTCA | 175 |
| m2-upper* | CACGAAACCTCTGACCTACCC | |
| m2-lower* | TCTGACCYGAMGACCCAACTA | 686 |
| m3-upper* | GTCTGGCTTGGGTCATCTCTT | |
| m3-lower* | GCTGCTGCTGTGGTCTTGGTC | 433 |
| m4-upper* | TGGGTCTTGTCCTTTGTGCTC | |
| m4-lower* | TTCATTGCCTGTCTGCTTTGTTA | 587 |
| m5-upper* | CTGGTCTCCTTCATCCTCTGG | |
| m5-lower* | CCTGGGTTGTCTTTCCTGTTG | 394 |
Y=mixed base code of CT, M=mixed base code of AC
sequences adapted from Wei et al. (23).
Mixing Experiments
Mixing experiments were performed to test the possibility of degradation of muscarinic receptors by proteases in the rat prostate. Ventral rat prostate was mixed in different proportions with rat heart, which is composed of almost entirely the m2 subtype, and rat parotid which is almost entirely the m3 subtype. Ratios of 2/3 prostate + 1/3 heart (or parotid), and 1/3 prostate plus 2/3 heart (or parotid) were used. The m2 receptor subtype was precipitated from mixing experiments containing prostate and heart while the m3 subtype was precipitated from the mixing experiments combining parotid and prostate tissue. Total prostate, heart, and parotid muscarinic receptors were also immunoprecipitated in the same experiments to determine the number of receptors added during the mixing experiments. For these experiments, a 10 µg/ml concentration of protein inhibitors was used. Each tissue was homogenized separately as above at a concentration of 100 mg/ml of TE. Following homogenization, the tissue homogenates were added together in the indicated ratios. The combined homogenate was then labeled with 3H-QNB and immunoprecipitated with the antibody to the appropriate muscarinic receptor subtype as described above.
RESULTS
Immunoprecipitation
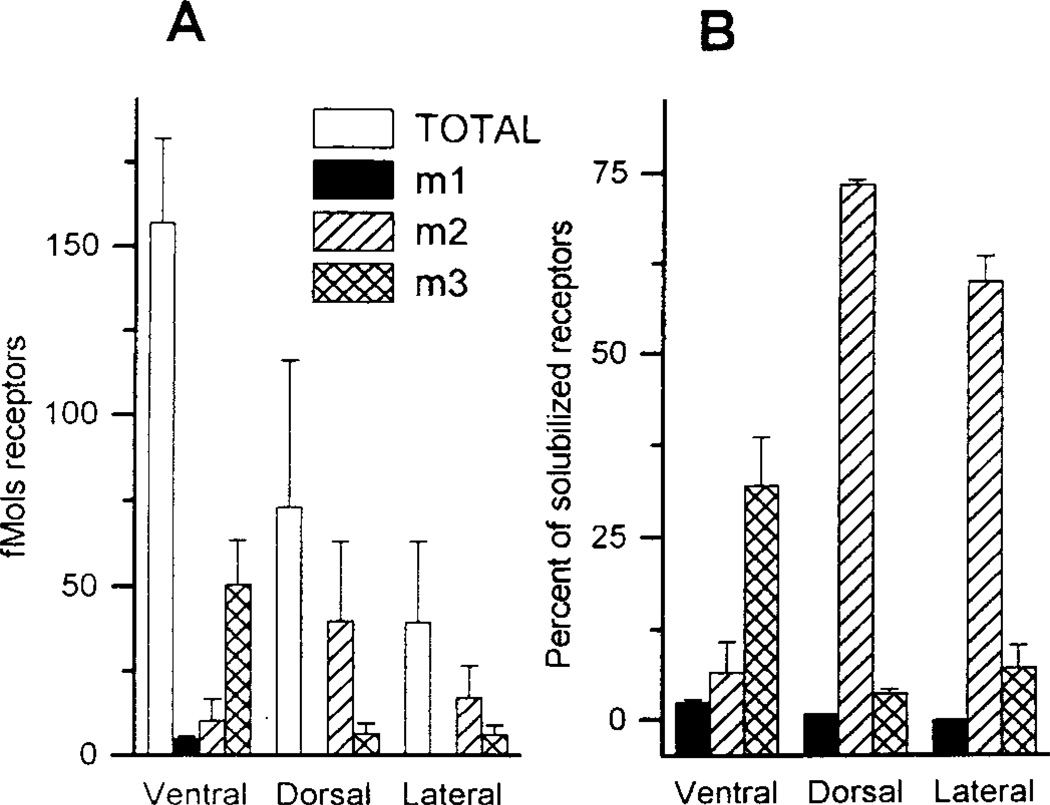
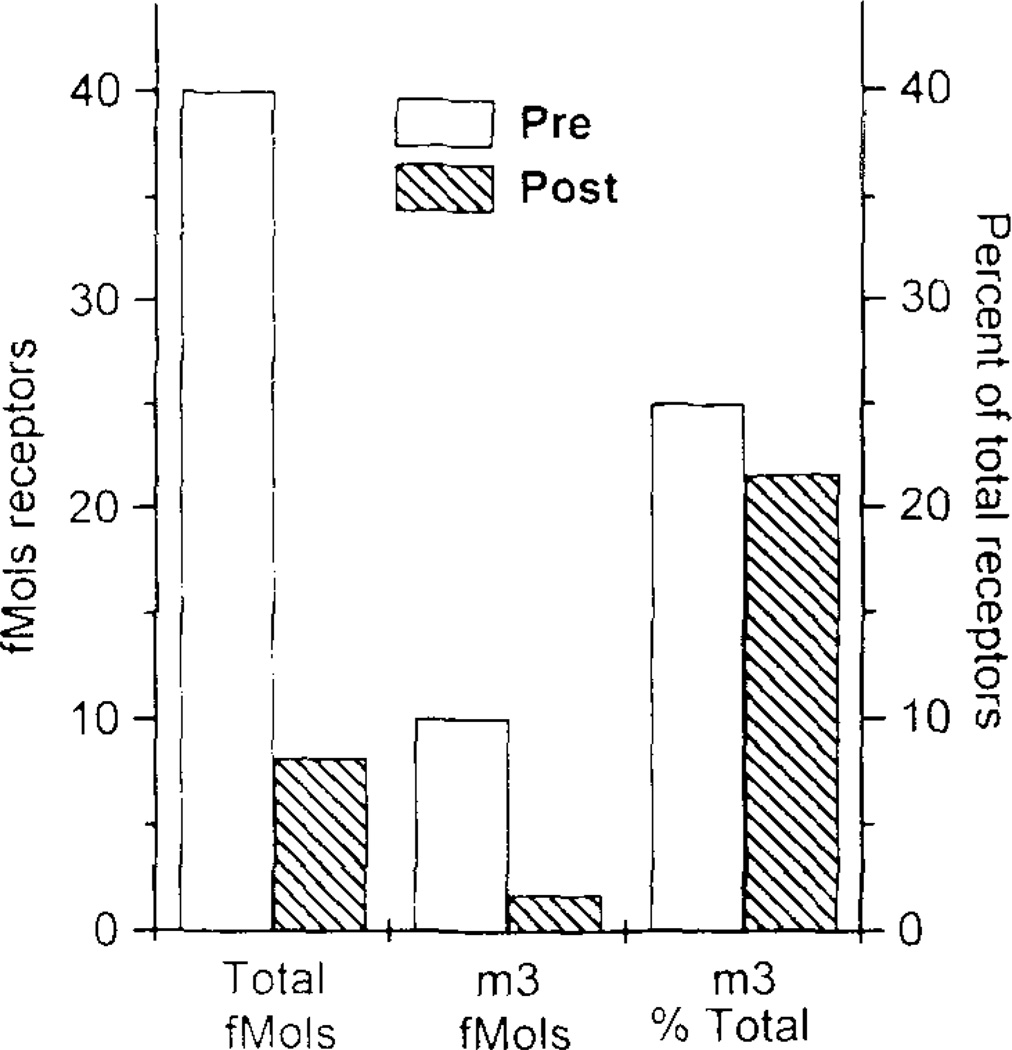
The m1, m2 and m3 receptor subtypes were detected in rat prostate tissues whereas the m4 subtype was not. Figure 1A shows the amount of each receptor subtype that was immunoprecipitated from each lobe in comparison to the total receptors solubilized from each tissue. For ventral prostate, tissue was pooled from several animals. Because of the much smaller amounts of ventral and dorsal prostate tissue in each animal, tissue was pooled from 10–15 rats for each triplicate determination. The large number of rats needed for each experiment in triplicate for these lobes limited the number of precipitations. It is apparent that these percentages for the individual subtypes do not add up to the total number of receptors solubilized. This issue is addressed in subsequent (4Uexperiments described below Figure 1B presents this same data normalized to percent of total receptors solubilized from each lobe. The m2 subtype is predominant in the dorsal and lateral lobes whereas the ventral lobe contains mostly the m3 subtype. In preliminary studies, we were unable to precipitate receptors from prostate in the absence of protease inhibitors (e.g. TE buffer alone). As the concentration of inhibitors was increased from 10 to 100 µg/ml TE, increasing amounts of receptor were precipitated. Greater protease inhibitor concentrations were not possible due to insufficient solubility of these agents. More receptors were precipitated when labeled with 3H-QNB prior to solubilization then when 3H-QNB labeling was performed after solubilization of receptors, but the ratio of receptor subtypes remained unchanged (Figure 2).
FIG 1.
Immunoprecipitation of m1, m2, and m3 muscarinic receptor subtypes from the three lobes of the rat prostate. Error bars = SEM from experiments with triplicate determinations; Ventral N=10 for total, N=2 for m1, N=5 for m2 and N=9 for m3. Dorsal and Lateral N=2 for total, m2 and m3, N=1 for m1. A. Y axis displays results expressed as fmol of muscarinic receptors per 100 mg wet weight of rat prostate tissue. B. Y axis shows amount of muscarinic receptors immunoprecipitated from the lobes of the rat prostate normalized to percent of total receptors solubilized. Values were normalized by dividing fmol of individual receptors precipitated by total fmol of receptors solubilized.
FIG 2.
Immunoprecipitation of the m3 muscarinic receptor subtype from the ventral lobe of the rat prostate labeled with [3H] QNB before solubilization (pre) or after(post). Left Y axis shows fmol of muscarinic receptors immunoprecipitated per mg protein of ventral prostate. Right axis shows fmol of m3 receptors as percent of total solubilized receptors.
Mixing experiments
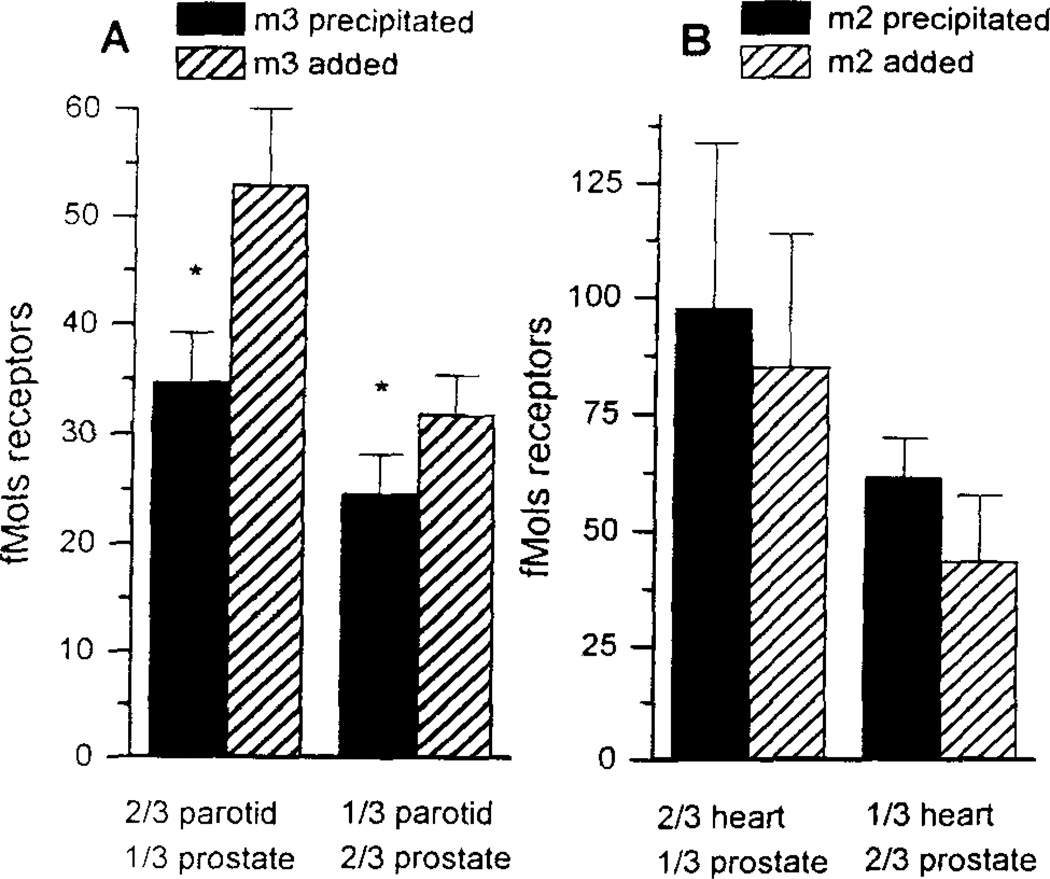
The ventral prostate has over twofold greater density of total solubilized muscarinic receptors than the other lobes (Fig 1A). However, we were able to immunoprecipitate proportionally fewer receptors from the ventral lobe than from the dorsal or lateral lobes. This is evident in figure 1B which shows that less than 50% of the total solubilized receptors could be immunoprecipitated in the ventral prostate with the m1 - m4 antisera whereas over 75% could be precipitated in the dorsal or lateral lobes. To determine whether this could be due to a selective degradation of the m3 compared to the m2 receptor by endogenous proteases in the ventral prostate, mixing experiments were performed as previously used for mixtures of rat heart and forebrain homogenates (21). The rat ventral prostate was used as the potential source of proteases, and was mixed with rat heart (m2) and rat parotid (m3) prior to immunoprecipitation of the specific receptor subtypes. Less m3 receptors were precipitated from rat parotid when mixed with the ventral rat prostate (Figure 3A). This was statistically significant in both ratios used. In contrast, there was no decrease in the fMol of m2 receptors precipitated from rat heart when mixed with rat prostate (Figure 3B).
FIG 3.
Immunoprecipitation of muscarinic receptors (in fmol) from different mixtures of rat parotid and ventral prostate using antibodies to the m3 (panel A) and m2 (panel B) muscarinic receptor subtype. Error bars represent SEM for separate triplicate determinations. N=3 for both m3 and m2 receptors * = p<0.05.
RT-PCR
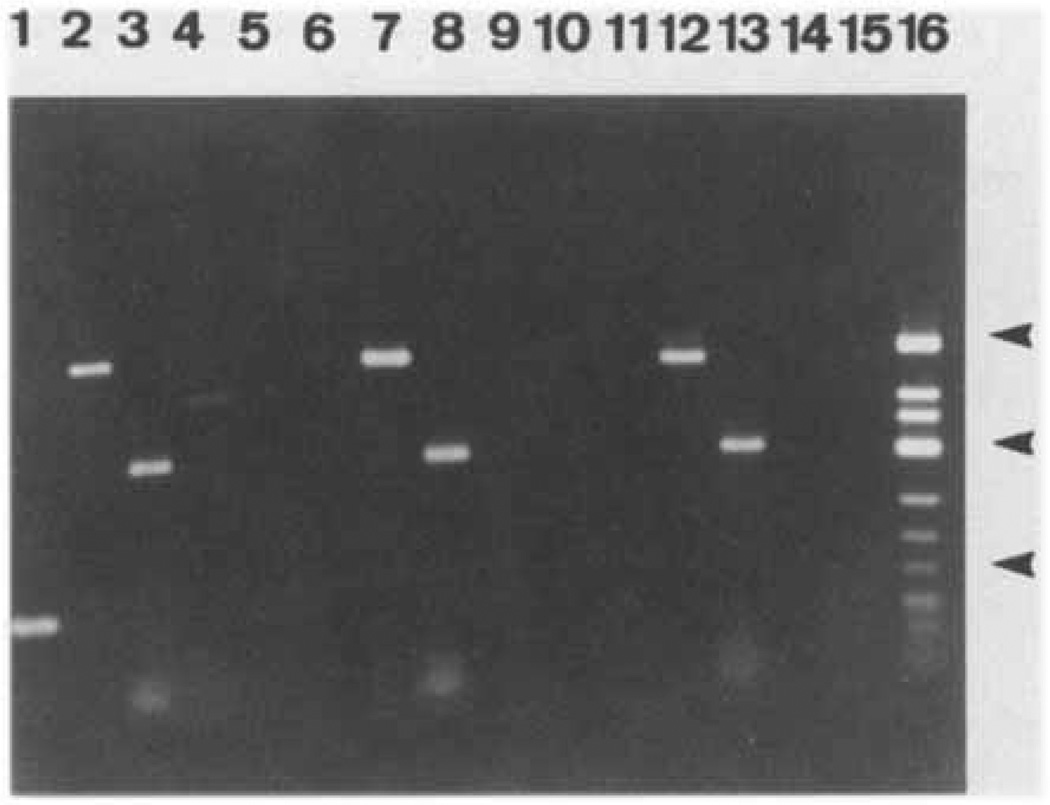
The primers used for RT-PCR for each of the m1-m5 receptor subtypes are shown in Table 1. Results using RNA isolated from the 3 lobes of the rat prostate are shown in Figure 4 and summarized in Table 2.
FIG 4.
Ethidium bromide stained agarose gel of RT-PCR products for the m1-m5 muscarinic receptors. Primers specific for muscarinic receptor subtypes were used to PCR amplify DNA products from a cDNA library prepared from the three lobes of the rat prostate. Ventral Prostate (lanes 1–5), Dorsal Prostate (lanes 6–10), Lateral Prostate (lanes 11–15). Lanes 1, 6, 11- m1 primers, lanes 2, 7, 12- m2 primers, lanes 3, 8, 13 - m3 primers, lanes 4, 9, 14 - m4 primers, lanes 5, 10, 15 -m5 primers - lane 16- molecular weight marker. Arrows on right; top, 725 bp; middle, 417 bp; bottom, 200 bp.
TABLE 2.
RT-PCR Results of Rat Prostate Lobes
| Lobe | m1 | m2 | m3 | m4 | m5 |
|---|---|---|---|---|---|
| Ventral | + | + | + | + | − |
| Dorsal | − | + | + | − | − |
| Lateral | − | + | + | − | − |
These results confirm the above results for immunoprecipitation of receptor protein. The m5 transcript was not amplified in any of the tissues. In the ventral lobe, transcripts for the m1-m4 subtypes were amplified whereas in the dorsal and lateral lobes only the m2 and m3 sets of primers amplified PCR products of the predicted size (Table 2). The negative control (no RT) yielded no products confirming the absence of DNA contamination in the RNA samples (not shown). Thus RT-PCR identification of subtype mRNA is completely consistent with the immunoprecipitation identification of subtype protein levels with the exception that RT-PCR identified the m4 transcript in the ventral lobes whereas the protein product was not detectable.
DISCUSSION
These results demonstrate a regional distribution in the subtypes of muscarinic receptors present in the rat prostate. Whereas the ventral lobe contains mostly the m3 subtype, the lateral and dorsal lobes are predominantly the m2 subtype. Other investigators have studied the muscarinic receptor composition of the rat prostate, but did not distinguish between individual lobes. When 3H-NMS is bound to homogenates of rat prostate ventral lobe, the Scatchard plots obtained are linear, suggesting a single class of binding sites (8). Muscarinic cholinergic receptors have previously been classified based on the affinities of somewhat selective antagonists into at least three subtypes, M1, M2, and M3 (18). Based on the inhibition of 3H-QNB binding by the selective muscarinic antagonists, atropine, pirenzepine and AF-DX 116, a predominance of the M3 muscarinic subtype is observed in whole rat prostate homogenates (7,9). If all three lobes were immunoprecipitated, the majority of receptors precipitated would be expected to be the m3 subtype because the ventral lobe comprises the vast majority of the wet weight of the rat prostate.
The results of immunoprecipitation using antibodies to the specific muscarinic receptor subtypes agrees with those of the RT-PCR except that PCR detected m4 receptor in the ventral lobe which could not be immunoprecipitated. This may indicate that the RNA for the m4 muscarinic receptor subtype is present but not translated. Another alternative is that the m4 receptor is present in the ventral lobe of the rat prostate, but is in such small quantities that it is not detectable with immunoprecipitation. This does not, however, rule out a functional role for the m4 receptor in this tissue.
Although the ventral lobe contains greater numbers of receptors than the other two lobes, we were able to immunoprecipitate proportionally fewer receptors from the ventral lobe than from the dorsal or lateral lobes. This is evident in figure 2 which shows that less than 50% of the total solubilized receptors could be immunoprecipitated in the ventral prostate whereas over 75% of total was precipitated from the dorsal or lateral lobes. Several possibilities must be considered in trying to explain this. Solubilizing receptors leads to a reduction in available binding sites, as has been shown by others (24,25) as well as shown in Figure 2. This was controlled for in these studies by labeling with 3H-QNB before solubilization of receptors. This is analogous to the urinary bladder, where labeling of muscarinic receptors after receptors are solubilized results in a marked decrease in the number of m3 receptors that can be precipitated (19).
Another factor to consider is the differential solubilization of receptor subtypes. Using muscarinic receptors transfected into insect salivary cells, Rinken et al (24) demonstrated differences in the solubility of the muscarinic subtypes, with the m3 subtype being the least soluble. One problem with applying this study to our data is that in the Rinken study the determination of the amount of receptors solubilized was made by binding to [3H]NMS after receptors were solubilized. It has already been determined in that paper and in our data that labeling after solubilization reduces the number of binding sites for ligands. These differences have not been established using immunoprecipitation of prelabeled receptors by subtype specific antibodies. Another question in applying Rinken’s data is whether the differences in solubilization are tissue specific. The differences in subtype solubilization were found using insect salivary gland cells. Whether this difference applies to other tissues is debatable. Our experience to date indicates that in precipitating the m3 receptor from other tissues including parotid, bladder and brain (19,21), we routinely achieve solubilization of 35–45% of total muscarinic receptors, and can then immunoprecipitate nearly 100% of the solubilized m3 receptors using the techniques applied in these studies. In the rat prostate it is clear that we are not achieving the same success with solubilization and/or precipitation, and that this is likely due to a tissue specific effect.
An explanation for the observed differences in precipitation of receptors from the ventral prostate is the effect of proteases. In our previous studies, we were unable to precipitate receptors from human prostate adenoma in the absence of protease inhibitors, e.g. TE buffer alone (20). In the current studies, as the concentrations of inhibitors were increased up to their solubility limit, increasing amounts of receptor were precipitated. It is possible that the extended assay procedure required for precipitation leads to degradation of some of the receptors in this protease-rich tissue, even in the presence of saturated concentrations of protease inhibitors, especially the in ventral lobe. Since the majority of muscarinic receptors in the ventral lobe are m3, one hypothesis to explain the decreased yield with immunoprecipitation in the ventral lobe is that the m3 receptors are more susceptible than the m2 subtype to degradation by the multitude of proteases found in the rat prostate. To test this hypothesis, we performed immunoprecipitation of rat parotid gland which contains almost exclusively the m3, and rat heart which contains almost exclusively m2 subtype of receptor, in the presence of homogenates of the ventral lobe of rat prostate.
The results of the mixing of parotid (m3) tissue with rat ventral prostate are consistent with the protease activity from the ventral prostate degrading a portion of the parotid m3 receptors such that they cannot be precipitated by the antibody used. The m3 antibodies used were raised to a synthetic peptide identical to a unique sequence (amino acids 561–578) at the extreme carboxy terminus of the m3 receptor (26). Since the binding pocket for antagonists has been localized to the second and third intracellular loop of the receptor (27) any proteolytic event between this binding pocket and the m3 antibody binding site at the extreme C-terminus would prevent the antibody from precipitating the radio-labeled (3H-QNB bound) portion of the receptor. This does not necessarily mean that the degraded receptor would be functionally inactive, as long as its G protein binding site is intact. The results with the m2 precipitation from heart mixed with rat prostate do not show a decrease in the number of receptors precipitated from the number expected. Thus, the m3 subtype appears to be selectively sensitive to protease degradation.
Muscarinic receptors have been localized to the prostate glandular epithelium in both humans and rats (8,20,28), and in the rat prostate play a role in secretion and growth (12). Differences in the regional composition of muscarinic receptors may give a structural basis for observed regional differences in composition and function in the rat prostate. Zinc is selectively taken up by the lateral lobe, but not by the other two lobes (15). Citric acid is found to be higher in concentration in the ventral prostate than the other lobes by a sixfold difference (29). There are many substances secreted by the rat prostate, and they vary by the lobe involved. Only the ventral lobe contains secretory acid phosphatase, prostatein, or prostate binding protein,-an androgen binding protein (16,17). In vivo in the rat prostate, the autonomic nervous system has been shown to be important for prostate growth and hypertrophy. McVary et al (12) performed a unilateral parasympathectomy which produced an ipsilateral decrease of only 8% in weight of the ventral lobe, and a contralateral increase of 24% due to prostatic hyperplasia. Little change was seen in the dorsal and lateral lobes.
Whereas the rat prostate is composed of predominantly m2 and m3 subtypes of muscarinic receptors, the human prostate adenoma is composed of predominantly the m1 subtype (20). The adenoma is taken from the time of transurethral resection of the prostate and represents the transition zone. Although this one zone of the human prostate is composed of a different subtype, the comparative composition and functional differences of the muscarinic receptors in the three zones as described by McNeal (30) is unknown. There is some evidence for differences in the response to alpha adrenergic stimulation between the three zones (31). The human prostate zones do however exhibit differential growth, and potential for carcinogenesis. Our lab has also characterized the muscarinic recptor subtypes in a prostate cancer cell line, the LnCAP cells. The LNCaP cell line is derived from a lymph node metastasis of prostatic carcinoma, and has been shown to synthesize PSA in vivo (32). The cells grow in response to exogenous carbachol, and contain mainly the m3 subtype of muscarinic receptor (13). Thus, although the human prostate tissue found in the transition zone has a different muscarinic subtype profile than the rat prostate, a prostate cancer cell line has a similar composition as the rat ventral lobe. How the other lobes of the human prostate, or prostate cancer tissue, compares in muscarinic subtype composition with rat prostate tissue is unknown.
Acknowledgments
This study was supported by NIH grant # RO1-DK43333 (MRR and GRL) a grant from the National Kidney Foundation of the Delaware Valley (MAP and MRR).
REFERENCES
- 1.Langworthy OR. Innervation of the pelvic organs of the rat. Invest. Urol. 1965;2:491. [PubMed] [Google Scholar]
- 2.Purinton PT, Fletcher TF, Bradley WE. Gross and light microscopic features of the pelvic plexus in the rat. Anat. Rec. 1973;175:697. doi: 10.1002/ar.1091750405. [DOI] [PubMed] [Google Scholar]
- 3.Vaalasti A, Hervonen A. Innervation of the ventral prostate of the rat. Am. J. Anat. 1979;154:231. doi: 10.1002/aja.1001540208. [DOI] [PubMed] [Google Scholar]
- 4.Hulsebosch CE, Coggeshall RE. An analysis of the axn population in the nerves to the pelvic viscera in the rat. J. Comp. Neurol. 1982;211:1. doi: 10.1002/cne.902110102. [DOI] [PubMed] [Google Scholar]
- 5.Wang J-M, McKenna KE, McVary KT, Lee C. Requirement of innervation for maintenance of structural and functional integrity in the rat prostate. Biol. of Reprod. 1991;44:1171. doi: 10.1095/biolreprod44.6.1171. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Pineeiro L, Dahiya R, Nunes LL, Tanagho EA, Schmidt RA. Pelvic plexus denervation in rats causes morphologic and functional changes in the prostate. J. Urol. 1993;150:218. doi: 10.1016/s0022-5347(17)35449-6. [DOI] [PubMed] [Google Scholar]
- 7.Latifpour J, Gousse A, Yoshida M, Weiss RM. Muscarinic receptors in diabetic rat prostate. Biochem. Pharm. 1991;42 suppl. S113 doi: 10.1016/0006-2952(91)90400-y. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro E, Miller AR, Lepor H. Down regulation of the muscarinic cholinergic receptor of the rat prostate following castration. J. Urol. 1985;134:179. doi: 10.1016/s0022-5347(17)47052-2. [DOI] [PubMed] [Google Scholar]
- 9.Yazawa H, Honda K. The M3-muscarinic cholinoceptor subtype in rat prostate and its down regulation by aging. Jap. J. Pharmacol. 1993;61:319. doi: 10.1254/jjp.61.319. [DOI] [PubMed] [Google Scholar]
- 10.Wang J-M, McKenna KE, Lee C. Determination of prostatic secretion in rats effects of neurotransmitters and testosterone. Prostate. 1991;18:289. doi: 10.1002/pros.2990180403. [DOI] [PubMed] [Google Scholar]
- 11.Smith ER, Ilievski V, Hadidian Z. The stimulation of canine prostatic secretion by pilocarpine. J. Pharmacol. Exp. Ther. 1966;151:59. [PubMed] [Google Scholar]
- 12.McVary KT, Razzaq A, Lee C, Venegas MF, Rademaker A, McKenna KE. Growth of the rat prostate gland is facilitated by the autonomic nervous system. Biol. Reprod. 1994;51:99. doi: 10.1095/biolreprod51.1.99. [DOI] [PubMed] [Google Scholar]
- 13.Smyth RJ, Ruggieri MR. Identification and characterization of muscarinic receptors in the LNCaP cell line. J Urol. 1995;153 suppl. 379A [Google Scholar]
- 14.Jesik CJ, Holland JM, Lee C. An anatomic and histologic study of the rat prostate. Prostate. 1982;3:81. doi: 10.1002/pros.2990030111. [DOI] [PubMed] [Google Scholar]
- 15.Gunn SA, Gould TC. A correlative anatomical and functional study of the dorsolateral prostate of the rat. Biochem. 1949;44:41. doi: 10.1002/ar.1091280105. [DOI] [PubMed] [Google Scholar]
- 16.Aumiller G, Seitz J. Protein secretion and secretory processes in male accessory sex glands. Int. Rev. Cytology. 1990;121:127. doi: 10.1016/s0074-7696(08)60660-9. [DOI] [PubMed] [Google Scholar]
- 17.Lea OA, Petrusz P, French F. Prostatein: a major secretory protein of the rat ventral prostate. J. Biol. Chem. 1979;254:6196. [PubMed] [Google Scholar]
- 18.Hulme EC, Birdsall NJM, Buckley NJ. Muscarinic recptor subtypes. Ann Rev Pharmacol Toxicol. 1990;30:633. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- 19.Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 20.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J. Pharmacol. Exp. Ther. 1995;273:959. [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggieri MR, Colton MD, Wang P, Wang J, Smyth RJ, Pontari MA, Luthin GR. Human prostate muscarinic receptor subtypes. J. Pharmacol. Exp. Therap. 1995;273:976. [PMC free article] [PubMed] [Google Scholar]
- 22.Luthin GR, Harkness J, Artymyshyn RP, Wolfe BB. Antibodies to a synthetic peptide can be used to distinguish between muscarinic acetylcholine receptor binding sites in brain and heart. Mol. Pharmacol. 1988;34:327. [PubMed] [Google Scholar]
- 23.Wei J, Walton EA, Millici A, Buccafusco JJ. m1-m5 muscarinic recptor distribution in rat CNS by RT-PCR and HPLC. J. Neurochem. 1994;63:815. doi: 10.1046/j.1471-4159.1994.63030815.x. [DOI] [PubMed] [Google Scholar]
- 24.Rinken A, Kameyama K, Haga T, Engstrom L. Solubilization of muscarinic receptor subtypes from baculovirus infected Sf9 insect cells. Biochem Pharmacol. 1994;48:1245. doi: 10.1016/0006-2952(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 25.Rinken A. Subtype-specific changes in ligand binding properties after solubilization of muscarinic receptors from baculovirus-infected Sf9 insect cell membranes. J. Pharmacol. Exp. Therap. 1995;272:8. [PubMed] [Google Scholar]
- 26.Wall SJ, Yasuda RP, Li M, Wolfe BB. Development of an antiserum against m3 muscarinic recptors distribution of m3 receptors in rat tissues and cloned cell lines. Mol. Pharmacol. 1991;40:783. [PubMed] [Google Scholar]
- 27.Kurtenbach E, Curtis CA, Pedder EK, Aitken A, Harris AC, Hulme EC. Muscarinic acetylcholine receptors. Peptide sequencing identifies residues involved in antagonist binding and disulfide bond formation. J. Biol. Chem. 1990;265:13702. [PubMed] [Google Scholar]
- 28.Lepor H, Kuhar MJ. Characterization and localization of the muscarinic cholinergic receptor in human prostatic tissue. J. Urol. 1984;132:397. doi: 10.1016/s0022-5347(17)49636-4. [DOI] [PubMed] [Google Scholar]
- 29.Humphrey GF, Mann T. Studies on the metabolism of semen: citric acid in semen. Biochem. 1949;44:97. [PubMed] [Google Scholar]
- 30.McNeal JE. Regional morphology and pathology of the prostate. American J. of Clinincal Pathology. 1968;49:347. doi: 10.1093/ajcp/49.3.347. [DOI] [PubMed] [Google Scholar]
- 31.Lepor H, Tang R, Meretyk S, Shapiro E. Binding and functional properties of alpha-1 adrenoreceptors in different regions of the human prostate. J. Urol. 1993;150:253. doi: 10.1016/s0022-5347(17)35457-5. [DOI] [PubMed] [Google Scholar]
- 32.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809. [PubMed] [Google Scholar]