Abstract
Since their introduction as ultrasound contrast agents, microbubbles have demonstrated the potential to revolutionise the use of ultrasound at the bedside. Aside from clinical application, where microbubbles are used to enhance ultrasonic assessment of myocardial perfusion, they have demonstrated potential in an exciting host of pre-clinical ultrasound imaging and therapeutic applications. These include the ability to target specific cellular markers of disease, provide dynamic blood flow estimation, deliver localised chemotherapy, potentiate the mechanisms of gene therapy, enhance lesion ablation through cavitation, and spatiotemporally permeabilise the blood-brain barrier. The unique and flexible construction of microbubbles not only enables a variety of ultrasound applications, but also opens the door to detection of microbubbles with modalities other than ultrasound. In this review, non-ultrasound imaging applications utilizing microbubbles are discussed, including MRI, PET, and DEI. These various imaging approaches illustrate novel applications of microbubbles, and may provide the groundwork for future multi-modality imaging or image-guided therapeutics.
Keywords: microbubbles, ultrasound contrast, contrast agents, MRI, PET, DEI
Introduction
Microbubbles have come a long way as ultrasound contrast agents since the observation of increased image contrast from hand-agitated saline in the 1960s.1 Today, microbubbles utilised for ultrasound imaging are typically encapsulated gas spheres filled with air or a high molecular weight gas such as a perfluorocarbon or sulfur hexafluoride.2 The use of a high molecular weight gas increases circulation time over nitrogen or air-filled agents.3 A thin shell is used to further stabilise the microbubble, and often consists of a lipid, protein, sugar, or polymer. Since micron-sized encapsulated microbubbles have rheological transit properties similar to erythrocytes, they are an ideal contrast agent for assessing blood perfusion.4
The density and compressibility of these gas-filled spheres provides them with unique acoustic properties which enables sensitive detection of small numbers of microbubbles with an ultrasound system.5 Microbubbles are currently used clinically in echocardiography and radiology imaging applications, 2, 6–8 as well as small animal imaging.9, 10 A highly compressible microbubble in a sound field will expand and contract rapidly in response to the acoustic pressure wave.11 This acoustic response is also hypothesised to be responsible for therapeutic effects observed with microbubbles combined with ultrasound such as enhanced vascular permeability,12, 13 enhanced drug and gene delivery,14–19 and enhanced local heating and ablation.20–22
Due to the gas-liquid interface of a microbubble shell in an aqueous in-vivo environment, as well as the possibility to incorporate a variety of materials into their lipid or protein shells, the imaging potential of microbubbles can be expanded beyond ultrasound. The implementation of microbubbles as contrast agents in other modalities is attractive for a number of reasons, including relatively innocuous nature of microbubble administration, their rapid biodegradation and clearance from the body, and their potential for combined use with targeted imaging or gene and drug delivery applications. In this review, we consider microbubbles that have been utilised for positron emission tomography (PET), magnetic resonance imaging (MRI), and diffraction-enhanced X-Ray imaging (DEI).
Imaging Applications of Microbubbles Beyond Ultrasound
MR
Magnetic resonance (MR) imaging utilizes the signal derived from water protons as augmented by high strength magnetic fields. Recently, there has been increased interest in alternatives to the standard gadolinium-based MR contrast due to their life-threatening side effects in patients with compromised renal function.23 The mechanism of MR contrast, however, is significantly different than ultrasound attenuation and scatter. Two major contrast mechanisms are involved, a T1, or spin-lattice mechanism that results in local signal enhancement, and T2, a spin-spin mechanism that results in local signal loss. Microbubbles’ suitability in MR studies is due to the induction of local magnetic susceptibility differences at the interface between the microbubble's paramagnetic gas core and the surrounding tissue, primarily a T2 effect.
A number of groups have demonstrated the potential utility of conventional ultrasound contrast agents in MR imaging. The signal changes induced have been shown to be directly proportional to the magnetic field strength, microbubble volume fraction, and microbubble size.24–27 While most of the MR-microbubble studies have been performed at high magnetic fields of 4.7 T and above,24, 27–30 Ueguchi et al. reported detecting Levovist® air microbubbles at 1.5 T using a phantom model.26 The majority of currently installed clinical scanners are 1.5T, limiting the utility of these conventional microbubbles, primarily due to the relatively small paramagnetic affect of the microbubble shell and contents..
Several groups have further enhanced the microbubble response by incorporating paramagnetic iron oxide particles into their shells, thereby enhancing the relaxation rates of the modified agent.31, 32 Table 1 compares the ΔR2* relaxation rates for standard and MR-enhanced microbubbles. Due to their ability to shorten T1 and T2 relaxation times, iron oxide nanoparticles have been investigated as MR contrast agents independent of any additional contrast mechanism or vehicle for delivery. However, because of adverse injection site reactions, the currently approved agents require slow infusion times on the order of 30 minutes. 33 When Chow et al. studied microbubbles entrapping iron oxide nanoparticles in vitro they discovered that the iron oxide nanoparticles were released into the surrounding liquid following ultrasound cavitation.29 Thus, embedding iron-oxide nanoparticles in microbubbles might provide a means for minimising adverse reactions, in addition to enabling monitoring of ultrasound-mediated local release of microbubble contents. In addition to increasing the transverse relaxation rate, Yang et al. reported that embedding superparamagnetic iron oxide nanoparticles into the microbubble polymeric shell enhanced ultrasound backscatter and provided longer MR-contrast enhancement in the rat liver.30
Table 1.
ΔR2* relaxation rates of various microbubbles
| Author | Bubble types | Model type | Magnetic Strength | ΔR2* ,s−1 | |
|---|---|---|---|---|---|
| Ordinary Microbubbles | Cheung | Air-filled Albumin MB* SonoVue ® (sulphurhexaflouride-filled lipid MB) | In vivo: rat brain | 7 T | 2.49 ± 1.00 2.41 ± 1.18 |
| Wong | Optison® (perflutren-filled albumin MB) | In vivo: rat liver | 7 T | 29.1 ± 1.6 (0.15 ml kg−1) 61.5 ± 12.9 (0.4 ml kg−1) |
|
| MR-enhanced Microbubbles | Chow | MION-entrapped polymeric MB† | In vitro: phantom | 7 T | ~300 (5% volume fraction) |
| Callot | 3He-filled lipid MB | In vitro: phantom | 2 T | 222 |
Microbubbles
Monocrystalline Iron Oxide Nanoparticles
Another option of microbubble modification is to fill the core gas with an MR-specific agent, such as a hyperpolarized gas. Using a hyperpolarized gas, such as 3He or 129Xe, with the appropriately tuned coil and MR transmit/receiver system, enables acquisition of a high SNR image from microbubbles, albeit at the expense of the loss of tissue signal. These gases have historically been used as inhaled contrast agents for visualization of the pulmonary tree and alveolar spaces and assessment of lung function.34 However, microbubbles loaded with hyperpolarized gases enable angiographic-type imaging as the microbubbles pass through the circulation. An example of the enhanced contrast provided by this technique can be seen in Figure 1.
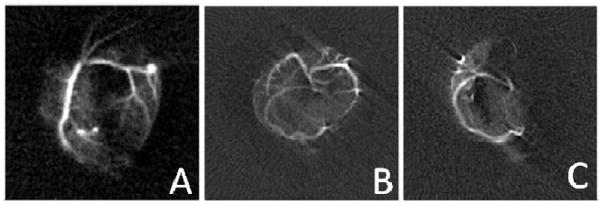
Figure 1.
MR images acquired using microbubbles with 3He gas cores as contrast agents. A) Image acquired after introduction of microbubbles into the left coronary artery. B) A transverse image projection acquired after microbubbles injected in both coronary arteries prior to the occlusion of the left coronary artery. C) A transverse image projection acquired after microbubbles injected in both coronary arteries after the occlusion of the left coronary artery. The field of view in these three images is approximately 14 cm. Adapted from 32. (Reprinted from Acad. Radiol., 9, (Suppl. 2), V. Callot, E. Canet, J. Brochot, H. Humblot, A. Briguet, H. Tournier and Y. Cremillieux, ‘Hyperpolarized helium3 encapsulated in microbubbles: a new class of blood pool MRI contrast agent’, S501–S503, 2002, with permission from Elsevier.)
Beyond simple image enhancement, microbubbles have found utility in functional measurements. For example, microbubbles have been proposed as pressure sensors by at least three different groups.24, 35, 36 As the microbubbles travel through the cardiac chambers, the pulmonary tree and the aorta, they can experience pressure changes of up to 120 mmHg in healthy patients. These pressure changes can be even higher in patients with systemic hypertension, pulmonary hypertension, valvular stenosis, or valvular insufficiency. These pressure gradients impart changes in the microbubbles’ volumes, which then translate into a change in their magnetic susceptibility. Using microbubbles as pressure sensors could allow them, in some instances, to replace catheterization with a significantly less invasive technique.
Efforts have been ongoing to develop dual modality MRI-US systems to enable combined imaging and therapeutic or interventional applications37–39. A dual modality agent would have obvious important utility in such a system. Furthermore, enhancing local drug delivery under direct MR observation would also be a useful clinical tool for a number of applications.
In summary, conventional microbubble contrast agents are of relatively limited utility at clinical imaging strengths as a replacement for conventional MR contrast agents. Modified microbubbles, however, may have great potential in a number of applications, including image enhancement, functional measurements, angiographic applications, and MR-guided ultrasound therapeutics.
PET
Radiolabelled microbubbles have been used to monitor bubble biodistribution since the development of the first ultrasound contrast agents.40 However the animals in these studies had to be sacrificed in order to make ex-vivo biodistribution measurements with a gamma counter. Recently, PET has been employed to detect radiolabelled microbubbles, which allows for real-time measurements and pharmacokinetic studies.41, 42
Tartis et al. reported the use of 18F-lipid-labelled microbubbles to monitor biodistribution of non-targeted microbubbles in a rat model immediately after injection and over several days.41 Additionally, using ultrasound radiation force followed by a destructive pulse, bubbles could be site-selectively disrupted in a rat kidney in order to study ultrasound mediated delivery via microbubble destruction. Though they were unable to report any significant intensity differences in their whole-body microPET image data between the treated and untreated kidneys, 90 minute acquisitions with a torso-only field of view, as well as ex-vivo studies both confirmed an increase in activity in the acoustically treated kidney.41 Images of the biodistribution of non-targeted microbubbles are illustrated in Figure 2.
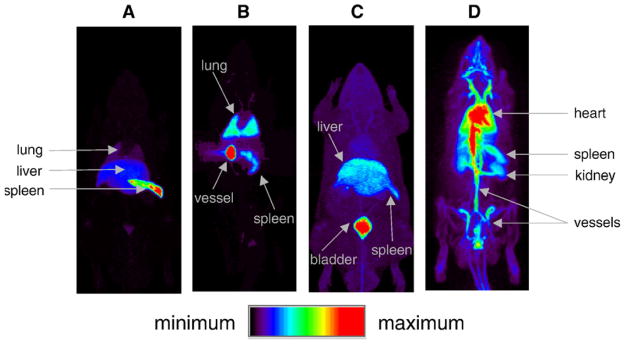
Figure 2.
Full-body maximum intensity projection PET images obtained from 90-minute continuous bed motion scans immediately following injection of radiolabelled FDP lipid formulations to assess biodistributions. A) Image after the introduction of 18F-labelled microbubbles results in accumulated activity in the lung, liver, and spleen, B) Image after the introduction of a higher concentration of 18F-labelled microbubbles results in accumulated activity in lungs, spleen, and a large vessel. C) Image acquired after injection of 18F-labelled FDP in ethanol resulting in the accumulated activity in the liver, spleen, and bladder and D) Image acquired after the injection of liposomal 18F-labelled FDP demonstrating a long circulation time. Reproduced from 41. (Reprinted from J. Control Release, 131, (3), M. S. Tartis, D. E. Kruse, H. Zheng, H. Zhang, A. Kheirolomoom, J. Marik and K. W. Ferrara, ‘Dynamic microPET imaging of ultrasound contrast agents and lipid delivery’, 160–166, 2008, with permission from Elsevier.)
Willman et al. extended the study of 18F- labelling using VEGFR2-targeted microbubbles, enabling biodistribution monitoring of molecularly targeted microbubble agents in a mouse.42 PET imaging of microbubbles enables sensitive and quantitative evaluation of microbubble biodistribution, which is of particular interest in studies of molecular targeted or acoustically concentrated microbubble agents. Acoustic concentration can be achieved with radiation force, which occurs during long ultrasound pulses with a low acoustic pressures, and can help localize targeted or drug-bearing bubbles at the endothelial wall.43, 44
DEI
Diffraction-enhanced imaging (DEI) is an x-ray based modality in development that provides better soft tissue contrast than conventional x-ray imaging with less radiation than computed tomography. DEI uses synchrotron-generated monochromatic x-ray beams and crystal analyzers to detect the refraction and diffraction of photons through tissue samples. The angular acceptance of the crystal-detection system is called the rocking curve, and has been demonstrated to have microradian angular sensitivity. This information is lost with plain and contrast-enhanced x-rays which only detect absorption and transmission. Microbubbles were established as feasible DEI scatter agents due to their gas-water interface by Arfelli et al., who used Levovist and Optison microbubbles .45 An example of the different contrast provided by microbubbles can be seen in Figure 3.
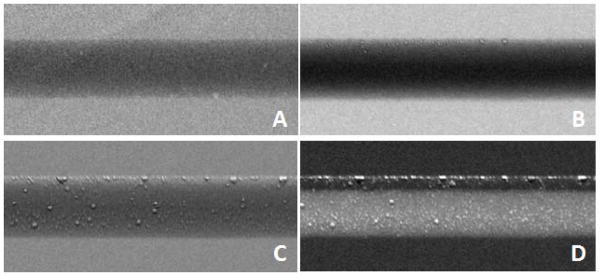
Figure 3.
Diffraction-enhanced x-ray images acquired with Levovist microbubbles. Images acquired in a phantom at 17 keV. A) The absorption image. B) Image acquired at the top of the rocking curve. C) Image acquired at the slope of the rocking curve. D) Image acquired at the far slope of slope of the rocking curve. Adapted from 45. (Reprinted from F. Arfelli, L. Rigon, R.-H. Menk and H.-J. Besch: ‘On the possibility of utilizing scattering-based contrast agents in combination with diffraction enhanced imaging’, Proc. SPIE, 2003, 5030, 10 with permission from SPIE.)
In a recent study by Faulconer et al., lipid-coated perflurocarbon microbubbles were also shown to act as candidate DEI contrast agents, with larger microbubbles providing increased contrast over smaller microbubbles.46 With further studies, microbubbles may become optimised for greater DEI scatter.
Discussion and Conclusion
Although the primary application of microbubbles as a contrast agent remains in ultrasound, microbubbles may also have future utility in MRI, PET, and DEI X-ray imaging.
In MRI imaging, microbubbles can be detected without modification, although their unenhanced contrast performance falls short of the standard gadolinium agents. However, the addition of a susceptibility agent to a bubble’s core or shell substantially enhances sensitivity. Hyperpolarized-gas filled microbubbles provide a unique mechanism of blood vessel imaging with low background signal from tissue. Since gadolinium-based contrast agents are contraindicated in patients with renal insufficiency, it is conceivable that microbubbles with hyperpolarised-gas cores or iron-oxide nanoparticles embedded in their shells might provide a safer alternative in the future. With the current development of MR-guided focused ultrasound systems for site-specific therapy, perhaps the most likely future multi-modality application of microbubbles is with this developing technology.
PET imaging has become one of the imaging modalities most commonly integrated into a multi-modality system, often coupled with CT and more recently with MR, to simultaneously acquire anatomical and molecular image data. Ultrasound could similarly provide anatomical imaging information, although due to the limited field of view of current clinical transducers, it is more likely that a PET-ultrasound combination would involve monitoring of ultrasound-microbubble mediated therapies.
DEI imaging is still in development, and is not yet being used clinically. Unenhanced microbubbles are one of the few biodegradable contrast agents to have demonstrated potential with this new imaging technique, so this may be one modality where unmodified microbubbles could be used as a contrast agent.
Although in none of these modalities do microbubbles provide the unique sensitivity as in ultrasound, where a single contrast agent can be detected, the readily modifiable construction of the microbubble enables further optimization of these vehicles to various modalities. Additionally, with increasing interest in microbubbles as drug carriers, there is greater motivation to track biodistribution of microbubbles with whole-body imaging systems. Microbubbles are still relatively new contrast agents, with commercial products being available for only approximately the last two decades. Currently available microbubbles are primarily designed for ultrasound imaging, and research is still ongoing to optimize their size distribution, gas core, and shell parameters, even for this modality. It is likely that future improvements will make microbubbles better suited for ultrasound imaging in the future, and future optimization could readily make microbubbles better suited for other modalities as well.
Based on studies to date and the direction of ongoing research, we anticipate that the microbubble will play an increasing role in future applications of multimodality imaging and therapy.
Acknowledgments
We appreciate the clinical perspectives and technical review provided by Yueh Lee, M.D., Ph.D.. Funding for the authors is provided by the Doris Duke Charitable Foundation (P.K.), the National Science Foundation (R.C.G.), and the National Institutes of Health (P.A.D.), grants R21EB005325, R01EB008733, and R01EB009066.
Footnotes
Disclosure: P.A.D. is on the scientific advisory board for Targeson, LLC.
References
- 1.Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol. 1968;3(5):356–366. doi: 10.1097/00004424-196809000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg BB, Raichlen JS, Forsberg F. Ultrasound Contrast Agents: Basic principals and clinical applications. London: Martin Dunitz; 2001. [Google Scholar]
- 3.Cohen JL, Cheirif J, Segar DS, Gillam LD, Gottdiener JS, Hausnerova E, Bruns DE. Improved left ventricular endocardial border delineation and opacification with OPTISON (FS069), a new echocardiographic contrast agent. Results of a phase III Multicenter Trial. J Am Coll Cardiol. 1998;32(3):746–752. doi: 10.1016/s0735-1097(98)00311-8. [DOI] [PubMed] [Google Scholar]
- 4.Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15(5):396–403. doi: 10.1067/mje.2002.117290. [DOI] [PubMed] [Google Scholar]
- 5.Klibanov AL, Rasche PT, Hughes MS, Wojdyla JK, Galen KP, Wible JH, Brandenburger GH. Detection of individual microbubbles of ultrasound contrast agents - Imaging of free-floating and targeted bubbles. Investigative Radiology. 2004;39(3):187–195. doi: 10.1097/01.rli.0000115926.96796.75. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann BA, Wei K, Lindner JR. Contrast echocardiography. Current Problems in Cardiology. 2007;32(2):51–96. doi: 10.1016/j.cpcardiol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Forsberg F, Piccoli CW, Merton DA, Palazzo JJ, Hall AL. Breast lesions: Imaging with contrast-enhanced subharmonic US - Initial experience. Radiology. 2007;244(3):718–726. doi: 10.1148/radiol.2443061588. [DOI] [PubMed] [Google Scholar]
- 8.Burns PN, Wilson SR, Simpson DH. Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol. 2000;35(1):58–71. doi: 10.1097/00004424-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Rychak JJ, Graba J, Cheung AMY, Mystry BS, Lindner JR, Kerbel RS, Foster FS. Microultrasound molecular imaging of vascular endothelial growth factor receptor 2 in a mouse model of tumor angiogenesis. Molecular Imaging. 2007;6(5):289–296. [PubMed] [Google Scholar]
- 10.Pollard RE, Dayton PA, Watson KD, Hu X, Guracar IM, Ferrara KW. Motion corrected cadence CPS ultrasound for quantifying response to vasoactive drugs in a rat kidney model. Urology. 2009;74(3):675–681. doi: 10.1016/j.urology.2009.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan KE, Allen JS, Dayton PA, Chomas JE, Klibanov AL, Ferrara KW. Experimental and theoretical evaluation of microbubble behavior: Effect of transmitted phase and bubble size. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2000;47(6):1494–1509. doi: 10.1109/58.883539. [DOI] [PubMed] [Google Scholar]
- 12.Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal, particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98(13):1264–1267. doi: 10.1161/01.cir.98.13.1264. [DOI] [PubMed] [Google Scholar]
- 13.Stieger SM, Caskey CF, Adamson RH, Qin S, Curry FR, Wisner ER, Ferrara KW. Enhancement of vascular permeability with low-frequency contrast-enhanced ultrasound in the chorioallantoic membrane model. Radiology. 2007;243(1):112–121. doi: 10.1148/radiol.2431060167. [DOI] [PubMed] [Google Scholar]
- 14.Lindner JR, Kaul S. Delivery of drugs with ultrasound. Echocardiography-a Journal of Cardiovascular Ultrasound and Allied Techniques. 2001;18(4):329–337. doi: 10.1046/j.1540-8175.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- 15.Bohmer MR, Klibanov AL, Tiemann K, Hall CS, Gruell H, Steinbach OC. Ultrasound triggered image-guided drug delivery. Eur J Radiol. 2009;70(2):242–253. doi: 10.1016/j.ejrad.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 16.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Advanced Drug Delivery Reviews. 2008;60(10):1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Advanced Drug Delivery Reviews. 2008;60(10):1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao SP, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound in Medicine and Biology. 1997;23(6):953–959. doi: 10.1016/s0301-5629(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 19.Miller DL, Bao S, Morris JE. Sonoporation of cultured cells in the rotating tube exposure system. Ultrasound Med Biol. 1999;25(1):143–149. doi: 10.1016/s0301-5629(98)00137-9. [DOI] [PubMed] [Google Scholar]
- 20.Tran BC, Seo J, Hall TL, Fowlkes JB, Cain CA. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control. 2003;50(10):1296–1304. doi: 10.1109/tuffc.2003.1244746. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko Y, Maruyama T, Takegami K, Watanabe T, Mitsui H, Hanajiri K, Nagawa H, Matsumoto Y. Use of a microbubble agent to increase the effects of high intensity focused ultrasound on liver tissue. Eur Radiol. 2005;15(7):1415–1420. doi: 10.1007/s00330-005-2663-7. [DOI] [PubMed] [Google Scholar]
- 22.Yu T, Fan X, Xiong S, Hu K, Wang Z. Microbubbles assist goat liver ablation by high intensity focused ultrasound. Eur Radiol. 2006;16(7):1557–1563. doi: 10.1007/s00330-006-0176-7. [DOI] [PubMed] [Google Scholar]
- 23.Heinz-Peer G, Neruda A, Watschinger B, Vychytil A, Geusau A, Haumer M, Weber M. Prevalence of NSF following intravenous gadolinium-contrast media administration in dialysis patients with endstage renal disease. Eur J Radiol. 2010;76(1):129–134. doi: 10.1016/j.ejrad.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Alexander AL, McCreery TT, Barrette TR, Gmitro AF, Unger EC. Microbubbles as novel pressure-sensitive MR contrast agents. Magn Reson Med. 1996;35(6):801–806. doi: 10.1002/mrm.1910350603. [DOI] [PubMed] [Google Scholar]
- 25.Dharmakumar R, Plewes DB, Wright GA. On the parameters affecting the sensitivity of MR measures of pressure with microbubbles. Magn Reson Med. 2002;47(2):264–273. doi: 10.1002/mrm.10075. [DOI] [PubMed] [Google Scholar]
- 26.Ueguchi T, Tanaka Y, Hamada S, Kawamoto R, Ogata Y, Matsumoto M, Nakamura H, Johkoh T. Air microbubbles as MR susceptibility contrast agent at 1.5 Tesla. Magn Reson Med Sci. 2006;5(3):147–150. doi: 10.2463/mrms.5.147. [DOI] [PubMed] [Google Scholar]
- 27.Wong KK, Huang I, Kim YR, Tang H, Yang ES, Kwong KK, Wu EX. In vivo study of microbubbles as an MR susceptibility contrast agent. Magn Reson Med. 2004;52(3):445–452. doi: 10.1002/mrm.20181. [DOI] [PubMed] [Google Scholar]
- 28.Cheung JS, Chow AM, Guo H, Wu EX. Microbubbles as a novel contrast agent for brain MRI. Neuroimage. 2009;46(3):658–664. doi: 10.1016/j.neuroimage.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 29.Chow AM, Chan KW, Cheung JS, Wu EX. Enhancement of gas-filled microbubble R(2)* by iron oxide nanoparticles for MRI. Magn Reson Med. 2009;63(1):224–229. doi: 10.1002/mrm.22184. [DOI] [PubMed] [Google Scholar]
- 30.Yang F, Li Y, Chen Z, Zhang Y, Wu J, Gu N. Superparamagnetic iron oxide nanoparticle-embedded encapsulated microbubbles as dual contrast agents of magnetic resonance and ultrasound imaging. Biomaterials. 2009;30(23–24):3882–3890. doi: 10.1016/j.biomaterials.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 31.Chawla MS, Chen XJ, Moller HE, Cofer GP, Wheeler CT, Hedlund LW, Johnson GA. In vivo magnetic resonance vascular imaging using laser-polarized 3He microbubbles. Proc Natl Acad Sci U S A. 1998;95(18):10832–10835. doi: 10.1073/pnas.95.18.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callot V, Canet E, Brochot J, Humblot H, Briguet A, Tournier H, Cremillieux Y. Hyperpolarized helium3 encapsulated in microbubbles: a new class of blood pool MRI contrast agent. Acad Radiol. 2002;9(Suppl 2):S501–503. doi: 10.1016/s1076-6332(03)80276-3. [DOI] [PubMed] [Google Scholar]
- 33.Neuwelt EA, Hamilton BE, Varallyay CG, Rooney WR, Edelman RD, Jacobs PM, Watnick SG. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009;75(5):465–474. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosbah K, Ruiz-Cabello J, Berthezene Y, Cremillieux Y. Aerosols and gaseous contrast agents for magnetic resonance imaging of the lung. Contrast Media Mol Imaging. 2008;3(5):173–190. doi: 10.1002/cmmi.252. [DOI] [PubMed] [Google Scholar]
- 35.Dharmakumar R, Plewes DB, Wright GA. A novel microbubble construct for intracardiac or intravascular MR manometry: a theoretical study. Phys Med Biol. 2005;50(20):4745–4762. doi: 10.1088/0031-9155/50/20/001. [DOI] [PubMed] [Google Scholar]
- 36.Morris RH, Bencsik M, Nestle N, Galvosas P, Fairhurst D, Vangala A, Perrie Y, McHale G. Robust spatially resolved pressure measurements using MRI with novel buoyant advection-free preparations of stable microbubbles in polysaccharide gels. J Magn Reson. 2008;193(2):159–167. doi: 10.1016/j.jmr.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz AC, van den Bosch MA, Rieke V, Dirbas FM, Butts Pauly K, Mali WP, Daniel BL. 3.0-T MR-guided focused ultrasound for preoperative localization of nonpalpable breast lesions: an initial experimental ex vivo study. J Magn Reson Imaging. 2009;30(4):884–889. doi: 10.1002/jmri.21896. [DOI] [PubMed] [Google Scholar]
- 38.Hesley GK, Gorny KR, Henrichsen TL, Woodrum DA, Brown DL. A clinical review of focused ultrasound ablation with magnetic resonance guidance: an option for treating uterine fibroids. Ultrasound Q. 2008;24(2):131–139. doi: 10.1097/RUQ.0b013e31817c5e0c. [DOI] [PubMed] [Google Scholar]
- 39.Hynynen K, McDannold N, Clement G, Jolesz FA, Zadicario E, Killiany R, Moore T, Rosen D. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain--a primate study. Eur J Radiol. 2006;59(2):149–156. doi: 10.1016/j.ejrad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Walday P, Tolleshaug H, Gjoen T, Kindberg GM, Berg T, Skotland T, Holtz E. Biodistributions of air-filled albumin microspheres in rats and pigs. Biochem J. 1994;299(Pt 2):437–443. doi: 10.1042/bj2990437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tartis MS, Kruse DE, Zheng H, Zhang H, Kheirolomoom A, Marik J, Ferrara KW. Dynamic microPET imaging of ultrasound contrast agents and lipid delivery. J Control Release. 2008;131(3):160–166. doi: 10.1016/j.jconrel.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willmann JK, Cheng Z, Davis C, Lutz AM, Schipper ML, Nielsen CH, Gambhir SS. Targeted microbubbles for imaging tumor angiogenesis: assessment of whole-body biodistribution with dynamic micro-PET in mice. Radiology. 2008;249(1):212–219. doi: 10.1148/radiol.2491072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shortencarier MJ, Dayton PA, Bloch SH, Schumann PA, Matsunaga TO, Ferrara KW. A method for radiation-force localized drug delivery using gas-filled lipospheres. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(7):822–831. doi: 10.1109/tuffc.2004.1320741. [DOI] [PubMed] [Google Scholar]
- 44.Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound Med Biol. 1999;25(8):1195–1201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 45.Arfelli F, Rigon L, Menk RH, Besch HJ. On the Possibility of Utilizing Scattering-based Contrast Agents in Combination with Diffraction Enhanced Imaging. Proceedings of SPIE. 2003;5030:10. [Google Scholar]
- 46.Faulconer LS, Connor DM, Mullin L, Tsuruta J, Dayton PA, Cole E, Zhong Z, Pisano ED. unpublished work. 2009 [Google Scholar]