Abstract
Background
Schistosoma mansoni is one of the parasites with high public and medical importance in Ethiopia. However, information is scarce about S. mansoni epidemiology in people living with higher risk of infection in Jimma town. This study was designed to determine point prevalence, intensity and risk factors of S. mansoni infection among residents nearby three rivers of Jimma town and assess the rate of Biomphalaria species shading cercariae from January to April, 2007.
Methods
A cross-sectional study was conducted in communities residing nearby three rivers of Jimma town. Structured questionnaires were used to collect data on socio- demographic and behavioral risk factors. After physical examination, stool samples were collected from 517 study participants and processed with Kato-Katz technique for microscopic examination and quantification of egg load. Snails were collected for identification of Biomphalaria species and then checked for cercarial shading.
Results
The prevalence of S. mansoni was 26.3 % with intensity ranging 24 to 936 eggs per gram of stool. Participants in the age group 10–19 years, OR = 2.19 (95% CI; 1.10 – 4.34), and those living near the Awetu River, OR = 2.67 (95% CI; 1.06 – 6.75), had higher risk of S. mansoni infection. Moreover, water contact while crossing a river, OR = 3.77 (95% CI; 1.79 – 7.95), and swimming, OR = 2.59 (95% CI; 1.37 – 4.91, was significantly associated with infection. Biomphalaria snails collected from Chore and Awetu Rivers shaded higher rate of cercariae compared with Kito River.
Conclusion
A moderate prevalence of S. mansoni infection was shown in the study population. Infection rate among the residents correlated with rate of cercarial shading Biomphalaria snails. Treatment of targeted groups, appropriate health education and environmental measures (e.g. snail control) are needed to improve the situation.
Keywords: Prevalence, Intensity, Schistosoma mansoni, Biomphalaria snails
Introduction
Schistosomiasis (bilharziasis) is a parasitic disease that has a worldwide public health importance. The disease is prevalent in 75 countries (1) and more than 650 million people live in endemic areas (2). Globally, about 200 million people are infected with schistosomiasis; 85% of which are living in sub-Saharan Africa. Among tropical diseases, the yearly estimated deaths of 200,000 are making schistosomiasis second only to malaria as a cause of mortality (3). Human schistosomiasis is initiated by exposure to water (while planting, fishing, washing, swimming and so on) containing the free living infective stage of the parasite (cercariea) released from the intermediate host (snail) (4).
In Ethiopia, Schistosoma mansoni and Schistosoma haematobium have significant medical and public health importance. S. mansoni is widespread and its presence has been recorded in all administrative regions and is rapidly spreading in connection with water resource development and intensive population movements (5). Two species of fresh water snails (Biomphalaria pfeifferi and Biomphalaria sudanica) are responsible for the transmission of S. mansoni in the country (6). The former is the most dominant and is found in a variety of habitats like pools, sewages, swamps, lakes, small and medium streams, irrigation canals and rivers (7). S. haematobium is restricted to lowland areas and has been so far reported from the Awash and Wabeshebele river basins and from Kurmuk at the Ethiopia/Sudan border. Bulinus abyssinicus and Bulinus africanus were incriminated as intermediate host of S. haematobium in Ethiopia and lower altitudes ranging from 300 to 700 meters were suitable for the establishment of these snails and maintenance of the parasite (6).
The World Health Organization recommends a strategy of controlling morbidity for both schistosomiasis and soil-transmitted helminthiasis so as to reduce consequences to the level that these diseases no longer constitute a public health burden. The strategy bases on the prevalence of infection on a given locality (8).
A review of literatures on prevalence of S. mansoni documented rates that range from less than 1% up to more than 90% in Ethiopia (6). And most of the studies measured the rate of infection in school children; but the epidemiology in the general population has not been adequately described. Jimma town is among the localities where S. mansoni and its intermediate host were previously documented (7). However, information is scarce on the epidemiology of infection in the area to plan effective prevention and control measures. This study aimed to determine the prevalence, intensity and risk factors of S. mansoni infection among residents of Jimma town and also assess the rate of Biomphalaria species shading cercariae.
Methods and Materials
A cross sectional study was conducted in Jimma town from January to April, 2007. The town is at mean altitude of 1780 meters above sea level with tropical climate of heavy rainfall and warm climate with a mean annual temperature of 24.9 °C. The total population of Jimma town was estimated to be 159,009 and the average family size was 6.3 (9). Residents living nearby rivers named; Awetu, Kito, and Chore were surveyed. The selection of the rivers was based on their ecological suitability for transmission of S. mansoni infection. The rivers Chore and Awetu join together and flow bisecting the town. Further downstream, they come together with Kito River, which passes crossing western part of the town. The flow of these rivers was persistent throughout the year except the part of Chore River, which dries up during a long dry season. An estimated 11, 000 people were living nearby these three rivers and were at higher risk of acquiring S. mansoni infection (Information obtained from Jimma Zone Health Office).
Minimum sample size was estimated to be 193 considering 14.8 % prevalence of S. mansoni (10), 5% precision and 95% level of confidence. However; in attempt to have better coverage of the study population with available resources, it was decided the maximum sample size to be 5% (550) of the population at risk. Thus, considering family size of 6.3, the number of households would be 87. With emphasis given to households living nearby water contact points along the rivers, 1334 houses were numbered. Then, systematic sampling technique was used to include all members from every 15th houses, which resulted a total of 517 residents to participate in this study. Individuals who had taken treatment for any intestinal parasites during the month prior to the survey and those who lived in the area for less than one year were excluded.
Study subjects were interviewed about demographic and behavioral risk factors using structured questionnaires. Physical examination was carried out by a nurse to assess signs and symptoms of S. mansoni infection. Furthermore, stool samples were collected and processed with Kato-Katz technique using template delivering 41.7 mg of stool plug (11). Then, slides were transferred to the laboratory of Jimma University and read once by the principal investigator. Conversions of egg counts into egg per gram (e.p.g) of stool were done by a factor of 24. Intensity of S. mansoni infection was estimated using e.p.g and classified as light (1–99 e.p.g), moderate (100–399 e.p.g) and heavy infections (>399 e.p.g) (8).
Residents were asked to indicate sites in the rivers where people often come in contact with water during washing, swimming, crossing and other activities. A scoop was used to collect snails from rivers at water contact points where at each site 30 minutes was spent to search and gather as many Biomphalaria species as possible. Morphological keys were used to identify Biomphalaria species as indicated (12); but other snails were not differentiated. Biomphalaria snails collected from each river were transferred in to labeled plastic buckets with water and vegetation (salad and cabbage). Then after, each snail were transferred into labeled vial containing aged water, exposed to light for one hour and checked for shading of cercariae. Attempt was not made to detect non-shedding infection; only Biomphalaria snails shading one or more cercariae with bifurcated tail were considered to have Schistosoma infection. Cercariae were detected using a microscope; however, definite identification of species was not possible due to constraint of resource.
Data was analyzed using SPSS version 13.0 and results were summarized using mean and percentages as appropriate. Differences in proportions and means were evaluated using chi-square and One-Way Anova, respectively. Bivariate and multivariate logistic regression analyses were used to assess the crude and adjusted effect of seemingly significant predicators of the S. mansoni infection. Odds ratio with 95% confidence interval was used to measure the strength of association and p-value of <0.05 was considered to be significant.
The study was approved by the Ethics committee of Medical Faculty, Addis Ababa University as well letter of support was obtained from Jimma Zone Health office. Study subjects were informed about the purpose of the study and oral consent was obtained from each study subject or their parents. Any information obtained from participants during the study was kept confidential. Participants found to be positive for intestinal parasites were treated with anti-parasitic drugs as recommended by the World Health Organization (8). Accordingly, those with S. mansoni infection were treated with praziquantel 40 mg/kg body weight, single dose.
Results
A total of 517 individuals participated in this study. The majority (49.3%) of them were residents around Chore River; whereas, participants nearby Awetu and Kito Rivers accounted 35.6% and 15.1 %, respectively. The mean age of participants was 22.3 and the male to female ratio was 1:2.1.
The cumulative point prevalence of S. mansoni infection in the study population was 136 (26.3%), and it peaked at 10–19 years of age. The intensity of S. mansoni infection ranges from 24 to 936 e.p.g. Among those infected subjects, the rates of light, moderate and heavy infections were 69 (50.7%), 54 (40.0%) and 13 (9.6%), respectively. The intensity of infection peaked at 10–19 years of age, which is similar to the prevalence rate. The arithmetic mean e.p.g was found to differ by gender in which males had higher intensity of infection compared to females (p= 0.03). However, the observed difference in arithmetic mean e.p.g among age groups was not significant (p = 0.43).
As presented in table 1, chi-square analysis shows statistically significant associations of S. mansoni infection with age, gender, religion and site of residence (p <0.05). Moreover, contact with river water while bathing, fetching, swimming, crossing, and washing clothes were all associated with S. mansoni infection (Table 2).
Table 1.
Sociodemographic characteristics and proportion of S. mansoni infection in different categories of the study subjects in Jimma town, 2007.
| Characteristics | Total (%) | Number (%) infected with S. mansoni |
Chi-square value |
P- value | |
| Residence site | |||||
| Kito | 78 (15.1) | 7(9.0) | 14.4 | 0.001 | |
| Chore | 255(49.3) | 77(30.2) | |||
| Awetu | 184 (35.6) | 52(28.3) | |||
| Age (years) | |||||
| < 10 | 148 (28.6) | 26 (17.6) | 27.7 | 0.000 | |
| 10 – 19 | 135 (26.1) | 57 (42.2) | |||
| 20 – 29 | 76 (14.7) | 20 (26.3) | |||
| 30– 39 | 63 (12.2) | 10 (15.9) | |||
| 40– 49 | 45 (8.7) | 12 (26.7) | |||
| ≥ 50 | 50 (9.7) | 11 (22.0) | |||
| Sex | |||||
| Female | 350 (67.7) | 79 (22.6) | 7.8 | 0.005 | |
| Male | 167 (32.3) | 57 (34.1) | |||
| Religion | |||||
| Orthodox | 248 (48) | 58(23.4) | 6.4 | 0.040 | |
| Muslim | 160 (30.9) | 39(24.4) | |||
| Protestant | 107 (21.1) | 39(35.8) | |||
Table 2.
Infection of S. mansoni in relation to river water contact activities in Jimma town, 2007.
| Characteristics | Total (%) | Number (%) infected with S. mansoni |
Chi-square value |
P- value | |
| Bathing in rivers | |||||
| No | 341(66.0) | 65(19.1) | 27.1 | 0.000 | |
| Yes | 176(34.0) | 71(40.3) | |||
| Fetching water from rivers | |||||
| No | 406(78.5) | 93(22.9) | 11.7 | 0.01 | |
| Yes | 111 (21.5) | 43(38.7) | |||
| Swimming habit | |||||
| No | 418(80.9) | 79(18.9) | 61.7 | 0.000 | |
| Yes | 99(19.1) | 57(57.6) | |||
| River water contact while crossing |
|||||
| No | 226 (43.7 | 23 (10.2) | 53.9 | 0.000 | |
| Yes | 291 (56.3) | 113(38.8) | |||
| Washing cloths in rivers | |||||
| No | 300 (58) | 56(18.7) | 21.5 | 0.000 | |
| Yes | 217(42) | 80(36.9) | |||
However, after adjustment for significantly associated variables using multivariate logistic regression analysis, residents nearby Awetu River were found to have higher risk of infection compared to those around Kito River, OR = 2.67 (95% CI; 1.06 – 6.75). The highest risk of S. mansoni infection was also shown in the age range 10–19 years compared with those less than 10 years of age, OR = 2.19 (95% CI; 1.10 – 4.34). After adjustment, the role of religion to influence rate of S. mansoni infection remained also significant. Similarly, contact with water while swimming, OR = 2.59 (95% CI; 1.37 – 4.91, and crossing rivers, OR = 3.77 (95% CI; 1.79 – 7.95), significantly raised the odds of S. mansoni infection compared to those with no habit of swimming and crossing, respectively (Table 3).
Table 3.
Multivariate logistic regression analysis on variables found significant predictors of S. mansoni infection by chi-square test in Jimma town, 2007.
| Characteristics | Crude OR (95% CI) |
Adjusted OR (95% CI) |
P - value* | |
| Residence site | ||||
| Kitto | 1 | 1 | ||
| Chore | 4.39(1.93–9.97) | 2.22(0.88–5.69) | 0.090 | |
| Awetu | 4.00(1.73–9.26) | 2.67(1.06–6.75) | 0.038 | |
| Age (years) | ||||
| < 10 | 1 | 1 | ||
| 10 – 19 | 3.43(1.99–5.91) | 2.19(1.10–4.34) | 0.025 | |
| 20 – 29 | 1.68(0.86–3.25) | 1.54(0.69–3.45) | 0.293 | |
| 30– 39 | 0.89(0.40–1.97) | 0.94(0.37–2.37) | 0.900 | |
| 40– 49 | 1.71(0.78–3.74) | 2.07(0.82–5.30) | 0.125 | |
| ≥50 | 1.32(0.60–2.92) | 1.53(0.61–3.85) | 0.365 | |
| Sex | ||||
| Female | 1 | |||
| Male | 1.78(1.18–2.67) | 1.66(0.99–2.80) | 0.054 | |
| Religion | ||||
| Orthodox | 1 | 1 | ||
| Muslim | 1.06(0.66–1.68) | 1.57(0.91–2.72) | 0.106 | |
| Protestant | 1.83(1.12–2.98) | 1.91(1.09–3.34) | 0.025 | |
| Swimming habit | ||||
| No | 1 | 1 | ||
| Yes | 5.82(3.65–9.30) | 2.59(1.37–4.91) | 0.004 | |
| River water contact while crossing |
||||
| No | 1 | 1 | ||
| Yes | 5.60(3.43–9.16) | 3.77(1.79–7.95) | 0.000 | |
| Washing cloths in rivers | ||||
| No | 1 | 1 | ||
| Yes | 2.54(1.71–3.80) | 0.88(0.45–1.72) | 0.707 | |
| Bathing in rivers | ||||
| No | 1 | 1 | ||
| Yes | 2.87(1.92–4.30) | 0.96(0.55–1.66) | 0.873 | |
| Fetching water from rivers | ||||
| No | 1 | 1 | ||
| Yes | 2.13(1.36–3.33) | 1.12(0.63–2.00) | 0.694 | |
P - value obtained in multivariable logistic regression analysis
Of the total study participants, 333 (66.4%) reported at least one clinical sign and symptoms of intestinal schistosomiasis. S. mansoni infection was detected in 90 (27%) of individuals with at least one clinical feature compared with individuals with no any clinical manifestation of schistosomiasis, 46 (25%) (p = 0.19). Abdominal pain was the most frequent complaint and reported in 73 (53.6%) of those with S. mansoni infection. On physical examination, splenomegaly or hepatomegaly was detected in 8 (1.5%) of the study participants. However, any of these clinical signs and symptoms was not significantly associated with rate of S. mansoni infection (p > 0.05).
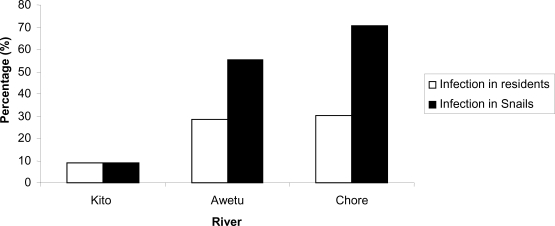
The rivers were observed to harbor snails of different genera and with varying degree of snail infestation. Among the rivers, Chore River was the most infested with Biomphalaria species. The cumulative rate of Biomphalaria species shading cercariae was 325 (58.0%). Rate of cercarial shading was observed to differ among the three rivers in which the highest rate was observed in snails collected from Chore River (Table 4). Trend in the rate of cercarial shading by Biomphalaria species coincides with rate of S. mansoni infection among residents nearby the corresponding river from where the snails were collected (figure 1).
Table 4.
Rate of cercarial shading Biomphalaria snails collected from rivers in Jimma town, 2007.
| River | Number (%) of collected Biomphalaria snails |
Number (%) of cercarial shading Biomphalaria snails |
Chi-square | P value |
| Chore | 302 (54.0) | 213 (70.5) | 84.8 | 0.000 |
| Awetu | 192 (34.3) | 106 (55.2) | ||
| Kito | 66 (11.8) | 6 (9.1) | ||
| Total | 560 (100) | 325 (58.0) |
Figure 1.
Rates of cercarial shading Biomphalaria snails and S. mansoni infection in residents nearby respective rivers in Jimma town, 2007.
Discussion
The point prevalence, intensity and risk factors of S. mansoni infection were determined in people dwelling around the three rivers (Chore, Kito and Awetu) in Jimma town. And also an attempt was made to assess the rate of cercarial shading status in Biomphalaria snails collected from the three rivers. The cumulative point prevalence of S. mansoni was 26.3 %, with intensity of infection ranging 24 to 936 e.p.g. In comparison, lower rate of infection (14.8%) was reported previously in the general population of Jimma town (10). This difference in prevalence rate may be because the study subjects were at relatively higher risk as households included had close contact with potentially infective rivers. Moreover, the effect of time and different laboratory methods used may have attributed for the observed disparity.
The prevalence and intensity of S. mansoni infection peaked among participants in the age range 10–19 years, and a similar finding was also reported in Kenya (13). On the other hand, a bimodal pattern in peak prevalence and intensity of S. mansoni infection was reported elsewhere (14). S. mansoni infection occurred irrespective of gender; in contrast to several results revealing the preponderance of infection among men (15–17). The shared water contact activities among both sex categories may justify the similar rate of infection in the current study. However, our result indicating the significance of religion to influence rate of S. mansoni infection deserves further investigation as similar observation is uncommon.
Snail collection has been proposed for identifying water bodies that are transmission sites for schistosomiasis. It was observed that rivers of Chore and Awetu were more infested with Biomphalaria snails in comparison with Kito River. This may be due to the fact that the former two rivers were containing adequate vegetation which favor the intermediate host to flourish as compared with Kito River, which is swampy and hence, unfavorable for snails.
As to the rate of infected intermediate host (Biomphalaria species), the majority (58.0%) of the snails collected from rivers were shading cercariae. A previous report from Jimma town showed a 50% rate of infection in the intermediate host (7). In contrast to this study, however, lower infection rate was shown in a study conducted on Lake Zway (0.5% – 6%) (18). This difference may be due to efficiency of parasite transfer from vertebrate to snail host, which is determined by factors including seasonal variation, snail density, prevalence and intensity of Schistosoma species and deposition rate of Schistosoma eggs in water bodies. It has been also noted that the rate of cercarial shading snails coincides with S. mansoni infection among residents living nearby corresponding rivers. However, Biomphalaria species was known to shade human and animal schistosomes and morphologic differentiation of S. mansoni from other cercariae is practically impossible. Thus, the possibility of shaded cercariae other than S. mansoni species could not be ruled out and this limitation should be taken in to account when interpreting the result of this study.
Overall, as classified by the World Health Organization (8), a moderate (10–50%) prevalence of S. mansoni infection was revealed among communities living nearby the three rivers of Jimma town and the rate of snails shading cercariae coincides with the rate of S. mansoni infection among residents. This preliminary data highlights swimming and walking through the rivers are significant risk factors for acquiring S. mansoni infection. Therefore, a multi-faceted control approach is direly needed to reduce the burden of S. mansoni in Jimma town. Interventions leading to reduction of water contact (swimming and crossing the rivers), improved environmental sanitation in the rivers' catchments, construction and appropriate use of latrine, safe water supply, snail control, health education and targeted chemotherapy of the affected communities should be systematically planned to make control efforts achievable.
Acknowledgments
We are very grateful to the study subjects with out whom this study would not have been realized. We also thank Jimma Zone Health Office and Jimma University for laboratory facilities provided, and finally Addis Ababa University for providing financial support made to conduct the study.
References
- 1.Gool TV, Vetter H, Vervoort T, et al. Serodiagnosis of imported schistosomiasis. J Clin Med. 2002;40(9):3432–3437. doi: 10.1128/JCM.40.9.3432-3437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan American Health Organization regional office of the World Health Organization Report, author. PAHO/ WHO preparatory meeting on epidemiological data needed to plan elimination of Schistosomiasis in the Caribbean. 2007. [23/10/2009]. http://www.who.int/schistosomiasis/resources/PAHO_report_Schistosomiasis_carribean.pdf.
- 3. [23/10/2009];World Health Organization Weekly epidemiologic record. 2006 81:145–164. http://www.who.int/wer/2006/wer8116.pdf. [Google Scholar]
- 4.Abebe F. Transmission dynamics of Schistosoma mansoni in an irrigation setting in Ethiopia. Ethiop J Health Dev. 1995;9(3):147–156. [Google Scholar]
- 5.Shibru T, Leykun J. Schistosomiasis and its distribution in Ethiopia and Eritrea. In: Hailu B, Shibru T, Leykun J, editors. Schistosomiasis in Ethiopia and Eritria. 2nd ed. Addis Ababa: Institute of Pathobiology Addis Ababa University; 1998. pp. 1–18. [Google Scholar]
- 6.Ali A, Erko B, Woldemichael T, Kloos H. Schistosomiasis. In: Berhane Y, Hailemariam D, Kloos H, editors. Epidemiology and Ecology of Health and Diseases in Ethiopia. 1st edition. Addis Ababa: Shama books; 2006. pp. 660–673. [Google Scholar]
- 7.Fekadu A, Mekuria L, Hailu B, et al. Malacology. In: Hailu B, Shibru T, Leykun J, editors. Schistosomiasis in Ethiopia and Eritria. 2nd edition. Addis Ababa: Institute of Pathobiology Addis Ababa University; 1998. pp. 114–122. [Google Scholar]
- 8.World Health Organization (WHO), author Prevention of schistosomiasis and soil transmitted helminthiasis: Report of WHO Expert Committee. WHO Technical Report Series 912. Geneva: 2002. [PubMed] [Google Scholar]
- 9.Federal Democratic Republic of Ethiopia, Central Statistical Authority of Ethiopia. National Statistics. Addis Ababa: Ethiopia; 2005. [Google Scholar]
- 10.Mengistu A, Gebre-Selassie S, Kassa T. Prevalence of intestinal parasitic infections among urban dwellers in southwest Ethiopia. Ethiop J Health Dev. 2007;21(1):12–17. [Google Scholar]
- 11.Cheesbrough M. Medical Laboratory manual for tropical countries: Part 1. 2nd ed. London: Cambridge University; 2001. pp. 216–239. [Google Scholar]
- 12.Brown DS. Fresh water snails of Africa and their medical importance. 2nd edition. London: Taylor & Francis; 1994. [Google Scholar]
- 13.Handzel T, Karanaja DM, Addiss DG, et al. Geographic distribution of schistosomiasis and soil transmitted helminthes in western Kenya. Am J Trop Med Hyg. 2003;69(3):318–323. [PubMed] [Google Scholar]
- 14.Shewakena F. S. mansoni in Jiga town, Gojam administrative Region. Ethiop J Health Dev. 1998;9(1):1–6. [Google Scholar]
- 15.Erko B, Medhin G, Berhe N, Abebe F, Gebre-Michael T, Gundersen SG. Epidemiological studies on intestinal schistosomiasis in Wondo Genet, southern Ethiopia. Ethiop Med J. 2002;40(1):29–39. [PubMed] [Google Scholar]
- 16.Tiruneh M, Fantahun M, Kassu A, Tiruneh G, Van Lieshout L. Schistosomiasis mansoni in school attenders and non-attenders in Northwest Ethiopia. Ethiop J Health Dev. 2001;15(2):117–123. [Google Scholar]
- 17.Birrie H, Abebe F, Gundersen SG, Medhin G, Berhe N, Gemetchu T. Epidemiology of schistosomiasis mansoni in three endemic communities in north-east Ethiopia: baseline characteristics before endod based intervention. Ethiop Med J. 1998;36:101–111. [PubMed] [Google Scholar]
- 18.Erko B, Balcha F, Kifle D. The ecology of Biomphalaria sudanica in Lake Ziway, Ethiopia. Afr J Ecol. 2006;44(3):347–352. [Google Scholar]