Abstract
Organophosphate compounds are the organic derivatives of Phosphorous containing acids and their effect on neuromuscular junction and Autonomic Synapses is clinically important. After exposure these agents cause acute and sub acute manifestations depending on the type and severity of the agents like Acute Cholinergic Manifestations, Intermediate Syndrome with Nicotinic features and Delayed Central Nervous System Complications. The patient reported here had severe Organophosphate Poisoning with various rare complications on a succession. This is the first report of Organophosphates Poisoning complicated by Intermediate Syndrome and Organophosphate Induced Delayed Polyneuropathy in Ethiopia and it is reported to increase awareness of health care workers on these rare complications of a common problem.
Introduction
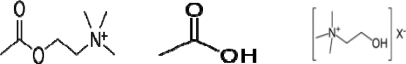
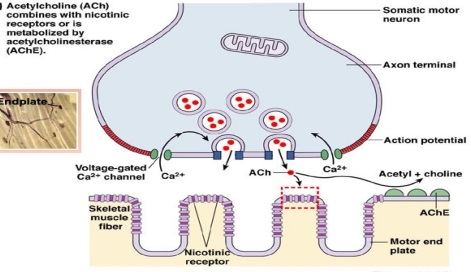
Organophosphate compounds are the organic derivatives of Phosphorous containing acids and their effect on Neuromuscular Junction and Autonomic synapses is clinically important. In the Neuromuscular Junction Acetylcholine is released when a nerve impulse reaches terminal axonal end and it diffuses across the Synaptic Cleft and binds to Cholinergic Nicotinic receptors on the muscle fibers, causing them to contract. The enzyme, Cholinesterase splits Acetylcholine into Acetic Acid and Choline, thus stopping its action (Figure 1). The end products of the metabolism of Acetylcholine are taken up by nerve fibers and resynthesized into Acetylcholine. In Organophosphate poisoning the Cholinesterase are phosphorylated by the Phosphate end of Organophosphates; then the net result is accumulation of excessive Acetyl Chlorine with resultant effect on Muscarinic, Nicotinic and central nervous system (Figure 2).
Figure 1.
Acetylcholine, acetic acid, and choline
Figure 2.
Organophosphate compound (OPC), the top and neuromuscular junction (NMJ), the bottom.
Following classical OP poisoning, three well defined clinical phases are seen: Initial Acute Cholinergic Crisis, the Intermediate Syndrome and Delayed Polyneuropathy (OPIDPN) .Here severe and prolonged cholinergic crisis with unusual complications, notably Intermediate Syndrome and OPIDPN are described in the same patient in different course of time. As far as the researcher's knowledge is concerned, this is the first report of Intermediate Syndrome and OPIDPN in Ethiopia.’
Case Report
The patient was admitted to TASH for three weeks and discharged with improved status but three days after discharged, he had new clinical manifestations and these details are described.
Immediate features and management
A nineteen years old male patient from Addis Ababa who ingested unquantified amount of Malathion, which was kept at home, in an attempt to commit suicide on January 3, 2007.
After he took the poison, he was found fallen and was given milk by the family members. Subsequently he lost consciouseness and was taken to a clinic on the same date where Atropine 15mg IV every 15 minutes , Ceftraxone 1gm IV every 12 hours and unquantified amount of Charcoal were adminstered. According to the referral and family informants, after three days he showed improvement with the recovery of consciousness and the medications were discontinued but he lost conscoiusiness again while he was still in the clinic and was referred to Tikur Anbessa specialized hospital(TASH) on January 6,2011.
On physicale examination at Tikur Anbesa Specialized Hospital (TASH) emergency room his blood pressure was 90/60mmHG, pulse rate 76/min, respiratory rate 16/min and temprature was 37.9°c. He had Rhonchi all over the chest, pupils were pinpointed and Glasgow Coma Scale (GCS) was 3/15. He also had paradoxic abdominal muscle movement. On investigations white blood cell count was 11,500/dl, Hematocrit 45.2%, Platele count 129,000/dl,Erythrocyte Sedimentation rate 24/hr and Random Blood Sugar 108mg/dl. However, Serum Transaminases, Alkaline Phosphatases and liver and renal function tests and Serum Electrolytes were in normal range.Futhermore, chest X-ray was normal and ECG showed prolonged QTc interval(49ms).Oxygen saturation was 80% with 6L/min flow of oxygen through nasal prongs but subsequently with face mask it was consistently above 92%.
He was admitted to the Medical Intensive Care Unit (MICU) and with an assessement of severe Organophosphate poisoning with Acute Cholinergic Crisis and Intermediate Syndrome and with Presumptive Diagnoses of type II respiratory failure.He was atropinized with Atropine 2mg stat and then 2 mg every 15 minutes, Charcoal 50 gm was given with in 4 hrs and 25 gm was continued every 6 hour. He had intensive monitoring of vital signs and organ functions. Ventilatory support was not started since ventilators were occupied by other patients but he was continued on large dose of Atropine(upto 90md/24hrs) and oxygen adminstration of 6L/min by face mask (Table 1).
Table 1.
Dose of atropine at TASH
| Time from date of malathion ingestion | Range of atropine dosage in 24 hrs |
| Day4–10 | 2 mg every 15–20 minutes |
| Day 11–14 | 2 mg every 1–4 hours |
| Day 15–19 | 1 mg every 1–6hrs |
| Day 20–22 | 1 mg every 12–24 hours |
| Day 23–25 | 0.4mg po QID |
| Day 25(discharged) | discontinued |
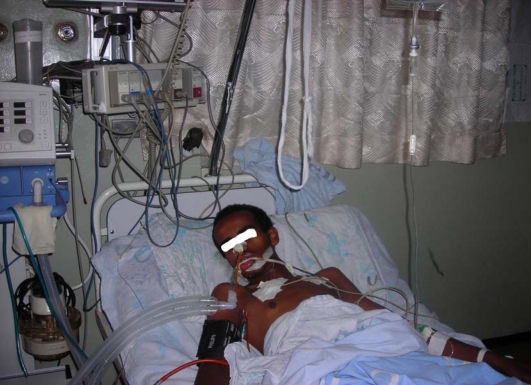
His GCS was 3/15 in the first 48hrs in the MICU after that he started to utter words but subsequently the level of consciuosness was waxing and waning.Therefore, he was continued with large dose of atropine with adminstration of 2mg every 15minutes and on the third day of MICU admission he was intubated and put on ventilatory support(Figure 3).In the meanwhile he developed Tonic Clonic Seizure and was started on Diazepam 5 mg IV stat and it was maintained with dose of 30mg in 1000cc of IV fluids every 12 hours for about 14 days and then discontiued after improvement of seizure.
Figure 3.
The patient with Organophosphate poisoning on mechanical ventilator at MICU of TASH
Cholinergic and central nervous system symptomes with pupillary constriction, respiratory hypersecreations and flactuating consciousness were pecuilar features with in the first three weeks; mental status was deteriorating when the dosage of Atropine was titrated down. By the end of three weeks mental status,pupilary signs ,hypersecreation improved; he was extubated from ventilator;subsequently Atropine and Diazepam were spaced and discontinued. Finally,at the end of third week he was discharged after psychiatric evaluation and caunselling was provided and was able to walk without support.
Post discharge manifestations
Three days after discharge from the hospital he devloped numbness of both feet which was followed by weakness of both lower extremities which progresssed to involve upper extremity and urinary and feacal incontinence.On physical examination he was conscious and oriented. Power on both lower extremity was 0/5, while on upper extremity it was 3/5. Deep tendon reflexes were depressed at both extremities while tone was reduced on the lower extremity and all modalities of sensory examinations were normal. Further investigations after discharge showed normal Hematological and blood chemistry tests; was negative for HIV and had normal Myelography and spine x-rays.In addition,nerve conduction test showed no motor response from Tibial and Peroneal nerves and there was severe reduction in amplitude of right Median motor nerve. However the sural and plantar sensory nerves were normal.Thus,severe motor axonal neuropathy sparing sensory nerves was concluded (Table 2).
Table 2.
Nerve conduction study
| Normal value | Patient value | ||
| Motor nerve conduction NCV(m/S) |
|||
| Tibial(R) | >48 | NR | |
| Tibial(L) | >48 | NR | |
| Peroneal ( R) | >44 | NR | |
| Peroneal(L) | >44 | NR | |
| Median (R) | >52 | NR | |
| Median (L) | >52 | NR | |
| CMAP | |||
| Tibial (R ) | >5 | NR | |
| Tibial (L ) | >5 | NR | |
| Peroneal ( R) | >2 | NR | |
| Peroneal ( L) | >2 | NR | |
| Median (R ) | >4 | 0.3 | |
| SENSORY NERVE CONDUCTION SNAP amplitude ( micro Volt) |
|||
| Sural Nerve (R ) | >5 | 6 | |
| Sural Nerve (L ) | >5 | 26 | |
| Medial plantar( R) | >10 | 28 | |
CMAP = Compound muscle action potential, NCV = Nerve conduction velocity; SNAP = Sensory nerve action potential; NR =No response.
Then physiotheray was started and subsequently his Sphincter function recovered. He showed improvement of upper extremity with which he started feeding himself but there was no improvement of lower extremity and is wheelchair dependent now.
Discussion
Organophosphate poisoning is one of the commonest types of poisoning in Ethiopia for suicidal intent and is one of the commonest causes of intensive care unit admissions and mortality (1, 2, 3, 4). In the case reported here it was severe poisoning and majority of complications described in literatures were observed in succession in this patient (6, 7, 8, 11) although confirmatory tests like RBC Cholinesterase level were not determined. One of the challenging situations in our case was intractably prolonged cholinergic features and central nervous system manifestations like coma and seizure. These manifestations were recurring and the patient was kept on high dose Atropine for three weeks. Similarly, Chia-Chang described in a case report of a 28 years Taiwanese woman a prolonged Cholinergic features for many days and needed Atropine up to 80 mg in an hour with a total dose of 11,665 mg in 17 days who also took Pralidoxime (11). Such delayed manifestations are reported to occur because of Acetylcholiesterase Enzyme (AChE) aging, poor rephosphorylation and decreased synthesis of new enzymes (6,10).
Our patient developed respiratory failure at the fourth day of poisoning which is typical duration for Intermediate Syndrome. Intermediate Syndrome occurs between the initial acute Cholinergic manifestations and the late Organophosphate Induced Delayed Neuropathy and was first described by wadia etal (9) but the name of the syndrome was given by Karalliedde (7). The basis for Intermediate Syndrome (the Nicotinic Syndrome) is that Nicotinic transmission requires inhibition of at least 80% of the synaptic AChE unlike the Muscarinic Synapses and nerve endings where AChE can be easily inhibited and the Nicotinic Syndrome occurs only in severe poisoning. The end result is hyperstimulation of the Neuromuscular Junction by excessive Acetylcholine, initially resulting in fasciculation, which later is followed by Neuromuscular Paralysis; the effect of intermediate syndrome may last for 2–18 days (7,8,9). In Intermediate Syndrome characteristically muscles of the neck, proximal limb, and the eyes, bulbar and respiratory groups are affected. In our patient respiratory muscle groups were affected causing respiratory failure but other groups of muscles have not been affected. In Organophosphate Poisoning respiratory failure is very important complication which can lead to significant morbidity and mortality. In addition to respiratory muscle weakness other contributing causes of respiratory failure could be respiratory tract infections, air way obstruction by secretions, Pulmonary Edema, and Central Respiratory Center Depression.
The late feature which occurred in our patient was sub acutely developed weakness of lower and upper extremity which was diagnosed to be Polyneuropathy clinically and in nerve conduction study. There has been diagnostic challenge in this phase as it was a rare phenomenon; Myelography and spine x-rays were ordered which turned out to be normal. OPC are known to cause delayed neurological complications, notably Organophosphates Induced Delayed Polyneuropathy (OPIDPN). OPIDPN is typically a subacute sensory motor nerve disease occurring 2–4 weeks after OPC poisoning and affects mainly the distal groups of muscles earlier. Spinal cord can also be affected in delayed neurologic manifestations Organophosphate associated Myelopathy (5,11) and this may be the cause of fecal and urinary incontinence in our patient although MRI was not done. The occurrence of OPIDN is said to follow the phosphorylation and subsequent ageing of an enzyme in axons called as Neuropathy Target Esterase (8). In this case the sub acute course of neurological manifestations coming after weeks of Organophosphate poisoning is classical for OPIDPN. Commonly the Clincopathology is sensory motor involvement of the peripheral nerves but pure or predominant motor axononal involvement similar to our patient has also been reported (14). The management in severe Organophosphates poisoning is supportive care of Homeostasis, administration of high dose of Atropine and rephosphorylation attempts by Oximes. Airway control and adequate Oxygenation are paramount in Organophosphate poisonings and Intubation may be necessary in cases of respiratory distress. Immediate aggressive use of Atropine may eliminate the need for intubation, like up 3–7mg bolus and 1–2 mg every 3–5min until desired effects are obtained. Adequate Atropinization is demonstrated by assessing a combination of signs including pupils, pulse rate, pulmonary secretions and mental state. It is not desirable to use any one criterion alone, because cases are seen where pupils do not dilate or pulse does not become fast in spite of adequate doses. Once Atropinized, a maintenance type dose at 1–3 mg 1/2 hourly is usually sufficient (12). The next unclear issue is about Oximes such as P2AM. P2AM is generally given in most intensive care units at a dose of 1 gm 4 to 6 hourly. Oximes displace the Organophosphates from the Acetylcholine Esterases and bind to the enzyme itself. Although these agents appear useful theoretically, in practice their effect is not confirmed in human studies to be useful. For instance in 1991, De Silva studied the treatment of Organophosphate poisoning with Atropine and 2-PAM and, later the same year, with Atropine alone. He found that Atropine seemed to be as effective as Atropine plus 2-PAM in the treatment of Acute Organophosphate Poisoning (13).Management of intermediate syndrome and OPIDPN is by early detection and supportive care like the case of respiratory failure management. There is no specific therapy for the late-onset Polyneuropathy due to Organophosphate compounds.
In conclusion, patients with severe Organophosphate Poisoning can have delayed Cholinergic manifestations which would need large dosage of Atropine administration and thorough evaluation of clinical features is needed to titrate the dose down and discontinue it. Moreover, careful follow-up is needed for the rare complications of Organophosphate Poisoning like Intermediate Syndrome and OPIDIN. Therefore, I recommend health care workers should be aware of these complications, their manifestations and how to manage them.
References
- 1.Abula T, Wondmikun Y. The pattern of acute poisoning in a teaching hospital, north-west Ethiopia. Ethiop Med J. 2006;44:183–189. [PubMed] [Google Scholar]
- 2.Mekonnen Desalew, Azaje Aklilu, Amare Amanuel, Melkie Addisu, Tesfaye Ethiopia Pattern of acute adult poisoning at Tikur Anbessa specialized teaching hospital, a retrospective study. Hum Exp Toxicol. 2011 Jul;30(7):523–527. doi: 10.1177/0960327110377520. [DOI] [PubMed] [Google Scholar]
- 3.Abebe M. Organophosphate pesticide poisoning in 50 Ethiopian patients. Ethiop Med J. 1991;29:109–118. [PubMed] [Google Scholar]
- 4.Mekuria Y, Tesfamariam T, Tefera S. Insecticides sold in the streets of Addis Ababa for domestic use and the possible health hazard associated with the practice. Ethiop J Health Dev. 1984;1:73–77. [Google Scholar]
- 5.Koelle GB. Pharmacology and toxicology of organophosphates and carbamates. 1992 [Google Scholar]
- 6.Singh S, Sharma N. Neurological syndromes following organophosphate poisoning. Neurol India. 2000;48:308–313. [PubMed] [Google Scholar]
- 7.Senanayake N, Karalliedde L. Neurotoxic effects of organophosphorus insecticides: An intermediate syndrome. N Engl J Med. 1987;316:761–763. doi: 10.1056/NEJM198703263161301. [DOI] [PubMed] [Google Scholar]
- 8.Wadia RS, Chitra S, Amin RB, Kiwalkar RS, Sardesai HV. Electrophysiological studies in acute Organophosphate poisoning. J Neurol Neurosurg Psychiatry. 1987;50:1442–1448. doi: 10.1136/jnnp.50.11.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh G, Khurana D. Neurology of acute organophosphate poisoning. Neurol India. 2009;57:119–125. doi: 10.4103/0028-3886.51277. [DOI] [PubMed] [Google Scholar]
- 10.Karalliedde L, Senanayake N. Organophosphorous insecticide poisoning. Br J Anaesth. 1989;63:736–750. doi: 10.1093/bja/63.6.736. [DOI] [PubMed] [Google Scholar]
- 11.Chia-Chang Chuang, Thy-Sheng Lin, Ming-Che Tsai. Delayed Neuropathy and Myelopathy after Organophosphate Intoxication. N Engl J Med. 2002;347:1119–1121. doi: 10.1056/NEJM200210033471421. [DOI] [PubMed] [Google Scholar]
- 12.Wadia RS. Treatment of Organophosphate Poisoning. Indian J Crit Care Med. 2003;7:85–87. [Google Scholar]
- 13.De Silva HJ, Wijewickrema R, Senanayake N. Does pralidoxime affect outcome of management in acute organophosphorus poisoning? Lancet. 1992;339:1136–1138. doi: 10.1016/0140-6736(92)90733-j. [DOI] [PubMed] [Google Scholar]
- 14.N Nand, HK Aggarwal, Komal Bharti, Chakrabarti D. Organophosphate Induced Delayed Neuropathy. JAPI. 2007 Jan;:55. [PubMed] [Google Scholar]