Abstract
Background
Globally, millions of people suffer from intestinal parasitic infections. These infections are among the most common resulting in considerable morbidity and mortality. In Ethiopia and particularly in Jimma and its surroundings intestinal parasitic infections are highly prevalent because of low living standards and poor environmental sanitation. The objective of the survey was to determine the prevalence and predictors of intestinal parasitosis among school children in four woredas of Jimma zone surrounding Gilgel gibe hydraulic dam and serve as a base line data to help evaluate health promoting activities for the future and monitor those already delivered to the community.
Methods
A cross-sectional study was carried out in October, 2008 in four Woredas of Jimma zone bordering Gilgel Gibe Dam. Children attending grades 1–8 in the schools located within 10 Kms ofthe Dam in the four bordering woredas and those living 30 Kms away from the shore line were the study subjects. Six hundred twenty four and 321 children were selected from the schools around Gilgel Gibe dam and from the schools in Bulbul, respectively. Data on background of participant was collected and stool specimen collected and processed. Data were filtered and entered into computer then analyzed using SPSS for windows version 13.0.1.
Results
Of the 937 selected individuals, 855 participated in the study giving a response rate of 91.2%. The prevalence of intestinal parasitosis was 47.1% where 174 (20%) had Ascaris lumbricoides monoinfection; 4.3% had dual infection involving Ascaris lumbricoides and hookworm and 0.2% had triple infection but all the infections were of light intensity. In addition, there was no association between prevalence of intestinal parasitosis with availability or regular use of latrine and clinical symptoms.
Conclusion
The prevalence and intensity of intestinal parasites in the study area is lower than national, urban and rural setting of Jimma zone. These might be due to a better awareness of the study community on prevention of intestinal parasitosis following increased health promoting activities in the area, delivered through various activities of Jimma Public health training program.
Keywords: intestinal parasitosis, Gilgel-Gibe, Southwest Ethiopia
Introduction
Globally, millions of people suffer from intestinal parasitic infections. More than 1.2 billion people are infected with Ascaris lumbricoides;740 million people with hookworm;795 million with Trichuris trichiura and 300 million with enterobiasis. (1, 2).These infections are among the most common in the world, being responsible for considerable morbidity and mortality. Intestinal parasitic infections are documented as serious public health problems because complications such as iron deficiency anemia, growth retardation in children and other physical and mental health problems with serious consequences may occur (3).
Intestinal parasites are highly prevalent in developing countries; this is said to be due to unsafe human waste disposal systems, inadequacy and lack of safe water supply, and low socioeconomic status (4, 5, 6). In sub-Saharan African countries; up to 250 million people are estimated to be infected with at least one or more species of intestinal nematodes (7). In Ethiopia, intestinal parasitic infections are highly prevalent because of low living standards, poor environmental sanitation, and other reasons mentioned above (4). Although the prevalence rates of individual parasites vary considerably with altitude in different parts of the country, several studies show that Ascaris lumbricoides is the most prevalent intestinal parasite, followed by Trichuris trichiura, and hookworm (4, 8–11). Previous studies conducted to evaluate the prevalence of intestinal parasitosis in Jimma zone showed high prevalence rate of intestinal infection in both urban and rural settings of the zone (12, 13, 14).
Studies to assess risk factors for intestinal parasitosis are very few, incomplete and inconsistent. One study which was done in children with age between 7–10 years in Turkey showed no association between intestinal parasitic infections and clinical symptoms (15). Another study which was done in children of aged 17 years and below from East African countries showed 50% prevalence rate and all infected children were asymptomatic at diagnosis (16). According to a study done in Jimma zone; there was association between sex and prevalence rates of mono-parasite but not poly-parasites. In the study, majority of the affected were females as compared to males (92.6 Vs 81.4%). The study showed no associations between rate of infection and family size, latrine usage, source of drinking water, and habit of wearing shoes (12).
The Gilgel Gibe Hydroelectric Dam is considered to be one of the largest development investments by the country. Cognizant of this the Jimma University has set out to make its contributions in terms of studying and recommending strategies to combat risk factors affecting health as a result of the dam. These multiple efforts would decrease the negative impact of the dam from water born infections to the surrounding community. One visible action by the University and in evidence to this commitment is the establishment of the Gilgel Gibe field epidemiology laboratory, which is being utilized by masters program in public health. This is instrumental not only in assessing and surveying the local health problems but also engaging graduate students in health promotion interventions of the community at large. In addition to this a number of studies on Malaria, schistosomiasis and even chronic non communicable disease are being carried out by various sections of the health science section of the university. However, there are no published data so far in any of these areas and particularly on intestinal parasitosis.
The objective of this survey was to determine the prevalence and predictors of intestinal parasitic infections among school children. In line with the various efforts of Jimma University mentioned above, this study is also hoped to serve as base line for future evaluation of interventions in the area and the generating hypothesis on effect of multiple health promotional intervention being carried out as described previously.
Subjects and Methods
A cross-sectional study was carried out in October 2008 in four Woredas of Jimma zone bordering Gilgel Gibe Hydroelectric Power to determine the prevalence and predictors of intestinal parasitic infections among school children. This study is conducted as sub-study to the main study; the objective of which was to determine the prevalence of intestinal schistosomiasis, and related factors such as risk behavior for infection among school children within 10 kilometers from shoreline of the dam, and survey the presence of suitable intermediate host to Schistosoma mansoni along the major water contact sites of the reservoir.
The Gilgel Gibe hydroelectric dam which started to collect water in 2001 and officially inaugurated in March 2002 is located 250 Kms Southwest of Addis Ababa and 75 Kms Northeast of Jimma City. The Dam covers an area of 51 square Kms at an altitude of 1670 meters above sea level, and holding around 668 million cubic meter of water. It is estimated to have the capacity of producing 40% of the country's hydroelectric power (184 Mega Watt). The four Woredas (administrative unit with a population of about 1,000,000) bordering the dam are Omonada, Sokoru, Tiruafeta and Kersa with 6, 4, 5, and 2 Kebeles within ten kilometers from the shore line of the dam and each kebele had one elementary school. The other study area was Bulbul, which is located in Kersa Wereda and 30 Kms away from the dam on the way to Jimma. It is an area where 1100 households displaced from the submerged by the dam water five years back have resettled.
The survey was carried out in selected schools within Kebeles of the four woredas of mentioned above. These woredas are close to Gilgel Gibe Hydroelectric Dam and have direct contact to the rivers of the dam. The schools surveyed are located within these particular Kebeles included in the Gilgel Gibe Project of Jimma University as well. To assess the impact of dam, a sample of school children from among community resettled from the four woredas to kersa Woreda, Bulbul Kebele were also included. Children attending grades 1–8 in the schools located adjacent to Gilgel Gibe dam in the four bordering woredas and those living 30 Kms away from the shore line were the study subjects. The inclusion criteria for the study were similar to the main study; these are
Children between the ages of 7–16, attending in a school within 10 Kms from the dam, or
Resettled Children of age 7–16, attending in a school in village 30 km away from the dam
A total of 5,721 children from schools within 10 Kms of the dam and 331 resettled children fulfilled the inclusion criteria. A total of 5,721 children within 10 Kms from the dam and 331 resettled children from the four Woredas bordering the dam fulfilled the inclusion criteria. Sample size calculation was done using Epinfo version 6.0 for cross-sectional study. Accordingly, prevalence of schistosomiasis in the four woredas (P1) was assumed to be 40% and prevalence of schistosomiasis in Bulbul (P2) was assumed to be 30 %( 17). With 95% confidence level and 80% power, and 10% non-response rate, total sample size was 937. Because of the large population of school children, sample size allocation; Gilgel Gibe: Bulbul was made as 2:1. Therefore, 624 children selected from the schools around Gilgel Gibe dam comprised cases and 312 children from the schools in Bulbul comprised controls. Using multistage sampling, four schools from Dogosso, Assendabo, Bore, Dimtu and two from Bulbul were selected from 17 schools. Proportional allocation of samples to each school and classes with in a school was made and lottery method was used to select samples from each class. All the resettled children (331) fulfilling the inclusion criteria in the two schools in Bulbul were included in the study.
Data on socio-demographic characteristics, clinical symptoms, availability and use of latrine were collected using a structured questionnaire prepared in Oromiffa. About 3 grams stool specimen was collected from each student using clean, dry, wide necked and leak proof plastic container. Upon delivery of the specimens the containers were labeled and each sample was Preserved with 5 to 10 ml of 10% formal saline solution and kept until examination. Stool samples were transported to Jimma University Parasitology Department Laboratory for analysis within 2 hours of collection. A portion of stool sample was processed by formol-ether concentration technique (box 1), for the presence of ova of intestinal parasites and intensity (density) of infection per gram of stool. The cut off point for classification of intensity of parasitic infection was set according to the thresholds proposed for use by a WHO Expert Committee in 1987 as shown in table 1 (18). Data was filtered and entered into computer then analyzed using SPSS for windows version 13.0.1
Table 1.
Classification of intensity of infection based on egg load, 2008.
| Parasite | light intensity infections |
moderate intensity infections |
heavy intensity infections |
|
Ascaris lumbricoides |
1–4,999 epg | 5,000–49,999 epg |
50,000 epg |
|
Trichuris trichiura |
1– 999 epg | 1,000–9,999 epg |
10,000 epg |
| Hookworms | 1–1,999 epg | 2,000–3,999 epg |
4,000 epg |
|
Schistosoma mansoni |
1– 99 epg | 100–399 epg | 400 epg |
Ethical clearance was obtained from Ethical Committee of Jimma University. Consent was obtained in a format prepared in the local language prior to sample collection and objective of the study were explained to each of the study subjects/guardians. Children who are found to be positive for intestinal parasites were treated with Albendazole 400 mg stat for helmenthic infections including Ascaris lumbricoides, hook worm, Trichuris trichiura and enterobiasis. Praziquantel 40mg per Kg was given for cases of schistosomiasis.
Box 1. Standard operational procedure for the Formol-Ether concentration technique, Jimma Gilgel Gibe area, Southwest Ethiopia, 2008.
Using a stick, emulsify about 1g (pea size) of feces in about 4ml of 10% formal water. Add more 3–4ml formal water
mix well by shaking and sieve into another tube made of glass or polypropylene
Add 3–4ml of ether (anesthetic). Stopper tube and mix for 1 min
loosen the stopper (there is pressure inside tube)
centrifuge immediately at 750–1000g(∼ 3000rpm) for 1 min
Using a stick, loosen the layer of fecal debris from the side of the tube and discard the supernatant, the sediment remain
Allow the fluid from the side of the tube to drain to the bottom. Tap the bottom tube to re suspend and mix sediment
Transfer a small portion of the sediment to a slide and cover it
Examine the preparation first with 10X and then 40X objective
Results
Of the 937 selected individuals, 855 actually participated on the study making the respondent rate 91.2% and among these females accounted 454(53.1%). The age distribution showed that the mean to be 8.9 years. The highest 253(29.9) were in 11–13 age group and the lowest were above 14 years of age accounted 59(6.9%). Respondent characteristics according to ethnicity showed Oromo to be the majority; accounting 769 (89.9%) and majority of the respondents; 749 (87.6%) were also Muslim by religion (Table 2).
Table 2.
Socio-demographic characteristics of in school children, Jimma Gilgel Gibe area, Southwest Ethiopia, 2008.
| Variables | Number (%) (n=855) |
Positive for any intestinal parasites Number (%) (n=406) |
p-value | |
| Sex | ||||
| Male | 401(46.9) | 190 (47.4) | >0.05 | |
| Female | 454(53.1) | 216 (47.6) | ||
| Age | ||||
| <8 | 75 (8.8) | 36 (48.0) | ||
| 8–10 | 468(54.7) | 227 (48.5) | >0.05 | |
| 11–13 | 253(29.6) | 117(46.2) | ||
| ≥ 14 | 59 (6.9) | 26 (44.1 | ||
| Ethnicity | ||||
| Amhara | 35 (4.1) | 17(48.6) | ||
| Oromo | 769( 89.9) | 362(47.1) | ||
| Tigre | 6(0.7) | 3(50.0) | >0.05 | |
| Gurage | 9(1.1) | 4(44.4) | ||
| others | 36(4.2) | 20(55.6) | ||
| Religion | ||||
| Orthodox | 87 (10.2) | 44(50.6) | ||
| Muslim | 749(87.6) | 353(47.1) | >0.05 | |
| Protestant | 16(1.9) | 8(50.0) | ||
| Catholic | 3(0.4) | 1(33.3) | ||
Females and males have similar pattern of parasite infections-216 (47.6%) and 190 (47.4), respectively. The prevalence of intestinal parasitosis was highest in the age group of 8–10 and lowest in those above 14 years; 48.5 and 44.1%, respectively. The prevalence in the different ethnic groups was similar where 17 of the 35 (48.6%) Amharas and 362 of the 769 (47.1%) Oromos were positive for intestinal Parasitosis. Most of the cases of intestinal parasitosis, 44 (50.6%) were among Orthodox Christians. Bi-variate analysis showed no association between the prevalence of intestinal parasites and socio-demographic variables (Table 2).
The spectrum of intestinal parasite showed that 47.1 % were positive for one or more intestinal parasite;174 (20.1%) had hookworm mono infection;126 (14.7%) had Ascaris lumbricoides mono-infection, 28(3.3%) had Trichuris trichiura mono infection and 4.3 % had dual infection involving Ascaris lumbricoides and hookworm and 0.2% had triple infection (Table 3).
Table 3.
spectrum of intestinal parasites among school children, Jimma Gilgel Gibe area, Southwest Ethiopia, 2008.
| Parasite | Number | Percent (%) |
| Any | 406 | 47.5 |
| Ascaris lumbricoides | 126 | 14.7 |
| Hookworm | 174 | 20.4 |
| Trichuris trichiura | 28 | 3.3 |
| other single infections (H.nana, enterobias, schistosoma mansoni) |
18 | 2.1 |
| Ascaris lumbricoides + hookworm | 37 | 4.3 |
| hookworm + other | 12 | 1.4 |
| Ascaris lumbricoides + other | 9 | 1.1 |
| hookworm+ Ascaris lumbricoides +other(triple) |
2 | 0.2 |
Participants who are positive for hookworm and/or Ascaris lumbricoides and / or Trichuris trichiura were categorized in to three groups; light, moderate and heavy intensity of infection and the result showed that all of the infections in the participants were light.
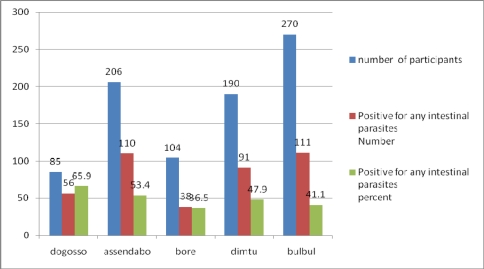
With regard to the distribution of intestinal parasitosis among the 855 respondents across the different schools 85 (9.9%), 206(24.1%) and 104 (12.2%) were from Dogosso and Assendabor respectively. Among these 406 (47.5%) were positive for one or more intestinal parasites and 65.9% of those from Dogosso were positive for one or more intestinal parasites. The lowest prevalence of intestinal parasites was found in Bore- only 38 of the 104 (36.5%) were positive (fig 1).
Fig 1.
Distribution of parasitosis across the different schools, Jimma, Southwest Ethiopia, 2008.
Respondents' characteristics according to latrine availability showed that 685 (80.1%) had latrine at home and 79.2% of all the respondents use latrine regularly. One hundred seventy (19.9%) had no latrine and never used latrine and the majority of these defecate in the bush while the rest in the garden. Of those positive for intestinal parasites 321 (79.1%) had latrine facility at home and 317 (78.1%) use latrine regularly (Table 4). Though individuals who don't have latrine at home 85(50%) were affected by intestinal parasitosis more than those who had latrine at home 321(46.9%), the Bi-variate analysis showed no association between the prevalence of intestinal parasitosis and availability and use of latrine (Table 4).
Table 4.
Availability and use of latrine Vs presence of any intestinal parasite in school children, Jimma Gilgel Gibe area, Southwest Ethiopia, 2008.
| Variable | Total | Positive for any intestinal parasite | P-value | |||
| Number | percent | Number | Percent | |||
| Is latrine available at home? | ||||||
| Yes | 685 | 80.1 | 321 | 79.1 | ||
| No | 170 | 19.9 | 85 | 20.9 | >0.05 | |
| Use of latrine | ||||||
| Regular (always) | 677 | 79.2 | 317 | 78.1 | >0.05 | |
| Sometimes or never | 178 | 20.8 | 89 | 21.9 | ||
| Total | 855 | 100.0 | 406 | 47.5 | ||
Six hundred ninety five (81.3%) of the subjects had previous history of abdominal pain and 351(41.1%) had pain during the study time. Five hundred fifty nine (65.4 %) and 103 (12.0%) had previous history of diarrhea and bloody diarrhea during the study time, respectively. Of those who had intestinal parasitosis (406) 82.3% had previous history of abdominal pain but only 39.2% had similar pain during the study period and 66% had previous history of diarrhea and only 2.5% had bloody diarrhea during the study time(Table 4).Bi-variate analysis showed no association between the prevalence of intestinal and clinical symptoms (Table 5).
Table 5.
Clinical symptoms Vs presence of any intestinal parasite in school children, Jimma Gilgel Gibe area, Southwest Ethiopia, 2008.
| Variable | Total | Positive for any intestinal parasite | ||||
| Number | Percent | Number | Percent | P-value | ||
| Previous history of abdominal pain | ||||||
| Yes | 695 | 81.3 | 334 | 82.3% | >0.05 | |
| No | 160 | 18.7 | 72 | 17.7% | ||
| Current history of abdominal pain | >0.05 | |||||
| Yes | 351 | 41.1 | 159 | 39.2% | ||
| No | 504 | 58.9 | 247 | 60.8% | ||
| Previous history of diarrhea | ||||||
| Yes | 559 | 65.4 | 268 | 66.0% | >0.05 | |
| No | 296 | 34.6 | 138 | 34.0% | ||
| Previous history of bloody diarrhea | ||||||
| Yes | 103 | 12.0 | 53 | 13.1% | >0.05 | |
| No | 752 | 88.0 | 353 | 86.9% | ||
| Current diarrhea | ||||||
| Yes | 111 | 13.0 | 58 | 14.3% | >0.05 | |
| No | 743 | 86.9 | 348 | 85.7% | ||
| Current bloody diarrhea | ||||||
| yes | 20 | 2.3 | 10 | 2.5% | >0.05 | |
| No | 835 | 97.7 | 396 | 97.5% | ||
Discussion
The prevalence of intestinal parasite in this study is 47.5%, and this figure is low as compared to the previous study done in Jimma zone, that showed prevalence of intestinal parasites to be as high as 83% and 86.2 in urban and rural setting respectively (12, 13). This difference may be due to the increased involvement of Jimma University researchers in this particular study areas resulting in improved awareness of the population and hence contributing to the lower prevalence of parasitic infections. Hook worm accounted the highest proportion of intestinal parasites (20.1%) followed by Ascaris lumbricoides (14.7%) and Trichuris trichiura (3.3%). These finding contrasts with previous study done in Assedabo where Ascaris lumbricoides was the leading (56.4%) followed by hookwork (25.5%) and Trichuris trichiura (21.6%) (12) and also with reports from studies done in other parts of Ethiopia(4,8–11).Although hookworm is the leading intestinal infection in this study, the overall proportion of hookworm is less than previous reports(12, 13, 14). The prevalence of ascaris and trichuris however, appears significantly lower than hookworm, this could be because of better awareness of the community towards the means of prevention of Ascaris lumbricoides and Trichuris trichiura infections which are oro-fecal compared to hookworm infection which is via the skin (bare footedness). This study showed no association between the prevalence of intestinal parasitosis and clinical symptoms, this is in accordance to other studies (15, 16).
This study assessed the prevalence, predictors and intensity of intestinal parasitic infection as well evaluated the impact of health related activities in the study zone on prevalence of infection by comparing the prevalence in this study to the previous reports at national, zonal and school level. Generally the study showed lower prevalence and intensity of intestinal parasites in the four woredas bordering Gilgel Gibe Hydroelectric Dam as compared to national level as well as urban and rural setting of Jimma zone. This may have been an effect of increased health promoting activities in the area which has resulted a better awareness in the community of prevention of intestinal parasitic infections. An active role have been played by the establishment of the Gilgel gibe project of Jimma University leading to increase interest and number of researchers conducting health related studies in the area and subsequent interventions. A de-worming program carried by the Zonal Health office ( personal communication) 1 month before stool sample collection may also contribute both to awareness in prevention as well lower infestation in the community. These multiple intervention are highly likely to have resulted in the lower infestation rate but would require further investigation to the actually verify that this is an effect of these intervention or some other factors.
Acknowledgments
We would like to acknowledge Professor Mathias Sibeck and Dr Anke Wanger of the Jimma and LMU link for facilitating the acquisition of funds to conduct this survey.
References
- 1.Intestinal nematodes; Harrison's principle of internal medicine. 17th edition. 2008. chap 210. [Google Scholar]
- 2.De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. (Medline) [DOI] [PubMed] [Google Scholar]
- 3.Evans AC, Stephenson LS. Not by drugs alone: the fight against parasitic helminths. World Health Forum. 1995;16:258–261. (Medline) [PubMed] [Google Scholar]
- 4.Tesfamichael T, Kloos H. Intestinal Prasitism. In: Zein AZ, Kloos H, editors. The Ecology of Health and Disease in Ethiopia. Addis Ababa: Ministry of Health; 1988. p. 214. [Google Scholar]
- 5.WHO Technical Report Series 749. Prevention and control of intestinal parasitic infections. Geneva: WHO; 1987. [PubMed] [Google Scholar]
- 6.World Health Organization, author. Basic Laboratory Methods in Medical Parasitology. Geneva: WHO; 1991. pp. 25–26. [Google Scholar]
- 7.Hall A, Suvenchan M. Intestinal Worms: Strategies to Control Disease. African Health. 1994;17:23. [PubMed] [Google Scholar]
- 8.Tedla S, Ayele T. Ascariasis Distribution in Ethiop Med J. 1986;24:79–85. [PubMed] [Google Scholar]
- 9.Mamo B, Assefa B, Lo CT. Intestinal helminths in Akaki town, withspecial emphasis on the epidemiology of S. Mansoni. Ethiop Med J. 1989;27:183–191. [PubMed] [Google Scholar]
- 10.Haile G, Jirra C, Mola T. Intestinal parasitism among Jiren Elementary and Junior Secondary School, South-western Ethiopia. Ethiop J Health Dev. 1994;8:37–41. [Google Scholar]
- 11.Woldemichael T, Assefa T, Seyoum S. Intestinal parasitism among the student population of Wonji-Shoa Sugar Estate. Ethiop J Health Dev. 1990;4:45–49. [Google Scholar]
- 12.Ali Ibrahim, Mekete Girma, Wodajo Negussie. Intestinal parasitism and related risk factors among students of Asendabo Elementary and Junior Secondary school, south western Ethiopia. Ethiop J Health Dev. 1999;13(2):157–162. [Google Scholar]
- 13.Mengistu Amare, Gebre-Selassie Solomon, Kassa Tesfaye. Prevalence of intestinal parasitic infections among urban dwellers in southwest Ethiopia. Ethiop JHealth Dev. 2007;21(1):12–17. [Google Scholar]
- 14.Haileamlak Abraham. Intestinal Parasites in Asymptomatic Children In Southwest Ethiopia. Ethiopia J Health Sci. 2005;15(2):107–118. [Google Scholar]
- 15.Limoncu M E, Kurt O, Gümüş M, Kayran E, Balcioğlu I C, Dinç G, Ozbilgin A. Is there an association between clinical symptoms and intestinal parasitic infections? Int J Clin Pharmacol Res. 2005;25(3):151–154. [PubMed] [Google Scholar]
- 16.Rice J E, Skull S A, Pearce C, Mulholland N, Davie G, Carapetis J R. Screening for intestinal parasites in recently arrived children from East Africa. Paediatr Child Health. 2003 Aug;39(6):456–459. doi: 10.1046/j.1440-1754.2003.00188.x. 12919501 (P,S,G,E,B) [DOI] [PubMed] [Google Scholar]
- 17.Birrie Hailu, Tedela Shibru, Erko Berhanu, Berehe Nega, Abebe Fekadu. Schistosomiasis in the Finchaa river valley, Wellega region, Western Ethiopia. Ethiop J Health Dev. 1993;7(1):9–15. [Google Scholar]
- 18.Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L. Guidelines for the evaluation of soil transmitted helminthiasis and schistosomiasis at community level. WHO/CTD/SIP/98.1. [AUGUST 12, 2010]. p. 30. www.who.int. [Google Scholar]