Abstract
The adaptability and the genomic plasticity of cancer cells, and the interaction between the tumor microenvironment and co-opted stromal cells, coupled with the ability of cancer cells to colonize distant organs, contribute to the frequent intractability of cancer. It is becoming increasingly evident that personalized molecular targeting is necessary for the successful treatment of this multifaceted and complex disease. Noninvasive imaging modalities such as magnetic resonance (MR), positron emission tomography (PET), and single-photon emission computed tomography (SPECT) are filling several important niches in this era of targeted molecular medicine, in applications that span from bench to bedside. In this review we focus on noninvasive magnetic resonance spectroscopy (MRS) and spectroscopic imaging (MRSI) and their roles in future personalized medicine in cancer. Diagnosis, the identification of the most effective treatment, monitoring treatment delivery, and response to treatment are some of the broad areas into which MRS techniques can be integrated to improve treatment outcomes. The development of novel probes for molecular imaging—in combination with a slew of functional imaging capabilities—makes MRS techniques, especially in combination with other imaging modalities, valuable in cancer drug discovery and basic cancer research.
Despite a reduction in the overall mortality from cancer, one in four deaths in the United States alone is still attributable to this disease.1 Functional imaging promises to play an important role in the management of cancer patients.
Magnetic resonance spectroscopy (MRS) techniques are based on the principle that it is possible to detect radiofrequency (RF) signals generated by magnetic nuclear spins of magnetic resonance (MR) active nuclei such as 1H, 31P, 13C, and 19F precessing in an external magnetic field B0. This detection is only possible after excitation with an RF pulse transmitted by an RF coil at the magnetic resonance frequency ω0. The magnetic resonance frequency ω0 is linearly dependent on B0 and on the gyromagnetic ratio of the nucleus γ, as ω0 = γB0. The MR signal intensity depends on the concentration and the gyromagnetic ratio of the nuclear spins and two rate constants that govern the time dependence of the magnetization signal: the spin-lattice or longitudinal relaxation time, T1, and the spin-spin or transverse relaxation time, T2. After acquisition of the free induction decay (FID) of these RF signals, the FID is Fourier-transformed, and phase and baseline corrections are performed to obtain an interpretable MR spectrum.
MRS provides information about the chemical environment of the nuclear spin such as number of chemical bonds, neighboring nuclei, and overall chemical structure. The presence of an electron cloud surrounding a particular nucleus creates an electronic shield that lowers the B0 magnetic field to which this nucleus would normally be exposed. This resonance frequency difference, which is also referred to as chemical shift, is expressed as parts per million or ppm, a value that is independent of the magnetic field strength. Chemical shift values in ppm thus provide information about the molecular group carrying a particular nucleus, and correspond to a change in the resonance frequency of this nucleus within the molecules, as a function of their chemical bonds. Chemical shifts are frequently reported relative to a reference resonance frequency, such as, for example, tetramethylsilane.
MR spectra can be acquired with or without spatial localization. In the absence of localization, the signal is acquired from the entire sensitive region of the coil that detects the RF signal. Localized spectra can either be acquired from a single volume element (single-voxel), or from multiple voxels (multi-voxel). In chemical shift imaging (CSI), phase-encoding gradients are incorporated to generate images of signals obtained at different chemical shifts by spatially encoding chemical shift information. Phase-encoding can be applied in one, two, or three spatial dimensions. CSI is especially useful in heterogeneous tissues such as tumors. Localized spectra can be processed to obtain images from individual metabolites.
Some of the applications of MRS in cancer are summarized in Table 1 and demonstrate the breadth of this modality in characterizing tumor metabolism, pH, hypoxia, drug delivery, treatment efficacy, and apoptosis. With its ability to identify different compounds by their chemical shifts, MRS is especially useful in studying metabolism. Due to aberrations in their genome and proteome, cancer cells exhibit a unique metabolic phenotype characterized by high glucose uptake, increased glycolytic activity and lactate production, decreased mitochondrial activity, low bioenergetic status, and aberrant phospholipid metabolism.2–4 Additionally, tissue-specific metabolites such as N-acetyl-aspartate (NAA) in the brain, and citrate in the prostate, display an MRS-detectable decrease as the cancer cell population in the tissue expands, thereby reducing the number of normal cells.4–6 Proton or 31P MRS or MRSI detection of these endogenous metabolites has proven useful in the diagnosis of cancer,7–14 and in monitoring anticancer therapy in some instances, as detailed in subsequent sections. Importantly, MRS characterization of the aberrant cancer cell metabolism has led to the identification of related enzymes as novel anticancer targets.3,15–18 MR biomarkers, such as the total choline (tCho) signal, are already being explored in the clinical setting for characterizing tumors and the response of tumors to anticancer therapy.19–21 Elevated metabolites in choline phospholipid metabolism present unique targets to exploit for molecular targeting, the outcome of which can be noninvasively detected and imaged by MRSI.15,22 Several pharmacological and molecular approaches are already being developed to target choline metabolism, and specifically choline kinase (Chk) activity, as discussed in detail below.
Table 1.
Commonly Studied MR-Detectable Nuclei
| Nucleus | γ [MHz/T] | Detected Metabolite or Property | Preclinically Available | Clinically Available |
|---|---|---|---|---|
| 1H | 42.58 | Total choline | Yes | Yes |
| Lactate | Yes | No | ||
| Lipid | Yes | Yes | ||
| N-acetyl-aspartate | Yes | Yes | ||
| Citrate | Yes | Yes | ||
| Extracellular pH (pHe) | Yes | No | ||
| Treatment efficacy | Yes | No | ||
| Detection of metastasis | Yes | No | ||
| Tissue oxygen (pO2) | Yes | No | ||
| 19F | 40.08 | Drug pharmacokinetics | Yes | Yes |
| pHe | Yes | No | ||
| pO2 | Yes | No | ||
| Enzyme activity | Yes | No | ||
| Labeled substrate utilization | Yes | No | ||
| 31P | 17.25 | Energy metabolism (NTP, PCr, Pi) | Yes | Yes |
| Intracellular pH (pHi) | Yes | Yes | ||
| Phospholipid metabolism | Yes | Yes | ||
| 13C | 10.71 | Labeled substrate (drug pharmaco kinetics, metabolic pathways) | Yes | Yes |
MR-detectable nuclei that are commonly studied are displayed in the order of their detection sensitivity, together with some of their preclinical and clinical applications in cancer. The detection limits of 1H and 19F MRS are typically within the millimolar range of the detected metabolite, with higher concentrations required for less sensitive nuclei such as 31P and 13C.
Abbreviations: NTPs, nucleoside diphosphates; PCr, phosphocreatine; Pi, inorganic phosphate.
Advances in pulse sequence design for MRS/I, development of novel substrates, probes, and drugs, and technological advances have further advanced and diversified the use of MRS/I in cancer discovery and treatment. Here we have reviewed some recent biomedical applications of 1H, 13C, 31P, and 19F MRS in preclinical models of cancer and have summarized new developments, such as the hyperpolarization of 13C spins to increase the MR detection sensitivity of 13C-labeled substrates.
1H AND 31P MRS OF CHOLINE PHOSPHOLIPID METABOLISM
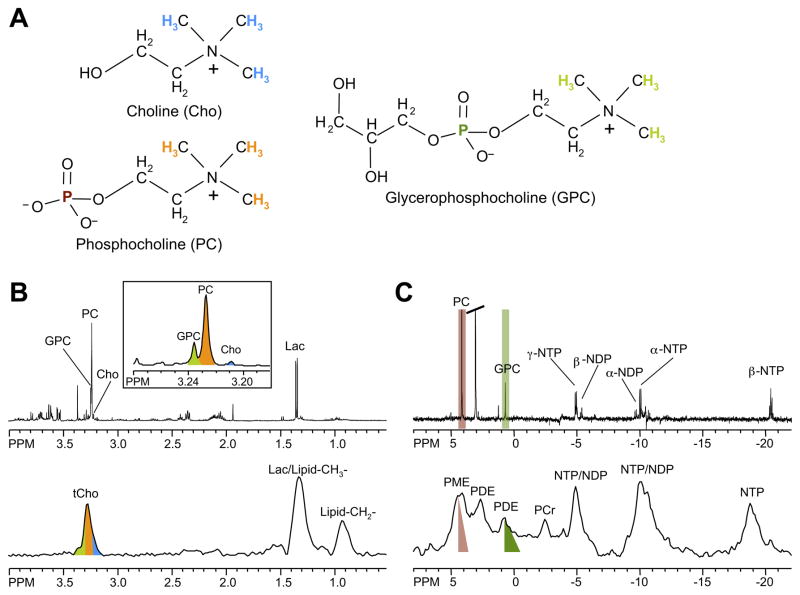
Choline phospholipid metabolism is profoundly altered in cancer cells.3,23–25 Almost every tumor type investigated has revealed elevated phosphocholine (PC) and increased tCho-containing metabolites.3,23–25 Malignant transformation has been found to alter the profile of the choline compounds glycerophosphocholine (GPC) and PC in breast26 and ovarian27 cancer cells. In high-resolution 1H and 31P MR spectra of breast26 and ovarian27 cell extracts, GPC was higher than PC in normal cells but switched to being lower than PC in cancer cells. Proton or 31P MRS can detect these endogenous metabolic changes in vivo, and in tumor tissue biopsies or cancer cells ex vivo, as shown for MDA-MB-231 human breast cancer cells and tumor xenograft models in Figure 1. Proton MRS signals from water-soluble choline metabolites arise between 3.2 and 3.3 ppm from the nine chemically equivalent protons in the choline –N(CH3)3 groups, which are displayed as color-coded 1H nuclei corresponding to MR signals with the same color-code in the representative spectra in Figure 1. Because nine protons contribute to this signal, it displays a higher signal intensity than the 1H signals in the methylene groups of the same choline metabolites, which only contain two equivalent protons each (see Figure 1). Using 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid as a chemical shift reference at pH 7.4, free choline (Cho) is detected at 3.21 ppm, PC at 3.23 ppm, and GPC at 3.24 ppm, in high-resolution 1H MR spectra of cell or tissue extracts (see Figure 1), or in high-resolution magic angle spinning (HR MAS) 1H MR spectra of biopsies (see Figure 2). The chemical structures of Cho, PC, and GPC are shown in Figure 1. It is not possible to resolve Cho, PC, and GPC in vivo, even at higher magnetic field strengths, because of the broader line widths that result from magnetic field inhomogeneities. Instead, a single tCho peak comprised of these three signals is detected, as shown in Figure 1. Clinical multicenter trials are currently underway to establish single-voxel 1H MRS, covering a breast lesion for breast cancer detection. Multi-voxel in vivo 1H MRSI of one or multiple slice(s) through a region of interest provides multiple spectra from a slice or volume of tissue, which can be processed to obtain the spatial distribution of tCho or other metabolites.
Figure 1.
(A) Chemical structures of the choline phospholipid metabolites free choline (Cho), phosphocholine (PC), and glycerophosphocholine (GPC). (B) High-resolution ex vivo 1H MR spectra of triple-negative human MDA-MB-231 breast cancer cell extracts (top) and in vivo 1H MR spectra of the same cell line grown as orthotopic tumor (bottom). (C) High-resolution ex vivo 31P MR spectra of triple-negative human MDA-MB-231 breast cancer cell extracts (top) and in vivo 31P MR spectra of the same cell line grown as orthotopic tumor (bottom). Cho, free choline; GPC, glycerophosphocholine; GPE, glycerophosphoethanolamine; DPDE, diphosphodiester; NDP, nucleoside diphosphate; NTP, nucleoside triphosphate; Lac, lactate; Lipid-CH2-, methylene groups of mobile lipids; Lipid-CH3-, methyl groups of mobile lipids; PC, phosphocholine; PE, phosphoethanolamine; PCr, phosphocreatine; Pi, inorganic phosphate; tCho, total choline-containing compounds (Cho+PC+GPC). The 1H and 31P nuclei in Cho, PC, and GPC and their respective 1H and 31P signals in the MR spectra are color-coded to identify the MR signals that arise from the corresponding nuclei.
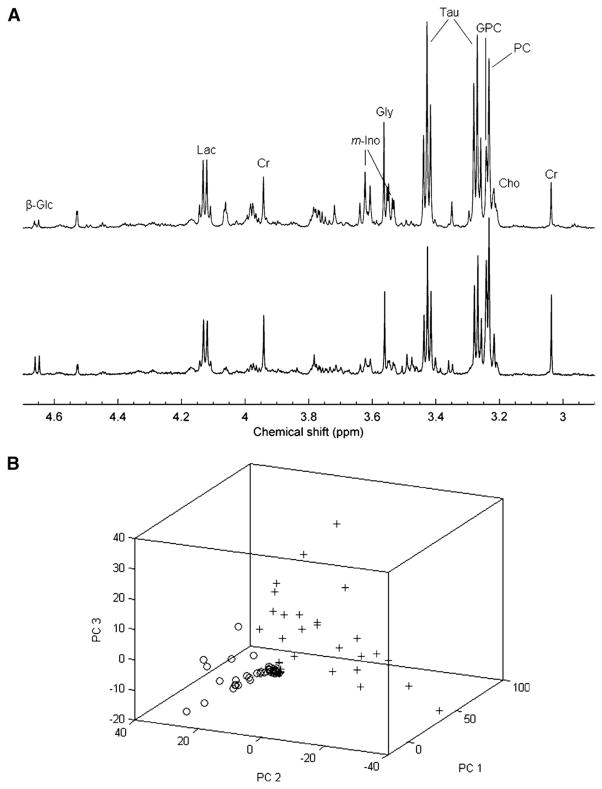
Figure 2.
(A) Region of representative HR MAS >1H MRS spectra selected for multivariate analysis, obtained from primary tumor tissue from patients with invasive ductal carcinoma grade III breast cancer. The top spectrum is derived from a patient diagnosed as hormone positive, with lymphatic spread, and the bottom spectrum from a hormone positive patient, without proven lymphatic spread. Glc, glucose; Lac, lactate; Cr, creatine; m-Ino, myo-inositol; Tau, taurine; GPC, glycerophosphocholine; PC, phosphocholine; Cho, choline. (B) Score plot of principal component 1 (PC1), principal component 2 (PC2), and principal component (PC3) from the principal component analysis of samples from 77 breast cancer patients. Samples from noninvolved adjacent tissue (0) are separated from the rest, thus demonstrating a marked metabolic difference from the malignant samples (+). However, invasive ductal carcinoma samples were interspersed, and there was no possibility to differentiate them from noninvolved adjacent tissue or cancer, emphasizing the need for more sophisticated analysis in order to achieve classification. Adapted with kind permission from Springer Science+Business Media: Bathen et al.65
Phosphorus-containing choline metabolites can also be detected with 31P MRS, as demonstrated in Figure 1. Following chemical shift calibration to a reference compound such as methylene diphosphonic acid at 18 ppm, a signal from PC at 3.9 ppm and GPC at 0.5 ppm is observed in high-resolution 31P MR spectra of cell or tumor extracts, as shown by color-coded signals and their corresponding 31P nuclei with the same color-code in the chemical structures. In the in vivo setting, a mixed phosphomonoester (PME) signal containing unresolved PC and phosphoethanolamine (PE) resonances, and a mixed phosphodiester (PDE) signal containing unresolved GPC and glycerophosphoethanolamine (GPE) resonances are observed in 31P MR spectra (see color-coding in Figure 1). Since in routine in vivo settings neither 1H nor 31P MRS are able to spectrally resolve Cho, GPC, and PC as individual signals, changes in PME, PDE, and tCho detected in vivo in 31P and 1H MR spectra often stem from concentration changes of a number of metabolites. Acquiring consecutive proton-decoupled 31P and 1H MR spectra can, in some cases, overcome this problem.28 A recent clinical study demonstrated the feasibility of partially resolving PC from PE, and GPE from GPC by using 1H-decoupled 31P MRS at 1.5 T29 and at the higher field strength of 3T that is also clinically available. In clinical studies 1H MRS is preferred over 31P MRS because of its higher sensitivity and standard availability on clinical scanners. A recent study reported a novel 1H to 31P polarization transfer method on a clinical 3T MR scanner that resulted in a more than twofold increase of signal-to-noise ratio compared to direct 31P MRS methods.30 Proton to 31P polarization transfer in this study was achieved by applying chemical shift selective refocusing pulses at 3T.30 These pulses canceled the homonuclear J-coupling effects that attenuated 31P signals from PE, PC, GPE, and GPC.30 Identification of PE, PC, GPE, and GPC in human brains was possible with this method, providing a voxel size of 2 × 2 × 2 cm3 in a three-dimensional MRSI data set.30
The molecular causes for the increased PC and tCho levels in cancer cells and tumors are an increased expression and activity of Chk,17 a higher rate of choline transport,31,32 and an increased phospholipase C and D activity.27,33 These enzymes, among others, constitute the biosynthetic and breakdown pathways of the major membrane phospholipid phosphatidylcholine (PtdCho).22 GPC, PC, and Cho are precursors and breakdown products of PtdCho.22 Chk3,15–18 and PtdCho-specific phospholipase D34 and C27 have recently been targeted by gene silencing or enzyme inhibition in studies of MRS-monitored, targeted anticancer therapies, as discussed in detail below. Growth factor signaling, cytokine action, oncogene activation, and chemical carcinogenesis impact on the enzymes in choline phospholipid metabolism.3,23,24
1H MRS OF MOBILE LIPIDS
In addition to choline metabolites, in vivo single-voxel 1H MRS and multi-voxel MRSI detect signals from lipid metabolism-related compounds, such as the methylene signal at 1.3 ppm, and the methyl signal at 0.9 ppm (see representative 1H MR spectra in Figure 1). These methylene and methyl signals originate from CH2 and CH3 groups, respectively, in fatty acyl chains of triacylglycerides that form mobile lipid droplets in the cytoplasm of intact cancer cells or in the intercellular space of solid tumors.35,36 Membrane lipids do not contribute to these lipid signals at 1.3 and 0.9 ppm because the low mobility of membrane lipids limits their detection by MRS in vivo.35,36 The CH2 lipid signal at 1.3 ppm overlaps with the lactate signal at 1.3 ppm, and requires spectral editing for the separation of these two signals.37–39 Additionally, mobile polyunsaturated fatty acyl chain signals can be detected at 5.4 and 2.8 ppm, and can be used to assess polyunsaturation of mobile lipids.36 However, the signal at 5.4 ppm may be difficult to detect because of its proximity to the large water signal at 4.7 ppm.36 Significantly higher levels of lipid have been detected in high-grade human gliomas compared to low-grade gliomas, suggesting a potential application of the lipid signal at 1.3 ppm in tumor grading.35 Intratumoral lipid droplets also have been shown to correlate with drug resistance or response.35 Cytoplasmic accumulation of triacylglycerides in cancer cells and tumors has been attributed to diverse biological processes such as hypoxia, degeneration of mitochondria, differentiation, growth arrest, and apoptotic cell death.35,36,40 Increased diacylglycerol and triacylglycerol biosynthesis in lipid metabolism can lead to the formation of triacyglycerides.36,40 The mobile lipid signal also has been observed to change with apoptosis, necrosis, or lipid droplet formation.41–43
31P MRS OF ENERGY METABOLISM AND PH
Because 31P MRS detects signals from energy metabolites and breakdown products such as nucleoside triphosphates (NTPs), nucleoside diphosphates (NDPs), phosphocreatine (PCr), and inorganic phosphate (Pi) (see Figure 1), it is ideally suited to investigate tumor energy metabolism in vivo. Importantly, tumor pH can be determined from the chemical shift of the Pi resonance as described below. However, its use has declined in recent years, especially for clinical studies, because of poor sensitivity. The bioenergetic state of cancer cells is relatively low because of the Warburg effect.44 The production of high-energy phosphates such as NTP and PCr depends on available glucose and oxygen, which are delivered to tumors through blood vessels. Therefore, energy metabolism is tightly coupled to tumor blood flow,45,46 and decreases in hypoxic regions. 31P MRS may be useful in detecting changes in tumor reoxygenation during radiation therapy that are mediated by changes in blood flow, as indicated in preclinical studies.47,48
The initial observation by Moon and Richards49 that the chemical shift of intracellular phosphates in whole blood is sensitive to pH led to the development of using the chemical shift of the Pi peak to measure tissue pH with 31P MRS. Although the Pi signal in tissues consists of both intra- and extracellular Pi, in tumors the Pi signal is primarily of intracellular origin,50 and the chemical shift of Pi reports intracellular pH (pHi). Since tumors are highly glycolytic, it was assumed for many decades that tumor pH was acidic, and indeed these assumptions were supported by electrode measurements of tumor pH.51 However, 31P MRS measurements of tumor pH revealed that tumor pHi was typically neutral or alkaline.52 The subsequent development of an extracellular pH (pHe) probe, 3-aminopropylphosphonate, 3-APP,53,54 enabled simultaneous detection of intra- and extracellular pH in tumors, and confirmed that the pHe of tumors is acidic, while the pHi is neutral-to-alkaline. The acidic pH within the tumor microenvironment can significantly influence several phenotypic characteristics, and the ability to measure tumor pH noninvasively is therefore important in oncological research. Acidic pHe can stimulate cancer cell invasion in culture.55 Chronic or acute treatment with bicarbonate can increase pH in vivo.56 A recent study demonstrated that mice treated with bicarbonate developed significantly fewer metastases.57 Intracellular pH has been measured in human cancers using 31P MRS,23 but probes to measure pHe in humans are not yet available. The low sensitivity of 31P MRS does not allow acquisition of spectra with high spatial resolution. To date, 31P MR spectra can be acquired from 3- to 4-mm thick tumor slices, or from large voxels of approximately 6 × 6 × 6 mm3 localized to the tumor.
To overcome the sensitivity limitations of 31P MRS, 1H and 19F MRS probes also have been developed to measure pHe.58–60 1H MRS probes are based on imidazole compounds and have been used to measure localized tumor pH using MRSI with spatial resolutions approaching 1 × 1 × 1 mm3.54,59,61 One imidazole-based compound, 2-imidazole-1-yl-ethoxy carbonyl propionic acid (IEPA), has been used to image tumor pHe in tumor models. Consistent with earlier findings, the probe reported an acidic and heterogeneous pHe.54,59
High-Resolution Magic Angle Spinning MRS
HR MAS 1H MRS is a relatively new technique for examining intact biological tissue ex vivo at high spectral resolution.6,62–64 HR MAS 1H MRS can be particularly useful in the clinic for analyzing the metabolome of biopsy specimens prior to pathological classification.6,62–64 By spinning the solid sample at a frequency of typically 1 to 70 kHz at the magic angle of θm, which is circa 54.74° and where cos2θm equals 1/3, with respect to the direction of the magnetic field, the normally broad lines become narrower, increasing the resolution for better identification and analysis of the spectrum.6,62–64 The major advantage of HR MAS 1H MRS is that, in contrast to high-resolution MRS of tissue extracts, the tissue can be used for subsequent histologic, biochemical, and genetic analyses.6,62–64 An example of HR MAS 1H MRS of clinical breast cancer specimens shown in Figure 2 demonstrates the sensitivity of this technique.65 A combination of principal component analyses and automated neural network applications is typically used to process the rich data sets obtained with HR MAS 1H MRS (see Figure 2).65 This approach was able to detect the metabolic phenotype of tumors, as evident from the derived score plot of three principal components shown in Figure 2, in which healthy adjacent breast tissue was clearly separated from cancer tissue, demonstrating a marked metabolic difference between healthy breast and breast tumor tissue.65 HR MAS 1H MRS may, in the future, be used to complement histopathological analysis for detecting and characterizing cancers.65
13C MRS TECHNIQUES TO DETECT LABELED SUBSTRATES
13C MRS is useful to detect 13C-labeled metabolites following administration of suitable 13C-labeled substrates in cancer cells and solid tumors. 13C MRS has been applied to study glycolysis, choline metabolism,22 or other metabolic pathways. 13C MRS is used to detect the incorporation of a 13C label, which is introduced into the system as a 13C-labeled substrate, within downstream metabolites and products. The flux of substrates through metabolic pathways can be derived by metabolic modeling. Unfortunately, direct detection of 13C nuclei by 13C MRS suffers from low sensitivity. The 13C MRS sensitivity can be improved by magnetization transfer techniques, such as nuclear Overhauser effect (NOE) methods, heteronuclear cross-polarization experiments,66 and indirect inverse detection methods.67 These methods improve the detection sensitivity of 13C nuclei making it comparable to that of 1H MRS, for in vivo studies. To enhance 13C signals, magnetization from neighboring protons is transferred through space using dipole-dipole spin coupling in NOE methods, or through chemical bonds using the J-coupling between 13C and 1H spins. The enhanced 13C signals are detected directly with broadband proton decoupling in direct detection methods, such as NOE, distortionless enhancement by polarization transfer (DEPT), insensitive nuclei enhanced by polarization transfer (INEPT), and heteronuclear cross-polarization. In indirect detection schemes such as heteronuclear multiple quantum coherence (HMQC) and heteronuclear single quantum coherence (HSQC) methods, the magnetization is transferred from 1H to 13C and then back to 1H. In HMQC and HSQC, the 13C signals are indirectly detected as 1H frequencies, achieving significantly enhanced sensitivity due to the higher gyromagnetic ratio γ of 1H.67
13C MRS OF 13C-LABELED GLUCOSE/LACTATE
Since a high glycolytic activity is a common feature of many cancers, 13C MRS of 13C-labeled glucose has been used to study glycolysis in tumors. Cancer cells undergo glycolysis even in the presence of oxygen,44,68 referred to as the “Warburg effect” after Otto Warburg who observed this phenomenon in 1930. Glycolysis is regulated by multiple oncogenes and signaling pathways.44 Glycolysis in cancer cells occurs under well-oxygenated conditions, in part, through the stabilization of hypoxia-inducible factor alpha (HIF-1α).68 Specifically, HIF-1α increases the formation of lactate dehydrogenase, which converts pyruvate to lactate; HIF-1α also activates pyruvate dehydrogenase, which converts pyruvate to acetyl-coenzyme A.69 Additionally, poor blood flow and the resulting hypoxia also contribute to increased anaerobic glycolysis in tumors.51,70 Increased expression of the glucose transporters GLUT-1 and -3, among others, in cancer cells also increases glucose uptake of tumors.71 Overall, the combination of increased glucose uptake with increased glycolysis results in cancer cells rapidly metabolizing glucose to form lactate. The kinetics of [3-13C]-labeled lactate formation can be determined by delivering [1-13C]-labeled glucose through an intravenous infusion. Determining the kinetics of 13C-labeled substrates over time facilitates the study of glucose uptake, delivery, and glycolytic breakdown, as well as lactate synthesis and clearance from the tumor.72 Lactate levels in tumors are determined by several factors, such as tumor hemodynamics, substrate supply, hypoxia, venous clearance, glucose supply, extent of necrosis, and degree of inflammatory cell infiltrate.73 For example, volume localized 13C MRS with 1H-13C cross polarization was applied to detect the conversion of [1-13C]-glucose to [3-13C]-lactate in a murine mammary carcinoma model, and demonstrated that decreasing tumor oxygenation correlated with increasing glycolytic rate.74 High-resolution 13C MRS studies of tumor or organ extracts are useful in animals infused with [1-13C]- or [U-13C]-labeled glucose.75 Such studies can reveal complex 13C-labeling patterns in several metabolites, providing insight into metabolic compartmentalization, shuttling of metabolites between cell types or organs, and metabolic fluxes.75
HYPERPOLARIZED 13C MRS
The use of hyperpolarized 13C-labeled substrates has revitalized 13C MRS studies because of the large increase in 13C detection sensitivity achieved by hyperpolarization.76,77 Dynamic nuclear polarization (DNP) for solution-state MRS (DNP-MRS) can, in theory, increase the 13C detection sensitivity of hyperpolarized 13C-labeled substrates and their metabolites by up to 10,000-fold.76,77 To achieve DNP, homogeneously distributed organic free radicals are added to the sample before cooling it, to fulfill the requirement for unpaired electrons.78 Once cooled and in the solid state, the high electron spin polarization of the sample is transferred to the nuclear spins by microwave irradiation.78 Subsequently, the sample is brought into a liquid solution after rapid dissolution.78 This method makes it possible to bring polarized, cold, solid samples into solution while preserving their nuclear polarization for a short time, which is sufficient for 13C MRSI,78 as evident in Figure 3.77 Complexities in achieving hyperpolarization in the solid state, the appropriate free radicals, and the limited number of molecules amenable reduce the actual, achievable increase in 13C detection sensitivity of hyperpolarized 13C-labeled substrates. DNP-MR spectrometers and hyperpolarizers are currently becoming commercially available. Elevated lactate and possibly alanine produced from hyperpolarized [1-13C]-labeled pyruvate are under investigation as noninvasive biomarkers to determine the presence and histologic grade of cancers in preclinical models,77 as shown in Figure 3. It may be possible to translate these preclinical studies into the clinical setting for the detection and management of cancer in humans.77 Parahydrogen can be used to hydrogenate multiple bonds in chemical structures containing enriched 13C isotopes (PASADENA) as an alternative mechanism to hyperpolarize 13C nuclei.79 Hyperpolarization based on parahydrogen requires 13C substrates with a particular chemical structure, limiting its general applicability compared to DNP-based methods.
Figure 3.
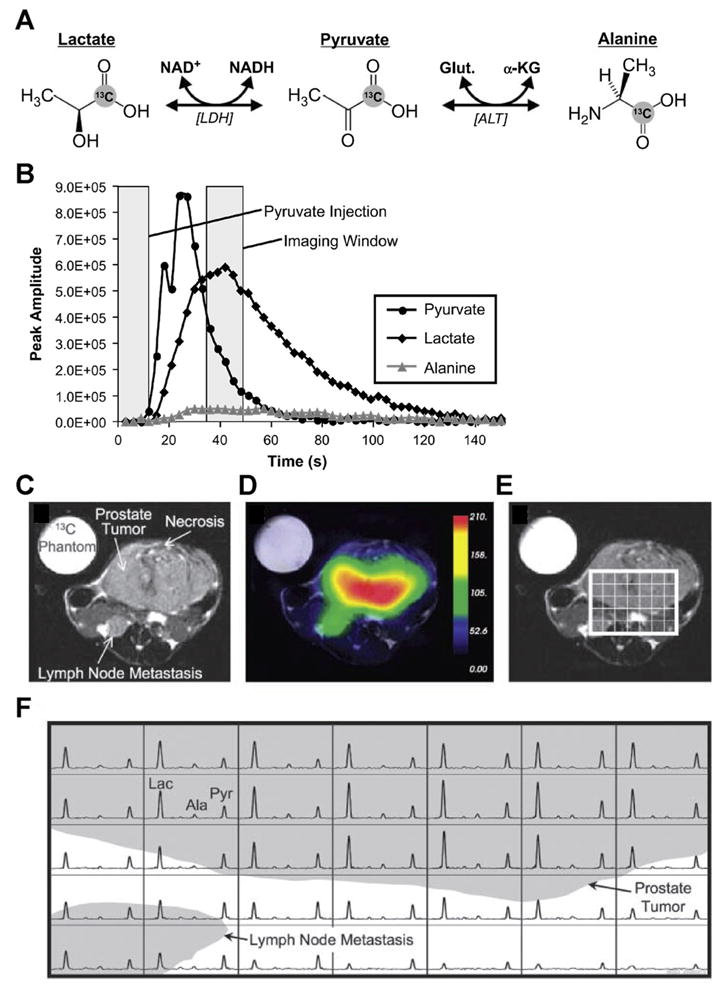
(A) Diagram of [1-13C]-pyruvate and its relevant metabolic pathways leading to [1-13C]-lactate and [1-13C]-alanine. (B) Peak height plots from hyperpolarized 13C spectra reveal the time course of hyperpolarized [1-13C]-pyruvate and its metabolic products following injection of 28 μmol of hyperpolarized [1-13C]-pyruvate at a constant rate from 0 to 12 s. (C) Axial T2-weighted 1H MR image showing the primary tumor and a lymph node metastasis in a ‘transgenic adenocarcinoma of mouse prostate’ (TRAMP) mouse with a high-grade primary tumor, and (D) overlay of an interpolated hyperpolarized [1-13C]-lactate image following injection of 28 μmol of hyperpolarized [1-13C]-pyruvate. After spatially zero-filling and voxel-shifting the 13C MR spectra to maximize the amount of tumor in the voxels, (E) a subset of the spectral grid was selected and (F) displayed. The three-dimensional MRSI was acquired with a nominal voxel size of 135 mm3, zero-filled to 17 mm3. Substantially elevated lactate was detected in the high-grade primary tumor compared with adjacent normal tissue. In addition, the metabolite signal is significantly lower in the necrotic regions of the primary tumor. Lac, lactate; Ala, alanine; Pyr, pyruvate. Adapted with permission from the American Association for Cancer Research.77
Recently hyperpolarized 13C-labeled bicarbonate was used to measure tumor pHe in vivo.80 This approach employs the cellular buffering system to determine pHe. DNP of 13C-bicarbonate increases the sensitivity of 13C detection dramatically, enabling 13C MRSI of tissues in vivo. The ratio of H13CO3− to 13CO2 provides a measure of tissue pHe, assuming a pKa of 6.17.80 pHe maps were obtained with a spatial resolution of 2 × 2 × 6 mm3 in initial preclinical studies.80 Since the bicarbonate probe is nontoxic, it may, in the future, be translated to the clinic to image pHe for oncological applications.
1H MRS IN CLINICAL DIAGNOSIS
Most clinical MR scanners have routine sequences for 1H MRS/I measurements.11,12,81–83 Quantitative 1H MRS and 1H MRSI measurements of tCho and tissue-specific metabolites are frequently implemented in the clinic, in addition to standard dynamic contrast-enhanced (DCE) MR imaging (MRI), to diagnose primary malignant tumors in brain,7–10 prostate,11 and breast.12–14,84 The addition of MRS to standard MRI techniques can significantly increase the sensitivity up to 88%, the specificity to greater than 90%, and the diagnostic accuracy up to 91%. Choline metabolites such as PC, GPC, and Cho, among others, provide robust biomarkers in human biopsy specimens ex vivo using high-resolution 1H MRS of extracts or HR MAS 1H MRS of intact specimens. Elevated tCho and PC concentrations have been used to identify meningiomas and recurrent astrocytomas in human brain tissue specimens,85 breast cancer in fine-needle aspirates of breast tumors,86 and prostatic carcinoma in postsurgical prostate tissue samples.6
The most striking and consistent difference between normal and tumor tissue is in levels of tCho; normal tissues display low tCho levels, whereas tumors display high tCho levels.12,82,83 Proton MRS and MRSI therefore have been applied clinically to assist in diagnosing cancer, and in specifying the margins of brain,7,87 prostate,82,83 and breast12,20,88 tumors, among others. Additionally, 1H MRS detection of tCho in the clinical setting can also help distinguish tumor recurrence from necrosis in the brain89,90 or prostate91 following anti-cancer therapy. Single-voxel 1H MRS or multi-voxel 1H MRSI are able to detect changes in tCho and other metabolites such as creatine, NAA, and lactate. Therefore, the specificity of detection can be improved by measuring and calculating metabolite ratios. In a recent in vivo study, all metabolite intensities detected in 1H MRSI data were translated into metabolomic profiles by applying prior knowledge from HR MAS 1H MRS data from the same tissue type.92 Malignancy indices were derived from the in vivo–detected prostate cancer metabolomic profiles, which linearly correlated with lesion size, and achieved up to 97% overall accuracy for detecting prostate cancer lesions.92
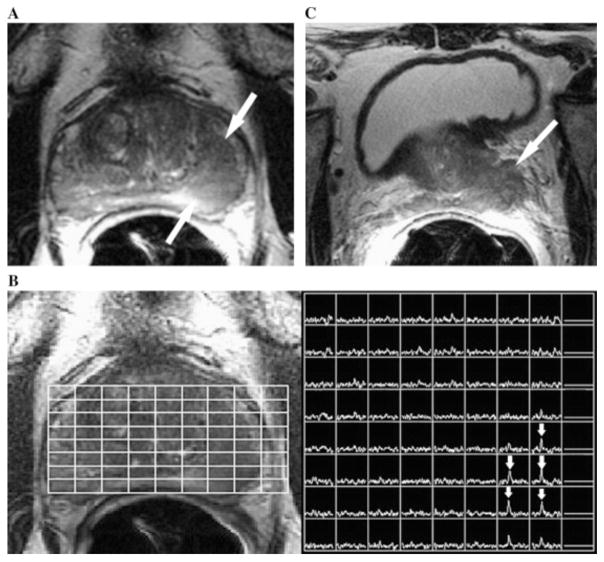
To identify suspicious lesions in the prostate, several MRI parameters, including T2-weighted contrast, T1-weighted DCE-MRI, and diffusion-weighted imaging (DWI) are often combined in current radiological practice. A high T2-weighted MRI signal is typically detected in normal prostatic tissue, whereas a low T2-weighted MRI signal has been shown to correlate with pathological prostatic tissue, as demonstrated in Figure 4.93 In spite of improved visualization of prostate morphology with T2-weighted MRI at 3T, there remains an urgent need for increased specificity and identification of “functional” cellular and metabolic characteristics of prostate tissue. As shown in Figure 4, this need can be satisfied by incorporating MRSI into the MR exam, where an elevated tCho signal was detected in a newly diagnosed patient with Gleason score 6 prostate cancer, serum prostate-specific antigen level of 4.6 ng/mL, and clinical stage of T2B.93,94 Inclusion of MRSI can augment the reliable outlining of tumor margins and tumor infiltration into healthy tissue. However, relatively high tCho signals may be detected in a few benign and highly proliferative lesions. Under such circumstances, a differential diagnosis should be based on the clinical data from other diagnostic scans.12,95 Additionally, single-voxel 1H MRS and multi-voxel 1H MRSI can be applied clinically in treatment planning for radiation96 or brachytherapy.97
Figure 4.
(A) Axial T2-weighted 1H MRI section in a 68-year-old man with newly diagnosed Gleason 6 prostate cancer, serum prostate-specific antigen level of 4.6 ng/mL, and clinical stage of T2B. A large focus (arrows) of reduced T2 signal intensity was observed in the left peripheral zone of the prostate. (B) Photomontage showing the axial T2-weighted 1H MR image on the left side with an overlaid grid that corresponds to the MRSI spectral array on the right side. Voxels corresponding to the focus of reduced T2 signal intensity displayed high total choline peaks (arrows), consistent with prostate cancer. (C) Axial T2-weighted 1H MRI section through the base of the prostate shows gross extracapsular extension of the tumor, with seminal vesicle invasion (stage T3B). The patient developed metastatic recurrence at 21 months after external beam radiotherapy. Adapted from Joseph et al93 with permission from Elsevier.
1H MRS MONITORING OF CANCER THERAPY: CLINICAL AND PRECLINICAL EXAMPLES
The identification, and effective neutralization, of targets and pathways that present a molecular Achilles heel of cancer cells form the basis of finding effective treatments against cancer. Factors complicating this endeavor arise because each cancer represents an individual disease with a unique molecular makeup and a genomic instability that facilitates adaptation and survival following anticancer treatments. As more critical targets in cancer cells are revealed, a transition is occurring in today’s anticancer therapies from the “sledgehammer” approach of conventional chemo- and radiotherapy toward specific molecular-targeted therapies. The availability of noninvasive imaging techniques is critically important for the success of these molecular-targeted treatments.98 Imaging is needed to select targeted therapies that would be the most effective against a particular cancer, and to detect the tumor response to a particular therapy.98 Advances in novel image-guided platforms such as nanoparticles, liposomes, and microencapsulation devices to deliver small interfering RNA (siRNA)99 or drugs to downregulate cancer-specific targets and pathways are also driven by the initial identification of these cancer-specific targets.
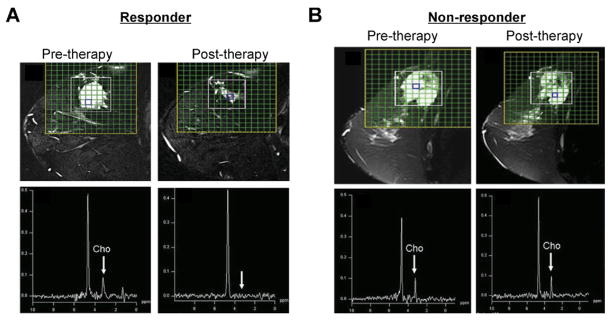
Detecting the early response of a tumor to traditional cytotoxic chemotherapeutic drugs is important to reduce damage to normal tissue in patients with nonresponding tumors, and to alter the therapeutic strategy early on, before completing a full course of treatment. Single-voxel 1H MRS and MRSI have shown utility in assessing treatment response in brain,10,100 breast,20 and prostate82 cancers. Multiple studies have identified changes in tCho as having a high likelihood of detecting early response in breast cancer.20,101 An example of detecting, and possibly predicting, tumor response based on the tCho signal was demonstrated in patients with locally advanced breast cancer who either responded (Figure 5A), or did not respond (Figure 5B), to neoadjuvant chemotherapy (NACT).102 An early response of prostate cancer to hormone-deprivation therapy82,83 and cryosurgery103 was detected by a decrease in [tCho/citrate] ratios quantified from 1H MR spectra. Anticancer treatments leading to apoptosis or necrosis induced characteristic changes in choline phospholipid metabolites.104 An early response to therapy in lymphomas was also associated with a reduction of tCho.105 Some cancers, eg, cervical cancer, did not exhibit a change in the tCho signal following neoadjuvant therapy and subsequent surgical resection of the tumors.106 However, these cervical cancers displayed a reduced tumor volume along with decreased triglyceride signal, without any difference in patient survival with or without neoadjuvant therapy, and there was no survival advantage associated with the reduced tumor volume or decreased triglycerides.106
Figure 5.
(A) A pre-therapy T2-weighted sagittal fat suppressed 1H MR image including MRSI grid is shown that was measured in a patient with locally advanced breast cancer who responded to neoadjuvant chemotherapy (NACT). The 1H MR spectrum on the left was obtained from a voxel with tCho signal prior to therapy. On the right, a post-therapy 1H MR spectrum obtained from the voxel highlighted in the 1H MR image is displayed that was obtained from the same patient after the third cycle of NACT and showed no tCho. (B) Pre-therapy T2-weighted sagittal fat suppressed 1H MR image with MRSI grid of a patient with locally advanced breast cancer who did not respond to NACT. The 1H MR spectrum on the left was obtained from a voxel highlighted in the above image showing tCho signal. On the right, a post-therapy T2-weighted sagittal fat suppressed 1H MR image, including a 1H MR spectrum obtained from the highlighted voxel, is displayed that was measured in the same patient after the third cycle of NACT showing tCho signal. These presented data suggest that the tCho signal can predict the response of breast cancer patients to NACT. Adapted with permission from John Wiley and Sons.102
Molecular-targeted preclinical studies demonstrate that caution must be exercised when using tCho and PC as surrogate markers to interpret tumor response. While a decrease of tCho and PC was observed following treatments against molecular targets such as mitogen-activated protein kinase (MAPK),107 fatty acid synthase,108 and Bcr-Abl tyrosine kinase,109 PC levels were found to increase following histone deacetylase (HDAC) inhibition.110 Therefore, a decrease of tCho or PC cannot always be associated with response, as this will depend on the target selected. However, PC levels did not change following HDAC inhibition in tumors in vivo.110 A novel fluorinated lysine derivative, which is a cleavable substrate for HDAC that can detect HDAC activity monitored by 19F MRS, was also described in the same study.110
The high PC levels in tumors are, in large part, caused by an increased expression and activity of Chk. Therefore, Chk presents an attractive molecular target for anticancer therapy.15,111–113 An advantage of targeting Chk is that its downregulation or inhibition will result directly in a decrease of PC and tCho, which can be detected noninvasively with 31P or 1H MRS.111,112 Novel pharmacological inhibitors, developed to target Chk,113 resulted in tumor growth arrest and apoptosis.18,111 RNA interference (RNAi) also has been used to silence Chk.15,112. RNAi is known to facilitate sequence-specific inhibition of gene expression.114 Small interfering RNA (siRNA), which is small double-stranded RNA of 19 to 23 nucleotides, can target any mRNA to which it is specific, and silence its expression.114 Silencing Chk-α with siRNA significantly reduced cell proliferation and increased differentiation in highly invasive and metastatic MDA-MB-231 human breast cancer cells but not in nonmalignant immortalized MCF-12A mammary epithelial cells.15 Gene therapy with an intravenously injected lentiviral vector, which was able to deliver Chk-specific short-hairpin RNA (shRNA) in a breast cancer model, was monitored noninvasively by single-voxel 31P MRS.112 In this study, a reduction of PC and PME was observed, which indicated that Chk was successfully downregulated by this lentiviral Chk-targeted gene therapy.112 Chk downregulation was accompanied by a reduction in cell proliferation and tumor growth, demonstrating the feasibility of future gene therapeutic approaches to target Chk in tumors and the ability to detect Chk targeting.112
With the implementation of hyperpolarization techniques enabling 13C MRSI, hyperpolarized 13C-labeled substrates, such as [1-13C]-pyruvate and [1,4-13C2]-fumarate, have been investigated for detecting treatment response in a murine lymphoma model.76,115 Tumor response was detected from the change in the flux of hyperpolarized 13C label between pyruvate and lactate in the case of [1-13C]-pyruvate, and an increase of [1,4-13C2]-malate production in the case of [1,4-13C2]-fumarate.76,115 The increase of [1,4-13C2]-malate production was attributed to an increase of necrosis. A recent study demonstrated that the tumor response to anticancer drugs that target phosphatidylinositol 3-kinase (PI3K) can be monitored by hyperpolarized [1-13C]-pyruvate-injected, and hyperpolarized [1-13C]-lactate-detected 13C MRS, through alterations in lactate dehydrogenase activity caused by PI3K pathway inhibition.116
A major problem in cancer chemotherapy is poor drug delivery. MRS can be used to directly measure pharmacokinetics of drugs that can be given at relatively high concentrations in the tumor.117,118 Most studies on MR pharmacokinetic measurements of tumors in vivo employ fluorinated drugs such as [5-19F]-fluorouracil (5-FU) detected by 19F MRS,119 because 19F MRS provides relatively high sensitivity combined with the absence of a background signal. In a recent study, the uptake and metabolism of the chemotherapeutic agent 5-FU was detected in liver metastases from colo-rectal cancer using 19F MRS.120 Successful image-guided delivery of a prodrug enzyme, bacterial cytosine deaminase (bCD), that converts nontoxic [5-19F]-fluorocytosine (5-FC) to 5-FU was recently reported in preclinical studies.121 Prodrug enzyme delivery was visualized by conjugating bCD to poly-L-lysine functionalized with biotin, rhodamine, and Gd3+-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) for optical and MR imaging.121 Damage from the active drug 5-FU was minimized in normal tissue and maximized in the tumor by timing 5-FC prodrug administration under image-guidance to coincide with maximum bCD concentration in the tumor and minimum bCD concentration in normal tissue.121 19F MRS was used to detect the conversion of the prodrug 5-FC to the active drug 5-FU.12
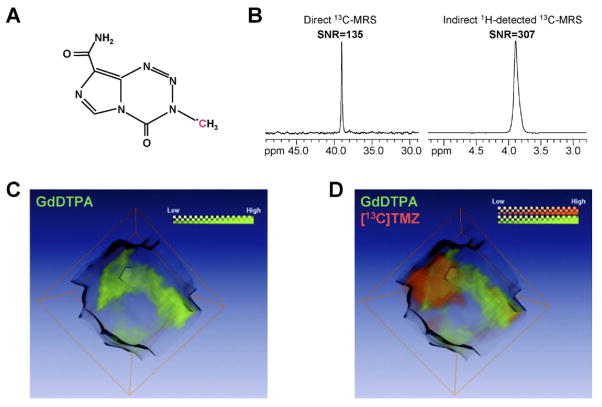
Drugs that do not contain 19F atoms may be altered in their physicochemical and pharmacological properties by chemical 19F-labeling.122 Therefore, 13C and 1H MRS methods also are being explored to detect drug uptake and distribution.123 Such approaches require the 13C-labeled drug to have at least one well-resolved, isolated 13C signal.124 The 13C-labeled drug has to be administered at relatively high doses to be within the detection sensitivity of MRS.124 The intratumoral distribution of the 13C-labeled anticancer agent temozolomide was recently imaged by 1H/13C MRS as shown in Figure 6.123 This 13C MRSI study revealed a heterogeneous delivery of temozolomide to the tumor123 (Figure 6). In the clinic, temozolomide is used as a chemotherapeutic drug to treat glioblastomas and anaplastic astrocytomas.125 These preclinical studies using 1H/13C MRSI to detect 13C-labeled temozolomide delivery in a brain tumor model suggest a potential use of future 1H/13C MRS/I techniques in detecting temozolomide delivery in human cancers.118
Figure 6.
1H/13C MRSI of 13C-labeled temozolomide ([13C]TMZ) in a human MCF-7 breast tumor xenograft, using the indirect detection technique, heteronuclear multiple-quantum coherence (HMQC). The same tumor was imaged using gadolinium(III) diethylene triamine penta-acetic acid (GdDTPA)-enhanced DCE-MRI. (A) The chemical structure of [13C]TMZ shows the 13C-labeled nucleus. (B) A significantly higher signal-to-noise (SNR) ratio was detected by indirect 1H-detected 13C MRS detection of [13C]TMZ using HMQC as compared to direct 13C MRS detected [13C]TMZ. (C) The corresponding GdDTPA contrast uptake map shown in green was reconstructed as a difference map between pre- and post-contrast acquisitions. The grayscale image represents the tumor. (D) The intratumoral 3D distribution of GdDTPA-contrast uptake (green) was co-registered with the HMQC CSI detected 3D distribution of [13C]TMZ shown in red. Green and red channels indicate the GdDTPA uptake and distribution of [13C]TMZ, respectively, and reveal a partial overlap of the GdDTPA-enhancing regions with tumor areas of high temozolomide uptake. Adapted with permission from John Wiley and Sons.123
CONCLUSION
The major strengths of MRS are the ability to provide a wide range of metabolic and functional information, and to seamlessly integrate with complementary MRI applications. MRS can fulfill important requirements in cancer discovery and treatment in the current era of personalized and targeted molecular medicine. MRS methods may provide an understanding of the effects of downregulating specific targets on downstream changes in physiology and metabolism, which are a rich source to mine for noninvasive biomarkers associated with molecular targets.
Sensitivity and spectral resolution continue to be limiting factors of today’s MRSI techniques. However, the availability of higher field strengths and novel techniques such as hyperpolarization can minimize these limitations. The multiparametric capabilities of MRS methods, and the ease of transitioning from bench to bedside and combining MRS methods with other imaging modalities, make MRS a valuable modality for molecular and functional imaging of cancer. MRS will continue to evolve as a cornerstone technique for personalized medicine in cancer.
Acknowledgments
Supported in part by National Institutes of Health Grants No. R01 CA134695, P50 CA103175, R01 CA73850, R01 CA82337, R01 CA136576, R01 CA138515, R21 CA140904, and R21 CA133600.
Footnotes
Financial disclosures: The authors have nothing to disclose.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Griffin JL, Kauppinen RA. Tumour metabolomics in animal models of human cancer. J Proteome Res. 2007;6:498–505. doi: 10.1021/pr060464h. [DOI] [PubMed] [Google Scholar]
- 3.Glunde K, Serkova NJ. Therapeutic targets and biomarkers identified in cancer choline phospholipid metabolism. Pharmacogenomics. 2006;7:1109–23. doi: 10.2217/14622416.7.7.1109. [DOI] [PubMed] [Google Scholar]
- 4.Costello LC, Franklin RB. ’Why do tumour cells glycolyse?’: from glycolysis through citrate to lipogenesis. Mol Cell Biochem. 2005;280:1–8. doi: 10.1007/s11010-005-8841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin JL, Kauppinen RA. A metabolomics perspective of human brain tumours. FEBS J. 2007;274:1132–9. doi: 10.1111/j.1742-4658.2007.05676.x. [DOI] [PubMed] [Google Scholar]
- 6.Swanson MG, Zektzer AS, Tabatabai ZL, et al. Quantitative analysis of prostate metabolites using 1H HR-MAS spectroscopy. Magn Reson Med. 2006;55:1257–64. doi: 10.1002/mrm.20909. [DOI] [PubMed] [Google Scholar]
- 7.Ross B, Michaelis T. Clinical applications of magnetic resonance spectroscopy. Magn Reson Q. 1994;10:191–247. [PubMed] [Google Scholar]
- 8.Nelson SJ. Analysis of volume MRI and MR spectroscopic imaging data for the evaluation of patients with brain tumors. Magn Reson Med. 2001;46:228–39. doi: 10.1002/mrm.1183. [DOI] [PubMed] [Google Scholar]
- 9.Howe FA, Barton SJ, Cudlip SA, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2003;49:223–32. doi: 10.1002/mrm.10367. [DOI] [PubMed] [Google Scholar]
- 10.Jenkinson MD, Smith TS, Joyce K, et al. MRS of oligodendroglial tumors: correlation with histopathology and genetic subtypes. Neurology. 2005;64:2085–9. doi: 10.1212/01.WNL.0000165998.73779.D9. [DOI] [PubMed] [Google Scholar]
- 11.Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24–0.7-cm3) spatial resolution. Radiology. 1996;198:795–805. doi: 10.1148/radiology.198.3.8628874. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs MA, Barker PB, Bottomley PA, Bhujwalla Z, Bluemke DA. Proton magnetic resonance spectroscopic imaging of human breast cancer: a preliminary study. J Magn Reson Imaging. 2004;19:68–75. doi: 10.1002/jmri.10427. [DOI] [PubMed] [Google Scholar]
- 13.Meisamy S, Bolan PJ, Baker EH, et al. Adding in vivo quantitative 1H MR spectroscopy to improve diagnostic accuracy of breast MR imaging: preliminary results of observer performance study at 4.0 T. Radiology. 2005;236:465–75. doi: 10.1148/radiol.2362040836. [DOI] [PubMed] [Google Scholar]
- 14.Stanwell P, Gluch L, Clark D, et al. Specificity of choline metabolites for in vivo diagnosis of breast cancer using 1H MRS at 1.5 T. Eur Radiol. 2005;15:1037–43. doi: 10.1007/s00330-004-2475-1. [DOI] [PubMed] [Google Scholar]
- 15.Glunde K, Raman V, Mori N, Bhujwalla ZM. RNA interference-mediated choline kinase suppression in breast cancer cells induces differentiation and reduces proliferation. Cancer Res. 2005;65:11034–43. doi: 10.1158/0008-5472.CAN-05-1807. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez de Molina A, Gallego-Ortega D, Sarmentero J, Banez-Coronel M, Martin-Cantalejo Y, Lacal JC. Choline kinase is a novel oncogene that potentiates RhoA-induced carcinogenesis. Cancer Res. 2005;65:5647–53. doi: 10.1158/0008-5472.CAN-04-4416. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez de Molina A, Gutierrez R, Ramos MA, et al. Increased choline kinase activity in human breast carcinomas: clinical evidence for a potential novel antitumor strategy. Oncogene. 2002;21:4317–22. doi: 10.1038/sj.onc.1205556. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Gonzalez A, Ramirez de Molina A, Fernandez F, Lacal JC. Choline kinase inhibition induces the increase in ceramides resulting in a highly specific and selective cytotoxic antitumoral strategy as a potential mechanism of action. Oncogene. 2004;23:8247–59. doi: 10.1038/sj.onc.1208045. [DOI] [PubMed] [Google Scholar]
- 19.Kurhanewicz J, Vigneron DB, Males RG, Swanson MG, Yu KK, Hricak H. The prostate: MR imaging and spectroscopy. Present and future. Radiol Clin North Am. 2000;38:115–38. doi: 10.1016/s0033-8389(05)70152-4. [DOI] [PubMed] [Google Scholar]
- 20.Meisamy S, Bolan PJ, Baker EH, et al. Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo (1)H MR spectroscopy—a pilot study at 4 T. Radiology. 2004;233:424–31. doi: 10.1148/radiol.2332031285. [DOI] [PubMed] [Google Scholar]
- 21.Baek HM, Chen JH, Nie K, et al. Predicting pathologic response to neoadjuvant chemotherapy in breast cancer by using MR imaging and quantitative 1H MR spectroscopy. Radiology. 2009;251:653–62. doi: 10.1148/radiol.2512080553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glunde K, Jie C, Bhujwalla ZM. Molecular causes of the aberrant choline phospholipid metabolism in breast cancer. Cancer Res. 2004;64:4270–6. doi: 10.1158/0008-5472.CAN-03-3829. [DOI] [PubMed] [Google Scholar]
- 23.Negendank W. Studies of human tumors by MRS: a review. NMR Biomed. 1992;5:303–24. doi: 10.1002/nbm.1940050518. [DOI] [PubMed] [Google Scholar]
- 24.Podo F. Tumour phospholipid metabolism. NMR Biomed. 1999;12:413–39. doi: 10.1002/(sici)1099-1492(199911)12:7<413::aid-nbm587>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Ronen SM, Leach MO. Imaging biochemistry: applications to breast cancer. Breast Cancer Res. 2001;3:36–40. doi: 10.1186/bcr268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999;59:80–4. [PubMed] [Google Scholar]
- 27.Iorio E, Mezzanzanica D, Alberti P, et al. Alterations of choline phospholipid metabolism in ovarian tumor progression. Cancer Res. 2005;65:9369–76. doi: 10.1158/0008-5472.CAN-05-1146. [DOI] [PubMed] [Google Scholar]
- 28.Albers MJ, Krieger MD, Gonzalez-Gomez I, et al. Proton-decoupled 31P MRS in untreated pediatric brain tumors. Magn Reson Med. 2005;53:22–9. doi: 10.1002/mrm.20312. [DOI] [PubMed] [Google Scholar]
- 29.Arias-Mendoza F, Payne GS, Zakian KL, et al. In vivo 31P MR spectral patterns and reproducibility in cancer patients studied in a multi-institutional trial. NMR Biomed. 2006;19:504–12. doi: 10.1002/nbm.1057. [DOI] [PubMed] [Google Scholar]
- 30.Klomp DW, Wijnen JP, Scheenen TW, Heerschap A. Efficient 1H to 31P polarization transfer on a clinical 3T MR system. Magn Reson Med. 2008;60:1298–305. doi: 10.1002/mrm.21733. [DOI] [PubMed] [Google Scholar]
- 31.Katz-Brull R, Degani H. Kinetics of choline transport and phosphorylation in human breast cancer cells; NMR application of the zero trans method. Anticancer Res. 1996;16:1375–80. [PubMed] [Google Scholar]
- 32.Eliyahu G, Kreizman T, Degani H. Phosphocholine as a biomarker of breast cancer: molecular and biochemical studies. Int J Cancer. 2007;120:1721–30. doi: 10.1002/ijc.22293. [DOI] [PubMed] [Google Scholar]
- 33.Noh DY, Ahn SJ, Lee RA, et al. Overexpression of phospholipase D1 in human breast cancer tissues. Cancer Lett. 2000;161:207–14. doi: 10.1016/s0304-3835(00)00612-1. [DOI] [PubMed] [Google Scholar]
- 34.Foster DA, Xu L. Phospholipase D in cell proliferation and cancer. Mol Cancer Res. 2003;1:789–800. [PubMed] [Google Scholar]
- 35.Gillies RJ, Morse DL. In vivo magnetic resonance spectroscopy in cancer. Annu Rev Biomed Eng. 2005;7:287–326. doi: 10.1146/annurev.bioeng.7.060804.100411. [DOI] [PubMed] [Google Scholar]
- 36.Hakumaki JM, Poptani H, Sandmair AM, Yla-Herttuala S, Kauppinen RA. 1H MRS detects polyunsaturated fatty acid accumulation during gene therapy of glioma: implications for the in vivo detection of apoptosis. Nat Med. 1999;5:1323–7. doi: 10.1038/15279. [DOI] [PubMed] [Google Scholar]
- 37.He Q, Bhujwalla ZM, Glickson JD. Proton detection of choline and lactate in EMT6 tumors by spin-echo-enhanced selective multiple-quantum-coherence transfer. J Magn Reson Series B. 1996;112:18–25. doi: 10.1006/jmrb.1996.0104. [DOI] [PubMed] [Google Scholar]
- 38.Melkus G, Morchel P, Behr VC, Kotas M, Flentje M, Jakob PM. Short-echo spectroscopic imaging combined with lactate editing in a single scan. NMR Biomed. 2008;21:1076–86. doi: 10.1002/nbm.1284. [DOI] [PubMed] [Google Scholar]
- 39.Thakur SB, Yaligar J, Koutcher JA. In vivo lactate signal enhancement using binomial spectral-selective pulses in selective MQ coherence (SS-SelMQC) spectroscopy. Magn Reson Med. 2009;62:591–8. doi: 10.1002/mrm.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engelmann J, Henke J, Willker W, et al. Early stage monitoring of miltefosine induced apoptosis in KB cells by multinuclear NMR spectroscopy. Anticancer Res. 1996;16:1429–39. [PubMed] [Google Scholar]
- 41.Al-Saffar NM, Titley JC, Robertson D, et al. Apoptosis is associated with triacylglycerol accumulation in Jurkat T-cells. Br J Cancer. 2002;86:963–70. doi: 10.1038/sj.bjc.6600188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barba I, Cabanas ME, Arus C. The relationship between nuclear magnetic resonance-visible lipids, lipid droplets, and cell proliferation in cultured C6 cells. Cancer Res. 1999;59:1861–8. [PubMed] [Google Scholar]
- 43.Schmitz JE, Kettunen MI, Hu DE, Brindle KM. 1H MRS-visible lipids accumulate during apoptosis of lymphoma cells in vitro and in vivo. Magn Reson Med. 2005;54:43–50. doi: 10.1002/mrm.20529. [DOI] [PubMed] [Google Scholar]
- 44.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okunieff PG, Koutcher JA, Gerweck L, et al. Tumor size dependent changes in a murine fibrosarcoma: use of in vivo 31P NMR for non-invasive evaluation of tumor metabolic status. Int J Radiat Oncol Biol Phys. 1986;12:793–9. doi: 10.1016/0360-3016(86)90038-6. [DOI] [PubMed] [Google Scholar]
- 46.Li SJ, Wehrle JP, Rajan SS, Steen RG, Glickson JD, Hilton J. Response of radiation-induced fibrosarcoma-1 in mice to cyclophosphamide monitored by in vivo 31P nuclear magnetic resonance spectroscopy. Cancer Res. 1988;48:4736–42. [PubMed] [Google Scholar]
- 47.Kallman RF. The phenomenon of reoxygenation and its implications for fractionated radiotherapy. Radiology. 1972;105:135–42. doi: 10.1148/105.1.135. [DOI] [PubMed] [Google Scholar]
- 48.Tozer GM, Griffiths JR. The contribution made by cell death and oxygenation to 31P MRS observations of tumour energy metabolism. NMR Biomed. 1992;5:279 – 89. doi: 10.1002/nbm.1940050515. [DOI] [PubMed] [Google Scholar]
- 49.Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973;248:7276–8. [PubMed] [Google Scholar]
- 50.Stubbs M, Bhujwalla ZM, Tozer GM, et al. An assessment of 31P MRS as a method of measuring pH in rat tumours. NMR Biomed. 1992;5:351–9. doi: 10.1002/nbm.1940050606. [DOI] [PubMed] [Google Scholar]
- 51.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–65. [PubMed] [Google Scholar]
- 52.Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991;64:425–7. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillies RJ, Liu Z, Bhujwalla ZM. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am J Physiol. 1994;267:C195–203. doi: 10.1152/ajpcell.1994.267.1.C195. [DOI] [PubMed] [Google Scholar]
- 54.van Sluis R, Bhujwalla ZM, Raghunand N, et al. In vivo imaging of extracellular pH using 1H MRSI. Magn Reson Med. 1999;41:743–50. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 55.Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996;14:176–86. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 56.Raghunand N, Mahoney B, van Sluis R, Baggett B, Gillies RJ. Acute metabolic alkalosis enhances response of C3H mouse mammary tumors to the weak base mitoxantrone. Neoplasia. 2001;3:227–35. doi: 10.1038/sj.neo.7900151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robey IF, Baggett BK, Kirkpatrick ND, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260–8. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gil S, Zaderenzo P, Cruz F, Cerdan S, Ballesteros P. Imidazol-1-ylalkanoic acids as extrinsic 1H NMR probes for the determination of intracellular pH, extracellular pH and cell volume. Bioorg Med Chem. 1994;2:305–14. doi: 10.1016/s0968-0896(00)82186-0. [DOI] [PubMed] [Google Scholar]
- 59.Bhujwalla ZM, Artemov D, Ballesteros P, Cerdan S, Gillies RJ, Solaiyappan M. Combined vascular and extracellular pH imaging of solid tumors. NMR Biomed. 2002;15:114–9. doi: 10.1002/nbm.743. [DOI] [PubMed] [Google Scholar]
- 60.Ojugo AS, McSheehy PM, McIntyre DJ, et al. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous (19)F and (31)P probes. NMR Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 61.Provent P, Benito M, Hiba B, et al. Serial in vivo spectroscopic nuclear magnetic resonance imaging of lactate and extracellular pH in rat gliomas shows redistribution of protons away from sites of glycolysis. Cancer Res. 2007;67:7638–45. doi: 10.1158/0008-5472.CAN-06-3459. [DOI] [PubMed] [Google Scholar]
- 62.Cheng LL, Burns MA, Taylor JL, et al. Metabolic characterization of human prostate cancer with tissue magnetic resonance spectroscopy. Cancer Res. 2005;65:3030–4. doi: 10.1158/0008-5472.CAN-04-4106. [DOI] [PubMed] [Google Scholar]
- 63.Sitter B, Lundgren S, Bathen TF, Halgunset J, Fjosne HE, Gribbestad IS. Comparison of HR MAS MR spectroscopic profiles of breast cancer tissue with clinical parameters. NMR Biomed. 2006;19:30–40. doi: 10.1002/nbm.992. [DOI] [PubMed] [Google Scholar]
- 64.Martinez-Bisbal MC, Marti-Bonmati L, Piquer J, et al. 1H and 13C HR-MAS spectroscopy of intact biopsy samples ex vivo and in vivo 1H MRS study of human high grade gliomas. NMR Biomed. 2004;17:191–205. doi: 10.1002/nbm.888. [DOI] [PubMed] [Google Scholar]
- 65.Bathen TF, Jensen LR, Sitter B, et al. MR-determined metabolic phenotype of breast cancer in prediction of lymphatic spread, grade, and hormone status. Breast Cancer Res Treat. 2007;104:181–9. doi: 10.1007/s10549-006-9400-z. [DOI] [PubMed] [Google Scholar]
- 66.Artemov D, Bhujwalla ZM, Glickson JD. In vivo selective measurement of (1-13C)-glucose metabolism in tumors by heteronuclear cross polarization. Magn Reson Med. 1995;33:151–5. doi: 10.1002/mrm.1910330202. [DOI] [PubMed] [Google Scholar]
- 67.van Zijl PC, Chesnick AS, DesPres D, Moonen CT, Ruiz-Cabello J, van Gelderen P. In vivo proton spectroscopy and spectroscopic imaging of [1-13C]-glucose and its metabolic products. Magn Reson Med. 1993;30:544–51. doi: 10.1002/mrm.1910300504. [DOI] [PubMed] [Google Scholar]
- 68.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–30. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 69.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 70.Vaupel P, Okunieff P, Kallinowski F, Neuringer LJ. Correlations between 31 P-NMR spectroscopy and tissue O2 tension measurements in a murine fibrosarcoma. Radiat Res. 1989;120:477–93. [PubMed] [Google Scholar]
- 71.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–62. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 72.Rivenzon-Segal D, Margalit R, Degani H. Glycolysis as a metabolic marker in orthotopic breast cancer, monitored by in vivo (13)C MRS. Am J Physiol Endocrinol Metab. 2002;283:E623–30. doi: 10.1152/ajpendo.00050.2002. [DOI] [PubMed] [Google Scholar]
- 73.Terpstra M, High WB, Luo Y, de Graaf RA, Merkle H, Garwood M. Relationships among lactate concentration, blood flow and histopathologic profiles in rat C6 glioma. NMR Biomed. 1996;9:185–94. doi: 10.1002/(SICI)1099-1492(199608)9:5<185::AID-NBM414>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 74.Nielsen FU, Daugaard P, Bentzen L, et al. Effect of changing tumor oxygenation on glycolytic metabolism in a murine C3H mammary carcinoma assessed by in vivo nuclear magnetic resonance spectroscopy. Cancer Res. 2001;61:5318–25. [PubMed] [Google Scholar]
- 75.Bouzier AK, Quesson B, Valeins H, Canioni P, Merle M. [1-(13)C]glucose metabolism in the tumoral and nontumoral cerebral tissue of a glioma-bearing rat. J Neurochem. 1999;72:2445–55. doi: 10.1046/j.1471-4159.1999.0722445.x. [DOI] [PubMed] [Google Scholar]
- 76.Day SE, Kettunen MI, Gallagher FA, et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007;13:1382–7. doi: 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- 77.Albers MJ, Bok R, Chen AP, et al. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res. 2008;68:8607–15. doi: 10.1158/0008-5472.CAN-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100:10158–63. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goldman M, Johannesson H, Axelsson O, Karlsson M. Hyperpolarization of 13C through order transfer from parahydrogen: a new contrast agent for MRI. Magn Reson Imaging. 2005;23:153–7. doi: 10.1016/j.mri.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 80.Gallagher FA, Kettunen MI, Day SE, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 2008;453:940–3. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
- 81.Gill SS, Thomas DG, Van Bruggen N, et al. Proton MR spectroscopy of intracranial tumours: in vivo and in vitro studies. J Comput Assist Tomogr. 1990;14:497–504. doi: 10.1097/00004728-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Kurhanewicz J, Vigneron DB, Nelson SJ. Three-dimensional magnetic resonance spectroscopic imaging of brain and prostate cancer. Neoplasia. 2000;2:166–89. doi: 10.1038/sj.neo.7900081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mueller-Lisse UG, Swanson MG, Vigneron DB, et al. Time-dependent effects of hormone-deprivation therapy on prostate metabolism as detected by combined magnetic resonance imaging and 3D magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;46:49–57. doi: 10.1002/mrm.1159. [DOI] [PubMed] [Google Scholar]
- 84.Jacobs MA, Barker PB, Argani P, Ouwerkerk R, Bhujwalla ZM, Bluemke DB. Combined dynamic contrast and spectroscopic imaging of human breast cancer. J Magn Reson Imaging. 2005;21:23–8. doi: 10.1002/jmri.20239. [DOI] [PubMed] [Google Scholar]
- 85.Lehnhardt FG, Bock C, Rohn G, Ernestus RI, Hoehn M. Metabolic differences between primary and recurrent human brain tumors: a 1H NMR spectroscopic investigation. NMR Biomed. 2005;18:371–82. doi: 10.1002/nbm.968. [DOI] [PubMed] [Google Scholar]
- 86.Mountford CE, Somorjai RL, Malycha P, et al. Diagnosis and prognosis of breast cancer by magnetic resonance spectroscopy of fine-needle aspirates analysed using a statistical classification strategy. Br J Surg. 2001;88:1234–40. doi: 10.1046/j.0007-1323.2001.01864.x. [DOI] [PubMed] [Google Scholar]
- 87.Li X, Lu Y, Pirzkall A, McKnight T, Nelson SJ. Analysis of the spatial characteristics of metabolic abnormalities in newly diagnosed glioma patients. J Magn Reson Imaging. 2002;16:229–37. doi: 10.1002/jmri.10147. [DOI] [PubMed] [Google Scholar]
- 88.Hu J, Vartanian SA, Xuan Y, Latif Z, Soulen RL. An improved 1H magnetic resonance spectroscopic imaging technique for the human breast: preliminary results. Magn Reson Imaging. 2005;23:571–6. doi: 10.1016/j.mri.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Wald LL, Nelson SJ, Day MR, et al. Serial proton magnetic resonance spectroscopy imaging of glioblastoma multiforme after brachytherapy. J Neurosurg. 1997;87:525–34. doi: 10.3171/jns.1997.87.4.0525. [DOI] [PubMed] [Google Scholar]
- 90.Laprie A, Pirzkall A, Haas-Kogan DA, et al. Longitudinal multivoxel MR spectroscopy study of pediatric diffuse brainstem gliomas treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:20–31. doi: 10.1016/j.ijrobp.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 91.Kurhanewicz J, Swanson MG, Nelson SJ, Vigneron DB. Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer. J Magn Reson Imaging. 2002;16:451–63. doi: 10.1002/jmri.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu C-L, Jordan KW, Ratai EM, et al. Metabolomic imaging for human prostate cancer detection. Sci Transl Med. 2010;2:1–7. doi: 10.1126/scitranslmed.3000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joseph T, McKenna DA, Westphalen AC, et al. Pretreatment endorectal magnetic resonance imaging and magnetic resonance spectroscopic imaging features of prostate cancer as predictors of response to external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:665–71. doi: 10.1016/j.ijrobp.2008.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jacobs MA, Ouwerkerk R, Petrowski K, Macura KJ. Diffusion-weighted imaging with apparent diffusion co-efficient mapping and spectroscopy in prostate cancer. Top Magn Reson Imagin. 2008;19:261–72. doi: 10.1097/RMR.0b013e3181aa6b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loening NM, Chamberlin AM, Zepeda AG, Gonzalez RG, Cheng LL. Quantification of phosphocholine and glycerophosphocholine with 31P edited 1H NMR spectroscopy. NMR Biomed. 2005;18:413–20. doi: 10.1002/nbm.973. [DOI] [PubMed] [Google Scholar]
- 96.Nelson SJ, Graves E, Pirzkall A, et al. In vivo molecular imaging for planning radiation therapy of gliomas: an application of 1H MRSI. J Magn Reson Imaging. 2002;16:464–76. doi: 10.1002/jmri.10183. [DOI] [PubMed] [Google Scholar]
- 97.Zaider M, Zelefsky MJ, Lee EK, et al. Treatment planning for prostate implants using magnetic-resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys. 2000;47:1085–96. doi: 10.1016/s0360-3016(00)00557-5. [DOI] [PubMed] [Google Scholar]
- 98.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–9. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13:372–7. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 100.Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology. 2002;222:715–21. doi: 10.1148/radiol.2223010558. [DOI] [PubMed] [Google Scholar]
- 101.Haddadin IS, McIntosh A, Meisamy S, et al. Metabolite quantification and high-field MRS in breast cancer. NMR Biomed. 2009;22:65–76. doi: 10.1002/nbm.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Danishad KK, Sharma U, Sah RG, Seenu V, Parshad R, Jagannathan NR. Assessment of therapeutic response of locally advanced breast cancer (LABC) patients undergoing neoadjuvant chemotherapy (NACT) monitored using sequential magnetic resonance spectroscopic imaging (MRSI) NMR Biomed. 2010;23:233–241. doi: 10.1002/nbm.1436. [DOI] [PubMed] [Google Scholar]
- 103.Kurhanewicz J, Vigneron DB, Hricak H, et al. Prostate cancer: metabolic response to cryosurgery as detected with 3D H-1 MR spectroscopic imaging. Radiology. 1996;200:489–96. doi: 10.1148/radiology.200.2.8685346. [DOI] [PubMed] [Google Scholar]
- 104.Evelhoch JL, Gillies RJ, Karczmar GS, et al. Applications of magnetic resonance in model systems: cancer therapeutics. Neoplasia. 2000;2:152–65. doi: 10.1038/sj.neo.7900078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwarz AJ, Maisey NR, Collins DJ, Cunningham D, Huddart R, Leach MO. Early in vivo detection of metabolic response: a pilot study of 1H MR spectroscopy in extracranial lymphoma and germ cell tumours. Br J Radiol. 2002;75:959–66. doi: 10.1259/bjr.75.900.750959. [DOI] [PubMed] [Google Scholar]
- 106.deSouza NM, Soutter WP, Rustin G, et al. Use of neo-adjuvant chemotherapy prior to radical hysterectomy in cervical cancer: monitoring tumour shrinkage and molecular profile on magnetic resonance and assessment of 3-year outcome. Br J Cancer. 2004;90:2326–31. doi: 10.1038/sj.bjc.6601870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beloueche-Babari M, Jackson LE, Al-Saffar NM, Workman P, Leach MO, Ronen SM. Magnetic resonance spectroscopy monitoring of mitogen-activated protein kinase signaling inhibition. Cancer Res. 2005;65:3356–63. doi: 10.1158/10.1158/0008-5472.CAN-03-2981. [DOI] [PubMed] [Google Scholar]
- 108.Ross J, Najjar AM, Sankaranarayanapillai M, Tong WP, Kaluarachchi K, Ronen SM. Fatty acid synthase inhibition results in a magnetic resonance-detectable drop in phosphocholine. Mol Cancer Ther. 2008;7:2556–65. doi: 10.1158/1535-7163.MCT-08-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klawitter J, Anderson N, Klawitter J, et al. Time-dependent effects of imatinib in human leukaemia cells: a kinetic NMR-profiling study. Br J Cancer. 2009;100:923–31. doi: 10.1038/sj.bjc.6604946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sankaranarayanapillai M, Tong WP, Yuan Q, et al. Monitoring histone deacetylase inhibition in vivo: noninvasive magnetic resonance spectroscopy method. Mol Imaging. 2008;7:92–100. [PubMed] [Google Scholar]
- 111.Al-Saffar NM, Troy H, Ramirez de Molina A, et al. Non-invasive magnetic resonance spectroscopic pharmacodynamic markers of the choline kinase inhibitor MN58b in human carcinoma models. Cancer Res. 2006;66:427–34. doi: 10.1158/0008-5472.CAN-05-1338. [DOI] [PubMed] [Google Scholar]
- 112.Krishnamachary B, Glunde K, Wildes F, et al. Noninvasive detection of lentiviral-mediated choline kinase targeting in a human breast cancer xenograft. Cancer Res. 2009;69:3464–71. doi: 10.1158/0008-5472.CAN-08-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lacal JC. Choline kinase: a novel target for antitumor drugs. IDrugs. 2001;4:419–26. [PubMed] [Google Scholar]
- 114.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–9. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 115.Gallagher FA, Kettunen MI, Hu DE, et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc Natl Acad Sci U S A. 2009;106:19801–6. doi: 10.1073/pnas.0911447106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ward CS, Venkatesh HS, Chaumeil MM, et al. Noninvasive detection of target modulation following phosphatidylinositol 3-kinase inhibition using hyperpolarized 13C magnetic resonance spectroscopy. Cancer Res. 2010;70:1296–305. doi: 10.1158/0008-5472.CAN-09-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Artemov D, Solaiyappan M, Bhujwalla ZM. Magnetic resonance pharmacoangiography to detect and predict chemotherapy delivery to solid tumors. Cancer Res. 2001;61:3039–44. [PubMed] [Google Scholar]
- 118.Kato Y, Holm DA, Okollie B, Artemov D. Noninvasive detection of temozolomide in brain tumor xenografts by magnetic resonance spectroscopy. Neuro Oncol. 2010;12:71–9. doi: 10.1093/neuonc/nop006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wolf W, Presant CA, Waluch V. 19F-MRS studies of fluorinated drugs in humans. Adv Drug Deliv Rev. 2000;41:55–74. doi: 10.1016/s0169-409x(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 120.van Laarhoven HW, Klomp DW, Rijpkema M, et al. Prediction of chemotherapeutic response of colorectal liver metastases with dynamic gadolinium-DTPA-enhanced MRI and localized 19F MRS pharmacokinetic studies of 5-fluorouracil. NMR Biomed. 2007;20:128–40. doi: 10.1002/nbm.1098. [DOI] [PubMed] [Google Scholar]
- 121.Li C, Penet MF, Winnard P, Jr, Artemov D, Bhujwalla ZM. Image-guided enzyme/prodrug cancer therapy. Clin Cancer Res. 2008;14:515–22. doi: 10.1158/1078-0432.CCR-07-1837. [DOI] [PubMed] [Google Scholar]
- 122.Reid DG, Murphy PS. Fluorine magnetic resonance in vivo: a powerful tool in the study of drug distribution and metabolism. Drug Discov Today. 2008;13:473–80. doi: 10.1016/j.drudis.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 123.Kato Y, Okollie B, Artemov D. Noninvasive 1H/13C magnetic resonance spectroscopic imaging of the intratumoral distribution of temozolomide. Magn Reson Med. 2006;55:755–61. doi: 10.1002/mrm.20831. [DOI] [PubMed] [Google Scholar]
- 124.Griffiths JR, Glickson JD. Monitoring pharmacokinetics of anticancer drugs: non-invasive investigation using magnetic resonance spectroscopy. Adv Drug Deliv Rev. 2000;41:75–89. doi: 10.1016/s0169-409x(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 125.O’Reilly SM, Newlands ES, Glaser MG, et al. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours. Eur J Cancer. 1993;29A:940–2. doi: 10.1016/s0959-8049(05)80198-4. [DOI] [PubMed] [Google Scholar]