Abstract
A single-laboratory validation study was conducted for a liquid chromatographic (LC) method for the determination of total and all-trans-lycopene in a variety of dietary supplements and raw materials. Gelatin-based and other water-dispersible beadlets, or tablets, capsules, and softgels containing such product forms, were digested with protease. Alginate formulations and the respective applications were treated with an alkaline sodium EDTA acetate buffer to release lycopene from the matrix. Lycopene and other carotenoids were extracted from the resulting aqueous suspensions with dichloromethane and ethanol. Oily product forms were directly dissolved in dichloromethane and ethanol. The extracts were chromatographed on an isocratic high-performance LC system using a C16 alkylamide modified silica column that provided satisfactory resolution of all-trans-lycopene from its predominant cis-isomers and separated the lycopene isomers from other carotenoids such as α- and β-carotene, cryptoxanthin, lutein, and zeaxanthin. The within-day precision relative standard deviation (RSD) for the determination of total lycopene ranged from 0.9 to 5.7% over concentration ranges of 50–200 g/kg for raw materials and 0.3–24 g/kg for dietary supplements. The intermediate precision RSD (total RSD) ranged from 0.8 to 8.9%. Recoveries obtained for beadlet and tablet material for the different extraction variants ranged from 95.0 to 102.1% at levels of 0.02–20 g/kg for tablets and from 95.0 to 101.1% at levels of 1–200 g/kg for beadlet material.
Lycopene is a naturally occurring carotenoid that has attracted considerable attention since several studies suggested a protective effect against prostate cancer (1–3). It is regarded as a strong antioxidant protecting cells from reactive oxygen species (4), which may explain possible health-promoting effects. Lycopene is commercially available in combinations with other carotenoids, e.g., β-carotene, lutein, and zeaxanthin, or plant extracts in a variety of dietary supplements. Unfortunately, versatile methods of analysis for lycopene in various formulations and matrixes have not been reported. Carotenoids are sensitive to light, heat, and oxygen and are able to form cis-isomers which usually show lower extinction coefficients compared with those of the all-trans-components (5). Various high-performance liquid chromatographic (HPLC) methods using silica, C-18, or even C-30 column materials have been reported for the determination of carotenoids and their isomers in complex matrixes (6–7). Only a few researchers have discussed the problems of sample preparation (8, 9) and quantification of cis-isomers (10). Quantification of lycopene cis-isomers is of great importance because recent studies showed differences in bioavailability compared with that of all-trans-lycopene. Schierle et al. (10) recently validated a method for the determination of total β-carotene and all-trans-β-carotene that used relative response factor (RRF) values for the major cis-isomers in dietary supplements and raw materials to avoid underestimation of the β-carotene content. In the present study, a versatile method was validated for quantification of lycopene by using RRF values for the determination of total lycopene and the major lycopene cis-isomers in a variety of dietary supplements and raw materials.
METHOD
Test and Negative Control Materials
Test materials.—Dietary supplements used in this study as test materials were obtained from commercial sources and were provided by AOAC INTERNATIONAL. The following selection represents the range of supplements currently available to the general public: (1) Softgels with lycopene in soybean oil; claim: 10% lycopene. (2) Beadlet raw material with lycopene of synthetic origin; claim: 5% lycopene. (3) Beadlet raw material with lycopene of natural origin; claim: 5% lycopene. (4) Beadlet raw material with lycopene of natural origin, vegetarian beadlet; claim: 5% lycopene. (5) Beadlet raw material with lycopene of natural origin, vegetarian beadlet; claim; 20% lycopene. (6) Beadlet raw material with lycopene of natural origin, vegetarian beadlet, allergen free; claim: 5% lycopene. (7) Beadlet raw material with lycopene of natural origin, vegetarian beadlet, allergen free; claim: 20% lycopene. (8) Beadlet raw material with lycopene of natural origin, water-dispersible beadlet; claim: 10% lycopene. (9) Multivitamin tablets; claim: 300 μg lycopene per tablet. (10) Softgel with lycopene and β-carotene in soybean and corn oil; claim: 10 mg lycopene per capsule. (11) Tablets with lycopene, β-carotene, α-carotene, lutein, and zeaxanthin; claim: 5 mg lycopene per tablet. (12) Tablets with lycopene of natural origin and herbs; claim: 1.5 mg lycopene per tablet. (13) Tablets with lycopene of synthetic origin, minerals, and herbs; claim: 2 mg lycopene per tablet. (14) Tablets with high lycopene content; claim: 25 mg lycopene per tablet. (15) Tablets containing lycopene, herbs, amino acids, fat-soluble vitamins, minerals, and isoflavones; claim: 10 mg lycopene per tablet. (16) Softgel capsules with lycopene, minerals, and herbs; claim: 1 mg lycopene per capsule.
Negative control materials.—(1) Placebo multivitamin tablets, DSM formula No. 80.23 (DSM Nutritional Products Ltd, Basel, Switzerland) containing vitamin A acetate, vitamin D3, vitamin E, ascorbic acid, thiamine mononitrate, vitamin B2, nicotinamide, pyridoxine HCl, vitamin B12, folic acid, pantothenic acid, biotin, vitamin K1, iron fumarate, magnesium oxide, potassium iodide, zinc sulfate monohydrate, manganese sulfate monohydrate, anhydrous copper sulfate, chromium chloride hexahydrate, anhydrous sodium selenite, sodium molybdate dihydrate, anhydrous calcium phosphate, potassium chloride, silicon dioxide, cellulose, stearic acid, magnesium stearate, and n-vinyl-2-pyrrolidon; coated with Sepifilm LP014 (Seppic, Inc., Fairfield, NJ). (2) Lycopene 10% WS (water-soluble) placebo beadlets without lycopene, Lot UT02111005, containing fish gelatin, saccharose, vitamin C palmitate, corn oil, DL-α-tocopherol, and water.
Lycopene beadlets, reference materials, and negative control materials were provided by DSM Nutritional Products Ltd. Test and negative control materials were stored at 5°C in the dark (refrigerator).
Principle
Gelatin-based and most other water-dispersible formulations, as well as finished products containing these formulations, are digested by an aqueous protease preparation. Alginate-based formulations and products thereof were disintegrated by an alkaline, EDTA buffer and protease treatment. The aqueous suspensions are extracted with dichloromethane and ethanol. Oily suspensions are dissolved directly in dichloromethane and ethanol. The extract is chromatographed isocratically on a C16 alkylamide spherical 5 μm HPLC column that separates the predominant geometrical isomers of all-trans-lycopene and its cis-isomers from each other and from other carotenoids such as α- and β-carotene, cryptoxanthin, lutein, and zeaxanthin.
Scope
The method is suitable for the determination of all-trans-lycopene and total lycopene. The term total lycopene comprises all-trans-lycopene and the major cis-isomers, 5-cis-lycopene, 9-cis-lycopene, 13-cis-lycopene, and 15-cis-lycopene. The method is applicable to the determination of concentrations of ≥0.1 mg all-trans-lycopene and the cis-isomers of lycopene in tablets and capsules, and >0.2% lycopene in oily or water-dispersible raw materials in the presence of other carotenoids such as β-carotene, α-carotene, and xanthophylls.
Apparatus
Balance.—Balance with readability of 0.01 mg, precision [standard deviation (SD)] of ±0.015 mg, and capacity of 205 g (AT261 DeltaRange, Mettler-Toledo, Nänikon-Uster, Switzerland); balance with a readability of 0.01 g, precision (SD) of ±0.005 g, and capacity of 2100 g (PM2000, Mettler-Toledo).
Spectrophotometer.—Wavelength range of 190–900 nm, fixed spectral bandwidth of 1.5 nm, wavelength accuracy of 0.07 nm (at 541.92 nm), and wavelength reproducibility of 0.01 nm (Vary Scan, Varian, Darmstadt, Germany).
Ultrasonic water bath.—150 W, 35 kHz, 4 L (TUC-160, Telsonic, Bronschhofen, Switzerland).
pH meter.—Model 713 (Metrom, Herisau, Switzerland).
Homogenizer.—750 W, 12 mm diameter dispersing aggregate (Polytron PT3100 drive unit and PT-DA-3012/2T aggregate; Kinematica, Lucerne, Switzerland).
Syringe.—Disposable, 2 mL (Henke-Sass, Wolff GmbH, Tuttlingen, Germany).
Filter.—Disposable, 0.45 μm pore size, 25 mm diameter, for organic solvents (Chromafil Type O-45/25, Macherey-Nagel, Düren, Germany).
HPLC system.—Consisting of quaternary pump, degasser, autosampler with thermostat, column oven with thermostat, diode-array detector 190–900 nm Agilent Series 1100 HPLC, and integrator (ATLAS™ Chromatography Data System, Thermo LabSystems, Manchester, UK, combined with a Microsoft® Windows 2000 Terminal Server, Citrix MetaFrame).
Suplex PKB-100 HPLC column.—5 μm, 250 × 4.6 mm (Cat. No. 58934, Supelco, Bellefonte, PA).
Reagents
All chemicals and solvents were analytical grade unless otherwise specified.
n-Hexane.—Purity of ≥99% [gas chromatographic (GC) grade]; available from Merck (Darmstadt, Germany).
Ethanol.—Purity of ≥99.5% (GC grade); available from Merck.
Methanol.—Purity of ≥99.8% (GC grade); available from Merck.
Dichloromethane.—Purity of ≥99.5% (GC grade); available from Merck. Discard 1 month after opening.
Acetonitrile.—Purity of ≥99.9% (GC grade); available from Merck.
N-Ethyldiisopropylamine.—Purity of ≥98% (GC grade); available from Fluka (Buchs, Switzerland).
2-Propanol.—Purity of ≥98% (GC grade); available from Fluka.
Water.—Distilled or demineralized.
Protex, 6 L.—Bacterial alkaline protease enzyme preparation in water Synonyms: subtilisin, IUB 3.4.21.62, CAS 9014-01-1. EINECS 2327522. Available from Genencor International, Inc. (Palo Alto, CA, www.genencor.com).
Ammonium acetate.—Purity of ≥98%; available from Fluka.
Butylated hydroxytoluene (BHT).—2,6-Di-tert-butyl-p-cresol; purity of ≥99%; available from Fluka.
Potassium dihydrogen phosphate.—Purity of ≥99%; available from Fluka.
Sodium hydroxide.—Purity of ≥98% (T grade); available from Fluka.
Disodium EDTA dihydrate.—Purity of ≥99%; available from Fluka.
Sodium dodecyl sulfate (SDS).—Purity of ≥99% (T grade); available from Fluka.
Ammonium acetate solution, 0.2%.—Dissolve 0.5 g ammonium acetate in 250 mL water. Store the solution at 5°C for no longer than 1 month.
NaOH solution, 4 M.—Dissolve 16 g sodium hydroxide in 100 mL water.
Mobile phase.—In a 1 L volumetric flask, dissolve 50 mg BHT in 20 mL 2-propanol, and add 0.2 mL N-ethyldiisopropylamine, 25 mL 0.2% ammonium acetate solution, 455 mL acetonitrile, and approximately 450 mL methanol. The mixture cools and contracts. Warm to room temperature, and dilute to volume with methanol. Discard after 2 days.
Alginate-based beadlet extraction solution.—In a 1 L Erlenmeyer flask, dissolve 68 g potassium dihydrogen phosphate and 10 g disodium EDTA dihydrate in approximately 900 mL water. Add approximately 18 g sodium hydroxide, and ultrasonicate the solution for 20 min until the sodium hydroxide is dissolved. Adjust the pH of the solution to 8.7 ± 0.3 with 4 M NaOH, and dilute to 1 L with water. Add 0.25 g SDS, and ultrasonicate for 20 min (will not completely dissolve).
Reference standard all-trans-lycopene.—Purity of ≥95% (HPLC grade); product code 0031 (Carote Nature, Lupsingen, Switzerland). Store under nitrogen at 5°C in the dark (refrigerator).
Calibration
Standard solution.—All-trans-lycopene at 3 μg/mL. Weigh approximately 6 mg all-trans-lycopene into a 100 mL volumetric flask, with an accuracy of 0.01 mg. Dissolve in 20 mL dichloromethane by treating in an ultrasonic bath for 30 s. Dilute to volume with dichloromethane to obtain a standard stock solution containing 60 μg/mL. From this standard stock solution, pipet an aliquot of 5 mL into a 100 mL volumetric flask. Dilute the contents of the flask to volume with n-hexane to obtain a standard solution containing all-trans-lycopene at 3 μg/mL in n-hexane–dichloromethane (95 + 5, v/v). Store standard stock solution at 5°C in the dark (refrigerator), and use on day of preparation.
-
Spectrophotometric purity (SP).—Measure the maximum absorbance of the standard solution, (a), versus n-hexane at approximately 471 nm. Calculate the SP of the lycopene standard as follows:
where AStd = absorbance of the standard solution, (a), at the maximum of approximately 471 nm, 20 000 = dilution factor, 3450 = E1%, 1cm of pure all-trans-lycopene in hexane, and W = weight, in mg, of the standard used for the standard stock solution.
The SP of the standard must be >95%.
-
Chromatographic purity (CP).—Inject at least 4 aliquots of 20 μL standard solution, (a), immediately after preparation into the HPLC system. Calculate the CP as follows:
Relevant peaks are all peaks in the chromatogram with the exception of any peaks due to the solvent.
The CP of all-trans-lycopene must be >95%.
-
Purity of the reference compound.—The calculation of the overall purity of the all-trans-lycopene content of the reference compound includes degradation products not absorbing in the 471 nm region (measured by visible spectroscopy; SP) and visible (colored) non-all-trans-lycopene compounds (detected by HPLC; CP). The purity of the reference standard is then calculated as follows:
where SP = spectrophotometric purity, CP = chromatographic purity, and 100 = scaling factor to express the purity in %.
The concentration of all-trans-lycopene in the standard solution is calculated as follows:
where W = weight, in mg, of the reference compound, P = purity, in %, and 200 = dilution and conversion factor. -
Calculation of the response factor (RF).—Calculate the RF of all-trans-lycopene from the averaged peak areas for the all-trans-lycopene peak divided by the calculated concentration of all-trans-lycopene in the standard solution as follows:
where RFall-trans-lycopene = response factor of the all-trans-lycopene, Aall-trans-lycopene = mean peak area, measured in area units (AU), of the all-trans-lycopene peak in the chromatograms of the standard, and Call-trans-lycopene = calculated concentration of all-trans-lycopene in the standard solution, in mg/L.
LC response of control solutions.—Control solutions are solutions of heat-isomerized all-trans-lycopene, the concentrations of which have been found to be stable at 5°C in the dark. It is not necessary to recalibrate the HPLC system if the previously determined RF has been shown to be valid by use of control solutions. (1) Preparation of LC control solution.—Dissolve 3 mg all-trans-lycopene reference substance and 1 g BHT in 10 mL dichloromethane in a 500 mL round-bottom flask, and add 200 mL n-hexane. Reflux the solution at 80°C in a water bath for 1 h. After the solution has cooled to room temperature, transfer the solution to a 500 mL volumetric flask, and dilute to volume with n-hexane. Dispense the solution, which is kept at room temperature in the dark overnight, into a large number of HPLC vials. Carefully seal the vials after filling them by using Teflon/silicone septa, and store the sealed vials at 5°C in the dark under refrigeration. (2) Use of the LC control solution.—Measure the initial total lycopene content immediately after preparation of the control solution during calibration of the HPLC system. Inject the control solution in parallel with the standard solutions, at least 6 aliquots of 20 μL, into the HPLC system. Calculate the mean lycopene content of the control solution from the resulting chromatograms, using the RRF values previously calculated. Subsequently inject the control solution together with each sample series. The RF is regarded as constant as long as the measured total lycopene content of the control solutions corresponds to the initial value within ±2%; otherwise the HPLC system must be recalibrated. (3) Linearity of the LC response.—Prepare calibration curves from lycopene with and without prior heat isomerization, using concentrations of 0.05–100 μg/L. For the heat-isomerized solution, dissolve 10 mg all-trans-lycopene and 100 mg BHT in 10 mL dichloromethane, in a 100 mL volumetric flask. Add 50 mL dichloromethane, and reflux at 80°C in a water bath for 2 h. Add approximately 30 mL dichloromethane, bring to room temperature, and dilute to volume with dichloromethane. In a second 100 mL volumetric flask, dissolve 10 mg lycopene reference compound in dichloromethane. Dilute these solutions according to Table 1 with the solvent mixture, ethanol–dichloromethane (1+1, v/v), to obtain the respective calibration solutions.
Table 1.
Dilution scheme for preparation of the calibration curve
| Solution to be diluted, μg/mL | Dilution, v + v | Final concn, μg/mL |
|---|---|---|
| 100 | —a | 100 |
| 100 | 1 + 1 | 50 |
| 100 | 1 + 4 | 20 |
| 100 | 1 + 9 | 10 |
| 50 | 1 + 9 | 5 |
| 20 | 1 + 9 | 2 |
| 10 | 1+ 9 | 1 |
| 5 | 1 + 9 | 0.5 |
| 2 | 1 + 9 | 0.2 |
| 1 | 1 + 9 | 0.1 |
| 0.5 | 1 + 9 | 0.05 |
— = Undiluted.
Inject the solutions into the LC system, and calculate the total lycopene content as described in the Calculations section. The response must be linear for all-trans-lycopene concentrations in the range of 0.1–50 μg/mL with a determination coefficient, R2, of >0.995 and the back-calculated concentration within the target of ±10% of the theoretical value.
Sample Preparation
Sample preparation is dependent on the physical form of the material, the claimed content of lycopene, and the weight of the dosage form.
Mean weight per unit.—Weigh 20 tablets or capsules, and calculate the mean weight, WD. Use the mean weight to calculate the lycopene content of the test material, in mg/unit (compare with Calculations section).
Number of tablets or capsules per assay.—To evaluate whether the recommended sample size of 3 units (tablets or capsules) is representative of the sample material, perform 3 assays in parallel, using 3 and 6 units, respectively. Report individual results. For determination of the mean total lycopene content, take the equivalent content of 3 tablets or capsules for each assay, and conduct 2 assays in parallel. Report the average result of the 2 assays.
Preparation of test sample.—Homogenize suspensions or emulsions by stirring, e.g., with a glass rod. Crush tablets to a powder by grinding between 2 halves of a folded weighing paper with a pestle. Empty capsules containing powder formulations and extract powder together with the capsule shells. Use beadlet raw materials and capsules containing liquid formulations as such. Weigh beadlet material in one step without prior stirring or shaking to avoid segregation.
Extraction
Protect extracts and solutions from direct sun and UV light.
Oily solutions and suspensions.—Prepare sample as described in the Sample Preparation section. Accurately weigh test portion equivalent to about 20 mg lycopene, add 250 mg BHT, and rinse into a 250 mL volumetric flask (V1) with 120 mL dichloromethane. Add 100 mL ethanol, and shake contents of flask. The mixture cools and contracts. Let mixture stand in the dark until room temperature is reached (approximately 2 h), dilute contents of flask to volume with dichloromethane, and shake flask vigorously. Dilute an aliquot in proportions of 1 + 9 with dichloromethane–ethanol (1 + 1, v/v) in a volumetric flask, e.g., dilute 5 mL (V2) to 50 mL (V3), and inject 20 μL into the HPLC system.
Gelatin-based and other water-dispersible beadlets, or emulsions.—Prepare sample as described in the Sample Preparation section. Accurately weigh test portion equivalent to about 10 mg lycopene into a 250 mL volumetric flask (V1). Add 250 mg BHT, 0.5 mL Protex 6L, and 15 mL water. Sway the flask in order to wet the contents, and place flask in an ultrasonic bath at approximately 50°C for 30 min, swirling flask for about 15 min. Add 100 mL ethanol to the warm suspension, and shake flask vigorously. Add 135 mL dichloromethane, and shake flask again. The mixture cools and contracts. Let mixture stand in the dark until room temperature is reached (approximately 2 h), dilute contents of flask to volume with dichloromethane, and shake flask vigorously. Dilute an aliquot in proportions of 1 + 9 with dichloromethane–ethanol (1 + 1, v/v) in a volumetric flask, e.g., dilute 5 mL (V2) to 50 mL (V3). If necessary, filter the solution through a 0.45 μm membrane. Inject 20 μL into the HPLC system.
-
Tablets and capsules containing gelatin-based and other water-dispersible beadlets with a test portion mass of <5 g.—Accurately weigh test portion of 3 tablets or capsules, prepared as in the Sample Preparation section, into a 250 mL volumetric flask (V1). Add 250 mg BHT, 0.5 mL Protex 6L, and 15 mL water. Sway the flask in order to wet the contents, and place flask in an ultrasonic bath at approximately 50°C for 30 min, swirling flask for about 15 min. Add 100 mL ethanol to the warm suspension, and shake flask vigorously. Add 120 mL dichloromethane, and shake flask again. If clumps form, homogenize with a rotation homogenizer, and rinse with 15 mL dichloromethane, combining the rinse with the contents of the volumetric flask. The mixture cools and contracts. Let mixture stand in the dark until room temperature is reached (approximately 2 h), dilute contents of flask to volume with dichloromethane, and shake flask vigorously; let solids settle. Proceed as follows: (1) Lycopene content of <10 mg in the test portion.—Use the supernatant without dilution. (2) Lycopene content of >10 mg in the test portion.—Dilute an aliquot (V2) of the supernatant with dichloromethane–ethanol (1 + 1, v/v) so that the lycopene content of the final solution is 1–10 μg/mL. V3 is the volume of the final solution.
Filter the solution through a 0.45 μm membrane, if necessary, and inject 20 μL into the HPLC system.
-
Tablets and capsules containing gelatin-based and other water-dispersible beadlets with a test portion mass of >5 g.—Accurately weigh test portion of 3 tablets or capsules, prepared as in the Sample Preparation section, into a tared 250 mL volumetric flask. Add 0.5 mL Protex 6L and 120 mL water. Sway the flask in order to wet the contents, and place flask in an ultrasonic bath at 50°C for 30 min, swirling flask for about 15 min. Add 150 mL ethanol to the warm suspension, cool to room temperature, and dilute contents of flask to volume with water. Weigh flask and contents (W1), and shake flask vigorously. Immediately pour 10 ± 2 g suspension into a tared 250 mL volumetric flask (V1), using a funnel. Weigh the transferred aliquot of the suspension (W2). Add 100 mL ethanol and 250 mg BHT, and shake flask. Add 120 mL dichloromethane, and shake flask again. If clumps form, homogenize with a rotation homogenizer, and rinse with 15 mL dichloromethane, combining the rinse with the contents of the volumetric flask. The mixture cools and contracts. Let mixture stand in the dark until room temperature is reached (approximately 2 h), dilute contents of flask to volume with dichloromethane, and shake flask vigorously; let solids settle. Proceed as follows: (1) Lycopene content of <25 mg in the test portion.—Use the supernatant without dilution. (2) Lycopene content of >25 mg in the test portion.—Dilute an aliquot (V2) of the supernatant with dichloromethane–ethanol (1 + 1, v/v) so that the lycopene content of the final solution is 1–10 μg/mL. V3 is the volume of the final solution.
Filter the solution through a 0.45 μm membrane, if necessary, and inject 20 μL into the HPLC system.
Alginate-based beadlets and tablets or capsules.—Accurately weigh test portion of beadlets equivalent to 10 mg, or 3 tablets or capsules, prepared as in the Sample Preparation section, into a tared 100 mL volumetric flask. Add 1 mL Protex 6L and 50 mL alginate-based beadlet extraction solution (see Reagents). Sway the flask in order to wet the contents, and place in an ultrasonic bath at 50°C for 30 min, swirling at about 15 min. Add 40 mL water to the warm suspension, cool to room temperature, and dilute contents of flask to volume with water. Weigh flask and contents (W1), and shake flask vigorously. Immediately pour 10 ± 2 g suspension into a tared 200 mL volumetric flask (V1), using a funnel. Weigh the transferred aliquot of the suspension (W2). Add 100 mL dichloromethane and 200 mg BHT, and shake flask. Add 70 mL ethanol, and shake flask again. If clumps form, homogenize with a rotation homogenizer, and rinse with 15 mL dichloromethane, combining the rinse with the contents of the volumetric flask. The mixture cools and contracts. Let mixture stand in the dark until room temperature is reached (approximately 2 h), dilute contents of flask to volume with dichloromethane, and shake flask vigorously; let solids settle. Proceed as follows: (1) Lycopene content of <10 mg in the test portion.—Use the supernatant without dilution. (2) Lycopene content of >10 mg in the test portion.—Dilute an aliquot (V2) of the supernatant with dichloromethane–ethanol (1 + 1, v/v) so that the lycopene content of the final solution is 1–10 μg/mL. V3 is the volume of the final solutions.
Filter the solution through a 0.45 μm membrane, if necessary, and inject 20 μL into the HPLC system.
Chromatography (HPLC)
Conditions.—Column: Suplex PKB-100 (Supelco) 5 μm, 250 × 4.6 mm; column temperature: 30°C; mobile phase: see Reagents; flow rate: 0.8 mL/min; pressure: approximately 33 bar; injection volume: 20 μL; autosampler temperature: 15°C; detection wavelength: 448 nm; and run time: 40 min.
Retention times.—All-trans-lycopene: approximately 15–20 min. Approximate retention times relative to all-trans-lycopene: all-trans-lutein, 0.45; all-trans-zeaxanthin, 0.47; all-trans-β-cryptoxanthin, 0.86; all-trans-lycopene, 1; coeluting 5-cis- and 9-cis-lycopene, 1.03; nonidentified cis-lycopene, 1.07; 13-cis-lycopene, 1.12; 15-cis-lycopene, 1.18; all-trans-α-carotene, 1.39; all-trans-β-carotene, 1.49; 9-cis-β-carotene, 1.60; 13-cis-β-carotene, 1.75; and 15-cis-β-carotene, 1.81.
Calculations
Calculate the contents of total and all-trans-lycopene in the test samples as follows:
Extraction variant (a), (b), (c)
Extraction variant (c), (d)
where Ctot and Ctrans are the total and all-trans-lycopene contents (mg/g or mg/unit); Aall-trans is the peak area of all-trans lycopene (AU); A5-cis/9-cis is the peak area of the coeluting 5-cis- and 9-cis-lycopene (AU); Ax-cis is the peak area of nonidentified cis-isomer(s) of lycopene (AU); A13-cis and A15-cis are the peak areas of 13-cis- and 15-cis-lycopene (AU, respectively; 1.3 and 1.4 are RRF values that were determined previously at DSM Nutritional Products Ltd; WD is the mean weight of a unit (tablet or capsule; g); m is the test portion amount (g); RFtrans is the RF of all-trans-lycopene (AU · L/mg); V is the theoretical volume in which the test portion is dissolved (L); V1 is the volume of the flask in which the test portion or aliquot is extracted with dichloromethane–ethanol (L; i.e., the first flask for extraction variants a, b, and c and the second flask for variants d and e); V2 is the volume of the aliquot that is diluted (L); and V3 is the volume to which the aliquot V2 is diluted (L). This is only applicable if the sample solution has to be diluted to reach the recommended concentration of 1–10 μg/mL.
W1 and W2 apply only to extractions (d) and (e). W1 is the weight (g) of the aqueous alcohol (d) or aqueous (e) suspension in the first volumetric flask, and W2 is the weight (g) of the aliquot of the suspension transferred to the second volumetric flask.
Procedures Used for Optimization and Validation
Selectivity.—The separation on the reversed-phase (RP)-HPLC system was compared with that on a normal-phase (NP) system (3 Nucleosil 300-5 HPLC columns in series, 5 μm, 250 × 4.6 mm, Cat. No. 720099.46, available from Macherey-Nagel). Mobile phase: 1.5 mL N-ethyldiisopropylamine in 1 L n-hexane, which showed an optimum resolution for lycopene isomers. A mixture of lycopene cis-trans isomers was prepared as described in the Calibration section (f)(3), Linearity of the LC response (concn = approximately 6 μg/mL). The mixture was injected into the RP-HPLC system of the present method and, in addition, into the NP system described by Schierle et al. (6). The peaks of the various lycopene isomers were identified on the basis of their UV-Vis spectra (11, 12) and by comparison with published elution orders (6, 10) in order to identify coelutions on the RP column.
Linearity.—Dilutions were prepared from solutions of heat-isomerized lycopene and almost pure all-trans-lycopene as described in the Calibration section (f)(3), Linearity of the LC response, of the present method. Total lycopene concentrations ranging from 0.05 to 100 μg/mL were injected into the HPLC system. From the resulting acceptable chromatograms, the total peak areas of all detected lycopene isomers (total lycopene) and of all-trans-lycopene were determined and plotted versus the respective concentrations. Curves were constructed by using the least-squares linear regression method.
Precision.—On 5 different days, 2 test portions from each of the 16 sample materials were analyzed according to the described method. The test portions consisted of approximately 100 mg sample (test materials 1–8) and 3 tablets or capsules (test materials 9–16). The work was performed by 2 technicians. Within-day and between-day precision values and HorRat values were calculated by 1-way analysis of variance (ANOVA; by using an Excel template provided by AOAC INTERNATIONAL).
-
Recovery.—Negative control materials were fortified in triplicate with isomeric mixtures of lycopene and analyzed according to the described method. The isomer mixtures were either generated from all-trans-lycopene by heat isomerization or obtained from beadlet material containing known amounts of cis-trans lycopene isomers.
Solutions of heat-isomerized lycopene were used for spiking placebo beadlets analyzed with extraction variant (b). In order to prepare the spiking solutions, 600 mg all-trans-lycopene and 0.5 g BHT were dissolved in 70 mL dichloromethane in a 150 mL volumetric flask. The solution was refluxed for 2 h at 80°C. After cooling to room temperature, the contents of the flask were diluted to volume with dichloromethane. Dilutions were prepared in dichloromethane–ethanol (1 + 1, v/v) to reach the described concentrations of 20 mg/L, 1 g/L, and 4 g/L. Aliquots of 10 mL of these spiking solutions were evaporated under reduced pressure in a volumetric round-bottom flask (rotary evaporator Rotavapor R-114 and water bath R-490, Büchi Labortechnik, Flawil, Switzerland) connected to a vacuum pump, absolute minimum pressure of 10 mbar (PC 5, Vacuubrand, Wertheim, Germany). Placebo beadlets (200 mg) were added, and the mixtures, representing fortification levels of 1, 50, and 200 g/kg, were analyzed according to extraction variant (b). This spiking technique could not be used for the other extraction variants because the lycopene remaining after evaporation of the spiking solution could not be dispersed homogeneously in the aqueous medium. Transfer of representative aliquots of the suspension to a second flask for further extraction was impossible.
To study the recovery of extraction variants (c), (d), and (e), negative control materials were spiked at high concentrations by adding weighed amounts of beadlets with known total lycopene contents. For spiking at low concentrations aqueous suspensions were prepared by weighing 100 mg Redivivo 5% TG (test material 2) into a 100 mL volumetric flask. After addition of 10 mg SDS, 0.5 mL Protex 6L, and about 50 mL water, the suspension was ultrasonicated at 50°C for 30 min. The homogenous suspension of the digested beadlet material was cooled to room temperature before the contents of the flask were diluted to volume with water; 2 mL (tablets) and 4 mL (beadlets) aliquots of this suspension were added by pipet to the blank matrixes.
Negative tablet control materials were fortified in test portions of 3 tablets, at total lycopene amounts of approximately 0.1, 1, and 100 mg/test portion representing lycopene contents of approximately 20 mg/kg, 200 mg/kg, and 20 g/kg.
Extraction efficacy.—In order to assess the extraction efficacy of extraction variant (e), 200 mg test material 4 (containing approximately 10 mg lycopene) was analyzed as such and, in addition, in a mixture with 3 placebo tablets and 200 mg placebo beadlets. The suspensions left after the extraction were filtered under reduced pressure through a 55 mm round filter (Schleicher & Schüll 576, black band). The residues were washed with 3 portions of approximately 5 mL ethanol to remove cortically adhering lycopene. The residues were then re-extracted and analyzed again by using extraction variant (e), including enzymatic digestion and extraction with dichloromethane and ethanol.
Effect of light during analysis.—In order to study whether light exposure during the extraction procedure gives rise to isomerization of lycopene, various samples were extracted and further processed either in a dark well-shaded fume hood or in diffuse daylight available in the laboratory with windows unshaded and fluorescent ceiling lamps on. Under these conditions, test materials 13 and 15 and, in addition, the residues of evaporated solutions of all-trans-lycopene and of heat-isomerized lycopene (prepared as described in the method) combined with placebo tablets were analyzed by using different extraction variants of the method. Direct irradiation by sunlight was avoided.
Results and Discussion
Optimization
-
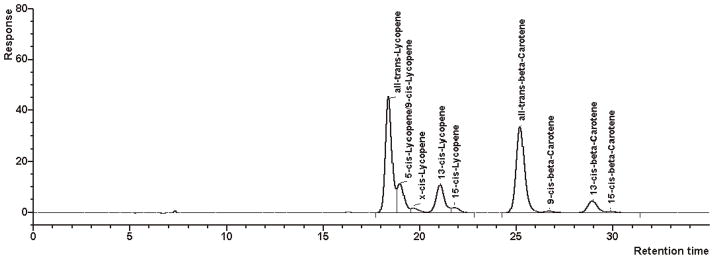
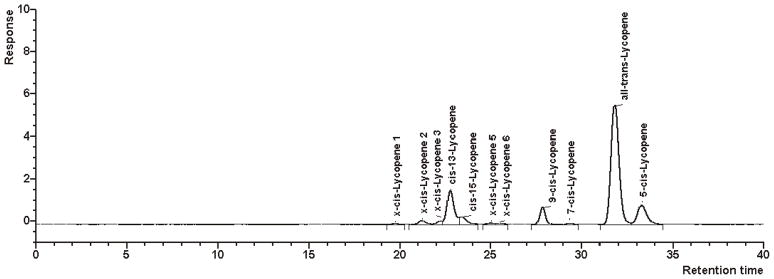
Selectivity.—We have shown previously that the RP system used in the present method separates lycopene from α- and β-carotene as well as from xanthophylls like lutein and zeaxanthin, and that it has a high selectivity for the β-carotene cis-trans-isomers (10). In order to evaluate the selectivity of this system for lycopene cis-trans-isomers, the separation of a thermally generated mixture of lycopene isomers achieved with this system (Figure 1) was compared with the separation obtained with an NP-HPLC system (Figure 2) that had previously shown excellent selectivity for the lycopene isomers (6).
The NP system separated all-trans-lycopene, 5-cis-, 9-cis-, 13-cis-, and 15-cis-lycopene. Additionally, at least 7 unidentified cis-isomers (x-cis-isomers) were detected (at 470 nm) in the chromatogram of the isomeric mixture, which amounted to about 6% of the total peak area (Table 2).
In comparison, the present RP system provided a lower resolution of the lycopene isomers; 5-cis- and 9-cis-lycopene coeluted on this column (Figure 1), and clearly fewer of the unidentified cis-isomers of lycopene were resolved (Table 2). These nonresolved cis-isomers may have coeluted mainly with 13-cis-lycopene because the proportions of all-trans-lycopene, of coeluting 5-cis- and 9-cis-lycopene, and of 15-cis-lycopene did not significantly differ between the 2 systems. Thus, the selectivity of the present RP-HPLC system provides a relatively good approximation of the cis-trans-isomer ratio of lycopene as well as determination of the total lycopene content.
Approximation of RRF values for cis-isomers of lycopene.—It is well documented that the spectrophotometric properties of carotenoids depend on the position and configuration of the double bonds. The cis-isomers especially show differences in the UV-Vis spectra and absorbance, compared with the all-trans-compounds. For quantitation of materials containing isomeric mixtures of lycopene, calibration with all-trans-lycopene leads to an underestimation of the total lycopene and the amounts of other major isomers, i.e., 9-cis-, 13-cis-, and 15-cis-lycopene. In order to enhance the accuracy of the determination, RRF values can be used to adjust for differences in relative absorptivity. Visible spectrophotometric data of mono-cis-isomers were available from earlier synthesis in the laboratories of Hoffman-La Roche DSM Nutritional Products Ltd. (11) and in an internal research report for approximation of RF values relative to all-trans-lycopene. The following RRF values were calculated from these data: 9-cis-lycopene, 1.1; 13-cis-lycopene, 1.3; and 15-cis-lycopene, 1.4. Subsequent isomerization experiments and HPLC analyses with diode-array detection (DAD) were carried out to confirm the calculated RRF values (data not shown). From these experiments, we calculated an RRF of 1.3 for the cis-isomer fraction, which comprised mainly 13-cis- and 15-cis-lycopene. This value was in good agreement with the RRF calculated from the spectrophotometric data (11). Because 5-cis- and 9-cis-lycopene coeluted on the Suplex PKB-100 column, only the RRF values for 13-cis- and 15-cis-lycopene were used in the present method for calculation of the total lycopene content. The amounts of the 5-cis-, 9-cis-, and x-cis-lycopene isomers present were calculated by using an RRF of 1.0.
-
Adequacy of sample size.—The homogeneity of the sample is of great importance in minimizing the variation of the analytical results. For tablet or capsule material it is necessary during sampling to ensure that the aliquot is adequate to represent the whole sample. Instead of homogenizing the whole batch of a sample material, we generally used 3 tablets or capsules, a quantity that was shown to be a representative aliquot. It has been our experience that grinding tablets containing beadlet product forms generally leads to segregated beadlets and nonhomogeneous aliquots taken from the ground tablet mass. In order to evaluate whether a sample amount of 6 capsules would show a further decrease in the variation of results, we analyzed 3 and 6 tablets of different sample materials with different lycopene contents for comparison.
Test materials 9, 12, and 15 (with low, medium, and high lycopene contents, respectively) were analyzed by using 3 single units per test portion according to the present method, i.e., extraction variant (c), 1, for samples 9 and 12, and extraction variant (c), 2, for sample 15, and the results were compared with those from an analysis of a test portion consisting of 6 units. Both test portions were analyzed in triplicate. No significant differences in total lycopene content were found with the 2-sided paired t-test (Table 3). The SD was not decreased by the use of 6 units instead of 3 units per sample portion. Therefore, a portion of 3 units can be regarded as an adequate aliquot for the analysis of tablets and capsules. These results are in good agreement with the data we obtained previously in our laboratory. Homogenization of the whole sample proved to be difficult. As previously mentioned, grinding of tablets can lead to segregation of the beadlet material, which will produce nonhomogeneity. Capsules containing liquids cannot be manually homogenized with good results. Thus, our method uses an adequate sample amount and includes the protease digestion step, which releases the analyte quantitatively from the tablet or capsule material.
-
Extraction efficacy of extraction variant (e).—The extraction variants used for gelatin-based and other water-dispersible beadlets, or for tablets and capsules containing such product forms, comprise digestion of the formulation with protease in water and subsequent extraction with organic solvents, i.e., extraction variants (b), (c), and (d). This procedure is routinely used in the analytical laboratories of DSM Nutritional Products Ltd for the determination of carotenoids in feed, food, and pharmaceutical formulations based on gelatin, pectin, gum arabic, and poly- and oligosaccharides.
Preliminary tests showed that extraction procedures (b) and (c) were not suitable for alginate-based beadlets; therefore, an alternative extraction method was developed, i.e., extraction variant (e). The results of the experiments (5 replicates) are presented in Table 4. In the case of beadlet material, the first extraction was almost complete, and only 0.2% lycopene was found after the second extraction. The measured extraction efficacy was slightly affected by the addition of placebo tablets. In this case, the second extraction released 1.4% of the totally extracted lycopene.
Column-to-column variation.—As described previously, the relative retention times of carotenoids on different Suplex PKB-100 columns are stable (10). We tested 3 used columns of different batches for stability of retention time. The absolute retention times for all-trans-lycopene ranged from 14.6 to 17.6 min. The relative retention times for all-trans-lycopene and the cis-isomers were nearly constant for 2 of the columns, and only a small deviation was obtained for the third column, which was only apparent for later-eluting 13-cis- and 15-cis-lycopene. Because the elution order did not change and almost no matrix interferences were detectable in that region of the chromatogram, peak assignment was not compromised by different columns.
Effect of light during the analysis.—Generally, under the influence of light and heat all-trans-lycopene can isomerize to cis-isomers, which are more readily oxidized (13, 14). In order to find out if room illumination affects the determination of lycopene, we extracted various samples in parallel under diffuse daylight (plus light from fluorescence tubes) and subdued conditions. As shown in Table 5, there was no consistent effect of this light on the total lycopene content and on the ratio of the lycopene isomers. Obviously, the effect of light, if any, was small compared with the effects of other factors on the precision of the method.
Figure 1.
Chromatogram obtained for heat-isomerized lycopene and a β-carotene control solution on a Suplex PKB-100 column (RP-HPLC).
Figure 2.
Chromatogram obtained for heat-isomerized lycopene and a β-carotene control solution on a Nucleosil 300-5 column (NP-HPLC).
Table 2.
Lycopene isomer ratios for a heat-isomerized mixturea
| Isomer | NP-HPLC, % | RP-HPLC, % | P-valueb |
|---|---|---|---|
| All-trans-lycopene | 59.0 ± 0.9 | 59.9 ± 0.7 | 0.03 |
| 5-cis- + 9-cis-Lycopene | 18.0 ± 0.3 | 17.5 ± 0.5 | 0.02 |
| 13-cis-Lycopene | 14.2 ± 0.8 | 16.1 ± 0.9 | <0.01 |
| 15-cis-Lycopene | 2.9 ± 0.4 | 3.0 ± 0.3 | 0.73 |
| Unidentified cis-lycopene isomers | 5.8 ± 0.3 | 3.5 ± 0.4 | <0.01 |
n = 6.
Two-sided paired t-test (P < 0.01).
Table 3.
Comparison of different sample sizes in the determination of total lycopene content
| Sample | No. of units | Total lycopene, mg/unit
|
RSD, %a | P-valueb | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Mean | ||||
| 9 | 3 | 0.469 | 0.455 | 0.465 | 0.463 | 1.5 | 0.10 |
| 6 | 0.465 | 0.439 | 0.450 | 0.451 | 2.9 | ||
| 12 | 3 | 1.54 | 1.62 | 1.71 | 1.63 | 1.6 | 0.48 |
| 6 | 1.80 | 1.64 | 1.67 | 1.70 | 4.9 | ||
| 15 | 3 | 4.12 | 4.12 | 3.92 | 4.06 | 3.2 | 0.66 |
| 6 | 4.16 | 4.03 | 4.08 | 4.09 | 1.6 | ||
RSD = Relative standard deviation.
Two-sided paired t-test (P < 0.01).
Table 4.
Extraction efficacy of extraction variant (e) for beadlet and tablet materiala
| Sample | Extraction | Lycopene, mg/test portion
|
Extraction efficacy, %b | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Mean | |||
| Beadlets | 1 | 11.8 | 11.1 | 9.8 | 11.0 | 9.6 | 10.7 | |
| 2 | 0.00 | 0.02 | 0.03 | 0.05 | 0.00 | 0.02 | 99.8 ± 0.2 | |
| Tablets | 1 | 10.1 | 11.2 | 11.4 | 10.3 | 10.3 | 10.7 | |
| 2 | 0.13 | 0.13 | 0.13 | 0.16 | 0.20 | 0.15 | 98.6 ± 0.3 | |
n = 5.
Sum for extractions 1 and 2 = 100%.
Table 5.
Stability of lycopene during sample preparation, evaluated by using different extraction variants
| Sample | Sample extraction variant | Total lycopene, mg/unit
|
All-trans-lycopene, %a |
||
|---|---|---|---|---|---|
| Dark | Light | Dark | Light | ||
| All-trans-lycopene + placebo tablets | (c), 2 | 4.91 | 4.91 | 97 | 97 |
| Isomerized lycopene + placebo tablets | (c), 2 | 5.17 | 5.00 | 76 | 80 |
| Test material 13 | (c), 1 | 1.70 | 1.70 | 77 | 74 |
| Test material 15 | (c), 2 | 3.91 | 3.98 | 80 | 77 |
| Test material 15 | (e), 1 | 3.87 | 3.88 | 77 | 75 |
Total lycopene = 100%.
Validation
Sixteen different sample materials, including beadlet raw materials, formulations, and finished products containing lycopene of natural and synthetic origin, were analyzed. The finished products included tablets, capsules, and softgels with claimed lycopene contents ranging from 300 μg to 25 mg per tablet or capsule. The 7 beadlet materials with a lycopene content between 5 and 20% included different formulations based mainly on gelatin, alginate, gum arabic, starch, and soy protein.
The HPLC system was calibrated at the beginning of the single-laboratory validation, and the RF of all-trans-lycopene (RFtrans) was calculated as described in the method. The SP, which is an indication of the absence of noncolored compounds not detectable at visible wavelengths, was determined in the reference substance to be 99.0% on the basis of the E1%, 1 cm value of 3450 for all-trans-lycopene in n-hexane (12). The HPLC-DAD analysis resulted in a CP value for all-trans lycopene of 95.8%, based on peak area comparison. Both purity data met the requirements of the method. The total purity, P, of the reference compound was calculated to 94.8%. The response of the HPLC system was controlled with a solution of heat-isomerized lycopene, which was measured on the day of calibration (resulting in an initial value) and at the beginning and end of each HPLC sequence run during subsequent days. The determined total lycopene content did not match the acceptance criterion (±2% of initial) only for one injection; however, because the second injection of that sequence was within the limits, the system was not recalibrated.
The calibration curves obtained for all-trans-lycopene and total lycopene content were linear between 0.1 and 50 μg/mL and showed linear coefficients of determination (R2) of >0.999 and 1.000, respectively. The instrumental limit of detection (LOD) was approximately 0.01 μg/mL, estimated as the concentration for which the peak signal of the all-trans-lycopene peak exceeds 3 times the baseline noise. The sensitivity of the method was decreased by using 448 nm as the wavelength of measurement instead of 472 nm, the lycopene absorbance maximum in the HPLC eluant. This wavelength of 448 nm was deliberately chosen to enhance the versatility of the method because a variety of carotenoids could be sensitively detected at 448 nm (10). The instrumental lower limit of quantitation (LLQ), estimated as the lowest concentration at which the back-calculated value from the regression curve was within ±10% of the theoretical value, was 0.05 mg/L for total lycopene and all-trans-lycopene.
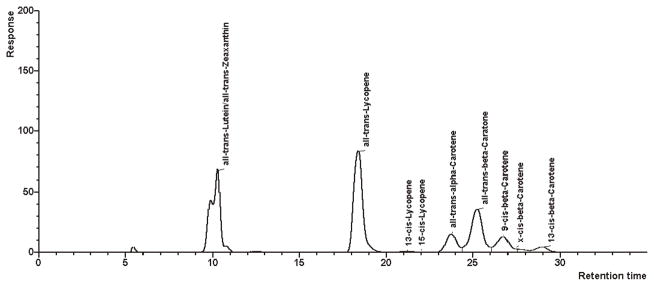
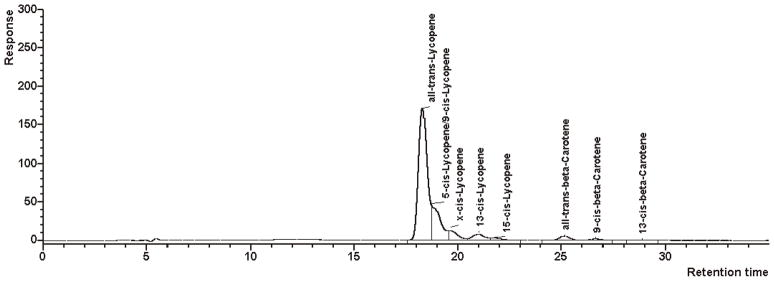
Two typical chromatograms of sample materials containing various carotenoids and only lycopene with small amounts of β-carotene are presented in Figures 3 and 4, respectively. Especially for samples containing higher amounts of cis-lycopene isomers, a good separation can be obtained. In samples with high amounts of all-trans-lycopene, small amounts (<3%) of 5-cis- and 9-cis-isomers may coelute (Figure 4).
Figure 3.
Chromatogram of a sample material containing various carotenoids.
Figure 4.
Chromatogram of a sample material containing mainly lycopene and small amounts of β-carotene.
The results from the precision experiments are presented in Tables 6 (total lycopene) and 7 (all-trans-lycopene). The total RSD, %, included the within-day and between-day variances (intermediate precision). The HorRat value estimates the accuracy of the intermediate precision with an acceptable range of 0.3–1.3 for single-laboratory validation studies (15). Table 6 shows that most sample materials reached the acceptance criterion, but samples 3 and 4 (beadlet materials) and samples 10 and 12 (tablets) had HorRat values of >1.3. In the case of sample material 3 (HorRat = 2.3), elimination of 2 outliers from the original data set did not diminish the high variance. We assume that extraction variant (b) was possibly not suitable for this sample material and that extraction variant (e) would have been a better alternative. Sample 4 had a HorRat value of 1.8. Outliers could not be identified. In this case, the within-day variation was even higher than the between-day variation (4.5 and 3.5%, respectively), possibly reflecting a lack of homogeneity or extraction problems. The analysis of sample material 10 (HorRat value = 2.3, no outliers) was difficult because clumps formed during sample preparation that could hardly be dispersed (by use of a Polytron homogenizer). This problem possibly contributed to the relatively high variation of the results. On the other hand, this sample material also showed high variation in the dose uniformity test (data not shown) and lack of homogeneity of the sample material may partly explain the results. Sample material 12 showed a deviating HorRat value of 1.5. No outliers were identified. We noticed after completion of the analyses, that these capsules probably contained beadlet material of sample material 3. These beadlets were not sufficiently extractable with extraction variant (b) (see above) and should have been analyzed with extraction variant (e). However, there was not enough material left for this additional analysis. For sample material 8 we obtained an almost linear decrease in total lycopene content after the original bag was unsealed, which may indicate instability of the sample material in air. The results from day 5 were excluded as outliers, and the between-day variation was acceptable (HorRat value = 1.2). The within-day variation was low (0.7%), indicating that the extraction method was suitable.
Table 6.
Precision data for the total lycopene content of the 16 test materials
| Test material | Extraction variant | Mean value, g/kg or mg/kga | Within-day RSD, % | Between-day RSD, % | Total RSD, % | HorRat |
|---|---|---|---|---|---|---|
| 1 | (a) | 107.1 | 0.9 | 1.4 | 1.70 | 0.6 |
| 2 | (b) | 55.1 | 0.4 | 1.1 | 1.2 | 0.4 |
| 3 | (b) | 50.8 | 2.7 | 6.7 | 7.3 | 2.3 |
| 4 | (e), 1 | 50.3 | 4.5 | 3.5 | 5.7 | 1.8 |
| 5 | (e), 1 | 235.9 | 0.9 | 0.6 | 1.0 | 0.4 |
| 6 | (e), 1 | 58.0 | 3.2 | 1.2 | 3.4 | 1.1 |
| 7 | (e), 1 | 239.2 | 0.6 | 0.4 | 0.8 | 0.3 |
| 8 | (b) | 101.6 | 0.7 | 3.2 | 3.3 | 1.2 |
| 9 | (c), 1 | 294.8 | 2.1 | 2.0 | 2.9 | 0.4 |
| 10 | (c), 2 | 11787.1 | 5.1 | 7.4 | 8.9 | 2.3 |
| 11 | (e), 1 | 9641.3 | 3.8 | 3.6 | 5.2 | 1.3 |
| 12 | (c), 1 | 1237.4 | 5.7 | 5.4 | 7.9 | 1.5 |
| 13 | (c), 1 | 1259.2 | 2.1 | 1.1 | 2.4 | 0.4 |
| 14 | (d), 2 | 23929.8 | 1.1 | 0.6 | 1.3 | 0.4 |
| 15 | (c), 2 | 2213.7 | 1.4 | 2.0 | 2.5 | 0.5 |
| 16 | (c), 1 | 1152.9 | 2.6 | 1.7 | 3.1 | 0.6 |
Test materials 1–8, g/kg; test materials 9–16, mg/kg.
The precision data for the determination of all-trans-lycopene (Table 7) were comparable to those obtained for total lycopene. In addition to the samples discussed above (sample materials 3, 4, and 10), samples 6 and 8 also had HorRat values of >1.3 and thus did not fulfill the acceptance criterion. This larger variance probably has chromatographic origins because 5-cis-lycopene and all-trans-lycopene were not completely separated; thus, differences in the integration of the all-trans-lycopene peak may have contributed to the variation.
Table 7.
Precision data for the all-trans-lycopene content of the 16 test materials
| Test material | Extraction variant | Mean value g/kg or mg/kga | Within-day RSD, % | Between-day RSD, % | Total RSD,% | HorRat |
|---|---|---|---|---|---|---|
| 1 | (a) | 88.8 | 2.3 | 0.8 | 2.4 | 0.8 |
| 2 | (b) | 44.8 | 0.8 | 2.0 | 2.1 | 0.7 |
| 3 | (b) | 41.4 | 10.4 | 17.5 | 20.3 | 6.3 |
| 4 | (e), 1 | 46.9 | 4.4 | 1.3 | 4.6 | 1.5 |
| 5 | (e), 1 | 223.5 | 1.0 | 1.2 | 1.6 | 0.6 |
| 6 | (e), 1 | 54.4 | 3.7 | 3.4 | 5.0 | 1.6 |
| 7 | (e), 1 | 224.7 | 0.6 | 1.2 | 1.3 | 0.5 |
| 8 | (b) | 90.1 | 2.6 | 5.0 | 5.6 | 2.0 |
| 9 | (c), 1 | 231.6 | 2.3 | 1.6 | 2.8 | 0.4 |
| 10 | (c), 2 | 10372.1 | 5.3 | 7.2 | 8.9 | 2.2 |
| 11 | (e), 1 | 8781.6 | 3.5 | 2.2 | 4.1 | 1.0 |
| 12 | (c), 1 | 1021.8 | 5.0 | 1.1 | 5.1 | 0.9 |
| 13 | (c), 1 | 944.7 | 1.5 | 1.9 | 2.4 | 0.4 |
| 14 | (d), 2 | 19363.0 | 1.7 | 1.1 | 2.0 | 0.6 |
| 15 | (c), 2 | 1669.7 | 1.9 | 3.4 | 3.9 | 0.7 |
| 16 | (c), 1 | 792.7 | 2.7 | 1.4 | 3.0 | 0.5 |
Test materials 1–8, g/kg; test materials 9–16, mg/kg.
Recovery was determined by adding lycopene to negative control material at concentrations of 0.02, 2, and 20 g/kg in tablets and 1, 50, and 200 g/kg in beadlet material. The recoveries, which ranged from 95.0 to 102.1% from tablets and from 95.0 to 101.1% from beadlets, were independent of the extraction variants and the lycopene concentrations used for fortification (Table 8).
Table 8.
Spiking materials used for recovery experimentsa
| Spiking material | Extraction variant | Spiking level, g/kg | Recovery, % |
|---|---|---|---|
| Tablets
| |||
| Test material 2 | (d), 2 | 20 | 96.8 ± 0.8 |
| Test material | 2 (c), 1 | 0.2 | 98.6 ± 2.8 |
| Suspension (test material 2) | (c), 1 | 0.02 | 98.4 ± 0.5 |
| Test material6 | (e), 2 | 20 | 96.9 ± 3.1 |
| Test material 6 | (e), 1 | 0.2 | 102.1 ± 2.0 |
| Suspension (test material 2) | (e), 1 | 0.02 | 95.0 ± 4.2 |
|
| |||
| Beadlets
| |||
| Heat-isomerized lycopene | (b) | 200 | 101.1 ± 0.2 |
| Heat-isomerized lycopene | (b) | 50 | 98.3 ± 0.3 |
| Heat-isomerized lycopene | (b)b | 1 | 98.3 ± 4.4 |
| Suspension (test material 2) | (e), 1 | 1 | 98.4 ± 1.4 |
| Test material 2 | (e), 2 | 200 | 95.0 ± 0.1 |
n = 3.
Without dilution.
Recommendations
We introduced for the first time RRF values for the quantitation of all-trans-lycopene and its major isomers in analyses of dietary supplements and raw material. Several parameters influence the repeatability and accuracy of the analysis. First, the different lycopene formulations available on the market cannot be analyzed with a single method, and different variants must be applied. In the case of alginate-based formulations, our standard extraction method failed. For these samples an extraction variant based on an alkaline phosphate buffer containing sodium EDTA and SDS was adapted. Although this extraction variant is also suitable for the extraction of other sample materials, we do not recommend this technique in general because this procedure is relatively tedious and most of the samples currently available on the market can be analyzed with the described extraction variants (b), (c), and (d). Extraction variant (e) is recommended if alginate-based formulations are to be analyzed or if extraction variants (b), (c), and (d) lead to significant deviations from the expected results (i.e., <90% of the expected value). In these cases, the analysis should be repeated with extraction variant (e). The results show that the different extraction variants can be successfully applied to a variety of different matrixes, and they cover a broad concentration range.
Contributor Information
André Müller, DSM Nutritional Products Ltd, Research and Development, Analytical Research Center, PO Box 2676, CH-4002 Basel, Switzerland.
Bernd Pietsch, DSM Nutritional Products Ltd, Research and Development, Analytical Research Center, PO Box 2676, CH-4002 Basel, Switzerland.
Nicole Faccin, DSM Nutritional Products Ltd, Research and Development, Analytical Research Center, PO Box 2676, CH-4002 Basel, Switzerland.
Joseph Schierle, DSM Nutritional Products Ltd, Research and Development, Analytical Research Center, PO Box 2676, CH-4002 Basel, Switzerland.
Edward H. Waysek, Caravan Ingredients, 100 Adams Dr, Totowa, NJ 07512
References
- 1.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. J Natl Cancer Inst. 1995;87:1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 2.Kucuk O, Sarkar FH, Sakr W, Khachik F, Djuric Z, Banerjee M, Pollak MN, Bertram JS, Wood DP., Jr Pure Appl Chem. 2002;74:1443–1450. [Google Scholar]
- 3.Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Exp Biol Med. 2002;227:845–851. doi: 10.1177/153537020222701002. [DOI] [PubMed] [Google Scholar]
- 4.Di Mascio P, Kaiser S, Sies H. Arch Biochem Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 5.Hengartner U, Bernhard K, Mayer K, Englert G, Glinz E. Helv Chim Acta. 2002;75:1848–1865. [Google Scholar]
- 6.Schierle J, Bretzel W, Bühler I, Faccin N, Hess N, Steiner K, Schuep W. Food Chem. 1997;59:459–465. [Google Scholar]
- 7.Unlu NZ, Bohn T, Francis DM, Nagaraja HN, Clinton SK, Schwartz SJ. Br J Nutr. 2007;98:140–146. doi: 10.1017/S0007114507685201. [DOI] [PubMed] [Google Scholar]
- 8.Periago MJ, Rincon F, Jacob K, Garcia-Alonso J, Ros G. J Agric Food Chem. 2007;55:8825–8829. doi: 10.1021/jf0705623. [DOI] [PubMed] [Google Scholar]
- 9.Hart DJ, Scott KJ. Food Chem. 1995;54:101–111. [Google Scholar]
- 10.Schierle J, Pietsch B, Ceresa A, Fizet C, Waysek EH. J AOAC Int. 2004;87:1070–1082. [PMC free article] [PubMed] [Google Scholar]
- 11.Hengartner U, Bernhard K, Mayer K, Englert G, Glinz E. Helv Chim Acta. 2002;75:1848–1865. [Google Scholar]
- 12.Britton G. In: Carotenoids. Britton G, Liaaen-Jensen S, Pfander H, editors. 1B. Birkhäuser Verlag; Basel, Switzerland: 1995. pp. 13–62. [Google Scholar]
- 13.Boskovic MA. J Food Sci. 1979;44:84–86. [Google Scholar]
- 14.Schieber A, Carle R. Trends Food Sci Technol. 2005;16:416–422. [Google Scholar]
- 15.http://www.aoac.org/dietsupp6/Dietary-Supplement-web-site/HorwitzValid.pdf