Abstract
Aims
Lower motor neuron damage to sacral roots or nerves can result in incontinence and a flaccid urinary bladder. We showed bladder reinnervation after transfer of coccygeal to sacral ventral roots, and genitofemoral nerves (L1, 2 origin) to pelvic nerves. This study assesses the feasibility of urethral and anal sphincter reinnervation using transfer of motor branches of the femoral nerve (L2–4 origin) to pudendal nerves (S1, 2 origin) that innervate the urethral and anal sphincters in a canine model.
Methods
Sacral ventral roots were selected by their ability to stimulate bladder, urethral sphincter, and anal sphincter contraction and transected. Bilaterally, branches of the femoral nerve, specifically, nervus saphenous pars muscularis [Evans HE. Miller’s anatomy of the dog. Philadelphia: W.B. Saunders; 1993], were transferred and end-to-end anastomosed to transected pudendal nerve branches in the perineum, then enclosed in unipolar nerve cuff electrodes with leads to implanted RF micro-stimulators.
Results
Nerve stimulation induced increased anal and urethral sphincter pressures in five of six transferred nerves. Retrograde neurotracing from the bladder, urethral sphincter, and anal sphincter using fluorogold, fast blue, and fluororuby, demonstrated urethral and anal sphincter labeled neurons in L2–4 cord segments (but not S1–3) in nerve transfer canines, consistent with rein-nervation by the transferred femoral nerve motor branches. Controls had labeled neurons only in S1–3 segments. Postmortem DiI and DiO labeling confirmed axonal regrowth across the nerve repair site.
Conclusions
These results show spinal cord reinnervation of urethral and anal sphincter targets after sacral ventral root transection and femoral nerve transfer (NT) to the denervated pudendal nerve. These surgical procedures may allow patients to regain continence.
Keywords: reinnervation, pudendal nerve, femoral nerve, urethral sphincter, anal sphincter, incontinence, spinal injury
INTRODUCTION
Urinary tract dysfunction occurs nearly universally following severe spinal cord injury (SCI), and is also manifest in patients with spina bifida and myelomeningocele. Early data identified renal disease as the major cause of death in this group of patients.1 More recent data points to pneumonia, accidents, and suicides.2 Although this indicates improved urologic care of these patients, their quality of life remains severely impaired. In a survey study of 681 patients with spinal cord injury, regaining bladder, and bowel function was rated of great importance to both paraplegics and quadriplegics. It was even rated as more important than regaining walking movement in paraplegics.3 The quality of life of these patients would be improved if restoration of urinary bladder emptying and control of urethral and anal sphincter function could be accomplished. At least one quarter of the patients seen at the Philadelphia Shriners Hospital for Children’s Spinal Cord Injury Center have acontractile bladders resulting from a lower motor neuron (LMN) lesion. This is especially prevalent in children that sustain lap belt injuries leading to cauda equina deficits. These patients cannot use functional electrical stimulation (FES) to induce bladder emptying because the neural connection between the spinal cord and the bladder has been disrupted. Current FES technology can only restore function in SCI patients with suprasacral upper motor neuron lesions.
Our ultimate goal is to develop a surgical approach to reinnervate the LMN-lesioned urinary bladder and sphincters so the patient can regain control of both bladder emptying and continence. To accomplish this, we developed a lower motoneuron lesioned canine model based on rhizotomy of nerve roots in the lumbosacral spine that innervate the bladder and both urethral and anal sphincters. While this does not mimic the mixed upper and lower motoneuron lesioned lower urinary tract commonly found with spinal cord injury, it does allow investigation of mechanisms of reinnervation in a pure lower motoneuron injury without the confounding issues of partially intact normal lower urinary tract motor innervation that a spinal injury model would produce. We have achieved considerable success in bladder reinnervation in this canine model using immediate repair of severed sacral nerve roots, transfer of coccygeal nerve roots to the severed sacral roots, and transfer of the genitofemoral (GF) nerve intra-abdominally to the vesicular branch of the pelvic nerve at varying time periods after bladder denervation.4–7 However, we have not yet addressed the issue of neurogenic sphincteric incontinence.
If both the detrusor muscle and urethral sphincter are reinnervated, bladder emptying and urinary continence should be expected. However, detrusor–sphincter dyssynergia may result should the detrusor and sphincter be stimulated simultaneously at the sacral spinal cord. An approach that we propose to minimize this involves selective targeting of the pelvic and pudendal nerves distal to the spinal cord and re-innervating them with nerves from other spinal cord segments. Having shown in prior studies that the bladder can be functionally reinnervated using heterotopic somatic nerve transfer to the pelvic nerve, we sought to determine whether urethral and anal sphincter muscles could be functionally reinnervated via transfer of motor branches of the femoral nerve to pudendal nerve branches in the perineum. We hypothesized that such reinnervation would allow a pathway by which urinary and fecal continence could potentially be restored. Specifically, we used the nervus saphenous pars muscularis, which is a femoral motor branch that innervates the sartorius muscle in dogs.8 This nerve is functionally equivalent to the sartorius motor branch of the femoral nerve in the human that also innervates the sartorius muscle and will be referred to as the femoral motor nerve in this report. This same approach of moving a femoral motor branch to pudendal nerve branches could also be used for reinnervation of the urinary or anal sphincters after a more restricted injury to the pudendal nerve.
MATERIALS AND METHODS
All studies were approved by the Temple University Institutional Animal Care and Use Committee in accordance with the laboratory animal care guidelines of both the U.S. Department of Agriculture and the Association for Assessment and Accreditation of Laboratory Animal Care. The study subjects were fully conditioned female mongrel hounds 6–12 months of age and 18–22 kg in body weight (Marshall BioResources, North Rose, NY). A total of nine canines were used. First, two canine cadavers were dissected after fixation to determine feasibility of the nerve transfer approach (Carolina Biological Supply, Burlington, NC). Then, three live dogs were used to surgically transfer, bilaterally, femoral nerve motor branches to pudendal nerve branches for a total of six nerve transfers. One normal control dog was utilized to assess, bilaterally, the typical innervation pattern of the sphincters from the spinal cord. We also included fluorogold cell counts from three sham operated, nerve intact controls. These nerve intact sham operated controls are the same animals as were included in a previous report.6
Cadaver Studies of Feasibility of Femoral Nerve Motor Branch Transfer to the Pudendal Nerve
A pilot project was performed in two canine cadavers to examine the feasibility of performing transfer of select femoral nerve motor branches to the pudendal nerve (Fig. 1). Although a transgluteal approach to the pudendal nerve with resection of the sacrotuberous ligament to access the pudendal nerve has been described,9 we determined in the cadaver dogs that the proximity of the femoral nerve motor branches in the anterior thigh to the pudendal nerve in the perineum is more feasible when using a perineal approach. Thus, we explored this perineal approach by exposing the pudendal nerve in Alcock’s canal (also known as the pudendal canal) located immediately medial to the ischial tuberosity.
Fig. 1.
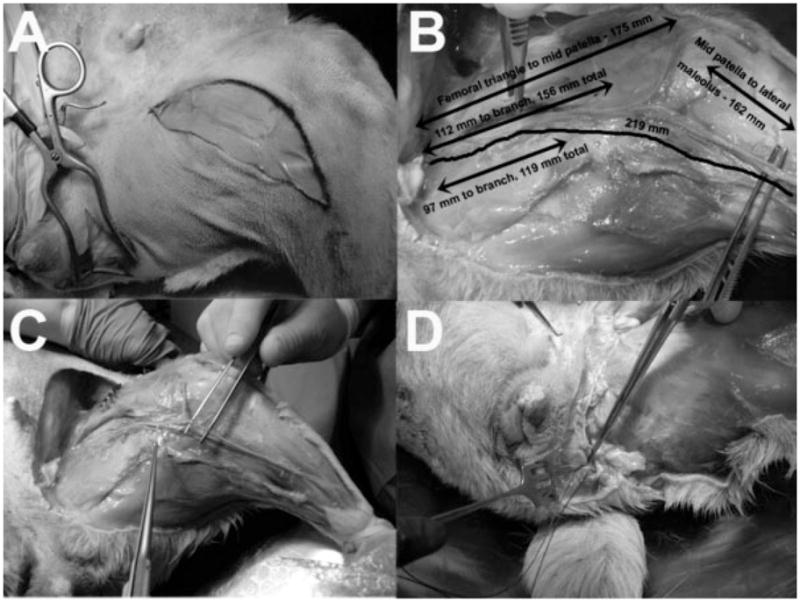
Feasibility study in cadaver dogs showing the dissection technique. This was performed prior to surgical procedures. A: Surgical incision sites on anterior thigh for access to the anterior femoral nerve branch and femoral nerve motor branches to the sartorius muscle and articularis genu muscle which is the nervus saphenous pars muscularis in canines,8 and to the lateral perineum for access to the pudendal nerve in Alcock’s canal. B: Various relevant dimensions of the femoral motor nerve branches. C: Mobilization of the femoral motor nerve is displayed. D: The femoral motor nerve reaches the pudendal nerve as it emerges from Alcock’s canal.
Surgical Preparation
The three live dogs to be used for surgery, as well as the normal control dog used for tract tracing, were fasted the day prior to surgery and administered antibiotics (30 mg/kg trimethoprim and 6 mg/kg sulfadiazine PO). A fentanyl patch (75–100 mg/hr for a 20 kg dog) was adhered to the shaved skin of the inner thigh and left in place for 3 days. Propofol (6 mg/kg iv) was administered to allow insertion of an endotracheal tube for isoflurane anesthesia (0.5–4% mean alveolar concentration) using oxygen as the carrier gas. Perioperative pain management included morphine (1 mg/L) in intravenous Ringers lactate at 60–100 ml/hr. Postoperative pain management included 2 mg/kg ketoprofen IM BID for 2 days beginning on the second day post surgery.
A Foley catheter was passed into the bladder through which bladder pressure was monitored. Balloon catheters interfaced with pressure transducers were placed in the rectum, as a surrogate for abdominal pressure and in the anal and urethral sphincters. The canine was repositioned to a supine jackknife position and longitudinal incisions were made in the perineum lateral to the midline between the anus and the ischial tuberosity. The pudendal nerves were located using the ischial tuberosity as a landmark, and stimulated with a hand-held monopolar electrode. This stimulation increased both the urethral and anal sphincter pressures and induced anal winking.
Sacral root transection, which induces bladder denervation, via loss of origin of the pelvic nerve as well as urethral and anal sphincter denervation, via loss of origin of the pudendal nerve, was performed as previously described.7 Briefly, with the animal in the prone position, a 30 degree V-laminectomy of the T7 vertebral body and a partial laminectomy of the T6 and S1 vertebral bodies was performed so that the S1 and S2 ventral roots innervating the bladder, urethral sphincter, and anal sphincter could be stimulated with a unipolar probe electrode. The two bilateral ventral roots that induced increased bladder, urethral sphincter, and anal sphincter pressure upon intraoperative electrical stimulation were transected and 15 mm long sections of the nerve roots were removed. Completeness of bladder denervation after root transection was confirmed by disappearance of bladder contractions upon stimulation of the entire conus medullaris with an epidural electrode placed in the midline under the T5 vertebral body. The incision was then closed in layers. For postoperative management of the neurogenic bladder, an abdominal vesicostomy was created as previously described.7,10
Transfer of Femoral Nerve Motor Branches to the Pudendal Nerve (Femoral NT)
To reinnervate the pudendal nerve, femoral motor nerve branches were surgically mobilized from their position on the distal anterior thigh and transferred to the pudendal nerve at its location in Alcock’s canal (femoral NT; Figs. 1 and 2). A femoral-pudendal nerve end-to-end anastomosis was created between the femoral nerve branches and the pudendal nerve branches under 10× magnification with approximation of the epineural layers using two to four 8-0 Vicryl sutures in an interrupted fashion. One centimeter from the anastomosis site, the transferred femoral nerve branches were enclosed in a unipolar 1.5 mm inside diameter nerve cuff electrode (Micro Probe, Inc., Gaithersberg, MD) with leads to a RF micro-stimulator (Alfred Mann Foundation for Scientific Research, AMF, Santa Clarita, CA.). The leads from the nerve cuff to the RF micro-stimulator were tunneled subcutaneously to the sacral area. The femoral nerve transfer procedure was carried out bilaterally in three canines.
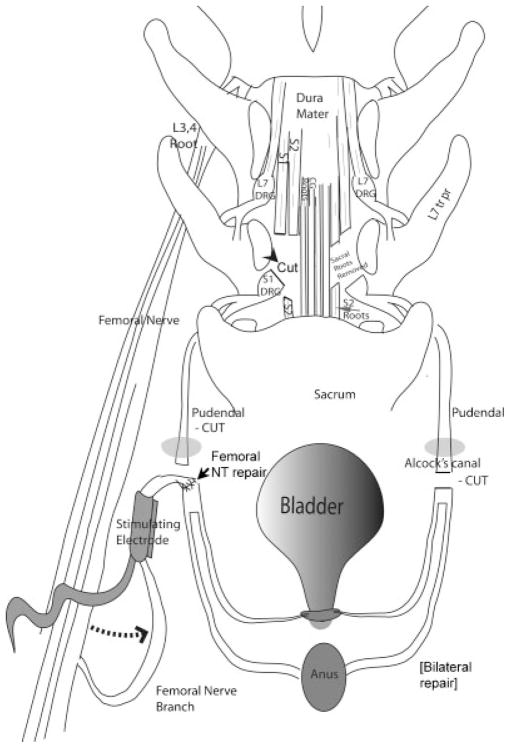
Fig. 2.
Diagram of the surgical transfer and repair methology. A spinal leminectomy was performed. Sacral ventral roots (S1, 2) were selected by their ability to stimulate sphincter contraction and transected. On the right side of the diagram, Alcock’s canal (gray oval) is shown. This is the site at which the pudendal nerve passes superficially into the perineum. On the left side of the diagram, the origin of nervus saphenous pars muscularis as part of the femoral nerve branches is shown. Bilaterally, motor branches of posterior branches of the femoral nerve were mobilized, transferred (dotted arrow) and attached by end to end anastomosis to the distal end of the transected pudendal nerves as they emerged from the pudendal canal in the perineum (as shown on the left), and enclosed in unipolar nerve cuff electrodes with leads to RF micro-stimulators. The external urethral spincter is shown at the base of the bladder, and the external anal sphincter is represented by the oval labeled as anus. The spinal cord laminectomy and root transections are the same as previously described and diagramed in Ruggieri et al.5. Abbreviatons: CG, coccygeal roots; DRG, dorsal root ganglion; L, lumbar toots; S, sacral roots; tr pr, transverse processes.
Transfer of the Genitofemoral Nerve to the Pelvic Nerve (GF-NT)
One of the canines receiving femoral nerve motor branch transfer to the pudendal nerve also underwent bilateral transfer of the genitofemoral (GF) nerve to the vesicular branch of the pelvic nerve leading to the urinary bladder as previously described.5,6 The GF nerves were mobilized bilaterally and attached to the distal severed end of the pelvic nerves leading to the bladder from the pelvic plexus by end-on-end anastomosis. The transferred GF nerve branches were also enclosed in unipolar nerve cuff electrodes with leads to RF micro-stimulators. The leads from the nerve cuffs to the RF micro-stimulators were tunneled to a subcutaneous location on the midline of lower abdomen above the pubic symphysis.
Functional Electrical Stimulation of the Transferred Femoral Motor and GF Nerves
The nerve stimulation system was activated at approximately monthly intervals post-operatively to determine functional reinnervation. For this procedure, the animal was anesthetized with isoflurane and catheters interfaced with pressure transducers were inserted into the bladder, external urethral sphincter, rectum, and anal sphincter. Three filling cystometries were performed to determine bladder capacity, the bladder was then filled to half of its capacity and the nerve stimulation system was activated. The implanted RF micro stimulation system produces asymmetric biphasic constant-current pulses with independently programmable pulse width, pulse amplitude, and frequency. Previous preliminary studies showed that optimal stimulation is obtained with a 200 μsec pulse width at 20 Hz (data not shown). FES of the implanted electrodes was achieved by activation of the RF micro-stimulator with the external coil during continuous monitoring of urodynamic pressures. Immediately before the animals were euthanized, the implanted nerve cuffs were located and the transferred nerves were evaluated for their ability to induce urethral and anal pressure by intraoperative electrical stimulation (2–6 V, 20 Hz, 1 msec square wave trains) using a unipolar probe electrode. In the animal with GF-NT, immediately before euthanization, the pelvic nerves leading to the bladder were also evaluated for their ability to induce bladder pressure and urine flow by intraoperative electrical stimulation.
Retrograde Neuronal Tracing
To confirm anatomically that the nerves responsible for innervating the external urethral sphincter and anal sphincter are under the control of transferred femoral motor nerve, we used lipophilic fluorochrome dye injections into the sphincters and allowed them to travel in a retrograde fashion to their respective innervating neuronal cell bodies located in the ventral horns of the spinal cord. Fluororuby and fast blue were injected into the external urethral and anal sphincters, respectively. Also, fluorogold was injected into urinary bladder. This was performed three weeks before euthanasia to allow sufficient retrograde travel. Fast blue (10% w/v, Sigma, St. Louis, MO, 50 μl/injection, 200 μl total per animal) was injected into the external urethral sphincter, fluororuby (4% w/v, Fluorochrome, LLC, Denver, CO, 50 μl/injection, 200 μl total per animal) into the anal sphincter skeletal muscle, and Fluorogold (4% w/v, Fluorochrome, LLC, Denver, CO) into the bladder, lateral to each ureteral orifice (50 μl/injection, 400 μl total per animal) as described previously.5–7
Animals were euthanized with an intravenous injection of 360 mg/kg sodium pentobarbital. The spinal cords, spanning from lower thoracic to coccygeal regions (16 cm length of cord), were collected at the time of euthanasia, fixed by immersion in 4% paraformaldehyde in 0.1 M phosphate buffer, and equilibrated in 30% sucrose in phosphate buffer for 3 days. The dorsal root entry zones were marked with an indelible histologic ink pen for segmental identification later in combination with cresyl violet detected morphological differences. The spinal cord was divided into separate segments of 2.5 cm in length and cryo-sectioned into 14 μm serial coronal section. Every 10th section was mounted onto charged slides (Fisher Plus). Fluorogold was distinguished from fast blue using a specific polyclonal antibody that detects the fluorogold protein (a Ki-67 antibody-W that reacts against the Ki-67 protein; Fluorochrome, LLC; 1:500 dilution in 0.4% Triton X-100 plus 1% goat serum, overnight at room temp), and then a secondary goat anti-rabbit antibody conjugated to a Cy2 (a green fluorescent tag; 1:100 in PBS for 2 hr, room temperature). For this, the spinal cord sections were first blocked with 10% goat serum in phosphate buffered saline (PBS) for 30 min at room temperature. All slides were washed in PBS and cover-slipped in 80% glycerol in PBS. We then evaluated the segments with microscopy for the presence of fluororuby (red), fast blue (blue), and fluorogold-Cy2 (green) labeled axonal cell bodies. Every fourth section was also stained with H&E for morphological verification of segment level.
Quantitative Analysis of Retrogradely Labeled Neurons in Spinal Cord
Spinal cord sections were analyzed quantitatively for the presence of retrogradely labeled motor neuronal cell bodies using a Nikon E800 microscope equipped with a X,Y motorized stage and interfaced with a quantification software-controlled computer system (Bioquant, Nashville, TN). The specific features that were utilized from this software included the irregular region of interest (ROI), topography, landmark, and object count array tools. Three sections per cord segment located 150 μm apart from each other were analyzed bilaterally for each animal using unbiased stereological counting methods in which an independent systematic sampling approach with a random start method was utilized as described previously.5,6,11 Specifically, spinal cord levels ranging from thoracic (T)12, lumbar (L)1, L2, L3, L4, sacral (S)1, S2, S3, coccygeal (Co)1 were assayed. Levels were verified by the indelible ink markings made on cords at the time of collection, as described above, that were still visible on the fluorescence-stained spinal cord sections, and by examination of adjacent cresyl violet stained spinal cord sections in order to maintain consistency in levels counted between animals. The ventral and intermediate gray matter areas of each of the different spinal cord segments were landmarked and circumscribed using a 4× objective (40× magnification) on live microscopic images displayed on a monitor, as shown in Fig. 6A and B. The average gray matter ROI assessed was 4.5 mm2 in size. Cell counting was then performed using a 40× objective (400× magnification) in sampling areas that were 0.015 mm2 in size (see small box indicated in Fig. 6D and G). Using the motorized stage, all fields were sampled systemically at 400× magnification within the larger ROI defined at the lower magnification per dog, per side, and per segment. To avoid bias in estimating the number of neurons, only retrogradely labeled cells in which the nucleus (unlabeled) was visible were counted. The sum of cells counted per retrograde dye was divided by the size of the larger ROI defined at the lower magnification to give an estimate of the number of cells per mm2, and the mean ± SEM per dog is presented. All counts were performed on coded preparations by one observer.
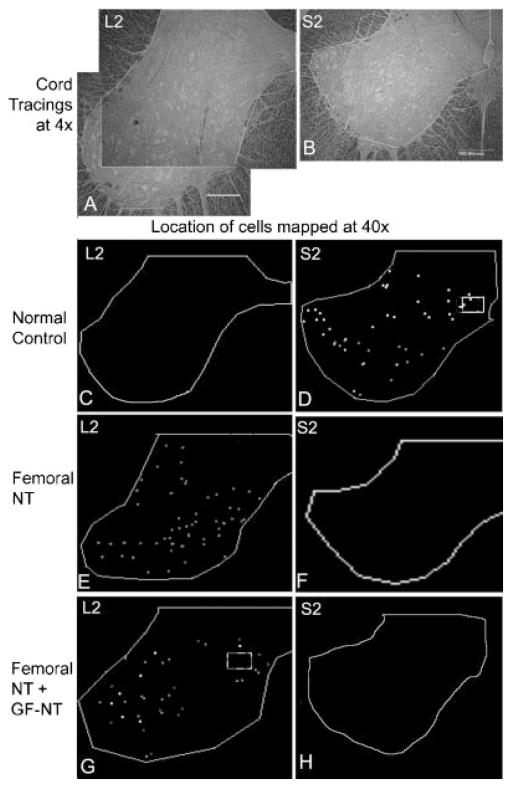
Fig. 6.
Location of neuronal cell bodies in the spinal cord retrogradely labeled with fast blue, fluororuby, or fluorogold after injection into the urethral sphincter, anal sphincter, or urinary bladder, respectively. A: Micrograph of an unstained bright-field section (taken with a ×4 objective) of a lumbar 2 (L2) spinal cord segment showing tracing around area in which neurons were counted. In this spinal cord segment, two larger areas were counted, as indicated, and the data combined. B: Micrograph of a sacral 2 (S2) spinal cord segment showing tracing around area analyzed (4.5 mm2). Scale bars in (A) and (B) = 500 μm. C, D: Representative tracings showing the location of fluororuby (red), fast blue (blue), and fluorogold (green after specific detection with Ki-67 antibody-W (Fluorochrome, LLC) and Cy2 secondary antibody) retrogradely labeled cell bodies in an S2 segment in a normal control. No retrogradely labeled neurons were detected in lumbar segments of normal controls. E, F: Representative tracings showing the location of retrogradely labeled cells in L2 and S2 segments in an animal in which nervus saphenous pars muscularis (L2–4) branches of the femoral nerve were transferred to the pudendal nerve (femoral-NT). No retrogradely labeled neurons were detected in sacral segments of Femoral-NT. G, H: Representative tracings showing the location of retrogradely labeled cells in L2 and S2 segments in an animal in which femoral nerve branches were transferred from the thigh to the pudendal nerve in Alcock’s canal (femoral-NT), and in which genitofemoral nerve branches were transferred intra-abdominally to the pelvic nerve branch to the bladder (GF-NT).
Postmortem Lipophilic Dye Tracing of Axons Across the Nerve Repair Site
Postmortem lipophilic dye tracing of the femoral NT to pudendal nerve surgical anastomosis site was also performed. After euthanasia, the nerve anastomosis site was located by following the lead from the RF micro-stimulators back to the nerve cuffs. Nerve segments approximately 1 cm proximal and 1 cm distal to the anastomosis site were removed and labeled with two different lipophilic dyes that diffuse through axonal bi-lipid membranes. Neurotrace DiI (red; N-228890; Molecular Probes of Invitrogen, Carlsbad, CA), was thinly coated onto small pieces of pulled glass micropipettes and inserted carefully using a dissecting microscope into the cut end of the transferred femoral nerve branch. In a similar fashion, Neurotrace DiO (green; N-22881; Molecular Probes of Invitrogen, CA) was inserted into the cut end of the pudendal nerve. This method of application labels axons from both the distal and the proximal end of the new nerve pathway across the anastomosis site. The nerve specimens were incubated at 37°C for 6 weeks to allow the lipophilic dyes to diffuse through the axonal membranes and along the axons. The nerves were then washed and cryo-sectioned without a sucrose incubation step, and visualized within 1 hr (in order to avoid non-specific diffusion of the dyes from the now sectioned nerves) using a Nikon epifluorescence microscope. Selected sections were also stained with hematoxylin and eosin (H&E).
RESULTS
Preliminary Feasibility Studies
Two canine cadavers were studied, with particular attention to the pudendal nerve and femoral motor nerve branches, which included the nervus saphenous pars muscularis to the sartorius muscle and its associated small branches to the articularis genu muscle. The location of these nerves, anatomical relationships to other structures, lengths, and possible rerouting pathways were examined. This allowed creation of our novel approach to pudendal reinnervation and a smooth intraoperative course. Figure 1 shows the dissection technique. The surgical incisions are shown in Figure 1A. These provide exposure of the femoral motor nerve branches for mobilization and tunneling along the crease between the inner thigh and the perineum to reach the pudendal nerve as it emerges from Alcock’s canal. Figure 1B shows the various relevant dimensions of the femoral nerve motor branches used for nerve transfer. Mobilization of these branches is displayed in Figure 1C. Figure 1D shows the femoral motor nerve branch reaching the pudendal nerve as it emerges from Alcock’s canal.
Figure 2 shows a cartoon of the above femoral NT procedure combined with a laminectomy to expose the cauda equina dorsally and transection of the S1 and S2 ventral roots that innervate the bladder and urethral and anal sphincters. This figure also shows the placement of stimulating electrodes onto the transferred femoral motor nerve branches after they were sutured to the pudendal nerve trunk. The transection and repair site of the pudendal nerves was prior to their branching into nerves to the external urethral sphincter and external anal sphincter.
Functional Studies
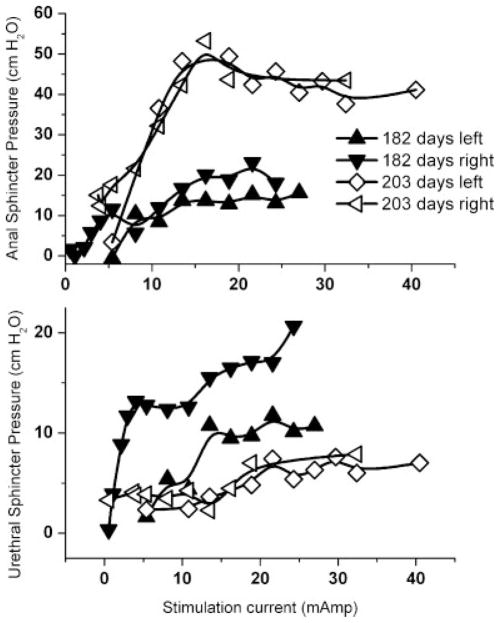
Representative urodynamic pressure responses to functional electrical stimulation of the transferred femoral motor nerve branches are shown in Figure 3. Functional responses are clearly demonstrated by increases in both urethral and anal sphincter pressures. In addition, there was visual evidence of anal winking during nerve stimulation. We also examined all dogs for knee function or gait changes throughout the course of the experiment and no dysfunction was observed. Representative examples of recruitment curves for increasing stimulation intensities performed at 182 and 293 days post operatively are shown in Figure 4. The mean maximal urethral pressure generated by activation of the RF micro-stimulator was 18.2 ± 4.6 cm H2O. The mean maximal anal sphincter pressure was 35.1 ± 9.5 cm H2O. Increased urethral and anal sphincter pressures during RF micro-stimulator activation were observed in five out of six femoral-pudendal nerve neo-anastomoses. Monthly post operative FES of the transferred femoral nerves first induced these increased urethral and anal sphincter pressures at 187, 120, and 72 days post operatively in the 3 dogs with nerve transfer. For the one animal that also received genitofemoral to pelvic nerve transfer, stimulation of the transferred genitofemoral nerves did not induce increased bladder pressure at any of the monthly postoperative activations of the implanted RF micro-stimulators nor with the unipolar probe electrode immediately prior to euthanasia.
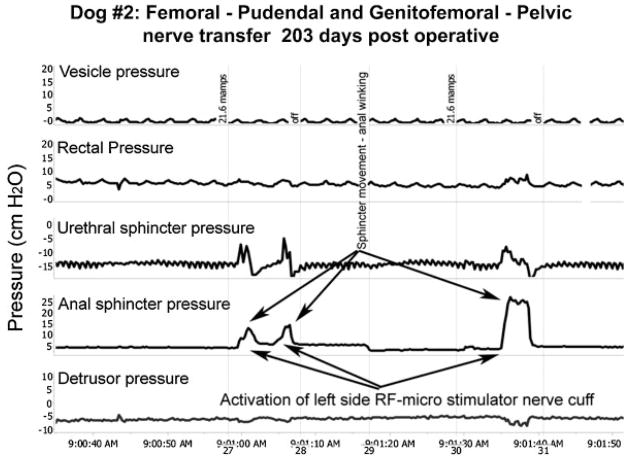
Fig. 3.
Functional electrical stimulation. Representative urodynamic pressure recordings during FES (arrows) via the implanted RF micro-stimulator with a lead to a unipolar nerve cuff electrode surrounding the transferred femoral motor nerve branches 1 cm proximal to the anastomosis with the pudendal nerve branches on the left side in dog #2. Foley balloon catheters (12 Fr, 5 ml balloon) were passed through the vesicostomy for bladder filling and through the urethra for vesicle pressure measurement using external transducers. Similar pressure transducers connected to the lumen of 10 Fr ballon catheters were passed into the rectum and into the urethral and anal sphincters for monitoring rectal, urethral sphincter and anal sphincter pressures. The bottom detrusor pressure trace is derived by subtracting the rectal pressure (as a surrogate for abdominal pressure) from the top trace for intravesical pressure. Similar increases in urethral and anal sphincter pressures during stimulation were observed for 5 of the 6 transferred femoral motor nerves.
Fig. 4.
Representative recruitment curves for anal and urethral sphincter pressure by increasing intensity of RF micro-stimulation of the transferred femoral motor nerves for both left and right side at 182 and 203 days post operatively.
Postmortem Lipophilic Dye Tracing Demonstrates Axons Traversing the Anastomosis Site
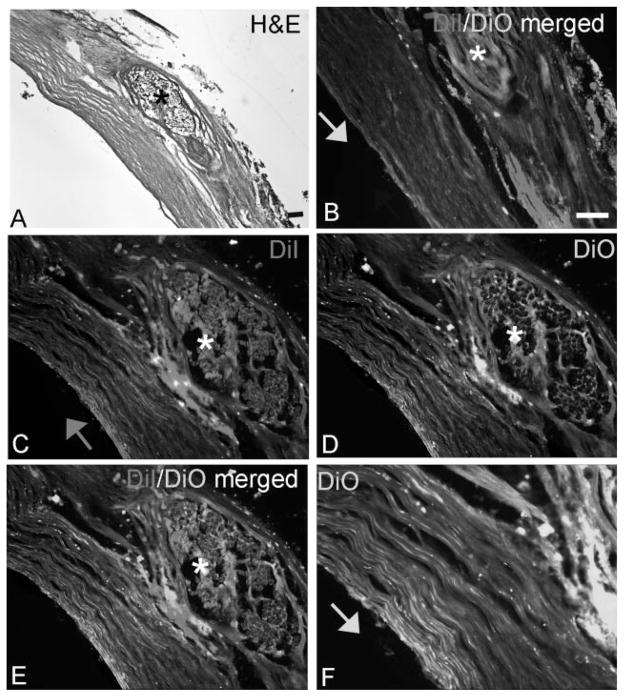
The femoral-pudendal nerve anastomosis sites were collected and analyzed histologically for the integrity of the repair site (Fig. 5). Two different postmortem lipophilic axonal labeling dyes were applied as a paste to either the distal (DiI, red) or proximal (DiO, green) ends of the repaired nerves 1 cm from the anastomosis site (Fig. 5B). Microscopic analysis of the sectioned site 6 weeks after this dye placement revealed the presence of both Di-labeled and DiO-labeled axons crossing the repair site (Fig. 5C–F). H&E staining of the sectioned nerves (Fig. 5A) also confirmed the presence of intact nerve tissue at the site of reanastomosis. The reanastomosis sites were definitively established by the presence of suture material (asterisks in Fig. 5).
Fig. 5.
DiI and DiO labeling confirmed axonal regrowth across the nerve repair site. A: H&E stained section showing a repaired nerve at the site of reanastomosis, as indicated by the suture (*). B: DiO (green axonal dye) was placed proximal to site of reanastomosis while DiI (red axonal dye) was placed distal to the site of reanastomosis, as indicated by the arrow. C: Higher power micrograph showing DiI-labeled axons crossing the repair site. D: Higher power micrograph showing Di-labeled axons crossing the repair site. E: Merged (C) and (D). F: Even higher power micrograph showing DiO-labeled axons crossing the repair site. Scale bar = 50 μm.
Retrogradely Labeled Axons are Visible in Spinal Cord Ventral Horn Segments
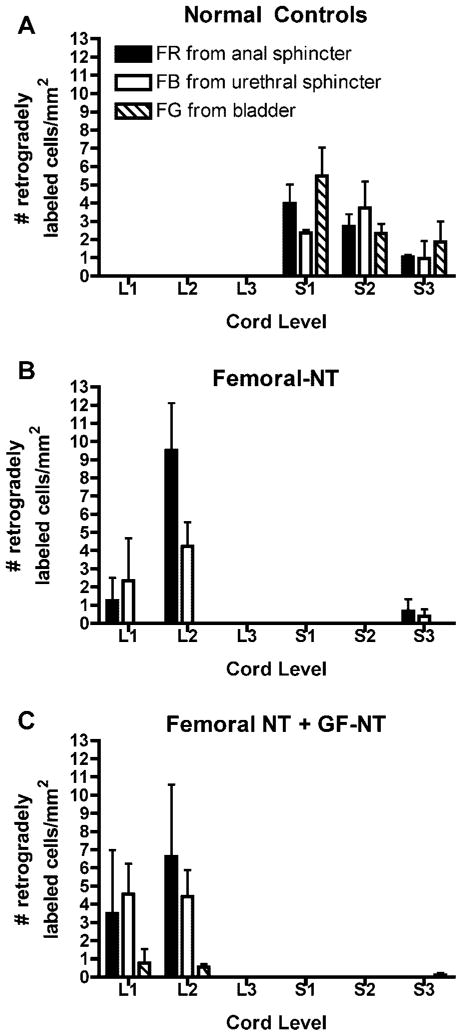
Examples of locations of the retrogradely labeled neuron cell bodies in lumbar (L1–3) and sacral (S1–3) spinal cord ventral horns are shown in Figure 6. Quantification and segmental localization of the labeled neuronal cells bodies are shown in Figure 7. No retrogradely labeled neurons were detected in lumbar segments of normal controls (Figs. 6C and 7A). Instead, fluororuby, fast blue, and fluorogold from the external urethral sphincter, anal sphincter, and bladder, respectively, were present in S1 and S2 segments, as well as some in S3 (Figs. 6D and 7A). No labeled motoneuron cell bodies were observed in coccygeal cord segments. These results confirm the normal spinal cord origin of the pudendal nerve to the upper sacral segments. The fluorogold labeled neuronal cell bodies from the bladder were located primarily (but not exclusively) in the intermediate zone of the ventral horn, while the sphincter neuronal cells bodies (fast blue and fluororuby labeled) were more localized to lamina 9 (Fig. 6D).
Fig. 7.
Graphs showing number of retrogradely labeled cells per mm2 in lumbar (L1, L2, L3) and sacral (S1, S2, S3) spinal cord segments in (A) a normal control, (B) the animals in which femoral motor nerve branches were transferred to the pudendal nerve (femoral-NT), and (C) the animal in which both femoral NT and genitofemoral-pelvic nerve transfer (GF-NT) was performed.
In contrast to the normal controls, in animals in which the nervus saphenous pars muscularis branches of the femoral nerve were transferred to the pudendal nerve (femoral-NT), many fast blue and fluororuby labeled neuronal cell bodies were located in upper lumbar spinal cord segments (Figs. 6E and 7B). This was expected because the femoral nerve originates from upper lumbar cord segments. These neuronal cell bodies were localized in both lamina 9 and the intermediate zone of the ventral horn (Fig. 6E). No retrogradely labeled neurons were detected in S1 or S2 sacral segments in femoral-NT animals (Fig. 6F). In the animal that received both femoral NT and GF-NT bilaterally, many fast blue and fluororuby labeled neuronal cell bodies were located in upper lumbar spinal cord segments (Figs. 6G and 7C). Also, a very low number of fluorogold retrogradely labeled neurons from the bladder were also detected in the S3 segment suggesting that this small number of nerve fibers were not transected during the S1 and S2 nerve transection at the onset of the experiment. Examination of lumbar spinal cord segments also revealed that both fluororuby and fast blue retrogradely labeled neurons had similar distributions in the ventral horn in femoral NT animals. Thus, the transferred lumbar motor neurons grew axons from the cut femoral nerve branch, across the nerve repair site, through the pudendal nerve stump to the urethral and anal sphincters.
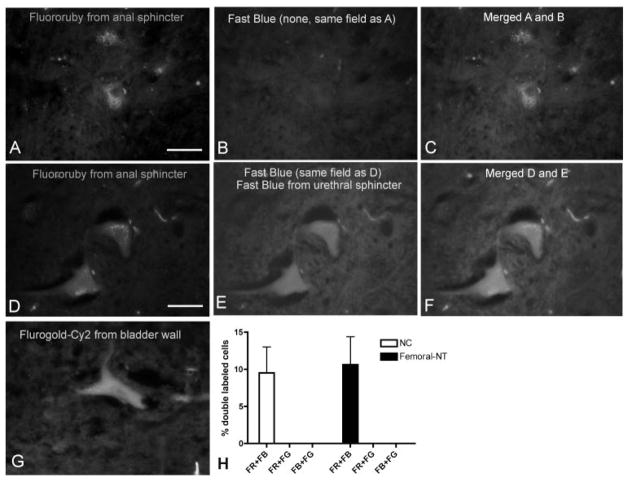
Examples of neuronal cell bodies in the spinal cord are shown in Figure 8. Most fluororuby-labeled neuronal cell bodies from the anal sphincter were not co-labeled with fast blue from the urethral sphincter, and vice versa (Fig. 8A–C). However, approximately 10% of the fluororuby-labeled neurons were double-labeled with fast blue (Fig. 8D–F). These results were similar in both normal controls and femoral NT animals (Fig. 8H). This indicates that approximately 10% of the motoneurons were dichotomized, innervating both urinary and anal sphincters. In contrast, fluorogold labeled neurons (Fig. 8G), labeled after injection of fluorogold into the bladder, were never co-labeled with either fluororuby or fast blue. This latter result indicates that the bladder wall has separate innervation patterns than the sphincters in that motoneurons do not dichotomize to innervate both the urinary bladder and either the urethral or anal sphincters.
Fig. 8.
Examples of neuronal cell bodies in the spinal cord retrogradely labeled with fluororuby (A, D), fast blue (B, E), and fluorogold-Cy2 (G) after injection into the external urethral sphincter, anal sphincter or urinary bladder, respectively. Fluororuby-labeled neurons shown in (A) are not double-labeled for fast blue (B) in the merged image (C), while fluororuby-labeled neurons shown in (D) are double-labeled with fast blue (E) in the merged image (F). The graph in panel (H) shows that the percent of double-labeled neurons in sacral segments of normal controls (NC) and in the lumbar segments of femoral to pudendal nerve transfer cords was similar. Scale bar = 50 μm.
DISCUSSION
While a cure for SCI-induced paralysis is certainly one of the ultimate goals of research in this area, an improvement in the quality of life by promoting functional recovery in the short term is a more realistic goal that may be achievable in a more immediate time frame. In patients that have sustained upper motor neuron lesions which spare the neuron cell bodies in S2–S4 spinal cord segments that innervate the lower urinary tract, stimulation strategies have been developed and put into clinical use that can achieve bladder emptying. These strategies do not rely on reinnervation, but on stimulation of existing intact neural pathways. However, with spinal cord or nerve injuries at or below the level of the conus medullaris, or in cases of upper motoneuron injuries with insufficient intact innervation, reinnervation strategies are required to achieve bladder emptying and continence because the sacral cord cell bodies innervating the bladder and sphincters are damaged or their axons are severed. We have had considerable success in functional reinnervation of the bladder with somatic nerve transfer techniques, and our objective with this study was to demonstrate reinnervation and functional control of the urethral and anal sphincters. To the best of our knowledge, this is the first report to document these.
Other investigators have attempted other somatic nerve transfers in hopes of reinnervating the pudendal nerve and its target organs, but have not been able to effect sphincter control. Sato attempted functional reinnervation with biceps femoris muscle nerve without electro stimulatory effect.12 We chose motor branches of the femoral nerve for somatic transfer given the length of these nerve branches available for anastomosis, and the relative lack of morbidity in sacrificing it for this purpose. We chose the saphenous nerve in these feasibility studies, due to its easy access on the anterior thigh and its inclusion of motor axons to the sartorius muscle in canines.8 However, in humans, other motor branches of the femoral nerve will have to be utilized for this type of nerve transfer, since the saphenous nerve is thought to be only sensory in humans. We observed no functional gait problems or knee dysfunction in any of the femoral-NT dogs, suggestive of compensatory action of the still innervated quadriceps femoris muscles.
Although it is agreed that the external sphincter is supplied by pudendal nerve fibers, some believe that other nerves may contribute to maintaining its closure function. Ali-El-Dein et al. explored the role of the urethral branch of the pelvic plexus on external urethral function and found that there was no significant effect on the distal urethral pressure profile after autonomic denervation.13 In our study, complete functional denervation of the urethral and anal sphincters was achieved by transecting the pudendal nerve root proximally before its division into the pelvic nerve and the pudendal nerve with branches supplying its respective sphincters. In our previous studies we confirmed that this completely severed the motor innervation of the bladder by the disappearance of bladder contractions upon stimulation of the entire conus medullaris with an epidural electrode placed in the midline under the L5 vertebral body.6,7 Because the implanted electrodes were placed on the transferred femoral nerve proximal to the pudendal nerve anastomosis, contraction in response to electrical stimulation of the transferred femoral motor nerve provides evidence of functional urethral and anal sphincter reinnervation via growth of the femoral nerve into the pudendal nerve.
We were able to document increased sphincteric pressures in five out of six transferred nerves. At a nerve growth rate of approximately 1 mm per day, sufficient time should have elapsed between the reinnervation surgery and the electrode stimulation for the nerve regrowth to reach the sphincters. All RF micro stimulators were removed from all animals after euthanization and all were found to be fully functional. Although electrode lead failure is a possible explanation for the one ineffective nerve, there is no evidence to support this possibility. Despite the inability of this one nerve transfer to induce sphincter contraction, the presence of fluoro ruby and fast blue were detected in lumbar spinal cord segments and DiI and DiO were detected across the anastomoses in this nerve showing histological evidence of reinnervation.
Approximately 10% of the retrogradely labeled motoneurons in our normal control dog were dichotomized, being dual labeled with both fast blue from the urethral sphincter and fluoro ruby from the anal sphincter (Fig. 8). Although dichotomizing dorsal root ganglion (afferent) neurons supplying sensory innervation to both the urinary bladder and distal colon have been described in the rat and mouse,14,15 our finding of dichotomizing neurons supplying motor (efferent) innervation to both the urethra and anal sphincters is novel. We also found that 10% of the motoneurons from the transferred femoral motor nerves in the three femoral NT animals were similarly dichotomized (Fig. 8). While this may be a spurious finding in this small series of animals, is it intriguing to speculate that these dichotomizing motoneurons innervating both the urethral and anal sphincters may have important functional significance.
Results of this study provide initial proof of concept that motor branches of the femoral nerve can be transferred successfully to the pudendal nerve. This finding provides a potentially viable clinical approach to reinnervating LMN-lesioned urethral and anal sphincters. In order to provide continence mechanisms, this new neural pathway would need to induce tone in the urethral and anal sphincters as well as induce acute sphincter contraction in reaction to increased abdominal pressure and relaxation during micturition. Although there is precedence in the literature for neuroplasticity in relearning to use reanastomosed nerves,16–18 it is not clear whether such a complex function as sphincteric control can be relearned with femoral nerve reinnervation of the pudendal nerve targets. However, if the new pathway cannot achieve continence, then the reanastomosed nerves can be directly stimulated using FES. Chronic electrical stimulation of the transferred gracilis muscle following graciloplasty of the anal19 and urethral20 sphincters has been reported to restore continence.
The evidence presented in this canine model of urethral and anal sphincter reinnervation by the transferred motor branches of the femoral nerve includes the following: (1) Increased urethral and anal sphincter pressure following electrical stimulation in five out of six nerve cuff electrodes implanted on the transferred femoral motor nerves, (2) abundant lumbar spinal cord cell bodies that were labeled in a retrograde fashion by fast blue injected into the external urethral sphincter and fluororuby injected into the anal sphincter, (3) the absence of any significant retrograde-labeled dye in the sacral spinal cord, and (4) the free diffusion of axonal labeling dye across the femoral-pudendal nerve anastomosis.
CONCLUSIONS
Femoral motor nerve branches to pudendal nerve transfer results in reinnervation of urethral and anal sphincters as determined by increased pressure during stimulation of implanted electrodes and retrograde dye labeling. The findings of this study pave the way for clinical application of this nerve transfer surgery in human patients with LMN lesions resulting in neurogenic sphincteric incontinence. These results, in combination with our previous studies on bladder reinnervation, provide direct evidence that bladder and sphincters can be reinnervated following a lower motor neuron lesion. Via a combined surgical approach, we may be able to provide a means by which patients with sacral spinal cord or nerve injuries can better control micturition, fecal continence, and improve their quality of life.
Footnotes
Dirk De Ridder led the review process.
Conflict of interest: None.
References
- 1.Hackler RH. A 25-year prospective mortality study in the spinal cord injured patient: Comparison with the long-term living paraplegic. J Urol. 1977;117:486–8. doi: 10.1016/s0022-5347(17)58506-7. [DOI] [PubMed] [Google Scholar]
- 2.Stover SL, Fine PR. The epidemiology and economics of spinal cord injury. Paraplegia. 1987;25:225–8. doi: 10.1038/sc.1987.40. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KD. Targeting recovery: Priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 4.Barbe MF, Ruggieri MR., Sr Innervation of parasympathetic postganglionic neurons and bladder detrusor muscle directly after sacral root transection and repair using nerve transfer. Neurourol Urodyn. 2011;30:588–605. doi: 10.1002/nau.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggieri MR, Braverman AS, D’Andrea L, et al. Functional reinnervation of the canine bladder after spinal root transection and genitofemoral nerve transfer at one and three months after denervation. J Neurotrauma. 2008;25:401–409. doi: 10.1089/neu.2007.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggieri MR, Braverman AS, D’Andrea L, et al. Functional reinnervation of the canine bladder after spinal root transection and immediate somatic nerve transfer. J Neurotrauma. 2008;25:214–24. doi: 10.1089/neu.2007.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruggieri MR, Braverman AS, D’Andrea L, et al. Functional reinnervation of the canine bladder after spinal root transection and immediate end-on-end repair. J Neurotrauma. 2006;23:1125–36. doi: 10.1089/neu.2006.23.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans HE. Miller’s anatomy of the dog. Philadelphia: W.B. Saunders; 1993. [Google Scholar]
- 9.Gustafson KJ, Zelkovic PF, Feng AH, et al. Fascicular anatomy and surgical access of the human pudendal nerve. World J Urol. 2005;23:411–8. doi: 10.1007/s00345-005-0032-4. [DOI] [PubMed] [Google Scholar]
- 10.Agelan A, Braverman AS, Dean GE, et al. Refinement in the management of the denervated canine urinary bladder using an abdominal vesicostomy. Ilar J. 2008;49:E8–14. doi: 10.1093/ilar.49.4.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouton PR. Principals and practices of unbiased sterology: An introduction for bioscienctists. Baltimore: The John Hopkins University Press; 2002. [Google Scholar]
- 12.Sato T, Konishi F, Kanazawa K. Muscle change after anal sphincter reinnervation by a normal somatic peripheral nerve: Preliminary study. Dis Colon Rectum. 1999;42:505–509. doi: 10.1007/BF02234177. [DOI] [PubMed] [Google Scholar]
- 13.Ali-El-Dein B, Ghoneim MA. Effects of selective autonomic and pudendal denervation on the urethral function and development of retention in female dogs. J Urol. 2001;166:1549–54. [PubMed] [Google Scholar]
- 14.Christianson JA, Liang R, Ustinova EE, et al. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128:235–43. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourol Urodyn. 2010;29:77–81. doi: 10.1002/nau.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaul JS., Jr Intrinsic motor recovery–a long-term study of ulnar nerve repair. J Hand Surg [Am] 1982;7:502–8. doi: 10.1016/s0363-5023(82)80048-8. [DOI] [PubMed] [Google Scholar]
- 17.Imai H, Tajima T, Natsumi Y. Successful reeducation of functional sensibility after median nerve repair at the wrist. J Hand Surg [Am] 1991;16:60–5. doi: 10.1016/s0363-5023(10)80014-0. [DOI] [PubMed] [Google Scholar]
- 18.Rosen B, Bjorkman A, Lundborg G. Improved sensory relearning after nerve repair induced by selective temporary anaesthesia - a new concept in hand rehabilitation. J Hand Surg [Br] 2006;31:126–32. doi: 10.1016/j.jhsb.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Baeten CG, Konsten J, Spaans F, et al. Dynamic graciloplasty for treatment of faecal incontinence. Lancet. 1991;338:1163–5. doi: 10.1016/0140-6736(91)92030-6. [DOI] [PubMed] [Google Scholar]
- 20.Janknegt RA, Baeten CG, Weil EH, et al. Electrically stimulated gracilis sphincter for treatment of bladder sphincter incontinence. Lancet. 1992;340:1129–30. doi: 10.1016/0140-6736(92)93153-e. [DOI] [PubMed] [Google Scholar]