Abstract
Motoneuronotrophic factor (MNTF) is an endogenous neurotrophin that is highly specific for the human nervous system, and some of the observed effects of MNTF include motoneuron differentiation, maintenance, survival, and reinnervation of target muscles and organs. MNTF is a neuro-signaling molecule that binds to specific receptors. Using In Silico Analysis, one of the active sites of MNTF was identified as an analog of six amino acids (GM6). The effect of chemically synthesized GM6 on ischemic stroke was studied in the middle cerebral artery occlusion (MCAo) mouse model. Mice were subjected to 1 hour of ischemia followed by 24 hours of reperfusion. Mice were injected intravenously with a bolus of GM6, at various doses (1 and 5 mg/kg) immediately after the start of reperfusion and examined for changes in physiological parameters, neurological deficits and infarct volume. GM6 was able to penetrate the blood brain barrier, and at both 1 and 5 mg/kg showed a significant protection from infarct damage, which translated to improvement of neurological deficits. Administration of GM6 demonstrated no changes in HR, BP, pO2, pCO2, or pH. A significant increase over the control group in CBF after reperfusion was observed with GM6 administration, which helped to mitigate the ischemic effect caused by the blockage of blood flow. The time window of treatment was assessed at various times following cerebral ischemia with GM6 demonstrating a significant protective effect up to 6–12 hours post ischemia. In addition, GM6 increased neurogenesis, and decreased apoptosis and inflammation in the mouse brain following cerebral ischemic injury. These data suggest that GM6 is neuroprotective to the brain following IV injection in the mouse model of MCAo.
Keywords: stroke, trophic factors, middle cerebral artery occlusion, mouse, neuroprotection
Introduction
Neuronotrophic factors (NTFs) are a specialized group of proteins which function to promote the survival, growth, maintenance, and functional capabilities of selected populations of neurons (Barde, Y.A., 1989; Barde, Y.A., 1988; and Thoenen, H., and Edgar, D., 1985). Studies have demonstrated that neuronal death occurs in the nervous systems of vertebrates during certain periods of growth and development (Burek and Oppenheim, 1996; Mennerick and Zorumski, 2000; Sendtner et al., 2000; Buss and Oppenheim, 2004). However, the addition of soluble neuronal trophic factors from associated target tissues serves to mitigate this phenomenon of neuronal death (Chau, R.M.W., et al., 1990; Kuno, M., 1990; Oppenheim, R.W., 1989; Krieglstein et al, 2002; Hennigan et al., 2007).
In the vertebrate neuromuscular system, the survival of embryonic motoneurons has been found to be dependent upon specific trophic substances derived from the associated developing skeletal muscles. Skeletal muscles have been shown, by both in vivo and in vitro studies, to produce substances which are capable of enhancing the survival and development of motoneurons by preventing the embryonic motoneurons from degeneration and subsequent, natural cellular death. (O’Brien, R.J. and Fischbach, G.D., 1986; Hollyday, M. and Hamburger, V., 1976). Similarly, several investigators have reported that chick and rat skeletal muscles possess certain trophic factors which can prevent the natural cellular death of embryonic motoneurons both in vivo and in vitro.(McManaman, J.L., et al., 1988; Oppenheim, R.W., et al., 1988; and Smith, R.G., et al., 1986). These skeletal muscle derived neuronotrophic factors have been demonstrated to be functionally different from other trophic factors such as Nerve Growth Factor (NGF), Ciliary Ganglion Neurotrophic Factor (CNTF), Brain-Derived Neurotrophic Factor (BDNF), and Retinal Ganglion Neurotrophic Factor (RGNTF) (Levi-Montalcini, R., 1982; Varon, S., et al., 1988; Barde, Y.A., 1989; McManaman et al., 1990; Chau, R.M.W., et al., 1991a; Chau, R.M.W., et al., 1991b).
More recently, the isolation and characterization of two motoneuronotrophic factors from rat muscle tissue with apparent molecular weights of 35 kD and 22 kD have been reported. (Chau, R.M.W., et al., 1992). The 35 kD protein was defined as motoneuronotrophic factor 1 (MNTF1) and the apparent 22 kD protein as motoneuronotrophic factor 2 (MNTF2). These two trophic factors have been demonstrated in vitro to support the growth and/or regeneration of both isolated anterior horn motoneurons and spinal explants of rat lumber spinal cord. A number of studies have demonstrated the efficacy of these MNTFs in various rat nerve systems, including the peripheral sciatic nerve, the peripheral musculocutaneous nerve, the cranial facial nerve, the cranial hypoglossal nerve, and the portion of the spinal cord that controls muscles in the neck, chest and upper limbs (Zhou et al 1993; Zhou et al 1992, Chau et al 1992; Wang et al., 1995). In the hemi-sectioned rat spinal cord model, MNTFs reduced inflammation, limited degeneration and enhanced regeneration of the grafted nerves (Wang et al., 1995). Furthermore, the wobbler mice with double recessive genes given one dose of 35μg/kg MNTF1 at the age of six weeks slowed the neurodegenerative genetic disease in this strain.
Subsequently, the cloning of human MNTF1 and its associated receptor from a human retinoblastoma cDNA library was reported (Chau et al., 1993). Human MNTF1 cDNAs were subcloned into expression vectors and the MNTF1 polypeptides contained in the expressed fusion proteins exhibited biological activity similar to that of the “native” MNTF1 protein in that they supported the in vitro growth of rat anterior horn motoneurons. The amino acid sequences of the human MNTF1 polypeptides were elucidated by direct protein sequencing. One of the MNTF1 polypeptides consisting of 33 amino acids was identified as MNTF33mer, and subsequently was successfully synthesized by solid phase chemistry. The synthesized MNTF33mer showed biological activity in various in vitro and in vivo functional assays. A number of studies have demonstrated the trophic and tropic efficacy of the synthesized MNTF33mer in well-established rat peripheral nerve model systems. In a rat sciatic nerve transection with a 8mm gap study, MNTF33mer treated animals have significant improvement of motoneuron regeneration in a dose response manner and promoted DRG neurons regeneration. In a transected femoral nerve rat model, the number of motoneurons projected correctly to muscle was enhanced in the MNTF33mer treated animals in a dose dependent manner. At the optimal dose, the number of motoneurons projected correctly to muscle was three times that of the motoneurons projected incorrectly to the skin.
In Silico Analysis was employed to search further for the active sites within the MNTF33mer molecule, and six active domain sites were identified. The smallest active site consists of 6 amino acids and hence was named MNTF6mer or GM6 (Chau, 2001). Recent studies have shown that GM6 had similar activity as the parent molecule (Chau, 2005; 2007). Studies with the synthesized GM6 also demonstrated similar trophic effects in a transected femoral nerve rat model. In a zebrafish bioassay, GM6 protected the organism from L-2-hydroxyglutaric acid (LGA) induced oxidative stress and apoptosis in the CNS, and reduced apoptosis by 85% in the midbrain. These studies suggest that MNTF33mer and its smaller analog GM6 provide protection from cell injury in a number of disease models.
Human MNTF1 is an endogenous neurotrophin, is highly specific for the human nervous system and it is expressed rapidly during the first trimester of human fetal development of the complete nervous system, peaking at week nine (Di and Huang, 1998). MNTF is a neuro-signaling molecule that binds on very specific receptors. The specific functions of MNTF, as demonstrated in animal and in vitro studies, include embryonic stem cell differentiation into motoneurons, motoneuron maintenance and survival, motor axon regeneration with guidance, and reinnervation of target muscles and organs (Chau et al., 1992). When the central nervous system (CNS) and peripheral nervous system (PNS) are under attack caused by diseases, disorders or injuries, MNTF creates a protective and permissive environment for nerve regeneration and repair that are neuroprotective, anti-apoptosis, anti-oxidation, anti-inflammation, and anti-scar.
Based on these data, we decided to test the ability of GM6 to protect the brain from acute ischemia and reperfusion injury.
2. Results
2.1. Blood brain barrier penetration of GM6
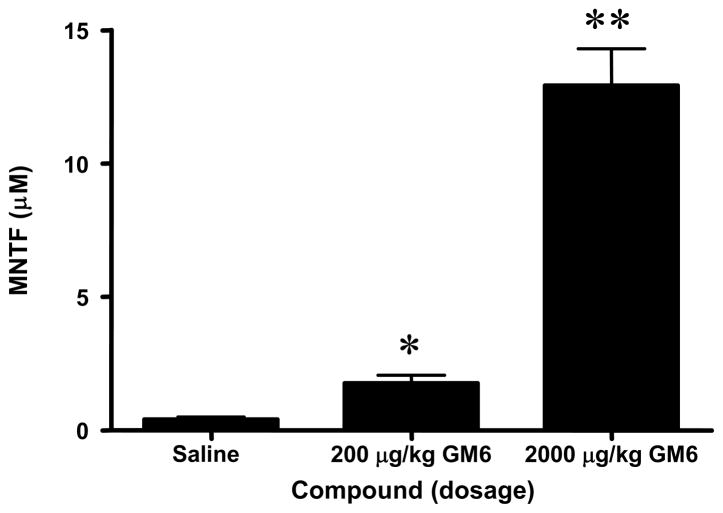
To measure GM6 levels in brain samples following intravenous (i.v.) administration, we used a competitive enzyme linked immunosorbent assay (ELISA). As shown in Figure 1, GM6 was detected in the cortex using the ELISA. The assay detected endogenous GM6 in the brain (0.4 μM) of the control animals. In the animals injected with 0.2 mg/kg of GM6 a 400% increase in GM6 was detected (1.760 μM) whereas injection of 2 mg/kg of GM6 gave rise to a 3000% increase in GM6 in the brain after 4 hours (12.92 μM). These data suggest that intravenous injection of GM6 allowed for distribution of the peptide in the brain.
Fig 1. Levels of GM6 in brain of mice.
C57BL/6 mice were injected with vehicle control (phosphate buffered saline), GM6 at 0.2 mg or GM6 at 2 mg/kg. The compounds were administered at time 0 and the brains were collected 4 hours later for analysis by ELISA. *p < 0.0001 compared to Saline; **p < 0.0001 for 2000 μg/kg compared to 200 μg/kg.
2.2. Dose effect of GM6 on ischemic injury
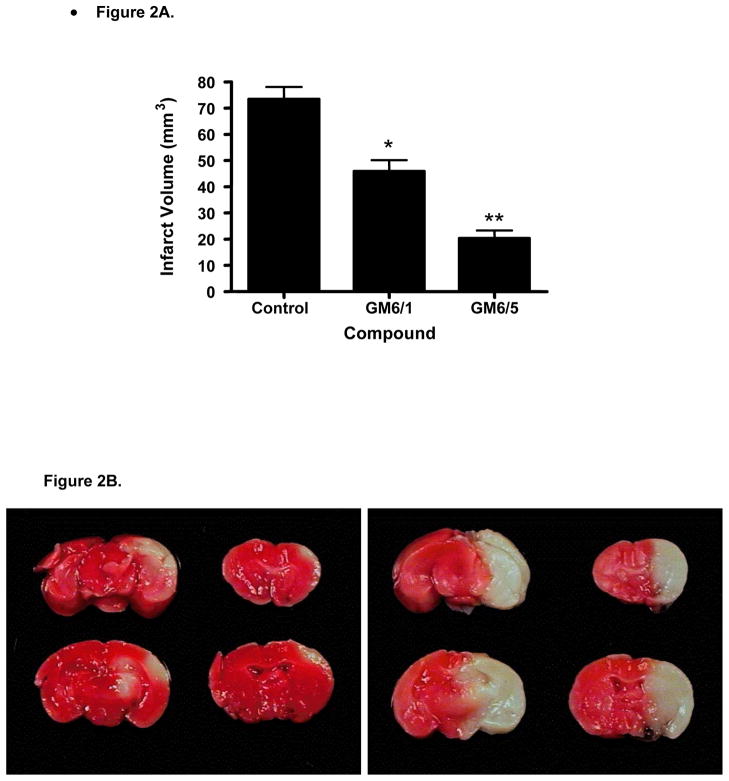
To determine the effect of GM6 on protection from ischemic injury, mice were subjected to 1 hr of ischemia followed by 24 hrs of reperfusion (Fig. 2). GM6 was administered i.v. at 1 or 5 mg/kg at the start of reperfusion, to ensure maximum protection. Compared with the vehicle-injected group, the infarct volume in the brains was significantly decreased in the GM6 treated groups (at both 1 and 5 mg/kg). GM6 showed a dose dependent reduction in lesion area. Infarct volumes vs. GM6 dosage was plotted in Figure 2A. The lesion areas were 73.37 mm3 for vehicle group, 45.93 mm3 and 20.29 mm3 for GM6 treated groups at 1 or 5 mg/kg, respectively. Thus, post ischemia i.v. administration of GM6 at 1 or 5 mg/kg resulted in 38% and 73% decrease in infarct volume respectively compared to vehicle. Figure 2B shows the damaged area present in animals subjected to either vehicle or 5 mg/kg of GM6.
Fig 2. Effects of GM6 on infarct volumes in the mouse following transient ischemia.
All mice were subjected to 1 hour of cerebral ischemia followed by 24 hours of reperfusion. A. Animals were injected with vehicle (control) or GM6 at 1 mg/kg or 5 mg/kg intravenously at the end of the ischemic period. Animals were sacrificed on day 2 and processed to determine the infarct volume. p< 0.0004 for GM6 (1mg/kg) compared to control; p < 0.0001 for GM6 (5mg/kg) compared to control. B. Representative pictures of brains from mice subject to 1 hr ischemia and 24 hr reperfusion. Animals were injected with GM6 (5 mg/kg) (left panel) or vehicle (right panel) at the end of ischemia.
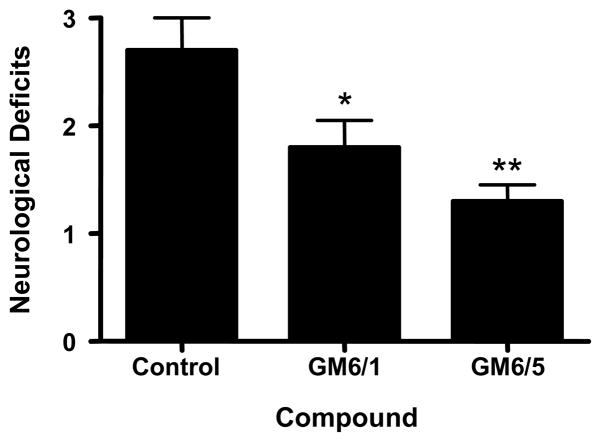
To determine the effect of GM6 on behavioral outcomes in mice following ischemic injury, animals were examined at 22 hrs for neurological deficits (Fig. 3). Animals were assessed for neurological deficits based on a scale of 0 to 4. Animals treated with GM6 showed a dose dependent decrease in neurological deficits (Vehicle, 2.7 ± 0.300; GM6 at 1 mg/kg, 1.8 ± 0.249; GM6 at 5 mg/kg, 1.3 ± 0.153). There were no significant differences in physiological parameters (mean arterial pressure, blood pO2, pCO2, and pH) between the vehicle and treated mice at baseline, during ischemia, or after reperfusion. A significant increase over vehicle group in cerebral blood flow after reperfusion was observed in the group treated with 5 mg/kg of GM6 (p<0.003) (Table 1).
Fig 3. Neurological deficits in mice subjected to GM6 treatment.
All mice were subjected to 1 hour of cerebral ischemia followed by 24 hours of reperfusion. Animals were injected with vehicle (control) or GM6 at 1 mg/kg or 5 mg/kg intravenously at the end of the ischemic period. Neurological deficits were measured at the end of reperfusion (22 hrs) as outlined in the Experimental Methods. *p <0.04 for GM6 (1mg/kg) compared to control; **p < 0.0006 for GM6 (5mg/kg) compared to control.
Table 1.
Cerebral blood flow after ischemic injury in GM6 treated mice.
| Compound (Group) | CBF after reperfusion | P value compare to vehicle |
|---|---|---|
| Vehicle | 84.9 ± 1.567 | |
| GM6 (1) | 89.6 ± 1.893 | P<0.07 |
| GM6 (5) | 91.2 ± 0.987 | P<0.003 |
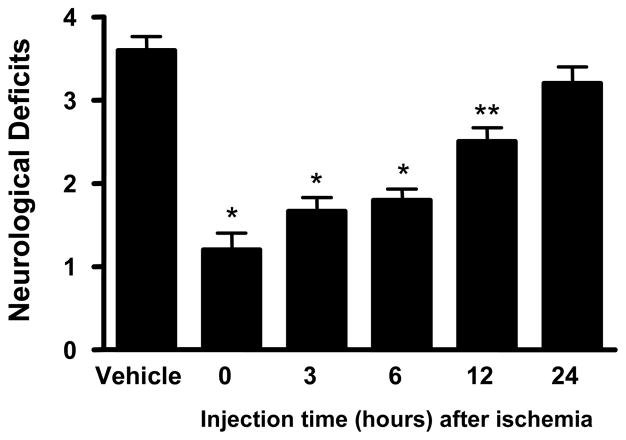
2.3. Time course of GM6 on ischemic injury
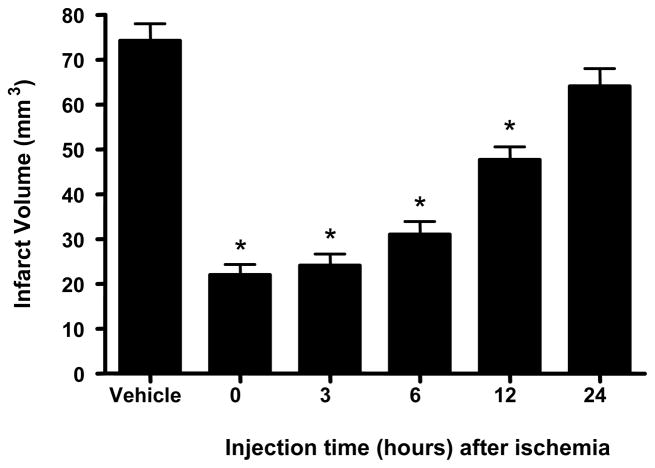
To determine the time course of protection afforded by GM6 in the mouse model of ischemia and reperfusion injury, 5 mg/kg of GM6 was selected because of the effect seen in the dosing studies. For these studies, mice were subjected to 1 hr of ischemia and 14 days of reperfusion for assessment. Animals were treated with GM6 at 0, 3, 6, 12 and 24 hrs after the end of the ischemic period and examined for infarct volumes and behavioral deficits (Figs. 4 and 5). Post ischemia i.v. administration of GM6 reduced the infarct volume in the animal brains (Fig. 4). GM6 administered initially at 0, 3, 6 and 12 hours after ischemia showed 70, 68, 58 and 36% reduction in lesion area, respectively. The infarct volume of the animals treated at 24 hours after ischemia, although decreased, did not show a significant difference from the vehicle treated animals.
Fig 4. Effects of GM6 on infarct volumes with various treatment times.
Mice were subjected to 1 hour of cerebral ischemia followed by 14 days of reperfusion. Animals were injected with vehicle (control) or GM6 at 5 mg/kg intravenously at various times after the end of the ischemic period. Animals were sacrificed on day 14 and processed to determine the infarct volume. *p<0.0001 for all groups compared to control except for t=24 h, p<0.0821.
Fig 5. Neurological deficits in mice subjected to time course of GM6 treatment.
All mice were subjected to 1 hour of cerebral ischemia followed by 14 days of reperfusion. Animals were injected with vehicle (control) or GM6 at 5 mg/kg intravenously at various times after the end of the ischemic period. Neurological deficits were measured at the end of reperfusion as outlined in the Experimental Methods. *p<0.0001 for all groups compared to control except for t=12 h (**p<0.0002) and t=24 h (p<0.1387).
Animals were assessed for neurological deficits based on a scale of 0 to 4. The neurological deficits for vehicle, or treated with GM6 at 0, 3, 6, 12 and 24 hours after ischemia were 3.6, 1.2, 1.67, 1.8, 2.5 and 3.2 respectively. Animals treated with GM6 showed a treatment time dependent decrease in neurological deficits (Fig. 5), with p<0.0001 for all treated groups compared to control except for t=12 h (p<0.0002) and t=24 h (p<0.1387). There were no significant differences in physiological parameters (mean arterial pressure, blood pO2, pCO2, and pH) between the vehicle and treated mice at baseline, during ischemia, or after reperfusion. However, GM6-mediated increase in cerebral blood flow after reperfusion was observed in the groups initially treated with 5 mg/kg of GM6 at 0 hr and 3 hr after ischemia compared to control (Table 2).
Table 2.
Cerebral blood flow after ischemic injury in mice treated with GM6 at various times after the ischemic period.
| Compound (Group) | CBF after reperfusion | P value compare to vehicle |
|---|---|---|
| Vehicle | 82.5 | |
| GM6 (0 hr) | 92.5 | p<0.0001 |
| GM6 (3 hr) | 87.3 | p<0.044 |
2.4. Effect of GM6 on neurogenesis, apoptosis and inflammation
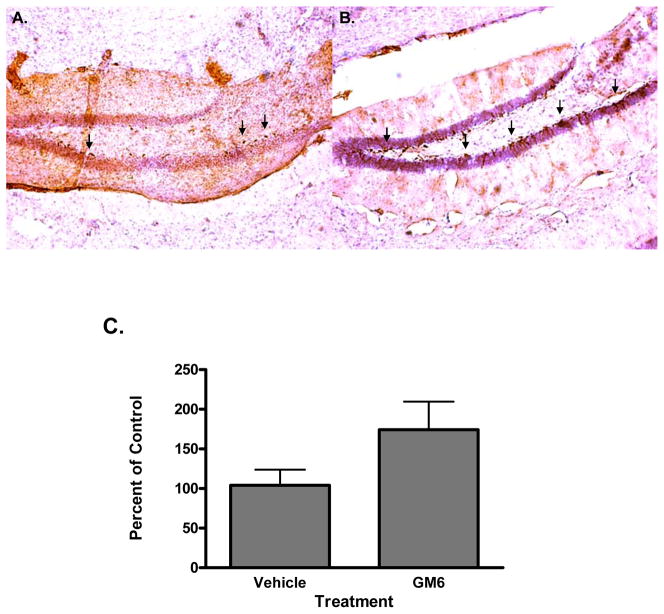
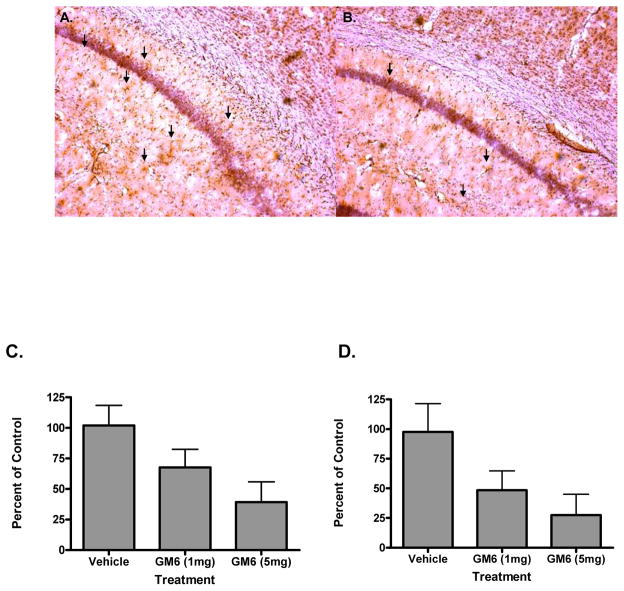
In an attempt to determine the mechanism of action of GM6 in the mouse brain following ischemic injury, animals were examined for neurogenesis, apoptosis and inflammatory markers. To study the effect of tGM6 on cell proliferation and neurogenesis, we identified proliferating cells by labeling with bromodeoxyuridine (BrdU). BrdU cells were detected in the hippocampus and dentate gyrus as well as in the cortex (Fig. 6A). Double labeling experiments with BrdU and doublecortin (DCX) indicated that the cells were neuronal in nature and mature neurons by staining with the neuron specific marker NeuN (Fig. 6B and data not shown). Figure 6C shows the quantitative assessment of the neurogenesis induced by GM6. The effect of GM 6 on cell death and inflammation were validated by measurement of apoptosis (TUNEL assay) and glial fibrillary acidic protein (GFAP) levels (Fig. 7). Both apoptosis and GFAP levels were attenuated in the GM6 treated animals in a dose dependent fashion (Fig. 7A and B). Quantitative analysis demonstrated a significant decrease in both following ischemia/reperfusion injury (Fig. 7C and D).
Fig 6. Effect of GM6 on neurogenesis.
Mice were subjected to 1 hr of ischemia followed by 14 days of reperfusion. Animals were injected with BrdU on day 7 for 3 days and examined for neurogenesis on day 14 of reperfusion. A. Tissue sections were immunostained with antibodies directed against BrdU. B. Tissue sections were immunostained with antibodies directed against NeuN. C. Quantitative analysis of neurogenesis in ischemic brain. *p<0.0001 for all groups compared to controls.
Fig 7. Effect of GM6 on apoptosis and inflammation.
Mice were subjected to 1 hr of ischemia followed by 24 hrs of reperfusion. A. Animals were examined at 24 hours of reperfusion for apoptosis using a TUNEL assay. B. Animals were examined at 24 hrs for the astroglial inflammatory marker GFAP. C. Quantitative analysis of apoptosis in the ischemic brain. D. Quantitative analysis of GFAP staining in the ischemic brain. *p<0.0001 for all groups compared to controls.
3. Discussion
Stroke is the third most common cause of death and the main cause of disability in the United States (American Heart Association, 2006). The outcome and infarction size after focal cerebral ischemia is determined by both “necrotic” cell death and by delayed neuronal cell loss in the borderzone of ischemia (programmed cell death or apoptosis) (Ekshyyan and Aw, 2004). Recent therapies have emerged to treat ischemic stroke. However, these treatments mostly dealt with dissolving the blood clot but did not address neuroprotection, reduction of behavioral deficit or brain infarct volume once the neuronal cell death cycle has been triggered (Grupper et al., 2007). Past and current neuroprotective strategies have been successful in animal models but have failed significantly in clinical trials (Young et al., 2007; Arumugam et al., 2008). Understanding the basic mechanisms that influence cell loss will help in the design of drugs and applications to reduce cell death associated with ischemic injury.
MNTF is a trophic factor that may provide protection from neurological diseases and allow for regeneration of neuronal tissue following injury (Zhou et al., 1993; Zhou et al., 1997). Recent studies have suggested that MNTF may be essential in the differentiation of embryonic stem (ES) cells into motor neurons (Zhou et al., 1994; Di et al., 1997; Di et al., 1998). Because of the expression pattern of MNTF during injury, its importance in neuronal protection and in differentiation of ES cells, we decided to test the efficacy of GM6, a six amino acid analog of MNTF, in an animal model of stroke. We showed that systemic administration of GM6 was capable of penetrating the blood brain barrier with reasonable efficiency. Subsequent studies performed here demonstrated the ability of GM6 to protect the brain from the detrimental effects of cerebral ischemia and reperfusion injury in an effective and efficient way. Intravenous administration of GM6 at 1 and 5 mg/kg single bolus dose demonstrated a dose dependent protective effect in the brain against ischemia/reperfusion injury by a decrease in infarct volume, improved behavioral attributes, and an increase in cerebral blood flow. In addition, we demonstrated that GM6 had a broad window of opportunity in the protection from ischemic injury. This may be due to several possibilities. First, GM6 showed an increase in cerebral blood flow following reperfusion suggesting it might be able to enhance reperfusion of the brain. This has been suggested in the endothelial nitric oxide synthase (eNOS) deficient mice that vascular nitric oxide (NO) production may be important for preservation of vascular function (Huang et al., 1996). Second, the effect of various MNTF analogs on ES cells suggests that MNTF may enhance neurogenesis in the brain following ischemic injury (Wang et al., 2007). Further studies are required to determine the mechanisms associated with GM6 and/or MNTF action in ischemic/reperfusion injury.
The rate of increase in cell proliferation that we find in the GM6 treated animals was greater than that seen in other models of neurogenesis (Sivilia et al., 2008). This suggests that GM6 helps not only to initiate but promote neural stem cells to proliferate and differentiate into neuronal cells. The increase is significantly greater than the vehicle treated animals. As suggested previously, the role of astrocytes in the inhibition of neurogenesis may be important and the reduction in glial response by GM6 may help to provide the niche necessary for new neuronal growth (Wachs et al., 2006). The underlying molecular and cellular mechanisms are still unknown however suggest that the neurotrophic factor basis to GM6 and MNTF may be involved in the potent proliferative and differentiation activity (Chau, 2007). One concern is that Brdu stains for a multitude of cell proliferation and may not necessarily indicate neurogenesis (Liu et al., 1998). However, it does indicate an increase in proliferation in the brain following injury. In addition, the increase in NeuN staining in the presence of GM6 suggests that at least some of the cells differentiate into neuronal like cells. The unique capabilities of GM6 to differentiate neural stem cells may provide a novel mechanism for treating ischemia and reperfusion injury. Not only can GM6 protect against neuronal injury but the induction of differentiation helps to stimulate recovery and repair to a greater extent.
Here we show that animals infused with GM6 demonstrated a reduction in apoptosis and inflammation. We found that in ischemia and reperfusion injury, apoptotic cells and intercellular apoptotic fragments were detectable at the border of the lesion in both vehicle and GM6 treated mice, and that GM6 reduced the amount of apoptotic cells. Cerebral ischemia results in necrotic cell death in the core of the infarct, and this is surrounded by apoptotic cells in the penumbra region (Rami et al., 2008). GM6 appeared to limit the extent of apoptotic cell death indicating that it provided neuroprotection. The preclusion of cell death paralleled the behavioral changes. Cerebral ischemia promotes apoptosis of neurons, oligodendrocytes, and astrocytes (Petito et al., 1998). Cell apoptosis begins as early as 4 hr after the trauma and peaks at 1–2 days post-injury but can continue to develop over time (Borsello and Forloni, 2007). Cytokine and inflammatory molecules have been shown to increase dramatically after ischemia, suggesting that they are involved in the apoptotic process (Rodríguez-Yáñez and Castillo, 2008). Studies have shown that cytokines (TNF-α, IL-1, IL-2, etc) and inflammatory mediators (reactive oxygen species, ROS) can contribute to the infarct volume (Rothwell and Luheshi, 1996; Wong and Crack, 2008). In addition, attenuation of inflammation can restrict the development of the lesion (Khan et al., 2004). We have shown that GFAP is dramatically elevated following ischemia and reperfusion injury (Kindy et al., 1992). In the acute period after ischemia, cytokines can also induce the expression of endothelial leukocyte adhesion molecules, which can activate neutrophils adhering to endothelial cells and damage the endothelium by releasing inflammatory mediators such as neutrophil elastase and reactive oxygen species (ROS) (Ducruet et al., 2008). The damage of endothelial cells leads to further ischemia by increasing the vascular permeability and decreasing the tissue blood flow (Kahles et al., 2007). Our data suggest that GM6 reduced the levels of apoptosis and inflammation in a dose dependent fashion and resulted in a reduction in lesion volume and improved physical outcome.
Our data establishes MNTF as a major factor in the pathogenesis of stroke. Alterations in MNTF expression or activity may affect the development and progression of stroke, and therapies to enhance MNTF expression or to provide MNTF following ischemic injury may attenuate the expression of the disease. These studies lay the groundwork for future studies to determine the beneficial effects of GM6 in stroke and other neurodegenerative disorders.
4. Experimental procedures
4.1. Animals
C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME), weighing 22–25 grams each were given free access to food and water before the experiment.
4.2. Experimental Groups
4.2.1. Blood brain barrier penetration
Animals were injected with a bolus i.v. via tail vein with vehicle or GM6 at 0.2 or 2 mg/kg. After 4 hrs, animals were perfused with cold PBS and then the cortex was examined for GM6 in the brain via ELISA. Four hrs was chosen as maximal accumulated dose of GM6 in preliminary studies (data not shown).
4.2.2. Dose effect of GM6
Animals were subjected to 1.0 h ischemia followed by 24 h reperfusion. Animals were randomly assigned to a vehicle group (n=10) or groups (n=10) treated with an intravenous injection of a 6-mer active component of MNTF (GM6) at a dose of 1 or 5 mg/kg. Formulation of GM6 (CS Bio Co., Menlo Park, CA, Catalog# CS1507, GMP013, lot C811) was performed by reconstituting GM6 with normal saline solution that was stored at 4°C. Vehicle control received phosphate buffered saline (PBS) solution. The bolus IV injections via tail vein were given immediately after the onset of reperfusion or at various times after reperfusion.
4.2.3. Time course of GM6 effect
Animals were subjected to 1.0 h ischemia followed by 14 days reperfusion. Animals were randomly assigned to a vehicle group (n=10) or one of the five treatment groups (n=10 for each group) that would receive an initial intravenous injection of GM6 at a dose of 5 mg/kg at 0, 3, 6, 12 or 24 hours after ischemia. Vehicle control received saline solution. The bolus i.v. injection of GM6 via tail vein was initiated in the different treatment groups at 0, 3, 6, 12, or 24 hours after the start of reperfusion and subsequently one additional dose of GM6 was i.v. injected every day for the next 3 days in all treatment groups. The investigators were blinded to the treatment groups.
4.3. Induction of Ischemia
The animals were anesthetized with halothane (1% in 70%/30% NO2/O2 by mask). Monitoring of mean arterial blood pressure (MABP) via tail cuff apparatus, and blood samples were collected to determine arterial pH levels and PaCO2 and PaO2. The MABP and heart rate were recorded using a Visitech System blood pressure monitor. Brain temperature was monitored using a rectal thermometer and thermistor probe inserted into the temporalis muscle. The animals’ body temperature was maintained at 37°C by using a water-jacketed heating pad. Brain temperature was monitored for 1 hour prior to ischemia to 6 hours following ischemia and was recorded at 30-minute intervals. Each mouse was anesthetized and the external carotid artery (ECA) and common carotid artery (CCA) were isolated (Gary et al., 1998; Mattson et al., 2000; Dubal et al., 2001; Ellsworth et al., 2003; Ellsworth et al., 2004). The left common carotid artery (CCA) was exposed through a midline incision in the neck. The superior thyroid and occipital arteries were electrocoagulated and divided. A microsurgical clip was placed around the origin of the external carotid artery (ECA). The distal end of the ECA was ligated with 6-0 silk and transected. A 6-0 silk was tied loosely around the ECA stump. The clip was removed and the fire-polished tip of a 5-0 nylon suture (silicone coated) was gently inserted into the ECA stump. The loop of the 6-0 silk was tightened around the stump and the nylon suture was advanced approximately 13 mm (adjusted for body weight) into and through the internal carotid artery (ICA) until it rested in the anterior cerebral artery (ACA), thereby occluding the anterior communicating and middle cerebral arteries. After the nylon suture was in place for 1 hour, it was pulled back into the ECA and the incision closed.
4.4. Histological Examination
For histological examination, the animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) 24 hours after ischemia was induced. The brains were transcardially perfused with 4°C, 10% phosphate-buffered saline (PBS). The brains were removed and chilled for 15 minutes at −20°C before being placed in a Rodent Brain Matrix. Coronal sections (1-mm thickness) were prepared and subjected to 2% triphenyltetrazolium chloride (TTC) staining at 37°C (Mattson et al., 2000). TTC stains live tissue (red) versus dead or dying tissue (white). Seven serial one-mm thick coronal sections through the rostral to caudal extent of the infarction were obtained from each brain, beginning two-mm from the frontal pole. The TTC stained sections were placed in 10% neutral buffered formalin and kept in darkness at 4°C for at least 24 hours. The infarct area in each section was determined with a computer-assisted image analysis system, consisting of a Power Macintosh computer equipped with a Quick Capture frame grabber card, Hitachi CCD camera mounted on an Olympus microscope and camera stand. NIH Image Analysis Software, v. 1.55 was used. The images were captured and the total area of damage determined over the seven sections. A single operator blinded to treatment status performed all measurements. The infarct volume was calculated by summing the infarct volumes of the sections. Infarct size (%) was calculated by using the following formula: (contralateral volume − ipsilateral undamaged volume) × 100/contralateral volume to eliminate effects of oedema.
4.5 ELISA Analysis
GM6 levels in samples were measured using the competitive ELISA kit. Coating buffer containing affinity purified rabbit anti-6Mer at 10μg/ml was coated on ELISA plate. 6Mer-biotin at 1μM (final dilution) was used in the assay. The known concentrations of GM6 (competitor) were used as reference standards in establishing the standard curve starting from 80μM and serially diluted 2-fold down to 0.625μM. Concentration of tested samples was estimated from their OD450 observation based on the standard curve. The brain tissue was prepared in cell lysis buffer containing 100 mM Tris/HCl, pH 7.0, containing 2% BSA, 1 M NaCl, 4 mM EDTA, 2% Triton X-100, 0.1% sodium azide and protease inhibitors (CompleteTM, Mini, Boehringer Mannheim). Homogenates were prepared in 10 volumes of buffer to tissue wet weight. The homogenates were centrifuged for 30 minutes at 14,000xg. The resulting supernatant was used for ELISA analysis with the appropriate volume adjustment. For the assay, the anti-6Mer pAb was diluted to 10μg/ml in ELISA coating buffer. The 96-well micro-titer plates were coated with 100μl/well of the diluted pAb. The plates were covered and kept refrigerated overnight. Next day, the plates were blocked with 200μl/well TBS with 3% BSA, and kept at RT for 60 min. The plates were washed 3X with TBST (TBS + 0.05% Tween 20). For each plate, lanes 1 and 2 were used for the standard curve. 100 μl of 1μM 6Mer-biotin was added to all wells except A1 and A2. 200μl/well of the standard mix of 1μM 6Mer-biotin with 80μM GM6 was added to A1 and A2. A serial 2-fold dilution from wells A1 and A2 down to wells H1 and H2 was performed by taking out 100μl sample from each well and mix it with next well—pipetting up and down at least 8 times. Test samples (brain homogenate) were diluted in Ab dilution buffer and mixed at 1:50 with the 1μM 6Mer-biotin in the wells. The plate was mixed on a shaker at 400rpm for 2 hours at RT. The samples were washed 6X with TBST. Streptavidin-HRP (10ml per plate) solution was prepared by diluting Streptavidin-HRP to 1:2000 in Ab dilution buffer. The plate was mixed on the shaker for another 1 hour at RT. The samples were washed 8X with TBST. 50μl/well substrate (TMBS, Genetel) was added and developed for 5 minutes. The reaction was stopped with 50μl 1M HCl and OD450 was measured immediately.
4.6 Measurement of Cerebral Blood Flow
Cerebral blood flow (CBF) was monitored by using a laser Doppler flowmeter (). The CBF values were determined as a percentage, because the values displayed by the laser Doppler flowmeter were not absolute. As described above, the animals were anesthetized with halothane (1% in 70%/30% NO2/O2 by mask) and were mounted in a stereotaxic frame and the probes were fixed to the head. In the hemisphere ipsilateral to the MCA occlusion, coordinates were as follows: point A, 0.5 mm posterior to the bregma and 2 mm lateral to the midline; point B, 1 mm posterior to the bregma and 1.2 mm lateral to the midline; point D, 1 mm anterior to the bregma and 1.7 mm lateral to the midline; and point C in the contralateral hemisphere, 1 mm posterior to the bregma and 2 mm from the midline. CBF was compared at 15 minutes prior to the onset of ischemia, during ischemia (15 minutes after the start of ischemia) before injection of test articles and at 30 minutes post injection (continuous measurements were taken from 15 minutes prior to ischemia to 30 minutes after the end of injection of the compound). Animals were re-anesthetized and the CBF was measured at 3 hours following reperfusion. The mean values before MCA occlusion were taken as baseline and the data thereafter were expressed as percentages of the baseline value.
4.7 Behavioral Assessment
Behavioral analysis (neurological deficit) was determined in the mice before and after ischemic injury (Dubal et al., 2001). Neurological scores were as follows: 0, normal motor function; 1, flexion of torso and contralateral forelimb when animal was lifted by the tail; 2, circling to the contralateral side when held by tail on flat surface, but normal posture at rest; 3, leaning to the contralateral side at rest; 4, no spontaneous motor activity.
4.8. Analysis of Neurogenesis, Apoptosis and Inflammation
BrdU administration was chosen to identify the lineage of newly generated cells as described previously (Kempermann et al.). Briefly, BrdU was administered intraperitoneally twice daily (75 mg/kg, Sigma, St Louis, MO, USA) for 3 days, and the rats were perfused 7 days after the last BrdU injection. Animals were then sacrificed and examined by immunohistochemistry. Sections were first incubated in 0.1 M PBS at room temperature for 30 min, followed by incubation at 37°C for 30 min with HCl 2 N. After rinsing in PBS for 20 min, the sections were incubated at 37°C for 4 min with pepsin 0.025%. Sections were then processed for BrdU visualization by using rat anti-BrdU diluted in PBS/Triton 0.3%. Cryosections were washed 3 times (5 min/wash) with tris-buffered saline (TBS, pH:7.4) buffer, followed by washing one time with 0.1% Triton-X100-TBS buffer for 5 min. Sections were then incubated in 3% activity. H2O2–TBS buffer for 30 min at room temperature to eliminate endogenous peroxidase Following 1hr blocking with 5.0% serum (goat), the sections were incubated overnight with primary antibodies: GFAP positive astrocytes (2E1; BD Biosciences, San Jose, CA, 1:200 dilution); DCX, (DCX, Santa Cruz Biotechnology, Santa Cruz, CA, 1:200 dilution); NeuN, (NeuN, Chemicon, Temecula, CA, 1:250 dilution). The next day, sections were washed three times (5min/wash) with 0.1% Triton-X100/TBS buffer to remove excess primary antibody. Thereafter, primary antibody was detected using HRP-conjugated rabbit VECTASTAIN ABC kit and DAB/substrate reagents (Burlingame, CA) according to the manufacturer’s instructions. At 24 hr ischemia, the terminal deoxynucleotidyl transferase-mediated dUTP-digoxygenin nick-end labeling (TUNEL) technique was used to detect apoptotic cells (ApopTag® Peroxidase In Situ Apoptosis Detection Kit, Chemicon International, Inc., Temecula, CA, USA). The preparation of tissue cryosections was the same as described above. TUNEL assay was performed according to the manufacturer’s instruction. In brief, tissue sections were fixed in 1% paraformaldehyde for 10 min at room temperature, and then post-fixed in pre-cooled ethanol: acetic acid (2:1) for 5 min at −20°C. Endogenous peroxidase was quenched in 3% H2O2 for 5 min at room temperature. Subsequently, sections were incubated with equilibration buffer for at least 10 seconds at room temperature, followed by incubation with terminal deoxynucleotidyl transferase in a humidified chamber at 37°C for 1 hr. The reaction was stopped by adding in stop/wash buffer for 10 min at room temperature. Sections were then incubated with anti-digoxigenin peroxidase conjugate in a humidified chamber for 30 min at room temperature and, developed with 3, 3′-diaminobenzidine (DAB) for 5 min at room temperature. Sections were counterstained with 0.5% methyl green and cover-slipped. Sections from 5–8 separate animlas were used to assess the number of TUNEL-positive cells.
4.9. Statistical Analysis
The results were expressed as the mean ± standard deviation (SD). The statistical significance of the results in the GM6 level in brain by ELISA, infarct volume, neurological deficit, physiological and histological data were analyzed using a one-way analysis of variance (ANOVA) followed by Fisher’s post hoc test. Repeated-measures ANOVA were computed on the monitoring data and the significance of the difference among groups were evaluated by Fisher’s post hoc test.
Acknowledgments
The authors wish to acknowledge Genervon Biopharmaceuticals for providing the MNTF 6-mer for the studies. We thank Dr. Sebastiano Gattoni-Celli and Dr. Pui-Chu Yu for reviewing the manuscript prior to submission and Eileen McFadden for assistance with preparation of the manuscript. Dorothy Ko hold equity in Genervon Biopharmaceuticals and Dr. Kindy holds equity in Neurological Testing Service, Inc. These studies were funded by Genervon Biopharmaceuticals, Inc.
Abbreviations
- MNTF
motoneuronotrophic factor
- MCAo
middle cerebral artery occlusion
- CBF
cerebral blood flow
- HR
heart rate
- BP
blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Heart Association. Heart Disease and Stroke Statistics — 2006 Update. http://www.americanheart.org/downloadable/heart/1136308648540Statupdate2006.pdf.
- Arumugam TV, Selvaraj PK, Woodruff TM, Mattson MP. Targeting ischemic brain injury with intravenous immunoglobulin. Expert Opin Ther Targets. 2008;12:19–29. doi: 10.1517/14728222.12.1.19. [DOI] [PubMed] [Google Scholar]
- Barde YA. What, if anything, is a neurotrophic factor? TINS. 1988;11:343–346. doi: 10.1016/0166-2236(88)90055-0. [DOI] [PubMed] [Google Scholar]
- Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–86. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- Burek MJ, Oppenheim RW. Programmed cell death in the developing nervous system. Brain Pathol. 1996;6:427–446. doi: 10.1111/j.1750-3639.1996.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Buss RR, Oppenheim RW. Role of programmed cell death in normal neuronal development and function. Anat Sci Int. 2004;79:191–197. doi: 10.1111/j.1447-073x.2004.00088.x. [DOI] [PubMed] [Google Scholar]
- Chau RMW, Wu XY, Zhao LP, Ren F, Jen JS. Neuronotrophic factor. Chin J Neuroanat. 1990;6:129–138. [Google Scholar]
- Chau RMW, Zhao LP, Jen LS. The effect of a 30 kDa protein from tectal extract on cultured retinal ganglion cell. Scient Sin. 1991a;B1:50–53. [Google Scholar]
- Chau RMW, Zhao LP, Jen LS. The effect of a 30 kDa protein from tectal extract on cultured retinal neuron. Sci China. 1991b;34:33–39. [PubMed] [Google Scholar]
- Chau RMW, Ren F, Huang W, Jen LS. Muscle neurotrophic factors specific for anterior horn motoneurons of rat spinal cord. Recent Advances in Cell And Mol Biol. 1992;5:89–94. [Google Scholar]
- Chau RMW. Polynucleotides encoding motoneuronotrophic factors. #6,309,877. US Patent. 2001
- Chau RMW. Methods and use of motoneuronotrophic factors. #6,841,531. US Patent. 2005
- Chau RMW. MNTF peptides and compositions and methods of use. #7,183,373. US Patent. 2007
- Di X, Huang W. Localization and morphometric study on motoneuronotrophic factor 1 and its receptor in developing chorionic villi of human placenta. Acta Anatomica Sinica. 1998;29:86–89. [Google Scholar]
- Di X, Huang WQ, Sun L. Immunohistochemical localization of c-fos p53 protein & MNTF1 receptor in early human placental villi. Acta Anatomica Sinica. 1997;28:404–406. [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducruet AF, Hassid BG, Mack WJ, Sosunov SA, Otten ML, Fusco DJ, Hickman ZL, Kim GH, Komotar RJ, Mocco J, Connolly ES. C3a receptor modulation of granulocyte infiltration after murine focal cerebral ischemia is reperfusion dependent. J Cereb Blood Flow Metab. 2008;28:1048–58. doi: 10.1038/sj.jcbfm.9600608. [DOI] [PubMed] [Google Scholar]
- Edgar D. Nerve growth factors and molecules of the extracellular matrix in neuronal development. J Cell Sci Suppl. 1985;3:107–13. doi: 10.1242/jcs.1985.supplement_3.11. [DOI] [PubMed] [Google Scholar]
- Ekshyyan O, Aw TY. Apoptosis in acute and chronic neurological disorders. Front Biosci. 2004;9:1567–1576. doi: 10.2741/1357. [DOI] [PubMed] [Google Scholar]
- Ellsworth JL, Garcia R, Yu J, Kindy MS. Time window of fibroblast growth factor-18-mediated neuroprotection after occlusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2004;24:114–123. doi: 10.1097/01.WCB.0000100063.36077.CD. [DOI] [PubMed] [Google Scholar]
- Ellsworth JL, Garcia R, Yu J, Kindy MS. Fibroblast growth factor-18 reduced infarct volumes and behavioral deficits after transient occlusion of the middle cerebral artery in rats. Stroke. 2003;34:1507–1512. doi: 10.1161/01.STR.0000071760.66720.5F. [DOI] [PubMed] [Google Scholar]
- Gary DS, Bruce-Keller AJ, Kindy MS, Mattson MP. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J Cereb Blood Flow Metab. 1998;18:1283–1287. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Grupper M, Eran A, Shifrin A. Ischemic stroke, aortic dissection, and thrombolytic therapy--the importance of basic clinical skills. J Gen Intern Med. 2007;22:1370–1372. doi: 10.1007/s11606-007-0269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennigan A, O’Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem Soc Trans. 2007;35:424–427. doi: 10.1042/BST0350424. [DOI] [PubMed] [Google Scholar]
- Hollyday M, Hamburger V. Reduction in naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976;170:311–320. doi: 10.1002/cne.901700304. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–7. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–6. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, Singh I, Singh AK. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res. 2004;76:519–27. doi: 10.1002/jnr.20087. [DOI] [PubMed] [Google Scholar]
- Kindy MS, Bhat AN, Bhat NR. Transient ischemia stimulates glial fibrillary acid protein and vimentin gene expression in the gerbil neocortex, striatum and hippocampus. Brain Res Mol Brain Res. 1992;13:199–206. doi: 10.1016/0169-328x(92)90027-9. [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K. TGF-β and the regulation of neuron survival and death. J Physiol. 2002;96:25–30. doi: 10.1016/s0928-4257(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Kuno M. Target dependence of motoneuronal survival: the current status. Neurosci Res. 1990;9:155–72. doi: 10.1016/0168-0102(90)90001-u. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. Developmental neurobiology and the natural history of nerve growth factor. Annu Rev Neurosci. 1982;5:341–62. doi: 10.1146/annurev.ne.05.030182.002013. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–78. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Zhu H, Yu J, Kindy MS. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J Neurosci. 2000;20:1358–1364. doi: 10.1523/JNEUROSCI.20-04-01358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaman JL, Crawford FG, Stewart SS, Appel SH. Purification of a skeletal muscle polypeptide which stimulates choline acetyltransferase activity in cultured spinal cord neurons. J Biol Chem. 1988;263:5890–5897. [PubMed] [Google Scholar]
- McManaman JL, Oppenheim RW, Prevette D, Marchetti D. Rescue of motoneuron from cell death by a purified skeletal muscle polypeptide: effects of the ChAT development factor, CDF. Neuron. 1990;9:155–172. doi: 10.1016/0896-6273(90)90142-3. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Neural activity and survival in the developing nervous system. Mol Neurobiol. 2000;22:41–54. doi: 10.1385/MN:22:1-3:041. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Fischbach GD. Modulation of embryonic chick motoneuron glutamate sensitivity by interneurons and agonists. J Neurosci. 1986;6:3290–6. doi: 10.1523/JNEUROSCI.06-11-03290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW, Haverkamp LJ. Neurotrophic interactions in the development of spinal cord motoneurons. Ciba Found Symp. 1988;138:152–71. doi: 10.1002/9780470513675.ch10. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci. 1989;12:252–5. doi: 10.1016/0166-2236(89)90021-0. [DOI] [PubMed] [Google Scholar]
- Petito CK, Olarte JP, Roberts B, Nowak TS, Jr, Pulsinelli WA. Selective glial vulnerability following transient global ischemia in rat brain. J Neuropathol Exp Neurol. 1998;57:231–8. doi: 10.1097/00005072-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Rami A, Bechmann I, Stehle JH. Exploiting endogenous anti-apoptotic proteins for novel therapeutic strategies in cerebral ischemia. Prog Neurobiol. 2008;85:273–96. doi: 10.1016/j.pneurobio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Yáñez M, Castillo J. Role of inflammatory markers in brain ischemia. Curr Opin Neurol. 2008;21:353–7. doi: 10.1097/WCO.0b013e3282ffafbf. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi GN. Brain TNF: damage limitation or damaged reputation? Nat Med. 1996;2:746–7. doi: 10.1038/nm0796-746. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Pei G, Beck M, Schweizer U, Wiese S. Developmental motoneuron cell death and neurotrophic factors. Cell Tissue Res. 2000;301:71–84. doi: 10.1007/s004410000217. [DOI] [PubMed] [Google Scholar]
- Sivilia S, Giuliani A, Del Vecchio G, Giardino L, Calza L. Age-dependent impairment of hippocampal neurogenesis in chronic cerebral hypoperfusion. Neuropath Appl Neurobiol. 2008;34:52–61. doi: 10.1111/j.1365-2990.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- Smith RG, Vaca K, McManaman J, Appel SH. Selective effects of skeletal muscle extract fractions on motoneuron development in vitro. J Neurosci. 1986;6:439–47. doi: 10.1523/JNEUROSCI.06-02-00439.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H, Edgar D. Neurotrophic factors. Science. 1985;229:238–42. doi: 10.1126/science.2409599. [DOI] [PubMed] [Google Scholar]
- Varon S, Manthorpe M, Davis GE, Williams LR, Skaper SD. Growth factors. Adv Neurol. 1988;47:493–521. [PubMed] [Google Scholar]
- Wachs FP, Winner B, Couillard-Despres S, Schiller T, Aigner R, Winkler J, Bogdahn U, Aigner L. Transforming growth factor-beta1 is a negative modulator of adult neurogenesis. J Neuropathol Exp Neurol. 2006;65:358–370. doi: 10.1097/01.jnen.0000218444.53405.f0. [DOI] [PubMed] [Google Scholar]
- Wang AM, Chau RMW, Chow SP, Zhang ZY, Li ZM. Effects of myogenic 22 and 35kD neurotrophic factors on axonal regeneration in free peripheral autografts into rat spinal cord. Chinese Journal of Spine and Spinal Cord. 1995;5:248–252. [Google Scholar]
- Wang Y, Jin K, Greenberg DA. Neurogenesis associated with endothelin-induced cortical infarction in the mouse. Brain Res. 2007;1167:118–122. doi: 10.1016/j.brainres.2007.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15:1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- Young AR, Ali C, Duretête A, Vivien D. Neuroprotection and stroke: time for a compromise. J Neurochem. 2007;103:1302–1309. doi: 10.1111/j.1471-4159.2007.04866.x. [DOI] [PubMed] [Google Scholar]
- Zhou MH, Wu XY, Ren F, Zhao LP, Huang WQ, Yang ZY, Ren LS. Effect of 22kD, 35kD molecules from skeletal muscle extract on ant. Horn motoneuron of lumbar spine in rat. Chinese Science Bulletin. 1992;37:1742–1745. [Google Scholar]
- Zhou MH, Yu WH, Reb F. Changes in MNTF and its receptor in tongue muscle post-denervation of the hypoglossal nerve. Acta Anatomica Sinica. 1993;24:391–395. [Google Scholar]
- Zhou MH, et al. Immunohistochemical localization of Motoneuronotrophic factor in fetal and neonatal rats. Acta Anatomica Sinica. 1994;25:189–192. [Google Scholar]
- Zhou MH, Wu X, Chen S. Distribution of MNTF1 in spinal cord and limb muscles of mice with motoneuron disease. Acta Academiae Medicinae Sinicae. 1997;19:171–178. [PubMed] [Google Scholar]