Abstract
Background
α-1-antitrypsin (A1AT) deficiency results from a genetic disorder at two common loci. Diagnosis requires quantitation of A1AT and subsequent identification of the specific variant. The current algorithm of laboratory testing for the diagnosis of A1AT deficiency uses a combination of quantitation (nephelometry), genotyping and/or phenotyping. We developed a novel multiple reaction monitoring LC-MS/MS method for simultaneous quantitation of A1AT and the identification of the two most common deficiency alleles present in 95% of the patients with A1AT deficiency.
Method
Serum samples (n=40) were digested with trypsin and appropriate 13C/15N-labeled standard peptides added. LC-MS/MS analysis was performed with a 0.5×150 mm C18 column and H2O:acetonitrile:n-propanol (A:98/1/1/0.2 and B:10/80/10/0.2; 12 μL/min) mobile phase in positive ion mode on a TSQ Quantum triple quadrupole MS system. The A1AT concentration was obtained by comparison to a calibration curve and the phenotype by the presence or absence of variant peptides. Results were compared to the current phenotyping assay by isoelectric focusing (IEF) and the immunonephelometry quantitative assay.
Results
For the A1AT allele detection, in 39 of 40 samples, the LC-MS/MS results were identical to those obtained by IEF gel electrophoresis. The single discrepant result was rerun by IEF at a lower dilution and the results were in concordance. The A1AT quantitation by LC-MS/MS also compared favorably with nephelometry.
Conclusion
This LC-MS/MS method correlates well with current phenotyping and nephelometric assays. It is a promising method with the potential to improve the laboratory diagnosis of genetic A1AT deficiency.
Keywords: α-1-antitrypsin deficiency, multiple reaction monitoring (MRM), mass spectrometry, LC-MS/MS, proteotypic peptides, IEF alternative
Introduction
Alpha-1-antitrypsin (A1AT) is a member of the serine protease inhibitor (SERPIN) family. A1AT is produced in the liver and transported to the lungs, where it functions to inhibit neutrophil elastase (1). Following binding, elastase cleaves a reactive loop of A1AT resulting in a portion of A1AT inserting into the β-sheet structure of elastase. A1AT is then trapped against elastase leading to lysosomal degradation of the A1AT/elastase complex (2–5).
A1AT is a relatively polymorphic gene, with over 100 polymorphisms identified (6). These mutations result in decreased plasma concentrations of A1AT (7). A lack of functional A1AT leads to uncontrolled proteolytic tissue damage mediated by elastase (8), a disorder known as A1AT deficiency. The two most common disease-associated alleles found in patients with genetic A1AT deficiency are the S and Z alleles. The S allele contains a substitution of valine for glutamate at codon 288 (E288V), resulting in production of an unstable transcript and decreased protein expression (9, 10). Individuals who are homozygous for the S allele are at risk for developing chronic obstructive pulmonary disease (COPD) due to proteolytic damage of the lung tissue. The Z variation consists of a glutamate-to-lysine substitution at codon 366 (E366K) (11). This mutation causes a conformational change such that A1AT polymers form within the hepatocytes. The polymerized A1AT is not efficiently secreted, leading to a decreased circulating concentration and an increased risk for COPD, similar to the S allele (11). The polymerization of the Z variant, however, also damages the hepatocyte (12). Individuals who are Z/Z homozygotes may also develop liver cirrhosis, possibly requiring transplantation (13).
The diagnosis of A1AT deficiency requires quantitation of circulating A1AT and confirmation of the presence of A1AT disease alleles. Quantitation may be accomplished by a variety of methods, although the most commonly-used technique is immunonephelometry and more recently antitryptic activity(14). Identification of the deficient alleles can be performed by two methodologies referred to as phenotyping and genotyping. Phenotyping uses isoelectric focusing (IEF) gel electrophoresis to separate the serum proteins based on charge. The specific A1AT alleles are identified based on migration patterns within the gel (15, 16). In contrast, genotyping uses molecular approaches to identify the mutations at the DNA level (17, 18). Some genotyping techniques, including allele-specific amplification and melting-curve analysis, detect only the S and Z alleles, while others, such as sequencing, have the ability to identify any mutation, although this is usually restricted to exons. The various phenotyping and genotyping assays have advantages and disadvantages. It is for this reason that most algorithms proposed for the diagnosis of A1AT deficiency recommend a combination of laboratory tests (19). Our laboratory has proposed an algorithm in which genotyping and A1AT quantification are performed as the first level of testing, with phenotyping used as a reflex test in cases where the A1AT concentration does not fall into the range expected for the given genotype (19). Given the various pieces of information required to make a diagnosis of A1AT deficiency, a multiplex assay capable of simultaneously quantitating A1AT and identifying the deficiency alleles would be a substantial improvement over the current laboratory testing paradigm.
Mass spectrometry (MS) is emerging as a powerful tool for the identification and quantification of human plasma proteins. Transferrin (carbohydrate deficient glycoprotein syndrome) and transthyretin (familial amyloidosis) provide good examples of MS applied to intact protein analysis (20, 21). Other groups have shown that peptides from protease digestions can be utilized to quantitate proteins utilizing LC-MS/MS (22–25). We have applied this approach for the simultaneous determination of A1AT deficiency and detection of S and Z A1AT alleles including the determination of zygosity.
Materials and Methods
Reagents
Research grade ammonium bicarbonate (NH4HCO3), trifluoroethanol (TFE), iodoacetamide (IAA), and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich. Dithiothreitol (DTT) and formic acid were purchased from Fluka. Fetal calf serum (FCS) was purchased from Invitrogen (Gibco 10437-028). TPCK treated trypsin (T-1426) was purchased from Sigma-Aldrich. Zwittergent detergent 3-16 (Z 3-16) was purchased from Calbiochem. Highly purified α-1-antitrypsin (A1AT) reference material was purchased from Athens Research & Technology (Cat # 16-16-011609, Athens, GA). Water, acetonitrile, n-propanol, and dimethylformamide (DMF) were HPLC grade. Isotopically labeled peptide standards were synthesized in the Mayo Proteomics Core and purity found by analytical HPLC to be ≥90%. Designations and labeling strategy is shown in Table 1. Stock peptide solutions were prepared in DMF at 1mM concentrations. A molecular weight of 52 kDa was utilized in all A1AT calculations(26). An extinction coefficient for A1AT of 4.33 (A280, 1%, 1cm) was utilized for quantitation of the protein standard (27).
Table 1.
Peptide sequences, labeling and MS/MS transitions monitored in the LC-MS/MS experiment. All peptides were synthesized in unlabeled and labeled forms by incorporation of a single 13C615N-leucine at the positions noted by bold underlined type in the Table.
| Peptide | Formulation | Designation | Sequence | Transition m/z |
|---|---|---|---|---|
| Proteotypic | Unlabeled | Proteotypic | SASLHLPK | 426.8 → 694.4 |
| Labeled | Proteotypic* | SASLHLPK | 429.5 → 700.2 | |
|
| ||||
| Wild Type Z | Unlabeled | WT-Z | AVLTIDEK | 444.8 → 718.4 |
| Labeled | WT-Z* | AVLTIDEK | 448.4 → 725.5 | |
|
| ||||
| Mutant Z | Unlabeled | Mu-Z | AVLTIDKK | 444.3 → 717.5 |
| Labeled | Mu-Z* | AVLTIDKK | 447.5 → 723.4 | |
|
| ||||
| Wild Type S | Unlabeled | WT-S | LQHLENELTHDIITK | 602.0 → 781.9 |
| Labeled | WT-S* | LQHLENELTHDIITK | 604.7 → 785.7 | |
|
| ||||
| Mutant S | Unlabeled | Mu-S | LQHLVNELTHDIITK | 592.0 → 766.9 |
| Labeled | Mu-S* | LQHLVNELTHDIITK | 594.5 → 770.5 | |
Trypsin Digestion Protocols
Lyophilized purified human A1AT was dissolved in water or FCS at appropriate concentrations. 5 μL of the A1AT solution (or 5 μL serum) was denatured with 25 μL TFE, diluted with 25 μL 100 mM NH4HCO3 after which 5 μL of 200 mM DTT was added and incubated for 30 min at 55ºC. The reduced samples were treated with 10 μL of 200 mM IAA and incubated for one hour at room temperature in the dark with shaking. The samples were diluted with 400 μL of 100 mM NH4HCO3 and treated with 25 μL of 1 g/L trypsin (~1:20 weight:weight ratio), and incubated for exactly six hours at 37ºC. Digestion was terminated with 10 μL of 10% formic acid (Fluka) after six hours. The prepared samples were stored at −20ºC prior to analysis.
Identification of signature tryptic A1AT peptides
The pure A1AT digest was analyzed by LC-MS/MS and signature peptides that contained the S and Z allele mutations were identified (see Supplemental Data Materials for LC-MS/MS conditions). For the S (E287V, LQHLVNELTHDIITK) and Z (E365K, AVLTIDKK) alleles, the choice of peptides was dictated by the location of the mutations. In addition, a proteotypic peptide (SASLHLPK) was selected for the quantitation of A1AT. A proteotypic peptide is a peptide that is unique to a single protein. SASLHLPK was selected because it does not contain any known polymorphisms, has an amino acid sequence specific to A1AT, does not contain any glycosylation sites and fragments readily to provide a good product ion for LC-MS/MS analysis. Fragment ions identified from the LC-MS/MS data of these three peptides determined the optimal transition states for A1AT quantitation and the S and Z variant analysis. All selected peptides were synthesized as unlabeled and 13C615N-leucine labeled for use as internal standards.
Study population
Serum samples (n=40) were obtained from individuals referred to the Mayo Clinic Immunology Laboratory for evaluation of possible A1AT deficiency. All samples were submitted for A1AT quantification and phenotyping. All samples were analyzed under an approved IRB.
LC-MS/MS analysis of patient serum samples
Serum was digested according to the method described above for purified A1AT substituting 5 μL serum for the 5 μL of purified A1AT. After digestion, dilutions of labeled peptides (designated with *) were added into the digest mixture for final concentrations of 2 μM (proteotypic*), 0.5 μM (Mu-Z*, WT-Z*), and 1 μM (Mu-S*, WT-S*). 2 μL was loaded onto the column and analyzed by LC-MS/MS as described in the Supplemental Data Materials.
Identification of S and Z A1AT alleles was determined by comparison of the response to labeled peptides which were used to authenticate retention times and instrument response.
Stock solutions of A1AT were obtained by dissolving highly purified lyophilized A1AT from Athens Research & Technology in FCS at 1.0-77μM. After digestion and LC-MS/MS analysis area ratios for the unlabeled and labeled proteotypic peptides were calculated. Calibration curves were constructed by plotting the peak area ratio against the A1AT concentration. Samples were bracketed by calibration curves pre- and post-samples. Intra-assay and inter-assay imprecision was determined by analyzing three samples at low, medium and high A1AT concentrations ten times each over four days. The LOQ was determined by serial dilutions of a standard solution. Six replicates at each dilution were measured and the lowest concentration giving a CV ≤10% was utilized for the LOQ.
Identification of S and Z A1AT alleles by phenotyping
Phenotyping of A1AT was performed using the Hydragel A1AT Isofocusing system (Sebia) according to manufacturer’s instructions. Briefly, serum proteins were separated by isoelectric focusing on agarose gels. A1AT was visualized by immunofixation using peroxidase-labeled A1AT anti-sera.
A1AT quantitation by nephelometry
A1AT was quantitated by nephelometry on a Behring Nephelometer II (Dade Behring, Inc) using the commercially available reagents and standards (Siemens; A1AT Reagent #PSAZ15 and Calibrator/Standard #OQIM15). All assays were performed according to manufacturer’s instructions.
Results
Phenotyping A1AT variants by mass spectrometry
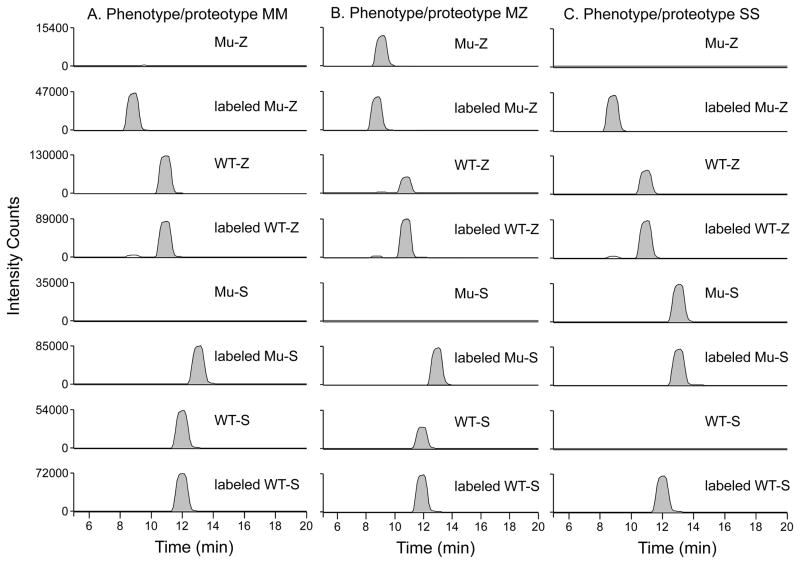
Supplemental Figure 1 indicates the sequence of A1AT and the tryptic peptides containing the E288V (S variant, designated Mu-S) and E366K substitutions (Z variant; Mu-Z). The corresponding wild-type peptides are designated WT-S and WT-Z respectively and indicate the M allele. The variant peptides, by virtue of the amino acid substitution, differ in mass and retention time from the wild-type peptides thereby allowing MS detection. The transitions monitored for each peptide and internal standard peptides are shown in Table 1. Figure 1 shows example chromatograms from control (M/M), M/Z and S/S phenotypes. In a normal individual only the wild-type alleles and no Mu-S or Mu-Z peptides are detected (Figure 1A). In contrast, in a patient who is an M/Z heterozygote the WT-Z, WT-S and Mu-Z peptides will be identified but no Mu-S (Figure 1B). An individual heterozygous for M/S shows the Mu-S, WT-S and WT-Z but no Mu-Z (data not shown). For individuals who are S/S homozygotes, only two peptides will be identified: the WT-Z and the Mu-S peptides (Figure 1C). The opposite would be true for a Z/Z homozygote (data not shown). By identifying each of the 4 peptides, we can determine if either of the common S and Z deficiency alleles are present.
Figure 1. Examples of LC-MS/MS chromatographs for various phenotypes.
Prototypic chromatographs are shown for an M/M homozygote (A), an M/Z heterozygote (B), and an S/S homozygote (C). An M/M homozygote is shown in panel (A) and no Mu-Z or Mu-S appears. An M/Z heterozygote in panel (B) shows WT-Z and Mu-Z but no Mu-S. The S/S homozygote is shown in panel (C) and indicates Mu-S but no WT-S or Mu-Z. The specific deficiency allele is easily read from the chromatographs.
As an initial validation of this method, 40 serum samples were analyzed by LC-MS/MS and by IEF gel electrophoresis (phenotyping). Of these 40 samples, 39 showed exact correlation between the allele identification obtained by the two methods (Supplemental Table 1). The single discrepant sample was identified as a Z/Z homozygote by IEF phenotyping, but as an M/Z heterozygote by LC-MS/MS. Further analysis of the LC-MS/MS data revealed that the wild-type M peptide peak was significantly smaller than generally observed in an M/Z heterozygote (data not shown). The sample was re-analyzed by IEF gel electrophoresis at a 1:5 instead of a 1:10 dilution, at which time a band representing the M allele was observed.
Absolute quantitation of A1AT by LC-MS/MS
Supplemental Figure 2 shows a calibration curve of the area ratio of unlabeled/labeled peptide versus μM A1AT using high purity A1AT from Athens Research & Technology (Supplemental Figure 3). Low, medium and high A1AT patient samples were each analyzed ten times on different days to assess the intra-assay and inter-assay imprecision of the LC-MS/MS method. The imprecision results are summarized in Table 2. The intra-assay imprecision (standard deviation*100/mean) was <5% for analysis of ten replicates. The inter-assay imprecision was <5% over a four day period. These values compare well to the nephelometric assay where intra-assay imprecision was 5.4 and 5.0% for 0.22 and 23 μM samples respectively and inter-assay imprecision was 5.3 and 3.2% for 19 and 41 μM samples respectively. The MS-based assay had an LOQ of 1.9 μM. Triplicate analysis of a 9.6 μM A1AT calibrator prepared from the Athens standard in FCS yielded 8.7±0.3μM (mean±SD) when measured by nephelometry indicating that our standard was within 10% of the nephelometry calibrators with a slight negative bias.
Table 2.
Intra- and inter-assay imprecision of the LC-MS/MS assay. Imprecision was determined three times over the course of four days using ten replicates at each concentration.
| Sample | Conc (μM) from Nephelometry | Intra-assay Conc (μM), Mean | Intra-assay Imprecision (%) | Inter-assay Conc (μM), mean | Inter-assay Imprecision (%) |
|---|---|---|---|---|---|
| Patient 1 | 48.8 | 66.1 | 4.3 | 63.6 | 3.1 |
| Patient 2 | 29.2 | 37.9 | 3.4 | 37.9 | 1.4 |
| Patient 3 | 4.6 | 6.4 | 4.1 | 6.3 | 2.6 |
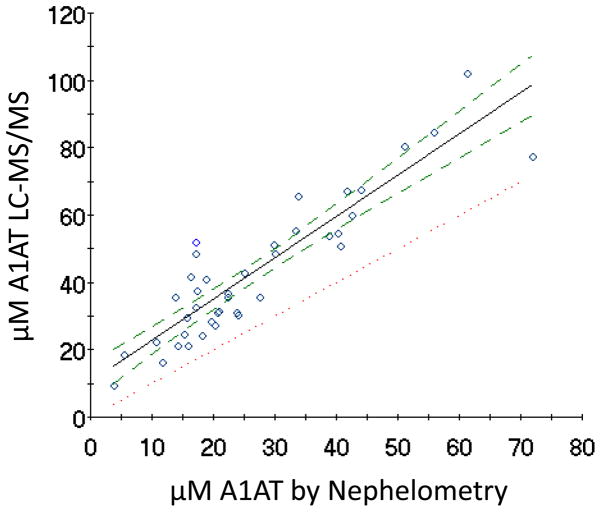
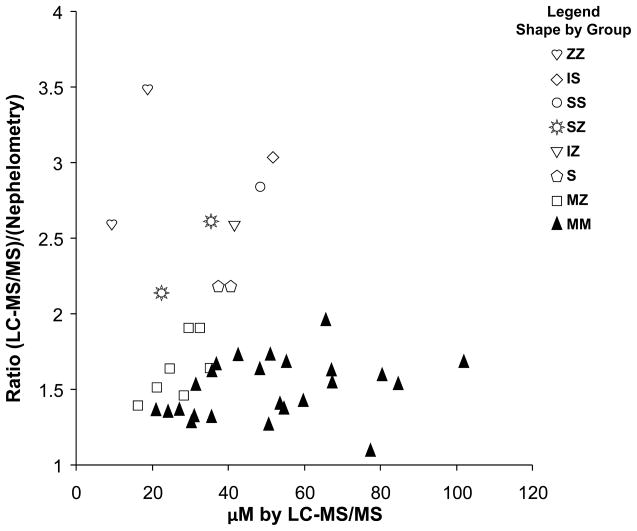
Sera from 40 patients encompassing a range of A1AT concentrations were analyzed by both LC-MS/MS and nephelometry. Figure 2 shows the relationship between the two methods. Figure 3 indicates that a bias exists in the LC-MS/MS method relative to the nephelometry method when the ratio of the two methods is plotted versus μM A1AT. A 1.5±0.2 (mean±SD) fold difference for controls is obtained with an even greater bias for samples containing variants. A Bland-Altman plot comparing the two methods is also illustrated in Supplemental Figure 4.
Figure 2. Comparison of A1AT quantitation between nephelometry and LC-MS/MS.
Ordinary least-squares regression analysis comparing the concentration of A1AT in serum samples (n=40) measured by LC-MS/MS using the proteotypic peptide as described in the Materials and Method section. The results from the LC-MS/MS method were compared to those obtained by nephelometry. The solid line indicates the regression and has a correlation coefficient of 0.91. The dotted line indicates perfect correlation. The dashed lines indicate the 95% confidence limits.
Figure 3.
Bias indicated by the ratio of μM A1AT determined by LC-MS/MS and nephelometry versus μM A1AT determined by LC-MS/MS. The shape indicates phenotype.
Discussion
Repeated attempts to analyze intact A1AT proved unsuccessful, probably due to the fact that A1AT is heavily glycosylated, in contrast to other proteins successfully analyzed in our lab (20, 21). In light of this, we developed a multiplexed LC-MS/MS method for detection of A1AT deficiency and associated allele identification. Our main goal in this work was to illustrate a method for accurately phenotyping A1AT. However, since multiple peptide targets can be analyzed in a MRM experiment A1AT quantitation is essentially free. The allele identification is by detection of the tryptic peptides that contain the S and Z variants and comparison to isotopically labeled internal standard peptides as a reference. For A1AT quantitation peak area ratios of the selected proteotypic peptide and internal standard peptide are compared to a calibration curve generated from authentic protein spiked into FCS at known concentrations. FCS was utilized as a background matrix to simulate human serum as no human serum completely deficient in A1AT is available. Supplemental Figure 5 indicates that no matrix effects results from the use of FCS.
The diagnosis of genetic A1AT deficiency requires more than quantitation alone as circulating concentrations of A1AT, an acute phase protein, may increase during infections, even in individuals with a true deficiency (19, 28). In contrast, concentrations may decrease in the absence of a genetic mutation, such as with an unrelated liver disease or protein-losing enteropathy(19). For these reasons, identification of the deficiency alleles and quantification are both important to establish a diagnosis of genetic A1AT deficiency. Current testing methodologies center on nephelometry for quantitation and phenotyping by IEF gel electrophoresis and DNA-based genotyping for detection of the deficiency alleles. Many laboratories also offer testing algorithms, generally recommending genotyping and quantitation, with follow-up by phenotyping in cases of discrepant results (6). Although well accepted, these testing methodologies and algorithms can be problematic. Most genotyping assays are designed to specifically detect only the S and Z mutations. This is a significant limitation, since other, rare deficiency alleles are not detected. Phenotyping has the advantage that many different alleles, not just Z and S, can be identified as long as the amino acid substitution results in an altered pI. Accurate identification of these variants requires well-characterized reference standards that are not commercially available for the rare deficiency alleles and must be developed and maintained by the individual clinical laboratory. In addition, phenotyping is a labor-intensive assay with relatively subjective interpretation. A comparison of genotyping and phenotyping revealed that approximately 70% of discrepancies between the 2 methodologies were due to phenotyping errors (19). The current diagnostic algorithms combine genotyping and phenotyping in a way to minimize the disadvantages of both methods(19). This, however, is also problematic. A major disadvantage of most algorithms is the requirement for two separate blood samples – whole blood for genotyping and serum for quantitation and phenotyping, if needed. This increases the volume of blood required for testing, which is a critical issue for the pediatric population as Z/Z homozygotes may present with neonatal hepatitis syndrome shortly after birth. The requirement of two samples also significantly increases the chances of a specimen mix-up. In addition, the genotyping assay has a relatively long turn-around-time of 3–4 days. For those samples requiring confirmation by phenotyping, there is an additional time delay, as long as 48 to 72 h, before the result is available to the clinician.
Our LC-MS/MS method for diagnosis of A1AT deficiency offers several advantages over current A1AT laboratory testing. The LC-MS/MS method is similar to the phenotyping assay in the respect that both methods identify the deficiency alleles based on analysis of the circulating A1AT protein. However, the LC-MS/MS method has the advantage of being much less labor-intensive and has the capability to both quantitate and phenotype A1AT in a single 20 min LC-MS/MS assay. This method also eliminates the subjective interpretation that surrounds phenotyping by electrophoresis. Despite these advantages, the LC-MS/MS method, in its current form only detects the common S and Z mutations. However, 95% of all A1AT deficient individuals are homozygous for the Z allele and would be detectable by this methodology (6). The LC-MS/MS method currently identifies M/M, M/S, M/Z, S/S, Z/Z and S/Z genotypes. Although rare, other mutations that result in the clinical disease A1AT deficiency have been described (26). Target peptides corresponding to the other rare deficiency alleles that phenotyping by IEF is capable of identifying will need to be added if the LC-MS/MS method is to completely replace phenotyping. For example, the next most common deficiency allele seen in our practice, the I allele, has an Arg63Cys substitution contained in the tryptic peptide ITPNLAEFAFSLYR(29). This conversion removes a tryptic cleavage site that would result in decreased amounts of this peptide after A1AT trypsin digestion. Additionally, seven known variants reside in the next tryptic peptide QLAHQSNSTNIFFSPVSIATAFAMLSLGTK including L65P, S69F, F75 deletion, S77F, A84T, G91E and T92I. However, this particular peptide and the adjacent tryptic peptide (ADTHDEILEGLNFNLTE IPEAQIHEGFQELLR) contain the known glycosylation sites (Asn70 and Asn170; in bold and underlined). Glycosylated peptides are not easily detected by mass spectrometry without prior removal of the glycosylation. We did not observe any of the tryptic peptides containing the glycosylation sites in our initial proteomics analysis (Supplemental Data Figure 1). Future work entails optimizing the methodology to expand the number of deficiency alleles detected by the LC-MS/MS method.
We initially thought some type of quantitation would be necessary to ascertain the S and Z variants. However, we found that the presence of signal corresponding to the variant peptides was sufficient diagnostically. So although not needed for quantitative purposes, the presence of internal standards serves to indicate only that the LC-MS/MS system is operating correctly. Utilizing Cohen’s kappa coefficient across the six genotypes of Supplemental Table 1 yields a kappa value of 0.96 indicating excellent agreement between IEF phenotyping and the LC-MS/MS phenotyping method (30).
Although in this work only a single transition was monitored for each peptide, future work should include additional transitions for each peptide to ensure specificity. We did not find any interferences in any of our studies utilizing a single transition. This assay has some advantage in this regard since A1AT is one of the 12 most abundant plasma proteins and only a tryptic peptide from another abundant plasma protein is likely to interfere.
The mutant Z peptide we eventually monitored was AVLTIDKK which is a missed cleavage. We monitored both AVLTIDK and AVLTIDKK initially. However the response for the fully tryptic AVLTIDK was much less abundant than the semi-tryptic AVLTIDKK and thus we settled on only looking at the missed cleavage peptide AVLTIDKK. Trypsin missed cleavages adjacent to basic residues is common and has already been noted in the literature(31–34). Our studies yielded similar results.
Although Figure 2 shows a good correlation between nephelometry and the MRM approach a 1.5±0.2 (mean±SD) fold positive bias does exist for control samples with a greater bias for variants (Figure 3). Utilizing a surrogate tryptic peptide for quantitation of intact proteins measures all forms of a protein, both intact and degraded forms. Thus, it is not surprising that the MRM-based approach would routinely measure a higher concentration than an antibody-based method which recognizes a single epitope that is absent in degraded forms of A1AT. The higher bias in the variants can be attributed to the presence of even more degraded A1AT relative to intact A1AT. A calibrator measured by both nephelometry and LC-MS/MS showed a slight negative bias error of 10% on our highly purified A1AT (See Supplemental Figure 3) indicating that the positive bias of the LC-MS/MS method is not due to any systematic error.
The single discrepant sample indicates some of the sensitivity inherent in the LC-MS/MS technique. Although we cannot confirm it, it is likely that the small amount of the M allele that was detected in this sample by LC-MS/MS was due to A1AT replacement therapy, a common treatment given to individuals who are homozygous for the Z allele. In this sample, the M protein was not detectable in the IEF analysis at the usual 1:10 sample dilution but reanalysis at 1:5 dilution showed M was present, confirming the LC-MS/MS results.
In conclusion, the LC-MS/MS method described here provides unambiguous phenotyping of A1AT and simultaneous quantification. As described, it is currently limited to the detection of the S and Z alleles which comprise 95% of all cases. However, we envision phenotyping of other alleles with appropriate modifications. The positive quantification bias we found for A1AT will no doubt exist for other proteins when this approach is compared to antibody based assays.
Supplementary Material
Acknowledgments
The authors wish to Jeanette Eckel-Passow for statistical help, David Barnidge, David Murray and Robin Karras for useful discussions and Diana Ayerhart for help in preparing figures. The Mayo Clinic College of Medicine, the Mayo Clinic Center for Individualized Medicine and the Mayo Clinic Research Committee provided financial support . Y. Chen was supported by a NIH Training Grant in Clinical Pharmacology (T32 GM08685). Yi Zhu was supported by the Robert and Arlene Kogod Center on Aging, Noaber Foundation and the Ted Nash Foundation. This work is also supported by generous gifts from Mr. and Mrs. Gordon C. Gilroy and the David Woods Kemper Memorial Foundation.
Abbreviations
- A1AT
α-1-antitrypsin
- LC-MS/MS
HPLC-tandem mass spectrometry
- MRM
multiple reaction monitoring, see Table 1 for peptide designations
Footnotes
Disclaimer: This manuscript has not been previously published in any format.
This is an un-copyedited authored manuscript copyrighted by The American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of 'Fair Use of Copyrighted Materials' (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article will be made available at http://www.clinchem.org 12 months after its publication in Clinical Chemistry.
References
- 1.Lee WL, Downey GP. Leukocyte elastase: Physiological functions and role in acute lung injury. Am J Respir Crit Care Med. 2001;164:896–904. doi: 10.1164/ajrccm.164.5.2103040. [DOI] [PubMed] [Google Scholar]
- 2.Carrell RW. Cell toxicity and conformational disease. Trends Cell Biol. 2005;15:574–80. doi: 10.1016/j.tcb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency--a model for conformational diseases. N Engl J Med. 2002;346:45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- 4.Lomas DA, Parfrey H. Alpha1-antitrypsin deficiency. 4: Molecular pathophysiology. Thorax. 2004;59:529–35. doi: 10.1136/thx.2003.006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlmutter DH, Joslin G, Nelson P, Schasteen C, Adams SP, Fallon RJ. Endocytosis and degradation of alpha 1-antitrypsin-protease complexes is mediated by the serpin-enzyme complex (sec) receptor. J Biol Chem. 1990;265:16713–6. [PubMed] [Google Scholar]
- 6.Brantly M. Efficient and accurate approaches to the laboratory diagnosis of alpha1-antitrypsin deficiency: The promise of early diagnosis and intervention. Clin Chem. 2006;52:2180–1. doi: 10.1373/clinchem.2006.078907. [DOI] [PubMed] [Google Scholar]
- 7.Brantly M, Nukiwa T, Crystal RG. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988;84:13–31. doi: 10.1016/0002-9343(88)90154-4. [DOI] [PubMed] [Google Scholar]
- 8.Stoller JK. Alpha1-antitrypsin deficiency. Thorax. 2004;59:92–3. doi: 10.1136/thorax.2003.017517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curiel DT, Chytil A, Courtney M, Crystal RG. Serum alpha 1-antitrypsin deficiency associated with the common s-type (glu264----val) mutation results from intracellular degradation of alpha 1-antitrypsin prior to secretion. J Biol Chem. 1989;264:10477–86. [PubMed] [Google Scholar]
- 10.Long GL, Chandra T, Woo SL, Davie EW, Kurachi K. Complete sequence of the cdna for human alpha 1-antitrypsin and the gene for the s variant. Biochemistry. 1984;23:4828–37. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- 11.Nukiwa T, Satoh K, Brantly ML, Ogushi F, Fells GA, Courtney M, Crystal RG. Identification of a second mutation in the protein-coding sequence of the z type alpha 1-antitrypsin gene. J Biol Chem. 1986;261:15989–94. [PubMed] [Google Scholar]
- 12.Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605–7. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 13.Needham M, Stockley RA. Alpha 1-antitrypsin deficiency. 3: Clinical manifestations and natural history. Thorax. 2004;59:441–5. doi: 10.1136/thx.2003.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roche D, Mesner A, Al Nakib M, Leonard F, Beaune P. Automated determination of serum alpha1-antitrypsin by antitryptic activity measurement. Clin Chem. 2009;55:513–8. doi: 10.1373/clinchem.2008.117002. [DOI] [PubMed] [Google Scholar]
- 15.Brantly M. Laboratory diagnosis of alpha-1-antitrypsin deficiency. In: Crystal RG, editor. Alpha-1-antitrypsin deficiency. New York: Marcel Dekker; 1995. pp. 45–60. [Google Scholar]
- 16.Kueppers F. Determination of alpha1-antitrypsin phenotypes by isoelectric focusing in polyacrylamide gels. J Lab Clin Med. 1976;88:151–5. [PubMed] [Google Scholar]
- 17.Braun A, Meyer P, Cleve H, Roscher AA. Rapid and simple diagnosis of the two common alpha 1-proteinase inhibitor deficiency alleles pi*z and pi*s by DNA analysis. Eur J Clin Chem Clin Biochem. 1996;34:761–4. doi: 10.1515/cclm.1996.34.9.761. [DOI] [PubMed] [Google Scholar]
- 18.von Ahsen N, Oellerich M, Schutz E. Use of two reporter dyes without interference in a single-tube rapid-cycle pcr: Alpha(1)-antitrypsin genotyping by multiplex real-time fluorescence pcr with the lightcycler. Clin Chem. 2000;46:156–61. [PubMed] [Google Scholar]
- 19.Snyder MR, Katzmann JA, Butz ML, Wiley C, Yang P, Dawson DB, et al. Diagnosis of alpha-1-antitrypsin deficiency: An algorithm of quantification, genotyping, and phenotyping. Clin Chem. 2006;52:2236–42. doi: 10.1373/clinchem.2006.072991. [DOI] [PubMed] [Google Scholar]
- 20.Bergen HR, 3rd, Zeldenrust SR, Butz ML, Snow DS, Dyck PJ, Dyck PJ, et al. Identification of transthyretin variants by sequential proteomic and genomic analysis. Clin Chem. 2004;50:1544–52. doi: 10.1373/clinchem.2004.033266. [DOI] [PubMed] [Google Scholar]
- 21.Lacey JM, Bergen HR, Magera MJ, Naylor S, O'Brien JF. Rapid determination of transferrin isoforms by immunoaffinity liquid chromatography and electrospray mass spectrometry. Clin Chem. 2001;47:513–8. [PubMed] [Google Scholar]
- 22.Bondar OP, Barnidge DR, Klee EW, Davis BJ, Klee GG. Lc-ms/ms quantification of zn-alpha2 glycoprotein: A potential serum biomarker for prostate cancer. Clin Chem. 2007;53:673–8. doi: 10.1373/clinchem.2006.079681. [DOI] [PubMed] [Google Scholar]
- 23.Carr SA, Anderson L. Protein quantitation through targeted mass spectrometry: The way out of biomarker purgatory? Clin Chem. 2008;54:1749–52. doi: 10.1373/clinchem.2008.114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoofnagle AN, Becker JO, Wener MH, Heinecke JW. Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin Chem. 2008;54:1796–804. doi: 10.1373/clinchem.2008.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hortin GL. A new era in protein quantification in clinical laboratories: Application of liquid chromatography-tandem mass spectrometry. Clin Chem. 2007;53:543–4. doi: 10.1373/clinchem.2006.083857. [DOI] [PubMed] [Google Scholar]
- 26.Cox DW. Alpha-1-antitrypsin deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic & molecular bases of inherited disease. 3. New York, NY: McGraw-Hill, Inc; 1995. pp. 4125–58. [Google Scholar]
- 27.Cowden DI, Fisher GE, Weeks RL. A pilot study comparing the purity, functionality and isoform composition of alpha-1-proteinase inhibitor (human) products. Curr Med Res Opin. 2005;21:877–83. doi: 10.1185/030079905X46395. [DOI] [PubMed] [Google Scholar]
- 28.Bornhorst JA, Procter M, Meadows C, Ashwood ER, Mao R. Evaluation of an integrative diagnostic algorithm for the identification of people at risk for alpha1-antitrypsin deficiency. Am J Clin Pathol. 2007;128:482–90. doi: 10.1309/44J4KBCFQ8E9D1B8. [DOI] [PubMed] [Google Scholar]
- 29.Mahadeva R, Chang WS, Dafforn TR, Oakley DJ, Foreman RC, Calvin J, et al. Heteropolymerization of s, i, and z alpha1-antitrypsin and liver cirrhosis. J Clin Invest. 1999;103:999–1006. doi: 10.1172/JCI4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-David A. Comparison of classification accuracy using cohen's weighted kappa. Expert Systems with Applications. 2008;34:825–32. [Google Scholar]
- 31.Siepen JA, Keevil EJ, Knight D, Hubbard SJ. Prediction of missed cleavage sites in tryptic peptides aids protein identification in proteomics. J Proteome Res. 2007;6:399–408. doi: 10.1021/pr060507u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen CY, Russell S, Mendoza AM, Meyer-Arendt K, Sun S, Cios KJ, et al. Improving sensitivity in shotgun proteomics using a peptide-centric database with reduced complexity: Protease cleavage and scx elution rules from data mining of ms/ms spectra. Anal Chem. 2006;78:1071–84. doi: 10.1021/ac051127f. [DOI] [PubMed] [Google Scholar]
- 33.Monigatti F, Berndt P. Algorithm for accurate similarity measurements of peptide mass fingerprints and its application. J Am Soc Mass Spectrom. 2005;16:13–21. doi: 10.1016/j.jasms.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Keil B. Specificity of proteolysis. New Your: Springer-Verlag; 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.