Abstract
We attempted to ascertain the effects of polyunsaturated fatty acids by conducting two studies in normal young men, in which monounsaturated fats were replaced by polyunsaturated fats within the guidelines of the American Heart Association step 1 diet. Study A employed a randomized parallel design in which subjects first consumed an average American diet (AAD) containing 37% of calories as fat (saturated fat, 16% calories; monounsaturated fat, 14% calories; and polyunsaturated fat, 7% calories). After 3 weeks, one third of the subjects continued with the AAD, one third switched to a step 1 diet in which total fat calories were reduced to 30% by replacing saturated fat with carbohydrate, and one third switched to a polyunsaturated fat-enriched (Poly) diet with the same 30% fat calories and a reduction of monounsaturated fat from 14% to 8% and an increase of polyunsaturated fat from 7% to 13% of calories. The randomized period lasted 6 weeks. Total and low-density lipoprotein (LDL) cholesterol levels on the step 1 and Poly diets were reduced compared with levels on the AAD (P < .001). Total and LDL cholesterol did not differ between the step 1 and Poly diets, although comparison between the two diets is limited by the small study groups. Serum apolipoprotein (apo) B levels fell on the Poly diet compared with the AAD. Total high-density lipoprotein (HDL), HDL2, and HDL3 cholesterol levels were not significantly affected by the diets. Postprandial lipid and lipoprotein concentrations did not significantly differ either. In study B, a randomized crossover design was used in which all subjects ate the step 1 and Poly diets for 5 weeks each with a 4-day break between diets. In the eight subjects studied, the values for fasting plasma total, LDL, and HDL cholesterol; triglycerides; apoB; and apoA-I were essentially identical at the end of each diet period. Postprandial triglyceride areas obtained after ingestion of a large, standard fat load were also the same. Finally, LDL apoB and HDL apoA-I turnovers were unaffected by replacement of monounsaturates with polyunsaturates. In summary our results indicate that modest exchanges of monounsaturated for polyunsaturated fats do not significantly affect LDL or HDL levels or metabolism, which supports the view that reducing saturated fats is the key to lowering total and LDL cholesterol.
Keywords: plasma lipids, lipoproteins, dietary fatty acids, LDL, HDL, saturated fat, monounsaturated fat, polyunsaturated fat
Reduction of dietary intake of saturated fatty acids appears to be the key to reducing plasma total and low-density lipoprotein (LDL) cholesterol (LDL-C) levels,1–3 and low–saturated fat diets are the cornerstone of the American Heart Association (AHA) –National Cholesterol Education Program (NCEP) dietary guidelines.4 While recent surveys indicate that consumption of saturated fatty acids has decreased significantly in the United States, total fat intake has changed little. The lack of a parallel reduction in total fat consumption may result, in part, from the longstanding habits and acquired tastes of the public. The relative constancy in total fat intake may, however, also reflect confusion as to the optimal replacement for calories previously derived from saturated fatty acids.
It is thus of interest that diets high in polyunsaturated fatty acids were shown several decades ago to lower plasma cholesterol levels significantly.5,6 The apparent ability of polyunsaturated fatty acids to independently lower total plasma cholesterol levels led physicians and clinical nutritionists to suggest diets high in polyunsaturated fats as a means of reducing risk for coronary heart disease. (The terms “independent” and “direct” are used to denote effects associated with replacement of carbohydrate by a particular class of fatty acids rather than the exchange of one fatty acid class for another.) Those early investigations measured only total plasma cholesterol concentrations, however, and later studies suggested that although polyunsaturated fatty acids could lower total cholesterol and LDL-C, their consumption might be associated with reductions in high-density lipoprotein (HDL) cholesterol (HDL-C) concentrations.7–13 Despite several recent studies demonstrating that moderate increases in dietary polyunsaturated fats do not reduce plasma levels of HDL-C,14–19 both the media and lay public remain confused. Adding further uncertainty, some12,15,16,20 but not all17–19,21 recent studies suggest that dietary polyunsaturated fatty acids do not reduce plasma LDL-C levels directly (that is, independent of their role as a substitute for saturated fats).
In parallel with the growing uncertainty concerning the efficacy of polyunsaturated fatty acids in lipid-altering diets, some studies in the last decade have suggested that monounsaturated fatty acids could reduce plasma total cholesterol and LDL-C concentrations directly.12,22 In addition, those studies demonstrated that HDL-C levels were unaffected by consumption of monounsaturated fatty acids. These findings were notable in their contrast to the classic studies of Keys et al1 and Hegsted et al,2 which indicated that monounsaturated fatty acids affected total cholesterol concentrations only if they were substituted for saturated fatty acids.
Thus, despite a very large literature focused on these issues, the answers to several salient questions remain unclear. The continued uncertainties also derive from the fact that in many studies numerous nutrient parameters were changed simultaneously, that the diets were often very high or very low in total fat, and that the quantities of polyunsaturated fatty acids provided were often well beyond the limits of practical diets. In an attempt to add clarity to this important issue, we conducted studies in which we made modest changes in the amounts of polyunsaturated and monounsaturated fatty acids while keeping the rest of the diet components (total and saturated fat, carbohydrate, and protein) constant. We also chose to design the diets so that they would otherwise fall within the guidelines of an AHA step 1 diet.4 The results of two such studies, in which polyunsaturated and monounsaturated fatty acids were varied by 6% of total energy intake while total fat and saturated fatty acid intakes were held constant, are reported here.
Methods
Two related studies were performed. Study A used a parallel randomized design to compare a high–saturated fat “average American diet” (AAD) with two diets low in saturated fatty acids but differing in their levels of polyunsaturated and monounsaturated fatty acids. Study B used a randomized crossover design to compare the two low–saturated fat diets directly. In the latter protocol, studies of LDL apolipoprotein (apo) B and HDL apoA-I metabolism were also performed.
Research Subjects
Study A
Thirty normal male medical and dental students 22 through 30 years of age were recruited from an initial population of 250 students with nonfasting plasma levels of total cholesterol between the 25th and 85th percentiles for their age group.23 None of the men had serious medical problems or were taking any medications that might affect plasma lipid levels. Before being accepted into the study the men were interviewed by the supervising registered dietitians. Potential subjects with excessive ethanol consumption or extreme dietary or exercise habits were excluded. Patterns of physical activity were also ascertained from the interview, and together with each man’s height, weight, and dietary history were used to estimate daily caloric requirements. Routine laboratory tests were performed several times during the study to ensure normal health status. The diet study was approved by the Institutional Review Board of Columbia University, and all subjects gave their written consent to participate. The men received no monetary compensation for participation.
Study B
An additional eight normal male medical and dental students were recruited from the same population used for study A. All aspects of the recruitment process were identical to those described above. The subjects who participated in the turnover studies received monetary compensation for their time.
Experimental Design
Study A
The study design included two diet periods. During a 3-week control period all the men consumed an AAD. The AAD contained 37% of the total calories as fat, with 16% saturated fatty acids, 14% monounsaturated fatty acids, and 7% polyunsaturated fatty acids. The AAD also contained 500 mg cholesterol/d. The 6-week randomized diet period began after the 3-week control period (Fig 1A). Plasma cholesterol levels obtained at the end of the baseline period were used to stratify the subjects for randomization to one of three dietary groups.
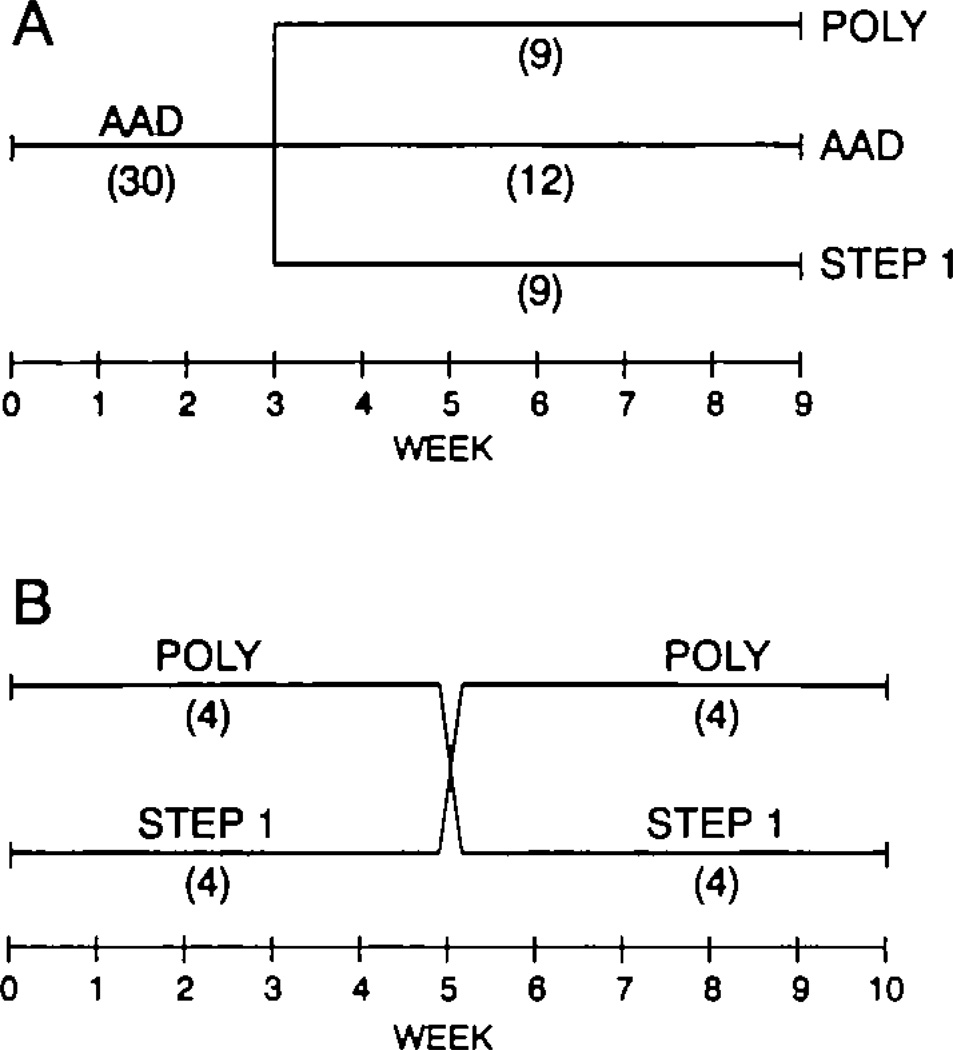
Fig 1.
Diagrams showing the two study designs. A, Study A used a randomized parallel design in which all subjects consumed an average American diet (AAD) for 3 weeks before being randomly assigned for an additional 6 weeks to a continued AAD; to the American Heart Association step 1 diet (STEP 1), in which 7% of calories from saturated fat was replaced by carbohydrate, with monounsaturated and polyunsaturated fats unchanged from AAD; or to a diet (POLY) in which 7% of calories from saturated fat was replaced by carbohydrate and 6% of calories from monounsaturated fat was replaced by polyunsaturated fat. B, Study B used a randomized crossover design in which all subjects were assigned randomly to either the step 1 or the Poly diet for 5 weeks and then switched to the other diet for an additional 5 weeks. There was a 4-day break between the two 5-week periods.
After randomization, one third of the men (the control group) continued to follow the AAD, one third switched to a typical AHA step 1 diet, and one third switched to a modified step 1 diet in which monounsaturated fatty acids were reduced and polyunsaturated fatty acids were increased (the “Poly” diet). Both the step 1 and Poly diets contained 250 mg cholesterol/d, and 30% of the total calories were consumed as fat. The step 1 diet contained 9% saturated fatty acids, 14% monounsaturated fatty acids, and 7% polyunsaturated fatty acids; the Poly diet contained 9% saturated fatty acids, 8% monounsaturated fatty acids, and 13% polyunsaturated fatty acids. The participants were urged to maintain their usual level of physical activity throughout the study. Only the dietary staff engaged in meal preparation and a single statistician were aware of the group assignments. Two 9-week studies were conducted, one in the spring (n=12) and one in the fall (n=18).
Study B
The study design included two 5-week diet periods (Fig 1B). The diets used were the AHA step 1 diet and the Poly diet from study A. The order in which the men ate the diets was randomly assigned. There was a 4-day break between diets.
Compositional Design and Delivery of Diets
Study A
The study diets contained foods prepared from fresh ingredients in accordance with computer-analyzed recipes and menu plans. The nutrient composition of the research diets was determined with NUTRITIONIST III software, version 3 (N-Squared Computing), with the data base expanded to include data on fatty acid composition from Handbook 8 of the US Department of Agriculture.24 The composition for each day’s meals, including protein, carbohydrate, fat, fatty acid classes, and cholesterol, matched the research protocol for the particular diet. Meals were prepared daily and individual portions were measured on a gram scale. Food samples from each of the three diets were collected and sent to Hazleton Laboratories America for compositional analysis. As in our previous studies, we used a 2-week menu cycle and served a mixed diet consisting of red meat (beef), poultry, fish (canned), dairy products, eggs, fruits, vegetables, a variety of complex carbohydrates, and desserts.25,26 The meals were designed so that the men were unaware of their own dietary group assignments. We served lunches and dinners in the student dining facility. Breakfasts were provided, along with snacks, in individual packages, and given after dinner. The men received all meals and snacks Monday through Friday. Breakfasts and lunches were also provided on weekends, but the men were allowed two self-selected weekend dinners for which they received dietary instruction. The subjects’ dietary compliance was checked by recording daily attendance at meals and inspection of trays. The men also completed a self-administered compliance form at each meal. Compliance forms and food records for weekend dinners were monitored by the supervising registered dietitian. On the basis of estimated daily caloric requirements, the men were assigned to one of two different caloric groups (2500 kcal/day or 2800 kcal/day) and were weighed every 2 weeks. Caloric intake was adjusted as necessary to maintain body weight.
Study B
The diet design, preparation, and service were essentially the same as for study A except that all meals and snacks were provided (7 d/wk). Lunches and dinners were eaten in the research diet facility of the Irving Center for Clinical Research. The men were weighed daily and calories adjusted as needed every 2 or 3 days.
Blood Sampling Protocol
Study A
Blood samples were obtained for the determination of plasma lipid, lipoprotein, and apolipoprotein levels at 1-week intervals during the control period and at 4, 5, and 6 weeks after the start of the randomized diet period. Fasting samples were obtained between 8 am and 9 am after a 12-hour overnight fast. In addition, postprandial samples were obtained just before and at 2, 4, and 6 hours after a standard lunch at the end of the randomized period. Blood samples were drawn into tubes containing EDTA (1.0 mg/mL) for plasma or into empty tubes for serum. The samples were placed immediately on ice and centrifuged at 2000 rpm for 20 minutes at 4°C within 1 hour of sampling. Plasma samples were stored at 4°C after the addition of aprotinin (trasylol, FBA Pharmaceuticals) and azide. Serum samples were stored in multiple aliquots at −70°C.
Study B
Three fasting blood samples were obtained during the final week of each diet period for determination of lipid, lipoprotein, and apolipoprotein levels. In addition, several samples were obtained during the first day of the LDL/HDL turnover studies and daily thereafter for 2 weeks (see below). On the final day of each diet period, the men ingested a standard liquid fat formula (105 gm fat per 2 m2 body surface), and blood was obtained just before and at 5 and 8 hours after they drank the formula. Blood was processed as described above.
LDL ApoB Turnover Studies
After each man had eaten a diet for 2 weeks, blood was obtained after an overnight fast for isolation of LDL by sequential ultracentrifugation.27,28 The isolated LDL was dialyzed against sterile saline and radiolabeled with 131I by using a modification of the iodine monochloride method.29,30 The labeled LDL was used within 24 hours. The men were admitted to the Irving Center for Clinical Research the day before the start of the turnover study. A saturated solution of potassium iodide, 3 drops PO BID, was started on admission and continued until 1 week after the end of the blood sampling period. The morning after admission, autologous 131I-LDL (25 µCi) was injected, and blood samples were obtained at 0, 2, 4, 6, 12, and 24 hours. The men were discharged from the hospital at that time but continued receiving the study diets as before. Fasting blood samples were obtained daily for the next 14 days (until the end of the study). LDL was isolated by sequential ultracentrifugation from each sample, and 131I-apoB specific radioactivity was determined by gamma counting and Lowry measurement of protein.28
HDL ApoA-I Turnover Studies
HDL was isolated by sequential ultracentrifugation after isolation of LDL as described above.27,31 Homologous, pure apoA-I (10 µg) was radiolabeled with 125I using a modification of the iodine monochloride method.29–31 125I apoA-I (100 µCi) was incubated with each man’s HDL overnight at 37°C, and the HDL was then reisolated by ultracentrifugation. Autologous HDL (containing 25 µCi of homologous apoA-I) was injected simultaneously with the 131I LDL, and blood samples were obtained as described above. HDL was isolated from each sample by ultracentrifugation, and 125I–apoA-I specific radioactivity was determined by gamma counting and Lowry measurement of protein. Because questions have been raised regarding the ability of radiolabeled homologous apoA-I to trace the autologous protein, we incorporated it into each subject’s own HDL before reisolation of the whole lipoprotein. In addition, the same method was used for each turnover study so that each subject’s studies were internally consistent.
Plasma Total and Lipoprotein Lipids
Plasma total cholesterol and triglyceride concentrations were measured after each sampling by enzymatic methods using an ABA-100 automated spectrophotometer (Abbott Laboratories). HDL-C was measured by the same enzymatic method after precipitation of apoB-containing lipoproteins with magnesium and dextran using reagents supplied by Sigma.32 LDL-C was estimated by using the Lipid Research Clinics method.33 Our laboratory participates in the quality control program administered by the Centers for Disease Control and Prevention (CDC), Atlanta, Ga. The interassay coefficients of variation were less than 3% for both cholesterol and triglyceride determinations.
Isolation of Plasma Lipoproteins
Very-low-density lipoproteins (VLDLs), intermediate-density lipoproteins (IDLs), LDLs, and HDLs were isolated by sequential ultracentrifugation using a 50.3-Ti rotor in an L8–80 Beckman ultracentrifuge.27 Cholesterol and triglycerides were measured in each fraction as described above. Phospholipids were determined by the method of Bartlett,34 and protein by the method of Lowry et al.35
HDL Subfractions
HDL2 and HDL3 cholesterol levels were determined after isolation of total HDL and HDL3 by sequential precipitation of plasma with heparin and manganese.36 All of these measurements were done at the end of the study using plasma samples that had been frozen at −70°C.
ApoB and ApoA-I
Serum apoB and apoA-I were measured by specific fluid-phase radioimmunoassays.37 Our laboratory participated in the Apolipoprotein Standardization Program administered by the CDC.38 All samples were assayed using single radioiodinated LDL and apoA-I tracers for serum apoB and apoA-I determinations, respectively. All samples from each individual were analyzed in the same assay for each apolipoprotein. The interassay coefficients of variation were 11% and 8% for apoB and apoA-I, respectively.
Statistical Analysis
Study A
For each subject, the value obtained at week 3 of the control period and the value obtained at the end of week 6 of the randomized diet period were used to determine the changes in concentration of total cholesterol, HDL-C, LDL-C, and each apolipoprotein. In the case of plasma triglycerides, the mean values obtained at weeks 4, 5, and 6 of the randomized period were used along with the value at week 3 of the control period. This strategy was chosen because we observed a greater week-to-week variability in plasma triglyceride concentrations. The HDL2 and HDL3 cholesterol levels were also analyzed by comparing the changes in values obtained at the end of the baseline and randomized periods. For each of these variables, the changes in the three groups were compared by ANOVA. If there was a significant (P < .05) diet effect, three group comparisons were made: between the change observed in the step 1 group and the change observed in the control (AAD) group; between the Poly and control groups; and between the step 1 and Poly groups. Since three comparisons were made, the critical probability value for the significance of each comparison was .05/3. Furthermore, because we conducted the study in two time periods, each ANOVA was performed with two fixed factors: diet (at three levels) and season (at two levels). Thus, we controlled for a seasonal effect (if any), although this effect was not our primary interest.
The values for total cholesterol, triglycerides, HDL-C, and LDL-C obtained just before and at 2, 4, and 6 hours after lunch at the end of the randomized periods were used to calculate postprandial areas for each variable. The areas for the three groups were compared by ANOVA, followed when appropriate by individual contrasts as described above.
The percent values for cholesterol, triglyceride, phospholipid, and protein levels were calculated in VLDL, IDL, LDL, and HDL isolated by ultracentrifugation from blood samples obtained fasting, just before lunch, and 4 hours after lunch at the end of the randomized period. The results were analyzed as described for the postprandial areas. These lipoprotein compositional data were obtained only in the subjects randomized to the step 1 diet or the Poly diet.
Study B
All data were first analyzed by ANOVA to test for carryover effects; none were observed. The diets were then directly compared by paired t tests. The kinetic data describing the turnover of plasma LDL apoB and HDL apoA-I were analyzed by using two-compartment models.28,31 Fractional catabolic rates (FCRs) were determined for each apolipoprotein. Total plasma turnover rates were estimated by multiplying the FCRs by the plasma pool of each apolipoprotein (plasma concentration × 0.45).
Results
The results of the diet compositional analyses are compared with our goals for each diet in Table 1. The same step 1 and Poly diets were used in studies A and B. It is clear that we were able to design and serve diets that met our goal of comparing two diets with different levels of monounsaturated and polyunsaturated fatty acids while holding total fat, saturated fat, and carbohydrates constant.
Table 1.
Study Diet Composition: Calculated vs Analyzed
| Nutrients | AAD | Poly | Step 1 |
|---|---|---|---|
| Fat | 35.2 (37) | 29.6 (30) | 29.8 (30) |
| Carbohydrate | 49.3 (48) | 55.2 (55) | 55.4 (55) |
| Protein | 15.5 (15) | 15.2 (15) | 14.8 (15) |
| Saturated fat | 13.9 (16) | 9.3 (9) | 9.5 (9) |
| Monounsaturated fat | 13.9 (14) | 8.8 (8) | 13.8 (14) |
| Polyunsaturated fat | 7.2 (7) | 11.9 (13) | 6.7 (7) |
| Cholesterol, mg/d | 581 | 310 | 389 |
AAD indicates average American diet; Poly, modified Step 1 diet with decreased monounsaturated and increased polyunsaturated fatty acids; and Step 1, American Heart Association Step 1 diet. Values are presented as percent of total calories except for cholesterol, and values in parentheses are calculated (see "Methods").
Study A
The clinical characteristics of the students randomized into each of the three diet groups in study A are depicted in Table 2. The groups were similar in age and body mass index, and they had similar plasma levels of total cholesterol and triglycerides, LDL-C, and HDL-C at the time of randomization (based on blood samples obtained at week 3 of the baseline period; Table 3).
Table 2.
Study A: Clinical Characteristics of Each Diet Group
| AAD | Poly | Step 1 | |
|---|---|---|---|
| No. of subjects | 12 | 9 | 9 |
| Age, y | 23.8 ± 1.5 | 25.9 ± 3.2 | 24.2 ± 1.1 |
| BMI, kg/m2 | 24.7 ± 1.8 | 24.0 ± 1.6 | 24.0 ± 1.6 |
AAD indicates average American diet; Poly, modified Step 1 diet with decreased monounsaturated and increased polyunsaturated fatty acids; Step 1, American Heart Association Step 1 diet; and BMI, body mass Index. All data are expressed as mean ± SD. See "Methods" for a description of study A.
Table 3.
Study A: Serum Lipid Levels at the End of the Baseline and Randomized Periods
| AAD | Poly | Step 1 | |
|---|---|---|---|
| Total cholesterol, mg/dL | |||
| Baseline | 176.6 ± 22.1 | 180.2 ± 16.8 | 180.1 ± 17.2 |
| Randomized | 180.7 ± 26.2 | 160.9 ± 15.1 | 162.6 ± 18.5 |
| Triglycerides, mg/dL | |||
| Baseline | 77.2 ± 26.2 | 69.2 ± 20.8 | 82.3 ± 16.2 |
| Randomized | 85.9 ± 30.5 | 82.3 ± 36.6 | 87.1 ± 13.9 |
| HDL cholesterol, mg/dL | |||
| Baseline | 50.5 ± 11.6 | 47.1 ± 9.7 | 46.1 ± 4.7 |
| Randomized | 49.8 ± 9.5 | 42.9 ± 9.0 | 43.3 ± 5.6 |
| LDL cholesterol, mg/dL | |||
| Baseline | 110.7 ± 20.9 | 119.3 ± 14.0 | 117.5 ± 19.3 |
| Randomized | 113.7 ± 22.2 | 101.5 ± 13.7 | 101.8 ± 19.0 |
| HDL2 cholesterol, mg/dL | |||
| Baseline | 11.7 ± 7.0 | 7.9 ± 6.5 | 4.7 ±3.1 |
| Randomized | 9.7 ± 5.1 | 6.3 ± 5.5 | 5.8 ± 2.2 |
| HDL3 cholesterol, mg/dL | |||
| Baseline | 41.3 ± 4.1 | 36.8 ± 5.1 | 39.4 ± 6.7 |
| Randomized | 38.2 ± 6.1 | 34.9 ± 4.2 | 38.2 ± 3.6 |
AAD Indicates average American diet; Poly, modified Step 1 diet with decreased monounsaturated and increased polyunsaturated fatty acids; Step 1, American Heart Association Step 1 diet; HDL, high-density lipoprotein; and LDL, low-density lipoprotein. Values are mean ± SD. See "Methods" for a description of study A.
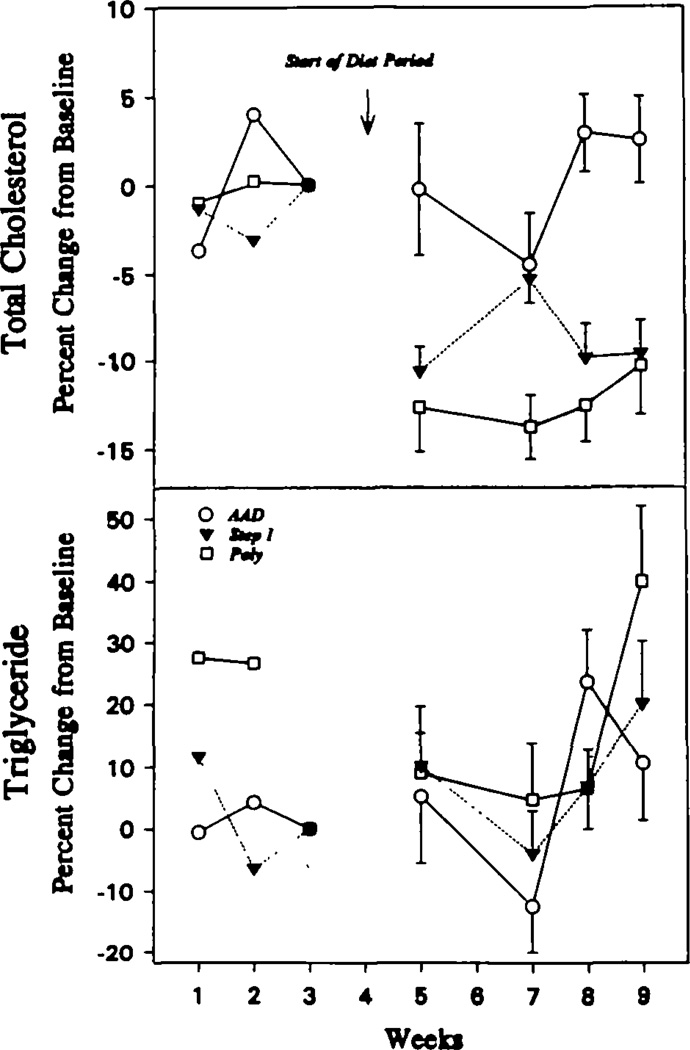
Fig 2 shows the effects of each diet on plasma total cholesterol and triglyceride levels. The data are presented as the mean percentage change at each sampling compared with the levels at the end of the baseline period. During the randomized diet period, both the step 1 and Poly diet groups had decreases in plasma cholesterol levels compared with the AAD group. The data suggest that the Poly diet caused a greater initial decline in total cholesterol than the step 1 diet, but by the end of 6 weeks there was no obvious difference between the two groups. Fasting triglycerides were more variable during the baseline period, and the levels of triglycerides tended to rise on all three diets during the randomized period.
Fig 2.
Total plasma cholesterol and triglyceride concentrations during study A at weeks 1,2,5, 7, 8, and 9 are platted as percent change from the values at the end of week 3 of the baseline period, during which all subjects ate the average American diet (AAD). The samples at week 5 were obtained 2 weeks after the start of the randomized diet periods. Subjects consuming the experimental diets (Step 1 or Poly; see “Methods” for definitions) had lower total plasma cholesterol levels by that time.
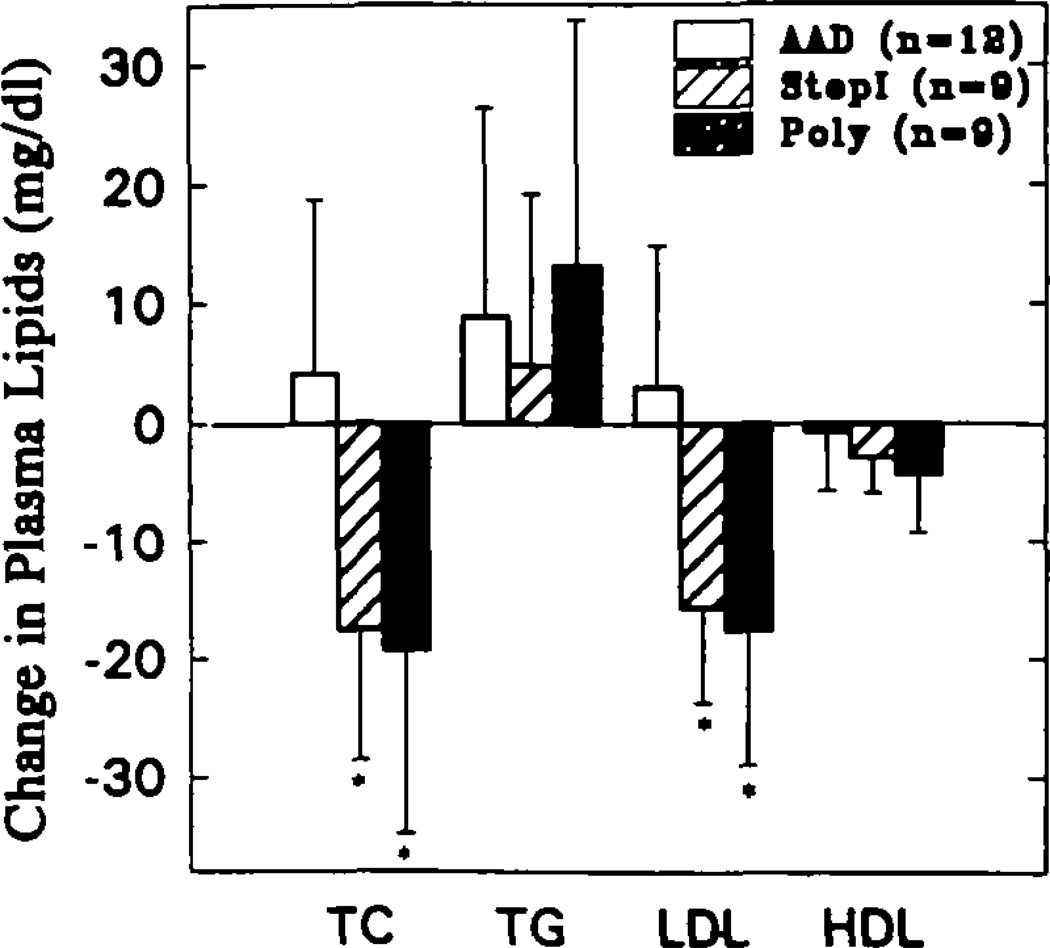
The reductions in fasting plasma total cholesterol levels (mean ± SD of the change between the end of the randomized period and the end of the baseline period) in both the step 1 group (17.6 ± 11.0 mg/dL; P < .01) and the Poly group (19.3 ± 15.5 mg/dL; P < .001) were significant compared with the slight rise in total cholesterol in the AAD group (4.1 ± 14.7 mg/dL) (Fig 3). These changes were equal to −9.8%, −10.7%, and 2.3% of the values for the baseline period in the step 1, Poly, and control groups, respectively. There was no obvious difference between the responses to the step 1 and Poly diets. The increases in fasting plasma triglyceride concentrations in the step 1 (4.8 ± 14.4 mg/dL) and Poly (13.1 ± 20.6 mg/dL) groups did not statistically differ from that in the AAD group (8.8 ± 17.5 mg/dL).
Fig 3.
Bar graph showing the plasma total (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol concentrations presented as the mean changes between the values obtained in study A at week 3 (end of the baseline period) and week 9 (end of the randomized period) for the average American diet (AAD), American Heart Association step 1 (Stepl), and Poly groups (see “Methods” for definitions of diet groups). Because of greater week-to-week variability, the change in plasma triglyceride (TG) levels was calculated from the difference between the value at week 3 and the mean of values obtained at weeks 7, 8, and 9. Total and LDL cholesterol levels were significantly lower in the step 1 and Poly groups compared with AAD (P < .001 for each comparison). There was no significant difference between the reductions in the step 1 and the Poly groups
Plasma concentrations of LDL-C also fell in both the step 1 (15.7 ± 8.0 mg/dL; P < .001) and Poly (17.7 ± 11.3 mg/dL; P < .001) groups compared with the control group’s increase (3.0 ± 11.9 mg/dL). These reductions were similar in the step 1 and the Poly diet groups (Fig 3). Plasma concentrations of total HDL-C tended to fall in all three groups during the randomized periods, but these reductions were not significantly different on either the step 1 (2.8 ± 3.1 mg/dL) or the Poly (4.2 ± 5.1 mg/dL) diet compared with the control group (0.7 ± 5.0 mg/dL) (Fig 3). In addition, the changes in HDL2 and HDL3 cholesterol levels, determined by sequential precipitation with heparin manganese, were not different among the three groups (Table 3).
Concomitant with the fall in fasting serum total cholesterol and LDL-C levels, fasting serum apoB levels also decreased significantly (21.4 ± 11.4 mg/dL; P < .01) in the Poly group compared with the control AAD group (Table 4). ApoB levels in the AAD group were constant (change between the end of baseline and end of randomization, −0.61 ± 11.5 mg/dL). Men eating the step 1 diet tended to have a reduction in apoB levels (11.2 ± 11.1 mg/dL; P=.051) compared with the control group. The lack of effect of either diet on plasma HDL-C levels was paralleled by the absence of effect of either the step 1 or the Poly diet on serum apoA-I levels compared with the control AAD group (Table 4).
Table 4.
Study A: Serum ApoB and ApoA-I Levels at the End of the Baseline and Randomized Periods
| AAD | Poly | Step 1 | |
|---|---|---|---|
| ApoB, mg/dL | |||
| Baseline | 104.0 ± 21.0 | 111.1 ± 23.6 | 108.0 ± 24.9 |
| Randomized | 103.4 ± 19.2 | 89.7 ± 14.4* | 96.8 ± 19.9 |
| ApoA-I, mg/dL | |||
| Baseline | 157.2 ± 38.6 | 179.1 ± 19.6 | 176.5 ± 29.0 |
| Randomized | 137.0 ± 29.9 | 169.3 ± 31.8 | 165.4 ± 30.2 |
Apo indicates apolipoprotein; AAD, average American diet; Poly, modified Step 1 diet with decreased monounsaturated and increased polyunsaturated fatty acids; and Step 1, American Heart Association Step 1 diet. All values are mean ± SD. See “Methods” for description of study A.
P < .01 for the change in apoB in the Poly diet group between the randomized and baseline periods compared with the change in the AAD between the same two periods.
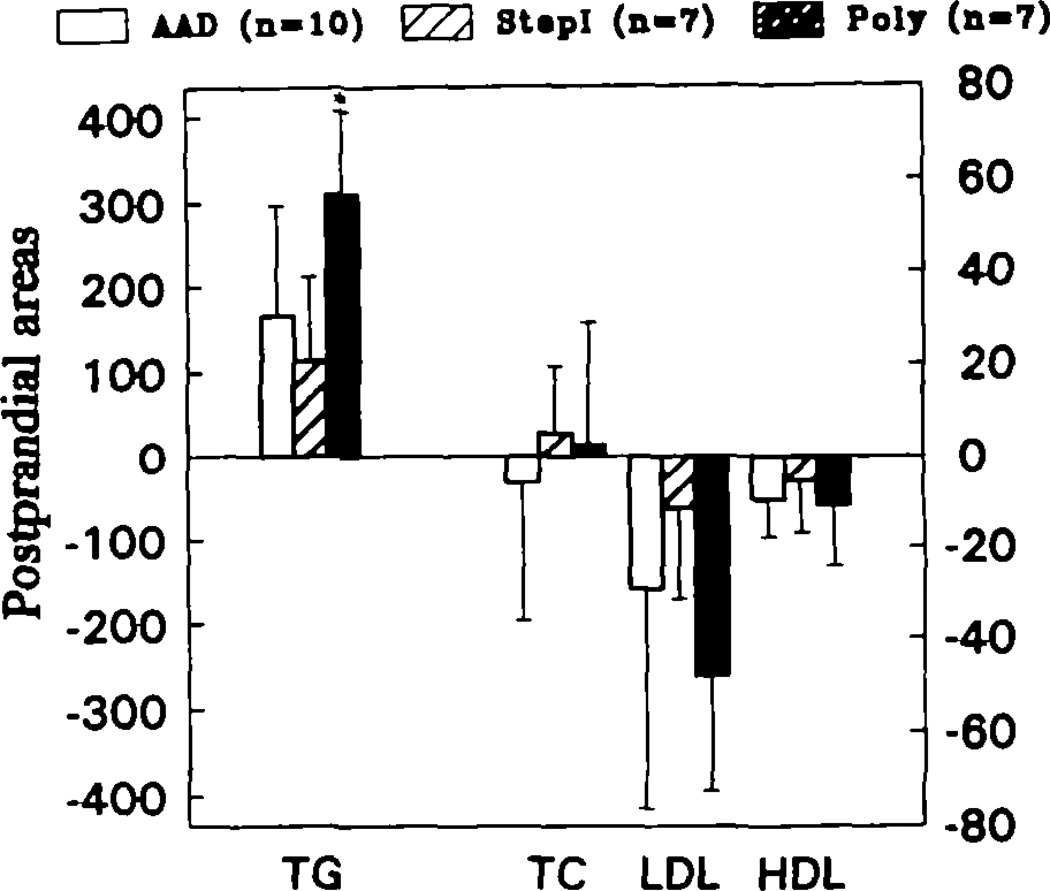
The postprandial areas of total plasma triglycerides and cholesterol, LDL-C, and HDL-C, determined from the levels just before and 2, 4, and 6 hours after ingestion of a representative lunch, are depicted in Fig 4. Postprandial responses of total cholesterol, LDL-C, and HDL-C did not differ among the groups. In contrast, the area for triglyceride above baseline (prelunch value) was significantly greater (P < .05) in the Poly group than in either of the other groups.
Fig 4.
Bar graph comparing the areas under the plasma concentrations of triglycerides (TG) and total (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol after study A subjects had eaten a lunch typical of each of the three diets. The areas were calculated from the changes at 2, 4, and 6 hours after lunch compared with prelunch levels. Postlunch TG levels were significantly greater in the Poly group (P < .05) than in either the average American diet (AAD) or the American Heart Association step 1 (Stepl) groups (see “Methods” for definitions of diet groups). There was no difference in postlunch TGs between the AAD and step 1 diets, and postlunch total, LDL, and HDL cholesterol levels did not differ in the three diet groups.
The mean percent composition of cholesterol, triglyceride, phospholipid, and protein in VLDL, IDL, LDL, and HDL isolated by ultracentrifugation at the end of randomized periods is presented in Table 5. We performed these measurements only in the Poly and step 1 groups. The data are for fasting samples, for samples obtained just before lunch, and for samples taken 4 hours after a lunch representative of each diet. We did not separate chylomicrons or their remnants from VLDL in the postprandial samples. As expected, all lipoproteins became relatively triglyceride enriched during the day as the subjects consumed dietary fat. There were no differences between the step 1 group and the Poly group in the composition of any of these lipoproteins at each time point.
Table 5.
Study A: Lipoprotein Composition Before Breakfast, Before Lunch, and 4 Hours After Lunch on the Poly and Step 1 Diets
| VLDL | IDL | LDL | HDL | |||||
|---|---|---|---|---|---|---|---|---|
| Poly | Step 1 | Poly | Step 1 | Poly | Step 1 | Poly | Step 1 | |
| Before breakfast | ||||||||
| Triglycerides | 34.6 | 35.9 | 13.4 | 13.8 | 6.3 | 5.9 | 3.5 | 3.6 |
| Total chol | 7.8 | 7.7 | 13.9 | 14.2 | 37.0 | 38.2 | 11.8 | 11.5 |
| Phospholipids | 41.3 | 41.4 | 50.1 | 49.1 | 30.3 | 30.0 | 21.1 | 20.4 |
| Protein | 16.3 | 15.0 | 22.7 | 22.9 | 26.3 | 26.0 | 63.6 | 64.6 |
| Before lunch | ||||||||
| Triglycerides | 38.4 | 42.8 | 15.9 | 16.4 | 5.5 | 5.6 | 4.3 | 4.7 |
| Total chol | 9.7 | 9.5 | 11.9 | 12.0 | 38.0 | 37.7 | 13.9 | 13.2 |
| Phospholipids | 33.5 | 31.1 | 44.9 | 46.8 | 27.6 | 27.5 | 19.6 | 21.0 |
| Protein | 18.4 | 16.6 | 27.2 | 24.8 | 28.9 | 29.2 | 62.2 | 61.2 |
| 4 Hours after lunch | ||||||||
| Triglycerides | 52.5 | 49.1 | 18.0 | 17.8 | 6.8 | 6.2 | 5.7 | 5.0 |
| Total chol | 8.4 | 8.4 | 11.7 | 13.6 | 36.6 | 37.9 | 13.1 | 13.1 |
| Phospholipids | 24.4 | 28.2 | 46.3 | 45.6 | 27.1 | 27.7 | 21.0 | 22.2 |
| Protein | 14.7 | 14.2 | 24.0 | 22.9 | 29.5 | 28.1 | 60.3 | 59.7 |
Poly indicates modified Step 1 diet with decreased monounsaturated and increased polyunsaturated fatty acids; Step 1, American Heart Association Step 1 diet; VLDL, very-low-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein; and chol, cholesterol. All data are given as percent total composition. See “Methods” for a description of study A.
Study B
The ages of the eight students recruited for the crossover design study averaged 24.5 ± 1.4 years (range, 23 to 27); their body mass indexes averaged 23.5 ± 1.9 kg/m2 (range, 19.4 to 25.2). The group was very similar to the larger group of subjects recruited for study A.
There were no obvious differences between the mean plasma levels of total cholesterol, triglycerides, LDL-C, or HDL-C measured at the end of each diet (Table 6). Similarly, serum levels of apoB and apoA-I did not differ between the step 1 and Poly diets. Triglyceride levels obtained over an 8-hour period after the ingestion of a standard liquid fat formula were the same at the end of each diet as well.
Table 6.
Study B: Lipids and Apolipoproteins at the End of Two Diet Periods
| Poly | Step 1 | |
|---|---|---|
| Total chol, mg/dL | 164.6 ± 21.6 | 167.3 ± 12.2 |
| Triglycerides, mg/dL | 93.8 ± 24.8 | 101.0 ± 39.4 |
| HDL-C, mg/dL | 42.4 ± 6.7 | 41.3 ± 6.1 |
| LDL-C, mg/dL | 103.5 ± 20.0 | 105.7 ± 13.4 |
| ApoB, mg/dL | 50.9 ± 6.3 | 53.7 ± 10.8 |
| ApoA-I, mg/dL | 121.2 ± 14.0 | 127.7 ± 18.7 |
| PPTG area, mg/dL per 6 h | 188.4 ± 122.8 | 242.1 ± 160.7 |
| LDL apoB FCR, pool/d | 0.34 ± 0.08 | 0.33 ± 0.08 |
| LDL apoB protein, mg/kg per d | 7.77 ± 1.67 | 7.84 ± 2.52 |
| HDL apoA-I FCR, pool/d | 0.23 ± 0.05 | 0.22 ± 0.06 |
| HDL apoA-I protein, mg/kg per d | 12.10 ± 1.99 | 12.21 ± 2.89 |
Poly indicates modified Step 1 diet with decreased monounsaturated and increased polyunsaturated fatty acids; Step 1, American Heart Association Step 1 diet; chol, cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; apo, apolipoprotein; PPTG area, area under the postprandial triglyceride curve; and FCR, fractional catabolic rate. See “Methods” for a description of study B. All values are expressed as mean ± SD of eight subjects, except for LDL apoB FCR and PR, which are based on seven subjects.
There was no discernible effect of diet on the fractional catabolism of either LDL or HDL. The mean LDL apoB FCRs were 0.34 ± 0.08 pool/d on the Poly diet and 0.33 ± 0.08 pool/d on the step 1 diet. The mean HDL apoA-I FCRs were 0.23 ± 0.05 pool/d on the Poly diet and 0.22 ± 0.06 pool/d on the step 1 diet. The concomitant lack of change in either serum apoB or apoA-I levels resulted in very similar total turnover rates for these apolipoproteins as well (Table 6).
Discussion
Historically, research that focused on the effects of dietary polyunsaturated fatty acids on plasma lipid and lipoprotein concentrations has yielded contradictory and confounding results. The early work of Ahrens et al5,6 indicating that increasing polyunsaturated fatty acids in the diet would lower plasma total cholesterol levels directly (when substituted for carbohydrate) was supported by the systematic studies of Keys et al1 and Hegsted et al.2 The regression equations describing the effects of dietary fatty acids on plasma cholesterol levels generated by their studies included negative coefficients of 1.31 and 1.65, respectively, for the effects of polyunsaturated fats. These equations, however, did not allow estimates of the individual effects of polyunsaturated fats on plasma LDL-C and HDL-C concentrations, and several studies published in the last 15 years have suggested that part of the reduction in total cholesterol observed with high polyunsaturated fat intake was the result of reductions in HDL-C.7–13 Although more recent studies have not demonstrated reduced HDL-C levels during consumption of dietary polyunsaturated fatty acids,14–19 resolution of questions related to the efficacy of polyunsaturated fats has been made more difficult by the fact that some of these same studies have also been unable to show that polyunsaturates directly lower LDL-C concentrations.12,15,16,20
Based on these contradictory data, we initiated two studies to address the question of the relative efficacies of low–saturated fat diets in which polyunsaturated or monounsaturated fatty acids predominated. We chose to use moderate levels of each fatty acid within the overall context of the recommended AHA step 1 diet.4 We used a randomized parallel design to compare each of our experimental diets with the AAD, and a randomized crossover design to compare the two diets directly. We also performed studies of LDL and HDL metabolism during the crossover study. Our findings indicated that in healthy normolipidemic young men, changing the source of 7% of calories from saturated fat to carbohydrate was associated with similar, significant reductions of total plasma cholesterol and LDL-C levels whether monounsaturated or polyunsaturated fatty acids predominated. In addition, we found that our Poly diet, with 13% of calories derived from polyunsaturated fat, did not affect plasma HDL-C levels compared with either a step 1 diet, with 7% of calories from polyunsaturates, or the AAD. Increasing dietary polyunsaturates from 7% to 13% of calories had no effect on HDL2 or HDL3 cholesterol or plasma apoA-I levels.
Based on the studies of Keys et al1 and Hegsted et al,2 we would have predicted that the Poly diet would affect plasma cholesterol concentrations via two mechanisms. The first significant factor would be the reduction of saturated fat (exchanged with carbohydrate), and the second would be the increase in the amount of polyunsaturated fat (exchanged with monounsaturated fat). Using their equations, however, we could account completely for the reductions in total cholesterol that we observed by using the regression coefficients for the exchange between saturated fat and carbohydrate (2.74 and 2.16, respectively) and assuming that polyunsaturated fatty acids were neutral. Our results are similar to those of Mattson and Grundy,39 who report that very large quantities of both polyunsaturated fats (primarily linoleic acid) and monounsaturated fats (primarily oleic acid) reduced total cholesterol and LDL-C concentrations to similar extents when exchanged with saturated fats. Our findings are also in accord with reports from Mensink and Katan15 and Dreon et al,16 whose experimental diets, in which polyunsaturated and monounsaturated fats were exchanged, were very similar to ours.
In contrast, Rassias et al21 reported that the addition of linoleic acid to an average diet (in exchange for carbohydrate) results in a significant drop in plasma total cholesterol and LDL-C levels despite an increase in total fat content of the diet. The contribution of polyunsaturated fatty acids to total calories in that study, however, increased from 6% to 23%, making direct comparison with our results difficult. Further support for a direct LDL-lowering effect of polyunsaturates can be found, however, in the recent meta-analysis by Mensink and Katan,3 in which they included the results of 27 trials with data for lipoprotein cholesterol concentrations. Their regression equation for LDL-C contains a statistically significant, negative coefficient of .55 for polyunsaturated fatty acids when they are used to replace carbohydrate as a source of calories. Finally, Hegsted et al40 analyzed the results of 155 metabolic studies in which lipoproteins were reported. These investigators found that LDL-C fell 0.77 mg/dL for every 1% increase in calories from polyunsaturated fats. In contrast, in both of these recent meta-analyses, monounsaturated fatty acids were found to have no significant LDL-C–lowering effect when used as a replacement for carbohydrate.
Why did we not see an effect of replacing monounsaturates by polyunsaturates on LDL-C? The regression equations derived by Mensink and Katan3 and Hegsted et al40 indicate that we might have expected that our Poly diet, in which we increased polyunsaturated fatty acids at the expense of monounsaturated fatty acids, would lower LDL-C about 3 to 5 mg/dL more than the step 1 diet. We would have needed approximately 80 to 100 subjects in each group in our parallel-design study to determine that difference between the two diets, and we would have needed more than 50 subjects to accurately assess a difference of 3 to 5 mg/dL between the diets in our crossover-design protocol. Clearly, our studies were too small to observe any such difference between the two groups; we could only demonstrate significant differences between each low–saturated fat diet and our AAD. We would note, however, that the turnover studies conducted in our crossover protocol suggested that fractional catabolism of LDL apoB was essentially identical on the two diets, as were serum apoB levels. In summary, meta-analyses3,40 indicate that polyunsaturated fatty acids have a modest cholesterol-lowering effect while monounsaturated fatty acids are neutral. Although our data, limited by sample size, do not contradict those findings, they indicate that adding modest quantities of polyunsaturated fatty acids to replace monounsaturates in an otherwise low–saturated fat diet is unlikely to result in clinically important reductions in LDL-C concentrations.
A review of the literature regarding the effects of polyunsaturated fats on HDL levels is confusing. Mensink and Katan3 derive positive regression coefficients for all fatty acid classes (compared with carbohydrates) regarding their effects on HDL-C levels. This means that reductions in dietary fat from any fatty acid class results in lower HDL-C levels. As the regression coefficient for polyunsaturated fatty acids was the least positive, isocaloric replacement of saturated or monounsaturated fatty acids by polyunsaturated fatty acids should result in lower HDL-C concentrations. However, the coefficients for polyunsaturated (.28) and monounsaturated (.34) fats were close, so that a 1% isocaloric exchange of polyunsaturates for monounsaturates would be predicted to reduce HDL-C by 0.06 mg/dL.3 For our diets, the prediction would be that HDL-C would be about 0.4 mg/dL lower on the Poly diet than on the step 1 diet: more than 200 subjects would be required, even with a crossover design, to see such a change. We actually found a somewhat larger, but clearly insignificant difference between the HDL-C levels on these two diets in the parallel study and essentially identical levels in the crossover-design study. The lack of any dramatic effect of increasing dietary poryunsaturated fat intake on HDL metabolism was supported by similar apoA-I levels in either study and by our inability to discern significant diet effects on HDL apoA-I turnover in the crossover protocol. Despite limitations imposed by sample size, our results, together with studies of similar size and design,15,16,20 support the view that modest increases in dietary polyunsaturated fatty acids will have minimal effects on plasma concentrations and metabolism of HDL. Of course, different results might be demonstrated in other study populations, including women and older men.
Finally, in an attempt to look beyond fasting plasma lipid and lipoprotein levels for effects of diets with different quantities of polyunsaturated and monounsaturated fatty acids, we determined lipid levels both after the subjects ate a standard step 1 or Poly lunch (study A) and after they ingested a standard high-fat liquid formula (study B). Although we saw the expected effects of both the standard lunch and the fat formula on plasma and lipoproteins lipids, there was little difference between the two diets. The only significant diet effect was a greater postlunch plasma triglyceride response on the Poly diet in study A. This may have been related to the greater fasting triglyceride level that was unexpectedly observed in the Poly group. On the other hand, this may have been a random occurrence among the many statistical contrasts that we conducted. In support of the latter view, we found that the Poly diet was associated with a nonsignificantly smaller triglyceride response to the fat formula in study B. In addition, fasting triglycerides in study B were insignificantly lower when the students ate the Poly diet.
There have been few diet studies in which postprandial lipid and lipoprotein levels have been determined, and most of those have focused on ω-3 fatty acids.41–43 Weintraub et al44 show that a diet high in ω-6 polyunsaturated fatty acids is associated with a low incremental triglyceride response to a fat-formula feeding compared with the response during consumption of a high–saturated fat diet. Although the fat formula they used was similar to ours, the diets differed significantly. In the study by Weintraub et al44 the saturated fat and polyunsaturated fat diets differed drastically in their saturated fat (28% versus 13% total calories) and polyunsaturated fat (2% versus 17%) contents. Thus, their study cannot be compared with ours, in which saturated fat was held constant, and monounsaturated and polyunsaturated fat levels were altered modestly. Similarly, the study by Demacker et al,45 in which lower 24-hour lipid and lipoprotein levels were observed during consumption of a high-polyunsaturated fat diet, differs from ours in both saturated and polyunsaturated fatty acid content. Thus, in contrast to other data in the literature confounded by reciprocal changes in saturated and polyunsaturated fats, our data suggested that neither polyunsaturated nor monounsaturated fatty acids affect the metabolism of postprandial lipoproteins when saturated fat and carbohydrates are held constant.
In conclusion, our studies indicated that within the overall guidelines for total and saturated fat intake proposed by the NCEP,4 a moderate, reciprocal change in monounsaturated and polyunsaturated fatty acid content has no clinically significant effect on plasma lipid and lipoprotein levels or metabolism in young healthy men. In particular, we could not demonstrate that increasing the polyunsaturated fat content of the diet (at the expense of monounsaturated fat) affected either LDL-C or HDL-C levels or metabolism. If, as suggested by this and other studies,15–20 these two fatty acid classes are relatively equivalent as replacements for saturated fats in many individuals, producers can more easily provide and consumers can prepare diets that will reduce the risk of coronary heart disease.
Acknowledgments
This work was supported by National Institutes of Health grants HL-39324, HL-36000, HL-21006, and RR-645 and by funds from Best Foods, Kraft Inc, and Bertolli USA Inc. The authors wish to thank the kitchen staff, including Roberta Holeman, Kelly Stennett, Maria Baldo, Ame Gilbert, Carmen Rodriguez, and Carla Carpentieri for outstanding contribution to these studies. We also thank Colleen Ngai and Minnie Myers for their invaluable laboratory work.
References
- 1.Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet, IV: particular saturated fatty acids in the diet. Metabolism. 1965;14:776–787. doi: 10.1016/0026-0495(65)90004-1. [DOI] [PubMed] [Google Scholar]
- 2.Hegsted DM, McGandy RB, Myers ML, Stare FJ. Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr. 1965;17:281–295. doi: 10.1093/ajcn/17.5.281. [DOI] [PubMed] [Google Scholar]
- 3.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. Arterioscler Thromb. 1992;12:911–919. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 4.The Expert Panel. Report of the National Cholesterol Education Program Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults. Arch Intern Med. 1988;148:36–69. [PubMed] [Google Scholar]
- 5.Ahrens EH, Jr, Blankenhorn DH, Tsaltas TT. Effect on human serum lipids of substituting plant for animal fat in diet. Proc Soc Exp Biol Med. 1954;86:872–878. doi: 10.3181/00379727-86-21260. [DOI] [PubMed] [Google Scholar]
- 6.Ahrens EH, Jr, Insull W, Blomstrand R, Hirsch J, Tsaltas TT, Peterson ML. The influence of dietary fats on serum-lipid levels in man. Lancet. 1957;l:943–953. doi: 10.1016/s0140-6736(57)91280-1. [DOI] [PubMed] [Google Scholar]
- 7.Tan MH, Dickinson MA, Albers JJ, Havel RJ, Cheung M, Vigne J-L. The effect of a high cholesterol and saturated fat diet on serum high-density lipoprotein cholesterol, apoprotein A-I, and apoprotein E levels in normo-lipidemic humans. Am J Clin Nutr. 1980;33:2559–2565. doi: 10.1093/ajcn/33.12.2559. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer El, Levy RI, Ernst ND, Van Sant FD, Brewer HB., Jr The effects of a low cholesterol, high polyunsaturated fat, and low fat diets on plasma lipid and lipoprotein cholesterol levels in normal and hypercholesterolemic subjects. Am J Clin Nutr. 1981;34:1758–1763. doi: 10.1093/ajcn/34.9.1758. [DOI] [PubMed] [Google Scholar]
- 9.Ehnholm C, Huttunen JK, Pietinen P, leno U, Mutanen M, Kostlainen E, Pikkarainen J, Dougherty R, Iacomo J, Puska P. Effect of diet on serum lipoproteins in a population with a high risk of coronary heart disease. N Engl J Med. 1982;307:850–855. doi: 10.1056/NEJM198209303071403. [DOI] [PubMed] [Google Scholar]
- 10.Jackson RL, Kashyap ML, Barnhart RL, Allen C, Hogg E, Glueck CJ. Influence of polyunsaturated and saturated fats on plasma lipids and lipoproteins in man 1–3. Am J Clin Nutr. 1984;39:589–597. doi: 10.1093/ajcn/39.4.589. [DOI] [PubMed] [Google Scholar]
- 11.Sirtori CR, Tremoli E, Gatti E, Gatti E, Montanari G, Sirtori M, Colli S, Gianfranceschi G, Maderna P, Dentone CZ, Testolin G, et al. Controlled evaluation of fat intake in the Mediterranean diet: comparative activities of olive oil and corn oil on plasma lipids and platelets in high-risk patients. Am J Clin Nutr. 1986;44:635–642. doi: 10.1093/ajcn/44.5.635. [DOI] [PubMed] [Google Scholar]
- 12.Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res. 1985;26:194–202. [PubMed] [Google Scholar]
- 13.Grundy SM, Nix D, Whelan MF, Franklin L. Comparison of three cholesterol-lowering diets in normolipidemic men. JAMA. 1986;256:2351–2355. [PubMed] [Google Scholar]
- 14.Weiswieler P, Janetschek P, Schwandt P. Influence of polyunsaturated fats and fat restriction on serum lipoproteins in humans. Metabolism. 1985;34:83–87. doi: 10.1016/0026-0495(85)90065-4. [DOI] [PubMed] [Google Scholar]
- 15.Mensink RP, Katan MB. Effect of a diet enriched with monounsaturated or polyunsaturated fatty acids on levels of low-density and high-density lipoprotein cholesterol in healthy women and men. N Engl J Med. 1989;321:436–441. doi: 10.1056/NEJM198908173210705. [DOI] [PubMed] [Google Scholar]
- 16.Dreon DM, Vranizan KM, Krauss RM, Austin MA, Wood PD. The effects of polyunsaturated fat vs monounsaturated fat on plasma lipoproteins. JAMA. 1990;263:2462–2466. [PubMed] [Google Scholar]
- 17.Iacono JM, Dougherty RM. Lack of effect of linoleic acid on the high-density-lipoprotein-cholesterol fraction of plasma lipoproteins. Am J Clin Nutr. 1991;53:660–664. doi: 10.1093/ajcn/53.3.660. [DOI] [PubMed] [Google Scholar]
- 18.Wardlaw GM, Snook JT. Effect of diets high in butter, corn, oil, or high-oleic acid sunflower oil on serum lipids and apolipoproteins in men. Am J Clin Nutr. 1990;51:815–821. doi: 10.1093/ajcn/51.5.815. [DOI] [PubMed] [Google Scholar]
- 19.Wardlaw GM, Snook JT, Lin M-C, Puangco MA, Kwon JS. Serum lipid and apolipoprotein concentrations in healthy men on diets enriched in either canola oil or sunflower oil. Am J Clin Nutr. 1991;54:104–110. doi: 10.1093/ajcn/54.1.104. [DOI] [PubMed] [Google Scholar]
- 20.Valsta LM, Jauhialnen M, Aro A, Katan MB, Mutanen M. Effects of a monounsaturated rapeseed oil and a polyunsaturated sunflower oil diet on lipoprotein in humans. Arterioscler Thromb. 1992;12:50–57. doi: 10.1161/01.atv.12.1.50. [DOI] [PubMed] [Google Scholar]
- 21.Rassias G, Kestin M, Nestel PJ. Linoleic acid lowers LDL cholesterol without a proportionate displacement of saturated fatty acid. Eur J Clin Invest. 1991;45:315–320. [PubMed] [Google Scholar]
- 22.Mensink RP, Katan MB. Effect of monounsaturated fatty acids versus complex carbohydrates on high-density lipoproteins in healthy men and women. Lancet. 1987;1:122–325. doi: 10.1016/s0140-6736(87)91965-9. [DOI] [PubMed] [Google Scholar]
- 23.The Lipid Research Clinics Program Epidemiology Committee. Plasma lipid distributions in selected North American populations: the Lipid Research Clinics Program Prevalence Study. Circulation. 1979;60:427–439. doi: 10.1161/01.cir.60.2.427. [DOI] [PubMed] [Google Scholar]
- 24.Consumer and Food Economics Institute. Comparison of Foods: Raw, Processed, Prepared. Washington, DC: US Government Printing Office; 1983. [Google Scholar]
- 25.Barr SL, Ramakrishnan R, Holleran S, Ginsberg HN. A 30% fat diet high in polyunsaturates and a 30% diet high in monounsaturates both lower total and low-density lipoprotein cholesterol levels in normal males. Arteriosclersis. 1990;10:872a. Abstract. [Google Scholar]
- 26.Ginsberg HN, Barr SL, Gilbert A, Karmally W, Deckelbaum RJ, Kaplan K, Ramakrishnan R, Holleran S, Dell RB. Reduction of plasma cholesterol levels in normal men on an American Heart Association Step 1 diet or a Step 1 diet with added monounsaturated fat. N Engl J Med. 1990;322:574–579. doi: 10.1056/NEJM199003013220902. [DOI] [PubMed] [Google Scholar]
- 27.Breckenridge WC, Little JA, Steiner G, Chow A, Poapst M. Hypertriglyceridemia associated with deficiency of apolipoprotein C-11. N Engl J Med. 1978;298:1265–1273. doi: 10.1056/NEJM197806082982301. [DOI] [PubMed] [Google Scholar]
- 28.Ginsberg HN, Le N-A, Gibson JC. Regulation of the production and catabolism of plasma low density lipoproteins in hypertriglyceridemic subjects: effect of weight loss. J Clin Invest. 1985;75:614–623. doi: 10.1172/JCI111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilheimer DW, Eisenberg J, Levy RL. The metabolism of very low density lipoproteins, I: preliminary in vivo and in vitro observations. Biochim Biophys Acta. 1972;26:212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- 30.McFarlane AS. Efficient trace labeling of proteins with iodine. Nature. 1958;182:53–57. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- 31.Le N-A, Gibson JC, Ginsberg HN. Independent regulation of plasma apolipoprotein CII and CIII concentrations In very low density and high density lipoproteins: implications for the regulation of the catabolism of these lipoproteins. J Lipid Res. 1988;29:669–677. [PubMed] [Google Scholar]
- 32.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 33.Lipid Research Clinic Program. Manual of Laboratory Operations. Vol 1, Lipid and Lipoprotein Analysis. Washington, DC: National Institutes of Health; 1974. pp. 75–628. Department of Health, Education, and Welfare publication 9NIHO. [Google Scholar]
- 34.Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 35.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Gidez LI, Miller GJ, Burstein M, Slagle S, Eder HA. Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res. 1982;23:1206–1223. [PubMed] [Google Scholar]
- 37.Gibson JC, Rubinstein A, Bukberg PR, Brown WV. Apolipoprotein E enriched lipoprotein subclasses in normolipidemic subjects. J Lipid Res. 1983;24:886–898. [PubMed] [Google Scholar]
- 38.Smith SJ, Cooper GR, Henderson LO, Hannon WH the Apolipoprotein Standardization Collaborating Group. An international collaborative study on standardization of apolipoproteins A-I and B. Clin Chem. 1987;33:2240–2249. [PubMed] [Google Scholar]
- 39.Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res. 1985;26:194–202. [PubMed] [Google Scholar]
- 40.Hegsted DM, Ausman LM, Johnson JA, Dallal GE. Dietary fat and serum lipids: an evaluation of the experimental data. Am J Clin Nutr. 1993;57:875–883. doi: 10.1093/ajcn/57.6.875. [DOI] [PubMed] [Google Scholar]
- 41.Harris WS, Connor WE, Alam N, Illingworth DR. Reduction of postprandial triglyeridemia in humans by dietary n-3 fatty acids. J Lipid Res. 1988;29:1451–1460. [PubMed] [Google Scholar]
- 42.Brown AJ, Roberts DCK. Moderate fish oil intake improves lipemic response to a standard fat meal. Arterioscler Thromb. 1991;11:457–466. doi: 10.1161/01.atv.11.3.457. [DOI] [PubMed] [Google Scholar]
- 43.Scanu AM, Edelstein C, Fless GM, Eisenbart J, Sltrin M, Kasawa B, Hinman J. Postprandial lipoprotein(a) response to a single meal containing either saturated or w-3 polyunsaturated fatty acids in subjects with hypoalphalipoproteinemia. Metabolism. 1992;41:1361–1366. doi: 10.1016/0026-0495(92)90108-m. [DOI] [PubMed] [Google Scholar]
- 44.Weintraub MS, Zechner R, Brown A, Eisenberg S, Breslow JL. Dietary polyunsaturated fats of the w-6 and w-3 series reduce postprandial lipoprotein levels. J Clin Invest. 1988;82:1884–1893. doi: 10.1172/JCI113806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demacker PNM, Rijnen IGM, Katan MB, Stuyt PM, Stalenhoef FH. Increased removal of remnants of triglyceride-rich lipoproteins on a diet rich in polyunsaturated fatty acids. Eur J Clin Invest. 1991;21:197–203. doi: 10.1111/j.1365-2362.1991.tb01809.x. [DOI] [PubMed] [Google Scholar]