Abstract
At sites of Mycobacterium tuberculosis (MTB) infection, HIV-1 replication is increased during tuberculosis (TB). Here we investigated the role of positive transcription elongation factor (P-TEFb), comprised of CycT1 and CDK9, as the cellular cofactor of HIV-1 Tat protein in transcriptional activation of HIV-1 in mononuclear cells from HIV-1-infected patients with pleural TB. Expression of CycT1 in response to MTB was assessed in mononuclear cells from pleural fluid (PFMC) and blood (PBMC) from HIV/TB patients with pleural TB, and in blood monocytes (MN) from singly infected HIV-1-seropositive subjects. We then examined whether the CDK9 inhibitor, Indirubin 3′-monoxime (IM), was effective in inhibition of MTB-induced HIV-1 mRNA expression. We found higher expression of CycT1 mRNA in PFMCs as compared to PBMCs from HIV/TB-coinfected subjects. MTB induced the expression of CycT1 and HIV-1 gag/pol mRNA in both PFMCs from HIV/TB subjects and MN from HIV-1-infected subjects. CycT1 protein was also induced by MTB stimulation in PFMCs from HIV/TB patients, and both MN and in vitro-derived macrophages. Inhibition of CDK9 by IM in both PFMCs from HIV/TB and MN from HIV-1-infected subjects in response to MTB led to inhibition of HIV-1 mRNA expression. These data imply that IM may be useful as an adjunctive therapy in control of HIV-1 replication in HIV/TB dually infected subjects.
Introduction
Tuberculosis (TB) remains the most common coinfection during HIV-1 infection worldwide,1and is associated with increased viral replication at time of diagnosis of TB,2 which is sustained even after Mycobacterium tuberculosis (MTB) infection has been treated.3 Pleural TB among HIV/TB dually infected subjects constitutes up to 10% of diagnosis,4 and study of mononuclear cells from sites of pleural TB is informative of the effect of immune pressure due to coinfection on HIV-1 dynamics. Both increased transcriptional activation of HIV-15 and enhanced predisposition to viral infection6 have been shown in pleural fluid mononuclear cells (PFMCs) from HIV/TB dually infected patients. Transcriptional activation of HIV-1 by MTB and its products appears to be the predominant mechanism of enhanced viral activity at sites of HIV/TB; however, the basis of increased HIV-1 replication in situ is not clear.
The positive transcription elongation factor b (P-TEFb), composed of cyclin-dependent kinase 9 (CDK9) and cyclin T1 (CycT1), is a cellular transcription elongation factor that stimulates the processivity of RNA polymerase II (RNAPII). P-TEFb is indispensable for transactivation of HIV-LTR by viral Tat protein and thereby for transcription of the HIV-1 genome.7–9 The regulatory component of P-TEFb, CycT1, is recruited to HIVLTR by Tat to enhance interaction of Tat with the viral transactivation responsive element (TAR) RNA. This leads to hyperphosphorylation of the CTD of RNAPII by the CDK9 subunit of P-TEFb, leading to transcriptional elongation of the integrated HIV-1 genome.10,11 Combinations of cytokines, namely tumor necrosis factor alpha (TNF-α), interleukin 2 (IL-2), and interleukin 6 (IL-6), have been found to increase P-TEFb activity in resting human CD4 T cells.12 Inhibitory cellular factors13 and cytokines, such as IL-10,14 decrease P-TEFb activity. The immunologic milieu at sites of HIV/TB dual infection is rich in MTB antigens and proinflammatory cytokines such as IL-6, and therefore it is likely that sites of active TB are conducive to activation of P-TEFb in both macrophages and T cells. With this rationale, blocking viral replication by inhibition of P-TEFb activity remains an attractive therapeutic possibility in the modulation of HIV-1 replication in HIV/TB dual infection; however, this has not been investigated to date.
Recently, selective inhibition of CDK9 of P-TEFb has been shown to potently inhibit HIV-1 replication in vitro.15 A number of agents have been introduced with the capacity to inhibit the kinase activity of CDK9.16 However, the potential for affecting the transcriptional activity of other cellular genes is limiting to their use. For example, the general CDK9 inhibitor flavopiridol inhibits several T cell activation genes and DNA synthesis in primary T cells.15 Indirubin 3′-monoxime (IM) derived from Indirubin, a component of Chinese traditional medicine [Danggui Longhui Wan] with demonstrated activity against chronic myelogenous leukemia,17 inhibits CDK9 and reduces HIV-1 replication with minimal cytotoxicity to healthy cells.18 Importantly, the anti-HIV-1 activity of IM has already been examined in primary blood mononuclear cells (PBMCs) from HIV-infected subjects and its inhibitory effect on viral replication confirmed.18
The dynamics of P-TEFb expression and whether CDK9 inhibitors are useful in the modulation of HIV-1 activity in HIV/TB dually infected subjects have not been studied. Here, we examined induction of P-TEFb expression in PFMCs from patients presenting with pleural HIV/TB dual infection, and assessed if IM may be useful in the inhibition of transcriptional activation of HIV-1 in PFMCs upon stimulation by MTB. As PFMCs are mainly comprised of T cells, to assess the effect of P-TEFb induction by MTB and the effect of its inhibition by IM on HIV activity in mononuclear phagocytes, PBMCs from HIV-1 singly infected subjects were also studied.
Materials and Methods
Study subjects
Patients with signs and symptoms consistent with TB who had moderate to large pleural effusions identified at Mulago Hospital, Kampala, Uganda were recruited. The study protocol was approved by the Ugandan National AIDS Research Subcommittee and the Institutional Review Board for Human Investigations at Case Medical Center, Cleveland, Ohio. Twenty HIV-1-infected subjects and eight HIV-1-uninfected subjects who met inclusion and exclusion criteria and in whom a diagnosis of pleural TB was established were enrolled. All patients underwent thoracocentesis for the diagnosis of pleural TB. The median age of patients was 37 (range 27 ∼ 56), and the male/female ratio was 3/1. In the HIV-infected group, the median CD4 count was 154/μl (range 8 ∼ 490/μl). HIV-1 viral load was assessed by Amplicor assay (Roche, Fullerton CA); median viral load in plasma and pleural fluid was 1.7×105 (range 1.9×103–1.7×106) and 3.2×105 (range 2.2×104–3.3×106), respectively.
In some experiments, HIV-1-infected subjects in Cleveland attending the Special Immunology Unit at University Hospitals/Case Medical Center were recruited, and underwent blood draw after IRB-approved consent.
Cell isolation and characterization
PFMCs and PBMCs were prepared by Ficoll Hypaque (Pharmacia Fine Chemicals, Piscataway, NJ) density gradient centrifugation as described.19 Cell viability was over 98% as assessed by trypan blue exclusion. Frequencies of macrophages and T cells in PFMCs and PBMCs were assessed by immunostaining and FACS analysis. Conjugated antibodies to CD14, CD4, and CD3 were as before.20 In PFMCs, frequencies of CD3–CD14+ macrophages were a mean of 1–3%, and that of CD3+CD4+ T cells was 45–55%. Frequencies of macrophages and T cells in PFMCs were similar in HIV/TB and TB patients with pleural TB. In PBMCs, frequency of CD3+CD14+ monocytes (MN) was 5–8% and did not differ between the two populations.
Blood MN from HIV-infected and -uninfected subjects were obtained by adherence from PBMCs as before.21 Purity of blood adherent MN was 85% in this population. In some experiments, monocyte-derived macrophages (MDM) were obtained by culture of adherent monocytes for 5 days in RPMI containing 5% pooled human serum (complete medium).
Stimulation of mononuclear cell cultures by MTB
PFMCs, MN, or MDM were cultured at 1×106/ml in complete medium. To some cultures MTB H37Rv lysate (H37RvL), a French Press preparation of MTB H37Rv (http://www.cvmbs.colostate.edu/microbiology/tb/requestinst.htm), was added at 0.1–1 μg/ml. Cultures were harvested at 4 or 24 h.
Measurement of CycT1 and HIV gag/pol mRNA expression in PFMCs
Total RNA was obtained from mononuclear cells as before,6 and real time RT-PCR using the Taqman methodology by an ABI 7700 thermo cycler (Applied Biosystems, Foster City, CA) was employed to quantify mRNA. Taqman primer sequences for CycT1 were forward primer –TGTTCGAGCAAGCAAGGACTT– and reverse primer AGGCTAAATGTGGTCAAATGCA–, and for HIV mRNA gag/pol Clade B were forward primer –CATGTTTTCAGCATTATCAGAAGGA– and reverse primer –CCACTGTGTTTAGCATGGTGTTTAA. In either case SYBERGreen (Applied Biosystems) was used for the detection of the PCR product. Primers and probes for TNF-α and HIV-1 mRNA gag/pol for Clade A/D were as before.22 Quantities of mRNA were determined using a dilution series of target cDNA in each assay. CycT1, TNF-α, and HIV-1 mRNA copies were corrected to the copy numbers of ribosomal 18S (R18) in the same sample and expressed as number of copies/1010 copies of R18 (equivalent to 1×106 cells).6
Western blot analysis for cyclin T1
Lysates of mononuclear cells were prepared in RIPA buffer and analyzed for protein content (Bio-Rad, Palo Alto, CA) as before.13 After SDS-PAGE analysis and transfer, blots were reacted to anti-CycT1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Stripped blots were re-probed with antibody to β-actin.
Statistical analysis
Comparison between the groups was by paired t-test. A p-value of 0.05 was considered significant. Correlation between parameters was by linear regression analysis.
Results
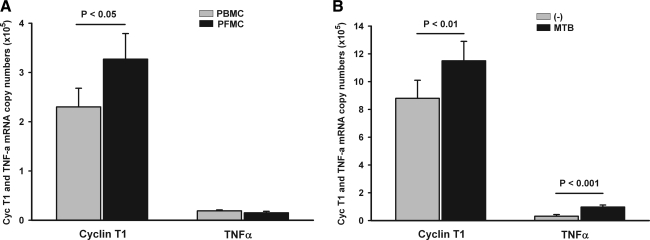
We first examined expression of CycT1 and TNF-α mRNA in PFMCs and PBMCs from HIV/TB dually infected subjects with pleural TB. PBMCs and PFMCs were assessed upon isolation from blood and pleural fluid. In PFMC expression of CycT1 mRNA was significantly higher than in PBMCs (p<0.05) (Fig. 1A). By contrast, levels of TNF-α mRNA in PFMCs and PBMCs were low and comparable. Interestingly, CycT1 was not induced significantly in PFMCs from HIV-uninfected patients (data not shown), possibly implicating additional immunologic components operative during HIV/TB infection as compared to TB alone. Induction of CycT1mRNA expression in PFMCs, i.e., the ratio of CycT1 mRNA in PFMCs over that in autologous PBMCs, correlated modestly with HIV mRNA in pleural fluid (r2=0.4). Overall, these data imply that the milieu at sites of pleural dual HIV/TB infection is conducive to up-regulation of P-TEFb expression in PFMCs.
FIG. 1.
Expression of cyclin T1 (CycT1) and tumor necrosis factor alpha (TNF-α) mRNA in pleural fluid mononuclear cells (PFMCs) and peripheral blood mononuclear cells (PBMCs) and their induction by Mycobacterium tuberculosis (MTB) H37Rv. PFMCs and PBMCs were isolated from subjects with pleural HIV/TB and assessed for CycT1 and TNF-α mRNA expression by real time RT-PCR. (A) Expression levels are compared between PFMCs (black bar) and PBMCs (gray bar) at the time of isolation of mononuclear cells from pleural fluid and blood (n=16). (B) Induction of CycT1 and TNF-α by MTB H37RvL (1 μg/ml) in PFMCs at 24 h is shown (n=12). Unstimulated (–) gray bar, MTB stimulated black bar.
Next, induction of CycT1 and TNF-α mRNA by MTB H37RvL was assessed in PFMCs in vitro. In preliminary dose–response data MTB H37RvL at 1 μg/ml was found to be optimal in the induction of CyclinT1 mRNA and protein. Therefore, PFMC cultures from HIV/TB patients received H37Rv L (1 μg/ml) or medium alone for 24 h. MTB-induced CycT1 mRNA expression in PFMCs increased by a median of 1.25 (range 1.1–2.4)-fold (p<0.01). TNF-α mRNA was also induced by a median of 3.7(1.1–7.5)-fold (p<0.001) (Fig. 1B).
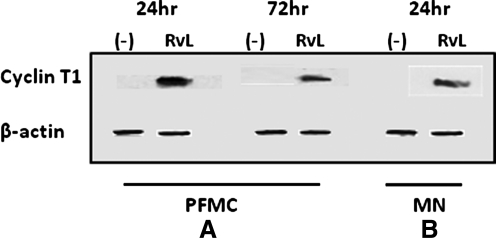
Induction of CycT1 by MTB H37RvL was also documented in PFMCs at the protein level by Western blot analysis (Fig. 2A). Interestingly, induction was optimal at 24 h and not at 72 h. As noted (Materials and Methods), PFMCs from HIV/TB patients with pleural TB are comprised mainly of T cells. However, the predominant mononuclear cell type at sites of pulmonary TB are macrophages,23 in particular immature macrophages. To ensure that MTB also induces CycT1 in MN and MDM these cell types were prepared and stimulated with MTB H37RvL in vitro. Cell lysates were prepared at 4 and 24 h. A representative experiment of CycT1 protein expression in MN is shown (Fig. 2B). Induction of CycT1 protein was optimal in MN at 4 and 24 h in MDM (data not shown) and MN, respectively.
FIG. 2.
Expression of CycT1 protein in PFMCs and mononuclear phagocytes. Mononuclear cells were stimulated with H37RvL or left unstimulated and assessed for CycT1 by Western blot analysis. (A) CycT1 expression is shown in PFMCs at 24 and 72 h. (B) CycT1 is induced in both monocytes (MN) and macrophages derived from monocytes (MDM).
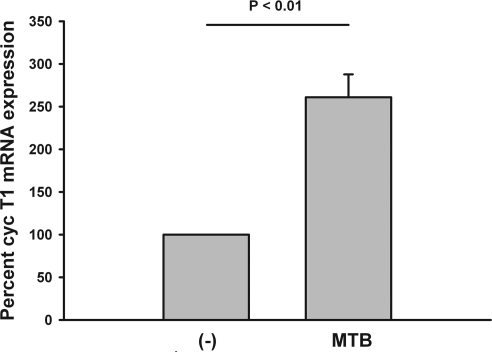
Next, adherent MN were isolated from HIV-1-infected subjects and stimulated with MTB H37RvL (1 μg/ml). CycT1 mRNA in cell lysates was assessed after 4 h of culture. Induction of CycT1 mRNA in MN from HIV-infected subjects was by 3-fold (p<0.01) (Fig. 3).
FIG. 3.
Expression of CycT1 mRNA in adherent MN from HIV-infected subjects. Adherent MN from five HIV-infected patients were prepared and stimulated with H37RvL (1 μg/ml). Induction of CycT1 mRNA expression at 24 h is shown. Data are shown as percent CycT1 activity in MTB-stimulated as compared to unstimulated (–) MN.
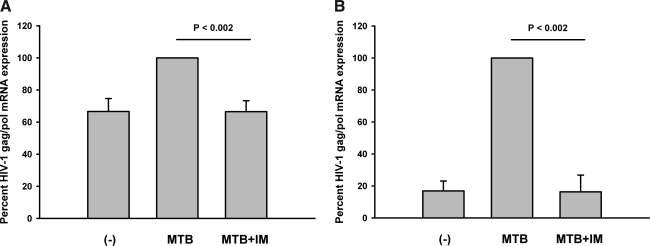
Overall, these data support an up-regulation in CycT1 expression by MTB and at sites of HIV/TB infection in both T cells and mononuclear phagocytes. However, the moiety of P-TEFb that ultimately activates gene transcription is CDK9. To assess the effect of inhibition of CDK9 on HIV-1 mRNA expression in PFMCs from HIV/TB patients, PFMCs were stimulated in vitro with MTB in the presence and absence of IM, and HIV-1 gag/pol mRNA was assessed. PFMC cultures received MTB H37Rv L (1 μg/ml) with and without IM at 0.5 or 2 μM/ml. Cultures were harvested at 24 h. In eight experiments, MTB induced HIV-1 gag/pol mRNA in PFMCs by a mean of 34% (p<0.004), and increased HIV-1 expression was inhibited back to levels in unstimulated PFMCs at both doses of IM. In the presence of IM at 0.5 μM/ml, the remaining HIV-1 gag/pol mRNA expression was reduced by a mean of 66% (p<0.002) (Fig. 4A). These doses of IM did not affect induction of host IL-2 mRNA expression in response to MTB (data not shown).
FIG. 4.
Expression of HIV-1 gag/pol mRNA in mononuclear cells induced by MTB is inhibited by IM. Mononuclear cells were stimulated with MTB H37RvL (1 μg/ml) in the presence or absence of IM, or left unstimulated (–). HIV-1 gag/pol mRNA was assessed at 24 h. (A) Results from PFMCs obtained from eight HIV/TB patients are shown. (B) Data for adherent MN from HIV-1 singly infected subjects (n=5) are shown.
To assess if IM also affects MTB-induced HIV-1 gag/pol mRNA expression in MN, adherent MN from five HIV-infected subjects were prepared. MTB H37RvL (1 μg/ml) with and without IM (0.5 μM/ml) was added to cultures. Control cultures received medium alone. After 24 h HIV-1 gag/pol mRNA for Clade B was assessed (Fig. 4B). In these experiments, induction of HIV gag/pol mRNA by MTB H37RvL was up by 80% and inhibited by IM almost completely (p<0.002).
Discussion
The cooperative binding of HIV-1 Tat and CycT1 to HIV-1 TAR activates CDK-9, which then phosphorylates RNAPII allowing increased processivity of the HIV-1genome. This step is critical to HIV-1 transcription; without P-TEFb HIV-1 transactivation is restrained to short unstable viral transcripts. Here we show that CycT1 mRNA is expressed in mononuclear cells at pleural sites of HIV/TB coinfection, and that MTB induces CycT1 in both PFMCs from HIV/TB patients and blood MN from singly HIV-1-infected subjects. Also, MTB induced CycT1 protein in both PFMCs from TB patients and monocytes/macrophages. Furthermore, inhibition of CDK9 by IM led to inhibition of MTB-induced HIV-1 transcription in PFMCs from HIV/TB and MN from HIV-1-infected patients. These data imply that IM may be useful in the control of HIV-1 replication in HIV/TB dually infected subjects.
Stimulation of primary T cells and macrophages results in the up-regulation of CycT1 and stabilization of CDK9, which in turn facilitate productive replication of HIV in infected mononuclear cells.24 Proinflammatory cytokines increase HIV transcriptional activity through both induction of CycT1,12 and directly by stimulation of CDK9 activity.25 Intracytoplasmic “free” CDK9 has been shown to interact with gp130, the common component of the receptor of the interleukin-6 (IL-6) family of cytokines.26 In this latter study, CDK9/gp130 interaction was increased further by IL-6 stimulation, and there was a synergism with IL-6 in the induction of IL-6 responsive reporter plasmids. It now appears that the IL-6 inducible transcription factor, STAT3, subsequent to its activation at the level of the IL-6 receptor, forms a complex with intracytoplasmic CDK9.27 It is possible that cytoplasmic CDK9 acts as a “shuttle” to deliver STAT3 to the nucleus. Levels of IL-6 are extremely high in pleural fluid of HIV/TB subjects (Z. Toossi, unpublished observations); therefore a cooperation between STAT3 and CDK9 facilitated by IL-6 may be a possible scenario in situ during TB. Whereas STAT3 binding sites have not been shown in HIV-LTR directly, signaling through IL-6 receptor involves activation of STAT3 and in a recent study inhibition of either IL-6 or STAT3 by sh RNA reduced HIV expression in mononuclear cells from cord blood.28 The role of CDK9/IL-6 interaction in HIV-1 gene expression needs to be further examined in PFMCs from HIV/TB patients.
Interestingly, induction of CycT1 protein in PFMCs was at earlier time points (24 h), and was not sustained, emphasizing that HIV-1 induction at sites of TB occurs as mononuclear cells are recruited and activated by MTB at sites of pleural TB. On the other hand, induction of CycT1 protein in 5 day in vitro-derived macrophages occurred with augmented kinetics as compared to blood MN. These latter data are consistent with findings previously reported.29 Differences of activation of components of P-TEFb in T cells and MN/macrophages from healthy subjects have been recently reviewed.30
Flavopiridol, an anticancer drug currently in clinical trials, and its “less cytotoxic” analogues bind and inhibit CDK9.15 However, flavopiridol interferes with key cellular processes, including T cell activation, at concentrations that inhibit HIV-1 replication.15 The toxicity profile of the derivatives of flavopiridol is currently under study. By contrast IM, derived from a Chinese traditional medicine,17 has been in use for years, and was found to be effective in the inhibition of HIV-1 gag/pol mRNA transcription in primary cells.18 Here, HIV-1 transcription was inhibited by IM in MTB-induced PFMCs from HIV/TB patients, with no effect on IL-2 expression. IM also inhibited MTB induction of HIV-1 mRNA in blood MN from HIV-1-infected subjects. This latter in vitro cell model better reflects events at pulmonary sites of dual HIV/TB infection, as mononuclear phagocytes comprise the majority of cells in the lung during pulmonary TB.23
Inhibition of HIV gag/pol mRNA in MN by IM was as efficient as that in PFMCs, considering baseline HIV-1 transcription. Due to the relatively “less activated” state of mononuclear cells in blood from singly HIV-infected subjects as compared to mononuclear cells at sites of dual HIV/TB infection, up-regulation of HIV-1 transcription by MTB was strong (by 80%) (Fig. 4B). However, almost all HIV-1 mRNA induction was inhibited by IM in blood MN from HIV-infected subjects. On the other hand, HIV-1 transcriptional activity was higher in unactivated PFMCs (Fig. 4A) and was induced by MTB by only by 34%, although it was efficiently inhibited by IM also. However, it is still possible that mononuclear phagocytes are more sensitive to inhibition of CDK9 than T cells upon activation. These issues need to be addressed more carefully in future studies of blood or tissue mononuclear phagocytes from TB patients.
Overall these data provide a framework with which to understand the basis of transcriptional activation of HIV at sites of MTB infection, and the possibility of using an inhibitor of P-TEFb as a therapeutic modality to reduce HIV-1 replication during HIV/TB infection. As shown here, IM, an agent with rather limited cytotoxicity, is effective in the inhibition of HIV-1 transcription. However, further investigations with mononuclear cells from HIV/TB patients with the more common form of pulmonary TB will be required before clinical trials to examine the effect of IM in the inhibition of HIV-1 transcription can be initiated.
Acknowledgment
This study was supported by NIH grants (HL-51636, AI-62516, AI-95383), by AmFAR (106386-33-RGGN), and by CFAR at CWRU (AI-36219).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dye C. Lonnroth K. Jaramillo E. Williams BG. Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ. 2009;87(9):683–691. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toossi Z. Mayanja-Kizza H. Hirsch CS, et al. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol. 2001;123(2):233–238. doi: 10.1046/j.1365-2249.2001.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kizza HM. Rodriguez B. Quinones-Mateu M, et al. Persistent replication of human immunodeficiency virus type 1 despite treatment of pulmonary tuberculosis in dually infected subjects. Clin Diagn Lab Immunol. 2005;12(11):1298–1304. doi: 10.1128/CDLI.12.11.1298-1304.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter C. Perenboom R. Mtoni I, et al. Clinical features of HIV-seropositive and HIV-seronegative patients with tuberculous pleural effusion in Dar es Salaam, Tanzania. Chest. 1994;106(5):1471–1475. doi: 10.1378/chest.106.5.1471. [DOI] [PubMed] [Google Scholar]
- 5.Toossi Z. Johnson JL. Kanost RA, et al. Increased replication of HIV-1 at sites of Mycobacterium tuberculosis infection: Potential mechanisms of viral activation. J Acquir Immune Defic Syndr. 2001;28(1):1–8. doi: 10.1097/00042560-200109010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Toossi Z. Mayanja-Kizza H. Baseke J, et al. Inhibition of human immunodeficiency virus-1 (HIV-1) by beta-chemokine analogues in mononuclear cells from HIV-1-infected patients with active tuberculosis. Clin Exp Immunol. 2005;142(2):327–332. doi: 10.1111/j.1365-2249.2005.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujinaga K. Cujec TP. Peng J, et al. The ability of positive transcription elongation factor B to transactivate human immunodeficiency virus transcription depends on a functional kinase domain, cyclin T1, and Tat. J Virol. 1998;72(9):7154–7159. doi: 10.1128/jvi.72.9.7154-7159.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garber ME. Wei P. KewalRamani VN, et al. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 1998;12(22):3512–3527. doi: 10.1101/gad.12.22.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali A. Ghosh A. Nathans RS, et al. Identification of flavopiridol analogues that selectively inhibit positive transcription elongation factor (P-TEFb) and block HIV-1 replication. Chembiochem. 2009;10(12):2072–2080. doi: 10.1002/cbic.200900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garriga J. Peng J. Parreno M. Price DH. Henderson EE. Grana X. Upregulation of cyclin T1/CDK9 complexes during T cell activation. Oncogene. 1998;17(24):3093–3102. doi: 10.1038/sj.onc.1202548. [DOI] [PubMed] [Google Scholar]
- 11.Wei P. Garber ME. Fang SM. Fischer WH. Jones KA. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92(4):451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 12.Ghose R. Liou LY. Herrmann CH. Rice AP. Induction of TAK (cyclin T1/P-TEFb) in purified resting CD4(+) T lymphocytes by combination of cytokines. J Virol. 2001;75(23):11336–11343. doi: 10.1128/JVI.75.23.11336-11343.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadlowsky JK. Nojima M. Schulte A. Geyer M. Okamoto T. Fujinaga K. Dominant negative mutant cyclin T1 proteins inhibit HIV transcription by specifically degrading Tat. Retrovirology. 2008;5:63. doi: 10.1186/1742-4690-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y. Rice AP. Interleukin-10 inhibits HIV-1 LTR-directed gene expression in human macrophages through the induction of cyclin T1 proteolysis. Virology. 2006;352(2):485–492. doi: 10.1016/j.virol.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Salerno D. Hasham MG. Marshall R. Garriga J. Tsygankov AY. Grana X. Direct inhibition of CDK9 blocks HIV-1 replication without preventing T-cell activation in primary human peripheral blood lymphocytes. Gene. 2007;405(1–2):65–78. doi: 10.1016/j.gene.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biglione S. Byers SA. Price JP, et al. Inhibition of HIV-1 replication by P-TEFb inhibitors DRB, seliciclib and flavopiridol correlates with release of free P-TEFb from the large, inactive form of the complex. Retrovirology. 2007;4:47. doi: 10.1186/1742-4690-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoessel R. Leclerc S. Endicott JA, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1(1):60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 18.Heredia A. Davis C. Bamba D, et al. Indirubin-3′-monoxime, a derivative of a Chinese antileukemia medicine, inhibits P-TEFb function and HIV-1 replication. AIDS. 2005;19(18):2087–2095. doi: 10.1097/01.aids.0000194805.74293.11. [DOI] [PubMed] [Google Scholar]
- 19.Lawn SD. Pisell TL. Hirsch CS. Wu M. Butera ST. Toossi Z. Anatomically compartmentalized human immunodeficiency virus replication in HLA-DR+ cells and CD14+ macrophages at the site of pleural tuberculosis coinfection. J Infect Dis. 2001;184(9):1127–1133. doi: 10.1086/323649. [DOI] [PubMed] [Google Scholar]
- 20.Vanham G. Penne L. Allemeersch H, et al. Modeling HIV transfer between dendritic cells and T cells: Importance of HIV phenotype, dendritic cell-T cell contact and T-cell activation. AIDS. 2000;14(15):2299–2311. doi: 10.1097/00002030-200010200-00011. [DOI] [PubMed] [Google Scholar]
- 21.Toossi Z. Sierra-Madero JG. Blinkhorn RA. Mettler MA. Rich EA. Enhanced susceptibility of blood monocytes from patients with pulmonary tuberculosis to productive infection with human immunodeficiency virus type 1. J Exp Med. 1993;177(5):1511–1516. doi: 10.1084/jem.177.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayanja-Kizza H. Wu M. Aung H, et al. The interaction of monocyte chemoattractant protein-1 and tumour necrosis factor-alpha in Mycobacterium tuberculosis-induced HIV-1 replication at sites of active tuberculosis. Scand J Immunol. 2009;69(6):516–520. doi: 10.1111/j.1365-3083.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- 23.Schwander SK. Sada E. Torres M, et al. T lymphocytic and immature macrophage alveolitis in active pulmonary tuberculosis. J Infect Dis. 1996;173(5):1267–1272. doi: 10.1093/infdis/173.5.1267. [DOI] [PubMed] [Google Scholar]
- 24.Marshall RM. Salerno D. Garriga J. Grana X. Cyclin T1 expression is regulated by multiple signaling pathways and mechanisms during activation of human peripheral blood lymphocytes. J Immunol. 2005;175(10):6402–6411. doi: 10.4049/jimmunol.175.10.6402. [DOI] [PubMed] [Google Scholar]
- 25.Brasier AR. Expanding role of cyclin dependent kinases in cytokine inducible gene expression. Cell Cycle. 2008;7(17):2661–2666. doi: 10.4161/cc.7.17.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falco GD. Neri LM. Falco MD, et al. Cdk9, a member of the cdc2-like family of kinases, binds to gp130, the receptor of the IL-6 family of cytokines. Oncogene. 2002;21(49):7464–7470. doi: 10.1038/sj.onc.1205967. [DOI] [PubMed] [Google Scholar]
- 27.Hou T. Ray S. Brasier AR. The functional role of an interleukin 6-inducible CDK9.STAT3 complex in human gamma-fibrinogen gene expression. J Biol Chem. 2007;282(51):37091–37102. doi: 10.1074/jbc.M706458200. [DOI] [PubMed] [Google Scholar]
- 28.Sundaravaradan V. Mehta R. Harris DT. Zack JA. Ahmad N. Differential expression and interaction of host factors augment HIV-1 gene expression in neonatal mononuclear cells. Virology. 2010;400(1):32–43. doi: 10.1016/j.virol.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Liou LY. Herrmann CH. Rice AP. Transient induction of cyclin T1 during human macrophage differentiation regulates human immunodeficiency virus type 1 Tat transactivation function. J Virol. 2002;76(21):10579–10587. doi: 10.1128/JVI.76.21.10579-10587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice AP. Herrmann CH. Regulation of TAK/P-TEFb in CD4+ T lymphocytes and macrophages. Curr HIV Res. 2003;1(4):395–404. doi: 10.2174/1570162033485159. [DOI] [PubMed] [Google Scholar]