Abstract
To assess the influence of mannosylated glycans on the immunogenicity of human immunodeficiency virus type 1 (HIV-1) Env proteins, we immunized mice with monomeric gp120 in the presence and absence of the mannose-binding protein, griffithsin (GRFT). For comparison, other groups of mice received the nonglycosylated HIV-1 Gag protein, with and without GRFT. Coimmunization with GRFT increased the anti-gp120 IgG reactivity significantly, but had no effect on the anti-Gag response. We also investigated the IgG response to GRFT and found that gp120, but not Gag, enhanced its immunogenicity. For both proteins, IgG1 antibodies dominated the IgG response, with IgG2b as the next most prevalent subclass. We conclude that gp120–GRFT complexes are more immunogenic than the free proteins, for both components, and that occluding the mannose moieties on monomeric gp120 can improve the humoral immune response to this protein.
Introduction
The envelope glycoprotein complex, Env, of human immunodeficiency virus type 1 (HIV-1) mediates virus entry into susceptible cells and is the only target for antibodies that can neutralize the virus (NAbs). No Env immunogen has been able to induce NAbs of sufficient potency and breadth to protect against virus transmission. A central element of vaccine design is therefore to engineer Env variants that specifically direct the antibody response to conserved neutralization epitopes.1
A related vaccine development goal is to improve the overall immunogenicity of Env. The outer Env glycoprotein gp120 is a weak immunogen in rodents and primates, even when delivered in experimental, effective adjuvants.2 Thus, the dose of gp120 used in human trials is 200–500 μg, given up to seven times.3–5 Even then, gp120-binding antibody titers decay rapidly, and NAb titers against primary isolates are negligible.2 In an attempt to mimic the native Env glycoprotein spike on the virion and expose predominantly the neutralization epitopes, various gp140 trimers have been produced.6,7 High quantities of gp140 are also required for immunization, which is a concern because good quality trimers can be difficult to manufacture in bulk. Hence there is a need to improve the immunogenicity of Env proteins in general, so that an effective immune response is induced by the lowest possible amount of protein. Moreover, a general increase in immunogenicity might reveal NAb activities that would otherwise fall below the level of detection.
Gp120 is an unusual immunogen. Half its mass consists of N-linked glycans, many of which contain terminal mannose moieties. It is also a biologically active protein that signals to cells of the immune system by binding to receptors, including mannose-binding C-type lectin receptors (MCLRs) such as DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin).2 These interactions can trigger the secretion of interleukin (IL)-10 and other cytokines, thereby modulating the activation of immune cells.2,8–10 In vitro, gp120 can have multiple, generally adverse, effects on various cells of the immune system at concentrations that might be similar to those present locally after immunization.2,11
We have shown that mannose-depleted gp120 is more immunogenic in mice, inducing higher gp120-binding antibody titers than the same amount of unmodified gp120 when delivered in Alum adjuvant.12 We sought to extend these observations by blocking gp120–MCLR interactions in a different way, i.e., by ligating the terminal mannose residues with the oligomannose-binding protein, griffithsin (GRFT). This small protein (a homodimer comprised of two 13-kDa subunits), isolated from the red algae Griffithsia sp., binds and cross-links oligomannose residues on gp120, and several other different virus envelope glycoproteins, via six carbohydrate-binding pockets per GRFT dimer.13–16 We first showed that like demannosylation, GRFT inhibits gp120 binding to DC-SIGN; we then immunized mice with gp120 in its presence and absence. The anti-gp120 IgG responses in the mice coimmunized with GRFT were stronger than in those given only gp120. However, GRFT did not enhance the murine IgG response to the nonglycosylated HIV-1 Gag protein, suggesting that its effect on the anti-gp120 response is mannose specific. We also observed that the proimmunogenic effect was mutual, in that gp120 but not Gag enhanced the response to GRFT, which is by itself a very poor immunogen (K.E. Palmer and A.B. Lasnik, unpublished observations). We discuss how the increased immunogenicity of gp120–GRFT complexes might shed light on the underlying mechanism and help Env vaccine design.
Materials and Methods
Reagents
Full-length, CHO-cell expressed gp120 (HIV-1JR-FL), the monoclonal antibody (MAb) PA-1, sCD4, and CD4-IgG2 were gifts from Dr. William Olson (Progenics Pharmaceuticals, Tarrytown, NY). The MAb b12 was donated by Dennis Burton (The Scripps Research Institute, La Jolla, CA), the MAbs 17b and 39F by James Robinson (Tulane University, New Orleans, LA), VRC-01 by Peter Kwong and John Mascola (Vaccine Research Center, Washington, DC), 2G12 by Hermann Katinger through the AIDS Research and Reference Reagent Program (ARRRP, Division of AIDS, NIAID, NIH), and HIV-Ig by the ARRRP. Purified recombinant p41-Gag (HIV-1HXB2), made by the Protein Purification Group at the National Cancer Institute (Frederick, MD), was donated by Robert Seder.17 The red-algal protein GRFT has been described elsewhere.13–15 We used plant-produced GRFT15 with a purity >99.5%. Both GRFT and p41-Gag (henceforth referred to as Gag) have been used in animal studies previously. Their endotoxin contents were <0.01 EU per mg of GRFT and <0.1 EU per mg of Gag. DC-SIGN-Fc was purchased from R&D Systems (Minneapolis, MN). Goat D7324 Ab was purchased from Aalto Bioreagents, Dublin, Ireland.
DC-SIGN-binding assay
The gp120-capture enzyme-linked immunosorbent assay (ELISA) adapted for DC-SIGN binding has been previously described.18 GRFT, serially diluted in TSM buffer (20 mM Tris, 150 mM NaCl, 1.0 mM CaCl2, 2.0 mM MgCl2) supplemented with 0.01% bovine serum albumen (BSA), was mixed with gp120 for 1 h, before the addition of HIVIg, 2G12, CD4-IgG2, 39F, PA1, b12, VRC01, and 17b (all at 0.1 μg/ml), the latter±sCD4 (at 1 μg/ml), or DC-SIGN-Fc (at 1.0 μg/ml) in TSM/5% BSA for 2 h. After three washes with TSM, supplemented with 0.05% Tween-20, horseradish peroxidase (HRP)-labeled goat-antihuman immunoglobulin G (IgG) (Jackson Immunoresearch) was added at a 1:5000 dilution (final concentration 0.2 μg/ml) in TSM/5% BSA for 30 min. The plates were washed five times with TSM/0.05% Tween-20 before colorimetric detection with a solution containing 1% 3,3′,5,5′-tetramethylbenzidine (TMB, Sigma-Aldrich, Zwijndrecht, The Netherlands), 0.01% H2O2 in 0.1 M sodium acetate, and 0.1 M citric acid. The colorimetric reaction was stopped by 0.8 M H2SO4 when appropriate, and absorption was measured at 450 nm.
Immunization of mice
Eight-week-old, female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were immunized (five per group) at weeks 0, 2, 4, and 6, and bled at weeks 4, 6, and 8. Drawn blood was allowed to clot for 1 h at room temperature. The serum was then collected and stored in aliquots at −20°C. In all primary and boosting immunizations, a 150 μl formulation containing either gp120 or Gag mixed with 250 μg of Alum (Alhydrogel; Accurate Chemical, Westbury, NY), with or without GRFT, was injected subcutaneously into the groin region. To make the immunizing mixture, GRFT (1.2 μg) was incubated at room temperature for 1 h with gp120 (5 μg) or Gag (2 μg) prior to Alum addition. The concentrations of gp120 and Gag were chosen to be equimolar, and were optimally immunogenic doses based on preliminary studies. GRFT was used at a 2-fold molar excess over gp120 and Gag. During the coincubation the GRFT concentration was 0.6 μM, which is two orders of magnitude above its Kd for binding to gp120.14
All experimental procedures were conducted in accordance with protocols approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee.
ELISA for anti-gp120, anti-Gag, anti-GRFT, and anti-gp120–GRFT complexes
The gp120-capture ELISA procedure has been described previously.19 Briefly, the plates were coated with 10 μg/ml of sheep Ab D7324 to the gp120 C-terminus (Cliniqa Corp, San Marcos, CA, Cat. #6205). Recombinant, CHO-cell expressed JR-FL gp120 [300 ng/ml in Tris-buffered saline (TBS)] was then captured onto the bound D7324.
The assay was varied to allow detection of Abs preferentially reacting with gp120–GRFT complexes. GRFT was allowed to bind to D7324-captured gp120, after which the plates were washed only once with TBS instead of the normal three times. Three conditions were tested at the same time: gp120 followed by incubation with GRFT at 600 ng/ml in TBS, gp120 followed by incubation with only TBS, and mock capture (no gp120) followed by GRFT at 600 ng/ml. After incubation with or without GRFT for 1 h at room temperature, the plates were washed three times with TBS and then treated as in the standard gp120 capture ELISA.
In another alternative procedure (used for gp120-binding IgG2a Abs, because the antimurine IgG2a Ab cross-reacts with the capture Ab, D732412), ELISA wells were directly coated with gp120 (5 μg/ml in carbonate buffer overnight). For anti-Gag IgG, and IgG subclass detection, the wells were directly coated overnight with Gag (1 μg/ml in carbonate buffer).
In all the above variant assays, mouse serum (3-fold serial dilutions from 1:100) was added to the gp120- or Gag-containing wells, followed by alkaline phosphatase (AP)-conjugated detection antibody (see below). The AMPAK Enzyme Amplification Kit (Dakocytomation, Ely, UK) and an E max precision microplate reader (Molecular Devices, Sunnyvale, CA) were used to provide a colorimetric endpoint (OD 490 nm).
The detection antibodies for antimouse IgG and IgG subclass antibodies have been described previously.12 Antimouse total IgG and IgG2a antibodies were detected with AP-conjugated polyclonal goat antimouse IgG and antimouse IgG2a (1:2000; AbD Serotec, Oxford, UK; Cat. # STAR117A and STAR 82A, respectively). To detect IgG1, IgG2b, and IgG3 Abs, we used unconjugated rat antimouse IgG1 (1 μg/ml, AbD Serotec, Cat. #MCA1289), IgG2b (2.5 μg/ml, BD Biosciences, Cat. #553392), and IgG3 (2.5 μg/ml, AbD Serotec, Cat. #MCA1292). The bound IgG1-, IgG2b-, and IgG3-specific Abs were then detected with a 1:30,000 dilution of AP-conjugated rabbit antirat IgG (Sigma, Cat. #A6066), a reagent we confirmed to be reactive with neither mouse IgG nor the sheep D7324 capture Ab.
To detect IgG specific for GRFT we used an ELISAs based on a fusion protein comprising the green fluorescent protein (GFP) fused to GRFT [2.5 μg/ml in phosphate-buffered saline (PBS)]. The GRFT-GFP was directly coated onto Nunc Maxisorp plates overnight at 4°C. The wells were blocked for 2 h at room temperature with 5% nonfat dry milk in PBS-T. Samples were serially diluted in PBS (2-fold steps starting at 1:150) and added for 2 h before washes and addition of the secondary antibody (goat antimouse IgG-HRP; Southern Biotech; 1:10,000 in PBS) for 1 h. All washes were performed with an automated plate washer (Immunowash, Bio-Rad). A colorimetric endpoint was derived by the use of KPL SureBlue TMB Microwell Peroxidase Substrate, and the reaction was stopped by the addition of 0.5 M H2SO4. The plates were read at 450 nm on a BioTek Synergy HT reader, and data were collected with Gen5 Software. The reciprocal endpoint dilutions at which the OD was equal to twice background were calculated.
Anti-GRFT IgG subclasses were quantified by the GFP-GRFT direct-coating assay described above. The appropriate secondary antibody (goat antimouse IgG1, IgG2a, IgG2b, or IgG3, all from Southern Biotech) was added at 1:10,000 in PBS for 1 h. Endpoint dilutions were calculated as for total IgG.
Statistical analyses
Area under the curve (AUC) was calculated in Prism (Graphpad). Differences between groups were compared by the Mann–Whitney U test (one tail). When the reactivities of the same sera with GRFT-complexed and -uncomplexed gp120 were compared, the Wilcoxon matched pairs test, giving significant effect of the sample pairing, was applied. The relationship between the responses to gp120 and GRFT in coimmunized animals was explored by Spearman rank correlation. The α level of significance was stipulated as p=0.05.
Results
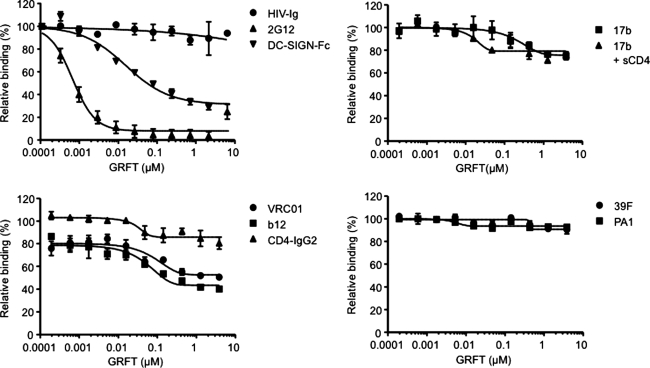
We first showed that GRFT inhibited the binding of both MAb 2G12 and the DC-SIGN-Fc protein to gp120, although the block of DC-SIGN binding was incomplete (Fig. 1). These findings are consistent with the known properties of GRFT.14 The 2G12 and DC-SIGN-Fc binding sites on gp120 both involve the terminal mannose moieties on N-linked glycans, and gp120 from which the mannoses have been removed enzymatically does not bind these ligands.10,20,21 GRFT did not affect the gp120 binding of HIV-Ig (polyclonal IgG prepared from HIV+ plasma) or of the two V3-directed gp120 MAbs 39F and PA1 (Fig. 1). Other epitopes were indirectly affected. Thus, CD4-IgG2 was slightly reduced (by ∼20%), but the binding of the two CD4BS MAbs b12 and VRC01 was reduced more, by ∼50% at the highest concentrations of GRFT and by ∼20% at the lowest (Fig. 1). The binding of MAb 17b to its CD4-induced epitope on gp120 was also slightly reduced (by ∼20%) by GRFT, both in the presence and absence of sCD4 (Fig. 1).
FIG. 1.
Partial block of gp120 binding to human DC-SIGN by griffithsin (GRFT). The binding to gp120 of HIV-Ig, 2G12, and DC-SIGN-Fc (upper left panel), 17b±sCD4 (upper right panel), VRC01, b12, and CD4-IgG2 (lower left panel), and 39F and PA1 (lower right panel) in the presence of varied concentrations of GRFT (x-axis) is normalized to that in the absence of GRFT (defined as 100%). The data are representative of two to three experiments. The means±SEM of two replicates in one ELISA are shown.
To further test the hypothesis that the immunogenicity of gp120 is impaired by its oligomannose moieties,10,12 we immunized mice with gp120 (5 μg) with and without a 2-molar excess of GRFT (1.2 μg), in Alum adjuvant. The GRFT preparation had been tested extensively to rule out nonspecific intrinsic inflammatory activity on cells of the human and animal immune systems.15 Nonetheless, we controlled for nonspecific effects by also immunizing mice with the nonglycosylated HIV-1 Gag protein (2 μg, an amount equimolar to the gp120 dose) with and without GRFT (1.2 μg).
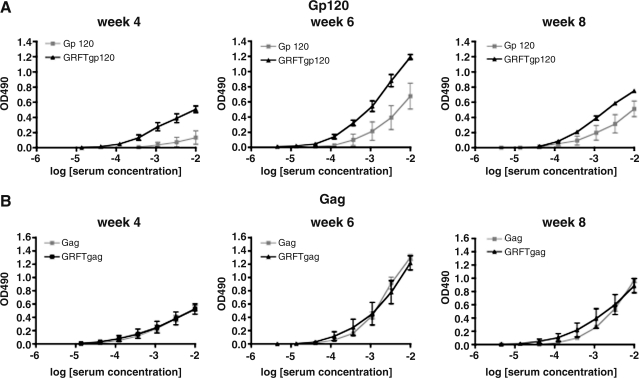
At all three time points tested, the mice immunized with gp120–GRFT had developed stronger anti-gp120 IgG responses than mice given only gp120 (Fig. 2A). The serum titration curves did not approach plateaus, which precluded the precise determination of mid-point titers. However, inspection of the curves suggests the anti-gp120 titer increase conferred by GRFT coimmunization is ∼10-fold. As an alternative way to assess the outcome of GRFT coimmunization, we calculated the area under the curve (AUC) for each mouse at each time point, a procedure that takes all data points into consideration. The AUC values were significantly higher at the two first time points and tended in the same direction at the last (week 4: p=0.0079, week 6: p=0.028, and week 8: p=0.11). In contrast to its enhancement of the anti-gp120 IgG response, GRFT had no effect on the corresponding Gag response (Fig. 2B). Thus, the AUC values were similar in the Gag and Gag–GRFT groups at all three time points (week 4: p=0.42, week 6: p=0.34, and week 8: p=0.50). The responses to gp120 and Gag peaked at week 6, with a clear decline at week 8; thus the immunizations at week 6 failed to boost the responses. This decline occurred both in the presence and absence of GRFT with both immunogens.
FIG. 2.
Effect of GRFT on the IgG responses to gp120 and Gag. The anti-gp120 (A) and anti-Gag (B) IgG responses are shown for three time points after immunization as background-corrected OD490 values. The animals received either gp120 or Gag with or without GRFT, as indicated. The data points represent the means±SEM for the five mice in each group.
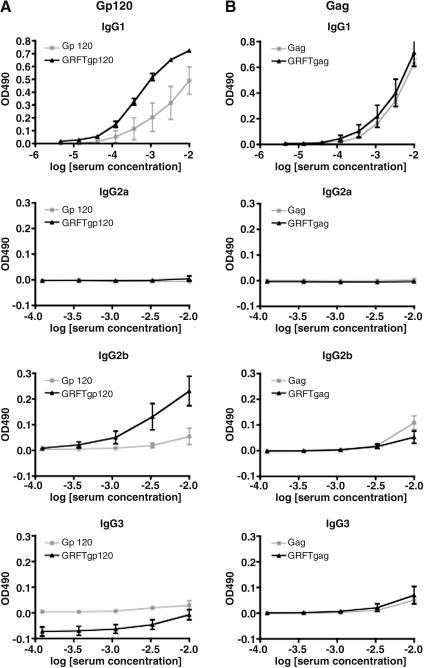
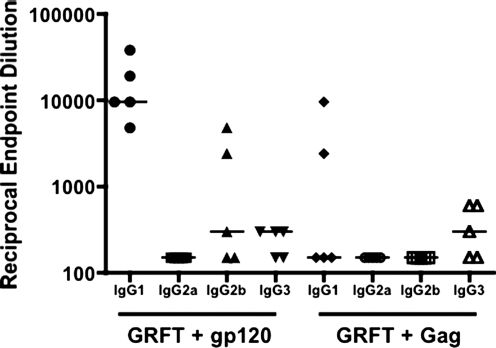
We also measured anti-gp120 IgG subclasses in the four groups of mice. IgG1 dominated the IgG response to gp120, IgG2b being the only other subclass detected, and only at week 6 (Fig. 3A). The IgG1 dominance and the complete absence of IgG2a reactivity indicate that the anti-gp120 response, including the enhancement by GRFT, was TH2-polarized. The increased anti-gp120 IgG response seen in the presence of GRFT was attributable to IgG1 and IgG2b; for IgG1 the difference was of borderline significance (p=0.075) and for IgG2b it was more substantial (p=0.0079). The IgG subclass response to Gag was even more dominated by IgG1, and it was unaffected by the presence of GRFT (Fig. 3B).
FIG. 3.
Isotype profile of the IgG response to gp120 and Gag in the presence and absence of GRFT. The IgG1, IgG2a, IgG2b, and IgG3 subclass responses at week 6 are shown as background-corrected OD490 values. (A) Anti-gp120; (B) anti-Gag. The data points represent the means (±SEM) for the five mice in each group. Note that the scales on the y-axes differ between the diagrams for IgG1, which is the dominant IgG subclass, and the minor subclasses, IgG2a, IgG2b, and IgG3.
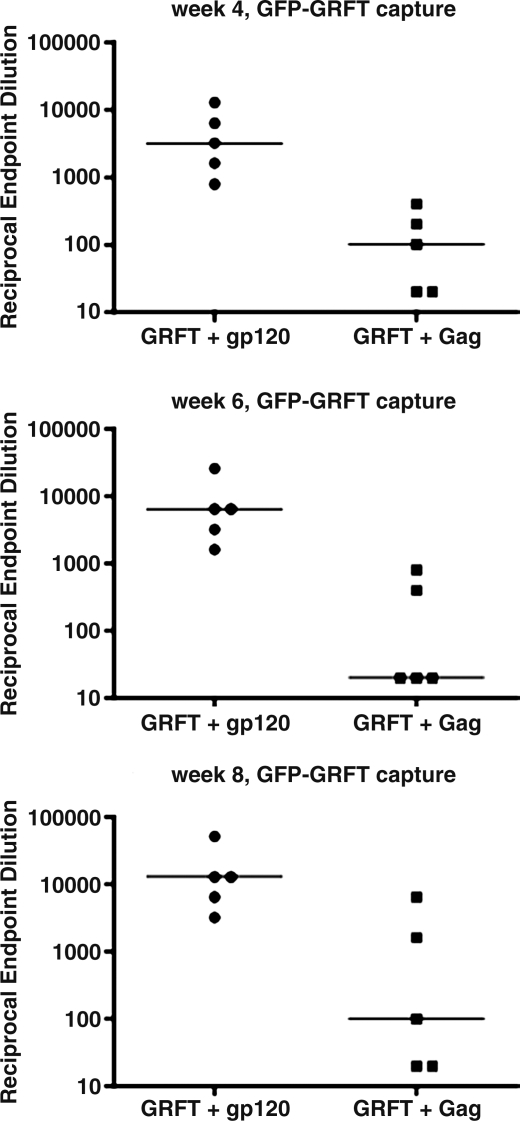
Since half the mice received GRFT together with either gp120 or Gag, we were also able to compare the effect of these proteins on the antibody response to GRFT (Fig. 4). Coimmunizing with gp120 yielded anti-GRFT IgG endpoint titers 100- to 1000-fold higher than with Gag. The difference was highly significant (p=0.0040 for each of the three time points). Unlike the IgG response to gp120 and Gag, the titers against GRFT did not decline from week 6 to 8 but increased modestly (∼2-fold).
FIG. 4.
IgG responses to GRFT in mice coimmunized with gp120 or Gag. The anti-GRFT IgG endpoint titers at the indicated time points were analyzed by GRFT-GFP direct-coating assay. The median values among the mice are indicated by horizontal bars.
The IgG subclass response to GRFT was also dominated by IgG1, with a smaller contribution from IgG2b (Fig. 5). The anti-GRFT IgG1 titers were significantly higher in the gp120–GRFT group than the Gag–GRFT group (p=0.016), while IgG2b responses were detectable only in the gp120–GRFT group (in three of five mice). When data for individual mice were inspected, we noted that the IgG2b responses to GRFT and gp120 arose in different animals (data not shown). IgG3 antibodies to GRFT were detected in three of five mice in both the gp120–GRFT and Gag–GRFT coimmunization groups, but no IgG2a was detected. Thus, overall, the gp120-enhanced GRFT immunogenicity was distinctly TH2-polarized.
FIG. 5.
IgG subclass responses to GRFT in mice coimmunized with gp120 or Gag. The anti-GRFT IgG endpoint titers were analyzed by direct-coating GFP-GRFT ELISA. Only results from week 8, when total IgG reactivity with GRFT was strongest, are shown.
To investigate the basis for the mutually enhanced immunogenicities of GRFT and gp120, we compared the antibody responses to the two antigens in each of the five coimmunized mice at week 6. There was no correlation between the anti-gp120 and the anti-GRFT responses (Spearman rank correlation, r=–0.41 and p=0.52). For example, the mouse with the strongest response to GRFT had the weakest one to gp120. The mutually enhanced immunogenicity was not therefore a simple reflection of generally elevated IgG responses to the gp120–GRFT complex.
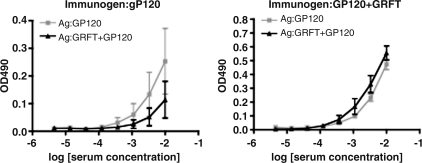
We redesigned the gp120-capture ELISA to allow detection of Abs that preferentially recognize gp120–GRFT complexes. In this analysis, we compared the sera from week 6 from the five mice that received both gp120 and GRFT with the corresponding sera from the five mice given only gp120. Gp120 was captured with or without the subsequent addition of GRFT (in 20-fold molar excess). As a control for nonspecific binding, GRFT was added to the plates in the absence of gp120. Neither group of sera at 1/100 dilution reacted in the GRFT control plates; the background-corrected mean OD490±SEM values were 0.012±0.011 for the gp120-immunized mice and 0.0060±0.0053 for mice coimmunized with gp120 and GRFT. This control experiment showed that only GRFT–gp120 complexes, and not nonspecifically adsorbed GRFT, contributed to the binding of Abs in the following ELISAs.
Sera from mice immunized with only gp120 reacted more weakly with gp120–GRFT complexes than with gp120 (p=0.031, Wilcoxon matched pairs test). Thus GRFT shields some of the gp120 epitopes recognized by Abs elicited by uncomplexed gp120. Conversely, the reactivity of sera from mice coimmunized with gp120 plus GRFT was marginally higher against gp120–GRFT complexes than against gp120 alone (p=0.16, NS, Wilcoxon matched pairs test) (Fig. 6). The reactivity with gp120–GRFT complexes, when normalized to reactivity with gp120 alone, was significantly higher for sera from the coimmunized mice than sera from mice given only gp120 (p=0.0040, Mann–Whitney U test). Hence the elicited Abs showed a net preference for the antigenic forms present in the immunogen. Any specific recognition of gp120–GRFT complexes by Abs in sera from coimmunized mice must, however, be modest. The higher relative reactivity of the sera from coimmunized mice with gp120–GRFT complexes than with gp120 may be at least partly attributable to Abs reactive with the GRFT moiety of the complex.
FIG. 6.
Antigenic basis for enhanced IgG responses in gp120–GRFT coimmunized mice. Recognition of gp120–GRFT complexes by week-6 sera from mice immunized with gp120 alone (left panel) or with gp120 plus GRFT (right panel). The data points show average OD490 after background subtraction±SEM as a function of log serum concentration. Serum reactivity with gp120 captured alone is represented by gray squares and with gp120–GRFT complexes by black triangles. Note that the scales on the y-axes differ in order to demonstrate the variations within the weaker responses (left-hand diagram, immunization without GRFT) and the stronger ones (right-hand diagram, immunization with GRFT).
Discussion
The principal observation of this study is that coimmunization with GRFT enhances the IgG response to gp120 in mice, the serum titration curves for the coimmunized mice being shifted ∼10-fold to the left. The enhancement of immunogenicity by GRFT was specific for gp120. There was no such effect in mice that received GRFT together with the nonglycosylated Gag protein. Hence it is reasonable to assume that the action of GRFT is mediated by its binding to the gp120 mannose moieties.
The particular mannose-dependent epitope recognized by 2G12, a prototypical cross-neutralizing Ab, is rarely immunogenic during infection, although Abs are readily elicited to other mannose epitopes.22,23 Obviously, blocking mannoses will not facilitate the elicitation of Abs with the rare 2G12 specificity, for which goal other means must be pursued.22–24 Here we focus on the general IgG response to gp120 that is directed to other, mannose-independent epitopes.
The present results are broadly consistent with those of our earlier study in mice, in which we showed that mannose-depleted gp120 transiently elicited ∼50-fold higher binding antibody titers than mannosylated gp120.12 However, the enhancement in that study was somewhat stronger than the modest and transient effect we report here. Although there could be advantages to occluding rather than digesting off mannose residues, there are also potential disadvantages. On the one hand, while deglycosylation could accelerate proteolysis of the immunogen, the formation of GRFT-gp120 complexes might protect gp120 and prolong its tissue half-life. On the other hand, the occluding molecule, GRFT, may attract some of the immune response to itself, at the expense of desired Ab responses to gp120. Furthermore, we found that GRFT partly blocks the binding of NAbs b12 and VRC01 to the CD4-binding site (CD4BS) on gp120, suggesting that the lectin would compromise the immunogenicity of this important epitope cluster. An explanation for the partial inhibition of b12 and VRC01 binding could be that GRFT interacts with the N-linked glycan at position 386. This glycosylation site is positioned at the rim of the CD4BS, and its elimination by mutagenesis is known to increase viral sensitivity to b12 neutralization.25–26
We did not attempt to measure NAb responses, because mice are not the best species for induction of NAbs and monomeric gp120 is a poor immunogen for this purpose. Our goal was to investigate influences on the overall immunogenicity of gp120, given that it is such an atypical, extensively glycosylated protein. Although there has been recent speculation that nonneutralizing antibodies to gp120 might play a role in protection from HIV-1 infection,27 we believe that the focus of HIV-1 vaccine research should remain squarely on the induction of NAbs with broad and strong activity. It seems highly unlikely that this goal would ever be achieved by using a monomeric gp120 protein. One alternative approach is the use of gp140 trimers, although that form of the protein is also inadequate for NAb induction at present. We do not yet know whether gp140 mannose moieties adversely influence the immune response to trimers, and hence whether GRFT coimmunization would increase their immunogenicity. However, the proportion of glycans present as unprocessed oligomannose forms is greater on trimers than on monomers when the two forms of Env are expressed in the same cells.28 A higher mannose content of trimers may therefore cause more profound biological effects, but it might also be harder to remove or block.
Whether occluding mannose moieties or removing them by enzyme treatment will be of practical benefit to Env vaccine development remains to be seen. In circumstances in which a complex protein is particularly hard to manufacture in bulk, any strategy that reduces the amount needed for each immunization could be useful. Adjuvants can do that,29,30 and we found that the beneficial effect of mannose depletion was observed only with Alum adjuvant, not with the more complex QS-21.12 However, there may be circumstances, for example, mucosal delivery, in which the use of certain adjuvants is precluded and where other approaches could be considered.
One potential consequence of occluding terminal mannose residues would be to reduce the uptake of gp120 into DCs via MCLRs and hence its presentation as an antigen. If such untoward effects do in fact occur, they must be overridden by proimmunogenic ones, since the net result was an enhancement of Ab reactivity, not a decrease. We can envisage three broad groups of mechanisms by which formation of a GRFT complex could increase the immunogenicity of gp120, but at present we cannot discriminate among them. The first is that GRFT, by binding to mannose moieties, inhibits the mannose-dependent interactions of gp120 with DC-SIGN and other MCLRs that would otherwise trigger the release of anti-inflammatory cytokines such as IL-10 from DCs, or impair the maturation of these cells.10 Enzymatic removal of the mannose moieties from gp120 had a marginally stronger effect on its immunogenicity, and at least some of the consequences of mannose depletion were mimicked by coadministering an anti-IL-10-receptor antibody with unmodified gp120.12
The second possibility is that GRFT binding prolongs the tissue half-life of gp120 by blocking proteolysis or by interfering with mannose-dependent scavenging systems. In principle, increasing the longevity of deposited gp120 could increase its immunogenicity.29 However, mannose-receptor-dependent clearance, which takes place mostly in the liver and spleen,31 may be more relevant when the immunogen is given intravenously than, as here, subcutaneously.
A clue about the third possible explanation is provided by the specific and reciprocal enhancing effects of gp120 and GRFT on the respective Ab responses. Thus both the anti-GRFT and the anti-gp120 titers were higher in the mice that received gp120+GRFT than in mice given, respectively, GRFT+Gag or gp120 without GRFT. Although the combination was more immunogenic than either protein individually, the resulting Abs mostly recognized the individual components with few, if any, appearing to be strictly complex specific (Fig. 6). What mechanisms could underlie a mutually enhanced immunogenicity that mostly yields Abs against the individual components of the complex? The size of an immunogen is an important factor.29 Antigen-presenting cells take up soluble proteins inefficiently, but the Alum adjuvant used in our experiments already promotes uptake by creating immunogen complexes of vastly increased size. Nonetheless, the array of epitopes available on Alum–immunogen complexes may differ when gp120 is presented with and without GRFT. An effect of this nature might be particularly important for the smaller protein, GRFT, akin to how conjugating short peptides to carrier proteins increases their immunogenicity. The response to the GRFT moiety may also have benefited from the T-helper cell epitopes present in gp120. We note that all responses to gp120, Gag and GRFT were IgG1-dominated and thus TH2-polarized. GRFT binds to gp120 with a stoichiometry of 10 to 1 and an overall Kd of ∼10 nM.14 In addition, GRFT may be able to multimerize gp120 molecules, thereby creating substantial aggregates that, regardless of any further agglomeration by Alum, present arrays of epitopes that favorably cross-link B cell receptors.29 Furthermore, some of the mannose residues may remain unblocked, and the net effect of aggregation could be to enhance antigen uptake into DCs, particularly for GRFT. Varying the molar ratio of the two components might create different forms of gp120–GRFT complexes and could enable the response to the more relevant component, gp120, to be optimized.
It is also noteworthy that mice immunized with only gp120 produced some Abs that could bind to uncomplexed gp120 but not to gp120–GRFT complexes in an ELISA. GRFT may compete with Ab binding either directly or by steric hindrance. In either case, such blocking effects on the immunogen may hinder the elicitation of certain Abs. Thus, the net enhancement of the anti-gp120 response by GRFT might carry the price of immunosilencing a subset of gp120 epitopes. In this regard, it is problematic that GRFT partly blocked the binding of the NAbs b12 and VRC01, which are directed to important, cross-reactive CD4BS epitopes on gp120. If GRFT complexes of Env proteins more relevant to NAb induction were tested as immunogens, this point should be addressed so that important neutralization epitopes remain unoccluded. For example, it may be advantageous to mutate glycosylation sites adjacent to neutralization epitopes on the edges of the glycan shield, while instead occluding the bulk of the glycans; eliminating the latter would expose peptidic epitopes that are inaccessible to Ab on the native protein.
Does the increase in the anti-GRFT response in the presence of gp120 refute arguments that gp120 might be immunosuppressive, reducing the antibody response to both itself and other coadministered immunogens?2,32 We believe the present data are not definitive on this issue, because the formation of the gp120–GRFT complex may eliminate at least some of the possible immunosuppressive effects of gp120, notably those mediated by its mannose moieties. Differently designed studies would be required to answer this point. It should be noted, however, that the unexpected converse enhancement of the response to GRFT by gp120 may constitute a disadvantage. Thus, the immunogen complexes would rapidly become coated by preexisting Abs during subsequent immunizations, which could further impede B cell responses to gp120.
We conclude that ligating terminal mannose residues on gp120 by the small algal lectin GRFT enhances the immunogenicity of gp120 in a manner that is compatible with, but may go beyond, the blocking of MCLR interactions.
Acknowledgments
We thank Bill Olson and Bob Seder for the generous gifts of gp120 and p41-Gag proteins, respectively, and Dennis Burton, James Robinson, Peter Kwong, and John Mascola for donating antibodies. We appreciate technical support from Samson Jacob. We are grateful to Bill Olson and Robin Shattock for helpful discussions. This work was funded by NIH Grants AI 45463, AI 36082, HIVRAD AI 082362, AI 076169, and by the Dutch AIDS fund (Amsterdam) Grant 2005021. R.W.S. is a recipient of Veni and Vidi fellowships from the Netherlands Organization for Scientific Research (NWO) and a Mathilde Krim fellowship from the American Foundation for AIDS Research (amfAR).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Walker LM. Burton DR. Rational antibody-based HIV-1 vaccine design: Current approaches and future directions. Curr Opin Immunol. 2010;22:358–366. doi: 10.1016/j.coi.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klasse PJ. Sanders RW. Cerutti A. Moore JP. How can HIV-type-1-Env immunogenicity be improved to facilitate antibody-based vaccine development? AIDS Res Hum Retroviruses. 2011 doi: 10.1089/aid.2011-0053. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert PB. Peterson ML. Follmann D. Hudgens MG. Francis DP. Gurwith M. Heyward WL. Jobes DV. Popovic V. Self SG. Sinangil F. Burke D. Berman PW. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 4.Gorse GJ. McElrath MJ. Matthews TJ. Hsieh RH. Belshe RB. Corey L. Frey SE. Kennedy DJ. Walker MC. Eibl MM. Modulation of immunologic responses to HIV-1MN recombinant gp160 vaccine by dose and schedule of administration. National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. Vaccine. 1998;16:493–506. doi: 10.1016/s0264-410x(97)80003-5. [DOI] [PubMed] [Google Scholar]
- 5.Wright PF. Mestecky J. McElrath MJ. Keefer MC. Gorse GJ. Goepfert PA. Moldoveanu Z. Schwartz D. Spearman PW. El Habib R. Spring MD. Zhu Y. Smith C. Flores J. Weinhold KJ. Comparison of systemic and mucosal delivery of 2 canarypox virus vaccines expressing either HIV-1 genes or the gene for rabies virus G protein. J Infect Dis. 2004;189:1221–1231. doi: 10.1086/382088. [DOI] [PubMed] [Google Scholar]
- 6.Binley JM. Sanders RW. Clas B. Schuelke N. Master A. Guo Y. Kajumo F. Anselma DJ. Maddon PJ. Olson WC. Moore JP. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol. 2000;74:627–643. doi: 10.1128/jvi.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X. Farzan M. Wyatt R. Sodroski J. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 2000;74:5716–5725. doi: 10.1128/jvi.74.12.5716-5725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghi P. Fantuzzi L. Varano B. Gessani S. Puddu P. Conti L. Capobianchi MR. Ameglio F. Belardelli F. Induction of interleukin-10 by human immunodeficiency virus type 1 and its gp120 protein in human monocytes/macrophages. J Virol. 1995;69:1284–1287. doi: 10.1128/jvi.69.2.1284-1287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessani S. Borghi P. Fantuzzi L. Varano B. Conti L. Puddu P. Belardelli F. Induction of cytokines by HIV-1 and its gp120 protein in human peripheral blood monocyte/macrophages and modulation of cytokine response during differentiation. J Leukoc Biol. 1997;62:49–53. doi: 10.1002/jlb.62.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Shan M. Klasse PJ. Banerjee K. Dey AK. Iyer SP. Dionisio R. Charles D. Campbell-Gardener L. Olson WC. Sanders RW. Moore JP. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 2007;3:e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klasse PJ. Moore JP. Is there enough gp120 in the body fluids of HIV-1-infected individuals to have biologically significant effects? Virology. 2004;323:1–8. doi: 10.1016/j.virol.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee K. Andjelic S. Klasse PJ. Kang Y. Sanders RW. Michael E. Durso RJ. Ketas TJ. Olson WC. Moore JP. Enzymatic removal of mannose moieties can increase the immune response to HIV-1 gp120 in vivo. Virology. 2009;389:108–121. doi: 10.1016/j.virol.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori T. O'Keefe BR. Sowder RC., 2nd Bringans S. Gardella R. Berg S. Cochran P. Turpin JA. Buckheit RW., Jr McMahon JB. Boyd MR. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 14.O'Keefe BR. Giomarelli B. Barnard DL. Shenoy SR. Chan PK. McMahon JB. Palmer KE. Barnett BW. Meyerholz DK. Wohlford-Lenane CL. McCray PB., Jr Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Keefe BR. Vojdani F. Buffa V. Shattock RJ. Montefiori DC. Bakke J. Mirsalis J. d'Andrea AL. Hume SD. Bratcher B. Saucedo CJ. McMahon JB. Pogue GP. Palmer KE. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci USA. 2009;106:6099–6104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moulaei T. Shenoy SR. Giomarelli B. Thomas C. McMahon JB. Dauter Z. O'Keefe BR. Wlodawer A. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between multivalent binding to carbohydrates and anti-HIV activity. Structure. 2010;18:1104–1115. doi: 10.1016/j.str.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tritel M. Stoddard AM. Flynn BJ. Darrah PA. Wu CY. Wille U. Shah JA. Huang Y. Xu L. Betts MR. Nabel GJ. Seder RA. Prime-boost vaccination with HIV-1 Gag protein and cytosine phosphate guanosine oligodeoxynucleotide, followed by adenovirus, induces sustained and robust humoral and cellular immune responses. J Immunol. 2003;171:2538–2547. doi: 10.4049/jimmunol.171.5.2538. [DOI] [PubMed] [Google Scholar]
- 18.Eggink D. Melchers M. Wuhrer M. van Montfort T. Dey AK. Naaijkens BA. David KB. Le Douce V. Deelder AM. Kang K. Olson WC. Berkhout B. Hokke CH. Moore JP. Sanders RW. Lack of complex N-glycans on HIV-1 envelope glycoproteins preserves protein conformation and entry function. Virology. 2010;401:236–247. doi: 10.1016/j.virol.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore JP. Jarrett RF. Sensitive ELISA for the gp120 and gp160 surface glycoproteins of HIV-1. AIDS Res Hum Retroviruses. 1988;4:369–379. doi: 10.1089/aid.1988.4.369. [DOI] [PubMed] [Google Scholar]
- 20.Calarese DA. Scanlan CN. Zwick MB. Deechongkit S. Mimura Y. Kunert R. Zhu P. Wormald MR. Stanfield RL. Roux KH. Kelly JW. Rudd PM. Dwek RA. Katinger H. Burton DR. Wilson IA. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 21.Sanders RW. Venturi M. Schiffner L. Kalyanaraman R. Katinger H. Lloyd KO. Kwong PD. Moore JP. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol. 2002;76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunlop DC. Bonomelli C. Mansab F. Vasiljevic S. Doores KJ. Wormald MR. Palma AS. Feizi T. Harvey DJ. Dwek RA. Crispin M. Scanlan CN. Polysaccharide mimicry of the epitope of the broadly neutralizing anti-HIV antibody, 2G12, induces enhanced antibody responses to self oligomannose glycans. Glycobiology. 2010;20:812–823. doi: 10.1093/glycob/cwq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunlop DC. Ulrich A. Appelmelk BJ. Burton DR. Dwek RA. Zitzmann N. Scanlan CN. Antigenic mimicry of the HIV envelope by AIDS-associated pathogens. AIDS. 2008;22:2214–2217. doi: 10.1097/QAD.0b013e328314b5df. [DOI] [PubMed] [Google Scholar]
- 24.Scanlan CN. Offer J. Zitzmann N. Dwek RA. Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature. 2007;446:1038–1045. doi: 10.1038/nature05818. [DOI] [PubMed] [Google Scholar]
- 25.Zhou T. Xu L. Dey B. Hessell AJ. Van Ryk D. Xiang SH. Yang X. Zhang MY. Zwick MB. Arthos J. Burton DR. Dimitrov DS. Sodroski J. Wyatt R. Nabel GJ. Kwong PD. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445:732–737. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders RW. van Anken E. Nabatov AA. Liscaljet IM. Bontjer I. Eggink D. Melchers M. Busser E. Dankers MM. Groot F. Braakman I. Berkhout B. Paxton WA. The carbohydrate at asparagine 386 on HIV-1 gp120 is not essential for protein folding and function but is involved in immune evasion. Retrovirology. 2008;5:10. doi: 10.1186/1742-4690-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes BF. Liao HX. Tomaras GD. Is developing an HIV-1 vaccine possible? Curr Opin HIV AIDS. 2010;5:362–367. doi: 10.1097/COH.0b013e32833d2e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doores KJ. Bonomelli C. Harvey DJ. Vasiljevic S. Dwek RA. Burton DR. Crispin M. Scanlan CN. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci USA. 2010;107:13800–13805. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachmann MF. Jennings GT. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 30.Evans TG. McElrath MJ. Matthews T. Montefiori D. Weinhold K. Wolff M. Keefer MC. Kallas EG. Corey L. Gorse GJ. Belshe R. Graham BS. Spearman PW. Schwartz D. Mulligan MJ. Goepfert P. Fast P. Berman P. Powell M. Francis D. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccine. 2001;19:2080–2091. doi: 10.1016/s0264-410x(00)00415-1. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ. Evers S. Roeder D. Parlow AF. Risteli J. Risteli L. Lee YC. Feizi T. Langen H. Nussenzweig MC. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295:1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 32.Toapanta FR. Craigo JK. Montelaro RC. Ross TM. Reduction of anti-HIV-1 Gag immune responses during co-immunization: Immune interference by the HIV-1 envelope. Curr HIV Res. 2007;5:199–209. doi: 10.2174/157016207780077057. [DOI] [PubMed] [Google Scholar]