Abstract
Background:
To compare oral midazolam (0.5 mg/kg) with oral butorphanol (0.2 mg/kg) as a premedication in 60 pediatric patients with regards to sedation, anxiolysis, rescue analgesic requirement, and recovery profile.
Materials and Methods:
In a double blinded study design, 60 pediatric patients belonging to ASA class I and II between the age group of 2–12 years scheduled for elective surgery were randomized to receive either oral midazolam (group I) or oral butorphanol (group II) 30 min before induction of anesthesia. The children were evaluated for levels of sedation and anxiety at the time of separation from the parents, venepuncture, and at the time of facemask application for induction of anesthesia. Rescue analgesic requirement, postoperative recovery, and complications were also recorded.
Results:
Butorphanol had better sedation potential than oral midazolam with comparable anxiolysis at the time of separation of children from their parents. Midazolam proved to be a better anxiolytic during venepuncture and facemask application. Butorphanol reduced need for supplemental analgesics perioperatively without an increase in side effects such as nausea, vomiting, or unpleasant postoperative recovery.
Conclusion:
Oral butorphanol is a better premedication than midazolam in children in view of its excellent sedative and analgesic properties. It does not increase side effects significantly.
Keywords: Anxiolysis, oral midazolam, oral butorphanol, premedication, pediatric anesthesia, sedation
Introduction
Planning and carrying out a smooth transition from an awake state to surgical anesthesia in a child's is a challenge for all the anesthesiologists. Preoperative anxiety can have negative physiological and psychological effects on a child.-[1] Various interventions used to allay the anxiety of a child during the perioperative period are sedative premedications, parental presence during induction, and preoperative preparation programs.[2,3] Sedation remains one of the widely used methods for decreasing anxiety in young children. The oral route remains the most accepted method of drug administration though various combinations of drugs and routes of administration are available.[4]
In this study, an attempt was made to compare midazolam premedication, a gold standard in pediatric patients, with butorphanol, an opioid agonist–antagonist as a premedication in children. Butorphanol has desirable sedative and analgesic properties, which are not yet fully explored in pediatric patients.
Materials and Methods
After obtaining approval from the hospital ethics committeeand written informed consent from parents, 60 pediatric patients, aged 2–12 years, of ASA grade I and II were included in the study. All these children were scheduled for elective surgery with anticipated duration of surgery between 30 min and 2 h. Patients with central nervous system disorders, obesity (weight > 95th percentile for age), gastrointestinal disorders that affect drug absorption, known adverse reaction to benzodiazepines/opioids were excluded from the study. If the premedication was incompletely ingested, the child was excluded from the study. The patients were randomly allocated into two groups using a computer based randomization list. Children in group I received midazolam 0.5 mg/kg orally, while group II received butorphanol 0.2 mg/kg orally. Due to unavailability of oral midazolam, parental preparation of midazolam was used to prepare the syrup. Both the drugs were diluted to a fixed volume with honey by a pharmacist to make it palatable. All the observers were unaware of the contents of the premedication. Demographic data (age, sex, and weight), baseline vital parameters (heart rate and blood pressure), anxiety scores, and sedation scores were recorded by the same investigator to minimize interobserver variability. Children were administered midazolam (group I) or butorphanol (group II), 30 min before induction of anesthesia. Hemodynamic parameters—pulse rate, noninvasive systolic and diastolic blood pressure, sedation score, and anxiety score were recorded at the time of separation from parents (20 min), venepuncture (25 min), and mask application (30 min).
General anesthesia was administered in a standardized manner. Rescue analgesia in the form of intravenous fentanyl (1 mcg/kg) was administered, if heart rate/blood pressure increased >25%. Postanesthesia recovery was assessed for a period of 24 h with respect to nausea, vomiting, recollection of venepuncture/facemask application, analgesic requirement, and unpleasant recovery (irritability and excessive crying).
The grading of sedation was based on four-point scoring system: 1–awake, 2–alert, 3–drowsy, and 4–asleep. Anxiolysis was graded based on four-point grading system: 1–panicky, 2–moaning, 3–composed, and 4–friendly.[4] Patients whose anxiolysis score was greater or equal to 3 were considered to have adequate anxiolysis, while patients with sedation score greater or equal to 3 were considered to have adequate sedation. Postoperative analgesia was assessed using modified pain score and rescue analgesia was administered using paracetamolsyrup 10 mg/kg.[5]
Data analysis for demographic variables was done with unpaired students-t test. Anxiolysis and sedation scores were compared using chi square analysis. Analgesia between both the groups was compared using Fischer's exact test. A P value < 0.05 was considered statistically significant.
Results
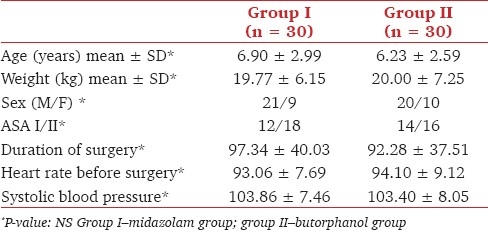
There was no statistically significant difference between the demographics (age, sex, ASA grade, and duration of surgery) and baseline vital signs (heart rate, BP, and SpO2) of the two groups. [Table 1]
Table 1.
Distribution of subjects according to demographic profile and vital signs
At the time of separation from parents, 30% of patients belonging to group I (midazolam) were adequately sedated, compared to 80% of patients in group II (butorphanol). The overall difference in sedation between the two groups was found to be statistically significant, as shown in Table 2. However, the difference in sedation scores at the time of interventions like facemask application and venepuncture was not statistically significant [Table 2].
Table 2.
Comparison of sedation score at the time of separation from parents, at venepuncture, and at facemask application

Patients in group I had a lower level of anxiety than group II after administration of premedication. At the time of separation from parents, 80% of children of group I had adequate anxiolysis when compared to 60% of the butorphanol group. The difference in anxiety between the two groups was not found to be statistically significant. At the time of venepuncture, 70% of the children of group I had adequate anxiolysis when compared to 37% of children of group II, which was statistically significant. 70% of the children in group I (midazolam) had adequate anxiolysis during facemask application, when compared to 30% of the children in group II (butorphanol). The difference in anxiety between the two groups was statistically significant [Figure 1].
Figure 1.
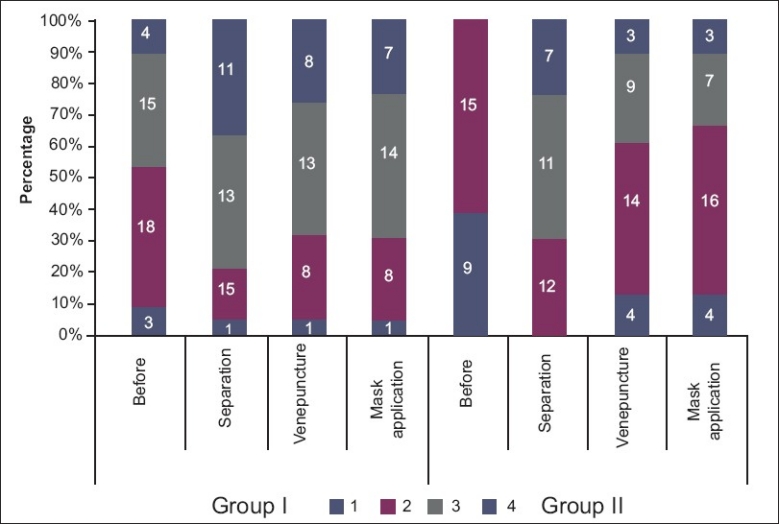
Comparison of anxiolysis score at the time of separation from parents, venepuncture, and separation. Group I–midazolam group; group II–butorphanol group
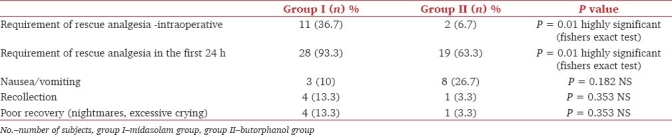
36.7% of children in group I (midazolam) needed rescue analgesia, when compared to 6.7% in group II, which was highly significant statistically [Table 3]. The differences in other parameters studied (recovery, nausea, and recollection) were statistically insignificant [Table 3].
Table 3.
Recovery profile and complications among the groups
Discussion
The quest for an ideal pediatric premedication drug is still on. Oral midazolam has been considered the gold standard for premedication in children. In a study conducted in US, more than 80% of anesthesiologists preferred midazolam as a premedication.[6] It has many desirable properties such as early onset and better level of sedation with no delay in recovery.[7] Although it has a number of beneficial effects, midazolam is far from an ideal premedication, especially with regards to confusion, long-term behavioral changes, and respiratory depression.[8,9] Numerous studies have been done comparing it with other drugs such as ketamine, clonidine, and dexmedetomidine, with mixed results.[6,9–16]
Butorphanol is a mixed agonist antagonist with intrinsic activity at mu-opioid receptors and a kappa agonistic activity. It has been used orally as an analgesic for chronic/cancer pain,[17] but there is only one study in literature on its efficacy as a pediatric premedication.[18]
The population sample studied was homogenous with regards characteristics such as age, weight, sex, anxiety, and sedation scores. Since oral preparations of midazolam are not widely available, we used the parenteral form of midazolamand mixed it in honey to make it palatable.[19]
Sedation and anxiety score were noted prior to premedication and at regular intervals—at the time of separation, venepuncture, and facemask application. The standard time of separation was taken as 30 min. Kain et al.[20] have shown that midazolam can be given as late as 10 min before separation with satisfactory results. Placebo group was not used for comparison, as the lack of placebo effect in this age group has been demonstrated previously.[21] The degree of sedation and anxiolysis in our study was far greater than that obtained by Feld et al..[22] This difference could be attributed to the fixed time of separation in our study as compared to their study, where separation occurred anytime between 30and 80 min after administration of oral midazolam. Such a wide time-interval limits interpretation of the pharmacodynamic effects of oral midazolam. Gopalvar et al. premedicated 706 children, between age of 6 months and 6 years, with midazolam and found that 24 of these children developed paradoxical reactions such as violent crying and were struggling within 10 min of the intravenous drug administration.[23] We did not encounter paradoxical reactions and this may be due to the smaller study size and the age group chosen. Paradoxical reactions are more common in the younger age group.
Singh et al. orally premedicated 60 pediatric patients with midazolam (0.5 mg/ kg) or butorphanol (0.2 mg/kg) and had results similar to that of our study.[18] They found that sedation score were comparable in both the groups at parental separation and venepuncture and the butorphanol group had better scores at the time of facemask application. Anxiety scores of both the groups were not compared in their study. Analgesic requirement was more in midazolam group (30%) when compared to butorphanol group (10%). There was no increase in side effects such as vomiting or delayed recovery in the butorphanol group. In our study, butorphanol provided better sedation at all levels with decreased analgesic requirement postoperatively. Anxiolysis in our study was better in the midazolam group at all intervals except at the time of separation from parents, when it was comparable in both the groups. The analgesic requirement in the patients of butorphanol group was less (6%) compared to the midazolam group (37%).
One of the most common side effects with the use of an opioid is nausea or vomiting.[24] We did not find a significant increase in the incidence of these side effects in the butorphanol group (27%) when compared to the midazolam group (10%). The limitations of our study are that postoperative recollection of perioperative events was unreliable in children < 5 years of age and that the sample size was small.
Conclusion
Butorphanol has better sedation potential than oral midazolam with comparable anxiolysis at the time of separation of children from their parents. However, midazolam is a better anxiolytic during venepuncture and facemask application. Butorphanol has additional analgesic property reducing need for supplemental analgesics and does not increase side effects such as nausea and vomiting.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kain Z, Mayes L, Caramico L, Hofstadter M. Distress during induction of anaesthesia and postoperative behavioural outcomes. Anesth Analg. 1999;88:1042–7. doi: 10.1097/00000539-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Kain Z, Mayes L, Wang S, Caramino LA, Hofstadter MB. Parental presence during induction of anesthesia vs.sedative premedication: Which intervention is more effective? Anesthesiology. 1999;89:1147–56. doi: 10.1097/00000542-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Messeri A, Caprilli S, Busoni P. Anesthesia induction in children: A psychological evaluation of the efficiency of parents’ presence. Pediatr Anesth. 2004;14:551–6. doi: 10.1111/j.1460-9592.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- 4.Kogan A, Katz J, Efrat R, Eidelman LA. Premedication with midazolam in young children: A comparison of four routes of administration. Paediatr Anaesth. 2002;12:685–9. doi: 10.1046/j.1460-9592.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 5.Wolf AR, Hughes D, Wade A, Mather SJ, Prys-Roberts C. Postoperative analgesia after pediatric orchiopexy: Evaluation of a bupivacaine morphine mixture. Br J Anesth. 1990;64:430–5. doi: 10.1093/bja/64.4.430. [DOI] [PubMed] [Google Scholar]
- 6.Kain ZN, Mayes LC, Bell C, Weisman S, Hofstadter MB, Rimar S. Premeditation in the United States: A status report. Anesth Analg. 1997;84:427–32. doi: 10.1097/00000539-199702000-00035. [DOI] [PubMed] [Google Scholar]
- 7.Bhakta P, Ghosh BR, Roy M, Mukherjee G. Evaluation of Intranasal midazolam for preanasthetic sedation in Paediatric patients. Indian J Anaesth. 2007;51:111–6. [Google Scholar]
- 8.Lönnqvist PA, Habre W. Midazolam as premedication: Is the emperor naked or just half-dressed? Pediatr Anesth. 2005;15:263–5. doi: 10.1111/j.1460-9592.2005.01600.x. [DOI] [PubMed] [Google Scholar]
- 9.Bergendahl H, Lonnqvist PA, Eksborg S. Clonidine in paediatric anaesthesia: A review of the literature and comparison with benzodiazepines for premedication. Acta Anaesthesiol Scand. 2006;50:135–43. doi: 10.1111/j.1399-6576.2006.00940.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt AP, Valinetti EA, Banderira D, Bertacchi MF, Simoes CM, Auler JO., Jr Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Paediatr Anaesth. 2007;17:667–74. doi: 10.1111/j.1460-9592.2006.02185.x. [DOI] [PubMed] [Google Scholar]
- 11.Ghai B, Grandhe RP, Kumar A, Chari P. Comparative evaluation of midazolam and ketamine with midazolam alone as oral premedication. Pediatr Anesth. 2005;15:554–9. doi: 10.1111/j.1460-9592.2004.01523.x. [DOI] [PubMed] [Google Scholar]
- 12.McCann ME, Kain ZN. The Management of Preoperative Anxiety in Children: An Update. Anesth Analg. 2001;93:98–105. doi: 10.1097/00000539-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Almenrader N, Passariello M, Coccetti B, Haiberger R, Pietropaoli P. Premedication in children: A comparison of oral midazolam and oral clonidine. Paediatr Anaesth. 2007;17:1143–9. doi: 10.1111/j.1460-9592.2007.02332.x. [DOI] [PubMed] [Google Scholar]
- 14.Bergendahl HT, Lönnqvist PA, Eksborg S, Ruthström E, Nordenberg L, Zetterqvist H, et al. Clonidine vs. midazolam as premedication in children undergoing adeno-tonsillectomy: A prospective, randomized, controlled clinical trial. Acta Anesthesiol Scand. 2004;48:1292–300. doi: 10.1111/j.1399-6576.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 15.Fazi L, Jantzen EC, Rose JB, Kurth CD, Watcha MF. A comparison of oral clonidine and oral midazolam as preanesthetic medication in the pediatric tonsillectomy patient. Anesth Analg. 2001;92:56–61. doi: 10.1097/00000539-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Tobias JD. Dexmedetomidine: Applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:1–17. doi: 10.1097/01.PCC.0000257100.31779.41. [DOI] [PubMed] [Google Scholar]
- 17.Sibille KT, Kindler LL, Glover TL, Gonzalez RD, Staud R, Riley JL. Individual Differences in Morphine and Butorphanol Analgesia: A Laboratory Pain Study. Pain Med. 2011;12:1076–85. doi: 10.1111/j.1526-4637.2011.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh V, Pathak M, Singh GP. Oral midazolam and oral butorphanol premedication. Indian J Pediatr. 2005;72:741–4. doi: 10.1007/BF02734144. [DOI] [PubMed] [Google Scholar]
- 19.Mishra LD, Sinha GK, Bhaskar Rao P, Sharma V, Satya K, Gairola R. Injectable midazolam as oral premedicant in pediatric neurosurgery. J Neurosurg Anesthesiol. 2005;17:193–8. doi: 10.1097/01.ana.0000181719.86978.05. [DOI] [PubMed] [Google Scholar]
- 20.Kain ZN, Mayes LC, Bell S, Wang SM, Rimar S, Weisman S, et al. Midazolam: Effects on amnesia and anxiety in children. Anesthesiology. 2000;93:676–84. doi: 10.1097/00000542-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Levine MF, Spahr-Schopfer IA, Hartley E, Lerman J, MacPherson B. Oral midazolam premedication in children: The minimum time interval for separation from parents. Can J Anaesth. 1993;40:726–9. doi: 10.1007/BF03009769. [DOI] [PubMed] [Google Scholar]
- 22.Feld LH, Negus JB, White PF. Oral midazolam preanesthetic medication in pediatric outpatients. Anesthesiology. 1990;73:831–4. doi: 10.1097/00000542-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Golparvar M, Saghaei M, Sajedi P, Razavi S. Paradoxical reaction following intravenous midazolam premedication in pediatric patients – a randomized placebo controlled trial of ketamine for rapid tranquilization. Paediatr Anaesth. 2004;14:924–30. doi: 10.1111/j.1460-9592.2004.01349.x. [DOI] [PubMed] [Google Scholar]
- 24.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–20. [PubMed] [Google Scholar]


