Abstract
Background:
Paravertebral block (PVB) has the potential to offer long-lasting pain relief because it can uniquely eliminate cortical responses to thoracic dermatomal stimulation. Benefits include a reduction in postoperative nausea and vomiting (PONV), prolonged postoperative pain relief, and potential for ambulatory discharge.
Aims:
To compare PVB with local infiltration for postoperative analgesia following modified radical mastectomy (MRM).
Methods:
Forty patients undergoing MRM with axillary dissection were randomly allocated into two groups. Following induction of general anesthesia in group P, a catheter was inserted in the paravertebral space and 0.3 ml/kg of 0.25 % of bupivacaine was administered followed by continuous infusion, while in group L, the surgical incision was infiltrated with 0.3 ml/kg of 0.25 % bupivacaine.
Statistical Analysis:
The statistical tests were applied as unpaired student ‘t’ test/nonparametric test Wilcoxon Mann Whitney test for comparing different parameters such as VAS score and consumption of drugs. The categorical variables such as nausea and vomiting scores, sedation score, and patient satisfaction score were computed by Chi square test/Fisher exact test.
Results:
VAS score was significantly lower in group P than in group L throughout the postoperative period. The mean alertness score (i.e., less sedation) was higher in group P in the postoperative period than group L. The incidence of PONV was less in PVB group.
Conclusion:
PVB at the end of the surgery results in better postoperative analgesia, lesser incidence of PONV, and better alertness score.
Keywords: Epidural catheter, modified radical mastectomy, paravertebral block, PCA, postoperative analgesia
Introduction
Although originally the main therapeutic indication for paravertebral block (PVB) was postoperative analgesia, the applications flourished to alleviation of angina pectoris, carcinoma pain, pain due to fractured neck of femur, causalgia, and post-traumatic sympathetic dystrophies. It was also used for treating supraventricular tachycardia, asthma, and pain of Herpes Zoster.[1] Its ease of performance and unilateral effect has made it an ideal block for unilateral surgeries such as mastectomy, hernias, and renal manipulations.[1] Continuous PVB has been used for pain relief following thoracotomy and mastectomy.[2,3]
Breast surgery is frequently associated with postoperative nausea and vomiting (PONV), pain, and painful restricted movements.[4] PVB has the potential to offer long-lasting pain relief because it can uniquely eliminate cortical responses to thoracic dermatomal stimulation.[5] Recently, PVB has been used for postoperative analgesia as well as sole anesthetic in patients undergoing breast surgery by several workers.[3–8] Benefits include a reduction in PONV, prolonged postoperative pain relief, and potential for ambulatory discharge.[7] It has also found to be effective in preventing chronic pain following mastectomy,[9–11] and it has been suggested that the risk of recurrence or metastasis is reduced in patients undergoing radical mastectomy (MRM) for breast cancer under PVB.[12] Local infiltration is a very old and proven method for analgesia. Its use is associated with a decrease in opioid requirement. Patients receiving local infiltration for thoracotomy have better respiratory outcomes.[13] Continuous infusion of local anesthetic at the incision site following MRM has been found to be useful.[14] With the advent of new analgesia regimes, PVB needs evaluation with comparative studies.
Materials and Methods
After approval of the Institutional review board, this prospective randomized study was conducted on 40 ASA grade 1–3 patients admitted to Maulana Azad Medical College and Lok Nayak Hospital for elective unilateral mastectomy under general anesthesia. The exclusion criteria were bilateral surgery, infection at block site, coagulation derangement or bleeding disorder, patient refusal, and allergy to amide group of local anesthetics. Patients were randomly allocated using computer generated numbers to one of the two groups comprising 20 patients each. In one set of patients (group P), a catheter was inserted in paravertebral space after induction and in other set of patients (group L), the surgical site was infiltrated after the completion of surgery.
On the night before the surgery, the patients were explained about the whole procedure and lean body weight (LBW) was calculated by the following formula:[15]
LBW (women) = (1.07 × weight (kg)) - 148 × weight (kg)2 /height (cm)2
All patients were premedicated with oral diazepam 10 or 5 mg, as per weight, 2 h prior to surgery. In the operation theater, patients were given morphine 0.1 mg/kg LBW intravenously (IV). Two minutes later, anesthesia was induced with thiopentone sodium sleep dose and vecuronium bromide 0.1 mg/kg LBW was used to facilitate the intubation of trachea. Lungs were ventilated with isoflurane and nitrous oxide in oxygen. Metoclopramide 0.2 mg/kg LBW IV was given 10 min before the end of the surgery.
In group P, following intubation, patient were turned lateral, with the side to be operated and blocked upwards. Under all aseptic precautions, a Touhy needle was inserted perpendicular to the skin 2.5 cm lateral from the cephalad edge of T3 spinous process. After the transverse process was contacted, the needle was withdrawn and redirected in the cephalad direction to walk off the transverse process. The ultimate goal was to insert the needle to a depth of 1 cm past the transverse process using loss of resistance technique.[3] A catheter was threaded and left 3–5 cm inside the space. A loading dose of 0.3 ml/kg LBW[1] of 0.25% bupivacaine was given through catheter and patient was turned supine. One hour after giving the loading dose an infusion of 0.25% bupivacaine was started @0.2 ml/kg/h and continued for next 20 h. Success of block was assessed by pin-prick after recovery from anesthesia. In group L, at the end of the surgery, the incision site was infiltrated with 0.25% bupivacaine 0.3 ml/kg LBW.
Patients were assessed for the following parameters after 30 min and subsequently after 2, 4, 6, 12, and 24 h after the reversal.
Pain
Visual analog score (VAS) score[16] was used to assess the pain scores in the patient at the above intervals . At any point of time if patient had VAS score > 3, intramuscular tramadol was administered 2 mg/kg LBW and continued for 8 h. Morphine was used as second line analgesic which was administered IV using patient controlled analgesia pump. If the patient had VAS score > 3, in between the subsequent visits, the patient was instructed to administer morphine 3 mg IV and lock out interval was fixed at 10 min.
Postoperative nausea and vomiting
PONV[6] was assessed at the same times. The time interval between the present and the previous visit was taken as the past time interval.
No nausea/vomiting in past time interval
Nausea in past time interval
Vomiting in past time interval
Nausea lasting more than 10 min or vomiting was treated with ondansetron 4 mg IV. The total consumption of antiemetics in the 24 h period was recorded.
Alertness using OAAS (Observer's Assessment of Alertness and Sedation Score)[16] was assessed at the same times as mentioned above.
Responds readily to name spoken in normal voice
Patient asleep but arousable to normal tone voice
Patient asleep but arousable to loud/repeated verbal stimulation
Patient asleep but arousable with mild shaking
Comatose patient.
Patient satisfaction score at 24 h[6]
Very unsatisfied
Somewhat satisfied
Acceptably satisfied
Very satisfied
The patients were evaluated for any complications or side effects up to 24 h after the surgery.
The statistical tests were applied as unpaired student ‘t’ test/nonparametric test Wilcoxon Mann Whitney test for comparing different parameters such as VAS score, consumption of post operative systemic analgesics, and antiemetics between two groups. The categorical variables such as PONV score, alertness score, and patient satisfaction score were computed by Chi square test/Fisher exact test. The comparison of the observations at different periods of time was assessed by paired ‘t’ test/nonparametric Wilcoxon rank sum test in each group. P < 0.05 was taken as cut point for level of statistical significance and the data were analyzed by using the SPSS statistical software.
Results
The two study groups were similar in age, weight, height, and ASA physical status distribution [Table 1]. VAS score was lower in group P than in group L throughout the postoperative period [Table 2 and Figure 1]. Mean postoperative requirement of tramadol in 24 h was 5.10 ± 2 mg/kg in group L with no requirement in group P (P < 0.001). Requirement of first dose of tramadol was seen in 55% of patients at the time of 1st assessment at 30 min following reversal in group L. 18 patients (90%) required tramadol in group L. Although two patients required morphine 3 mg IV bolus in addition to tramadol in group L, however this was not statistically significant.
Table 1.
Demographic profile

Table 2.
Visual analog score at rest

Figure 1.
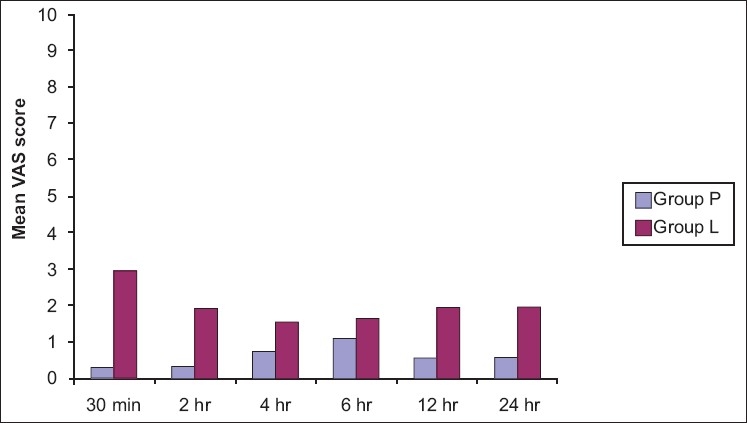
Mean Visual Analogue Scale Score at rest at various time intervals
The mean alertness score was more in group P in the postoperative period than group L; however, they were statistically significant at 2, 4, and 6 h [Table 3]. Lower mean PONV score was seen in group P; however, the scores at 30 min and 12 h only were statistically significant [Table 4]. Requirement of antiemetics (ondansetron) in 24 h was 5.40 ± 4.160 mg in group L and 0.4 ±1.23 in group P (P = 0.00) [Figure 2]. The requirement of first dose of ondansetron was seen in the immediate postoperative period, i.e., within 30 min in nine patients in group L. Two patients required ondansetron at 30 min and 2 h in group P.
Table 3.
Sedation score

Table 4.
Postoperative nausea and vomiting score
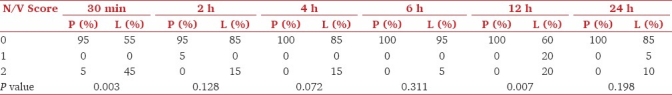
Figure 2.
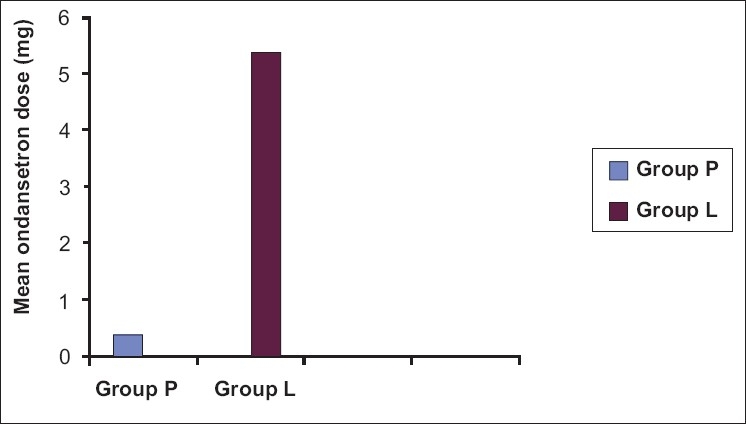
Mean postoperative ondansetron requirement in mg/patient in 24 hrs
Discussion
Regional anesthesia using PVB is an ideal alternative to general anesthesia for breast cancer surgery. The relative containment of the paravertebral space limits anesthetic diffusion, providing prolonged afferent blockade, and excellent surgical analgesia in both the inpatient and outpatient setting.-[7] D’Ercole et al. reported the use of PVB to achieve effective surgical anesthesia and postoperative pain control for breast cancer surgery in a pregnant patient requiring MRM with axillary dissection.[8] Several typesof pain syndrome are described: phantom breast pain, pain inor around the scar, pain in the chest wall, and pain in the arm. Phantom sensations after mastectomy have been studied extensivelyby Kroner and colleagues[9,10] ,with an incidence varying from about 13% at 3 weeks to17% at 6 years. Kairaluoma et al.[11] conducted a study on 60 patients scheduled for breast cancer surgery and randomly gave single injection PVB at T3 with bupivacaine 5 mg/ml (1.5 mg/kg) or saline before GA. A 1-year follow-up was performed and found that PVB reduced the prevalence of postoperative chronic pain.
In a retrospective analysis, Aristomenis et al. suggested that PVB for breast cancer surgery reduces the risk of recurrence or metastasis during the initial years of follow-up. This can be ascribed to the fact that the process of surgery inevitably induces a profound neuroendocrine, metabolic, and cytokine responses. A consequence of this stress response is transient perioperative inhibition of immune function. PVB analgesia reduces the need for opioids, which themselves impair immune function. Inhibition of the surgical stress response by PVB might attenuate perioperative factors that enhance tumor growth and spread.[12]
The results of our study demonstrated that thoracic PVB resulted in superior postoperative pain relief compared with GA, followed by local infiltration when used for MRM. 18 patients (90%) in group L required tramadol. The two patients who did not require tramadol were aged 60 and 65 years old. Requirement of first dose of tramadol was seen in 55% of patients at the time of 1st assessment at 30 min following reversal in group L. No patient in group P required tramadol. In a study, 81% patients undergoing MRM under GA (without local infiltration) required immediate postoperative analgesia (within 30 min) in contrast to 12.5 % of patients undergoing MRM under PVB[7]. Tramadol was chosen as the rescue analgesic as it is known to cause minimal sedation and respiratory depression.
In addition, there are other advantages of PVB for women undergoing breast surgery such as reduced incidence of PONV.-[1,2,7] Lönnqvist et al. prospectively evaluated complications after PVB (thoracic and lumbar) in 367 patients (319 adults, 48 children) and observed the following frequency of complications: vascular puncture 3.8%, hypotension 4.6%, pleural puncture 1.1%, and pneumothorax 0.5%.[17] We had no complication.
PONV complicate between 20% and 50% of all operative procedures.[18] The incidence is greater in patients undergoing general anesthesia, in female patients, in patients experiencing postoperative pain, and in women undergoing breast surgery.[18–21] A 59% incidence of nausea and vomiting during the 24-h interval after breast cancer surgery with general anesthesia has been reported.[20]
In our study, in group L, 75% patients required antiemetics compared to group P in which 10% patients required antiemetics. This is in contrast to a study by Coveney et al. in which 20% incidence was noticed in PVB group and 39% in GA group.[7] However, they used propofol as the induction agent which may have resulted in a lower incidence of PONV in the GA group. They used single-shot PVB as compared to continuous PVB in our patients.
OASS were found to be consistently higher in group P, i.e., patients were less sedated than group L patients. The higher levels of sedation in group L can be ascribed to the use of tramadol and morphine as rescue analgesics. Patients undergoing surgery under GA with PVB had lower pain score, PONV, and sedation. These are criteria favorable for better patient comfort and early discharge from hospital.
To conclude, PVB with general anesthesia is associated with better postoperative analgesia, lower incidence of PONV, and better alertness score compared to general anesthesia with local infiltration.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Richardson J, Lönnqvist PA. Thoracic Paravertebral block. Br J Anaesth. 1998;81:230–8. doi: 10.1093/bja/81.2.230. [DOI] [PubMed] [Google Scholar]
- 2.Matthews PJ, Govenden V. Comparison of continuous paravertebral and extradural infusions of bupivacaine for pain relief after thoracotomy. Br J Anaesth. 1989;62:204–5. doi: 10.1093/bja/62.2.204. [DOI] [PubMed] [Google Scholar]
- 3.Buckenmaier CC, 3rd, Klein SM, Nielsen KC, Steele SM. Continuous Paravertebral Catheter and Outpatient Infusion for breast surgery. Anaesth Analg. 2003;97:715–7. doi: 10.1213/01.ANE.0000075836.53838.EB. [DOI] [PubMed] [Google Scholar]
- 4.Pusch F, Freitag H, Weinstabl C, Obwegeser R, Huber E, Wildling E. Single injection Paravertebral block compared to general anaesthesia in breast surgery. Acta Anaesthesiol Scand. 1999;43:770–4. doi: 10.1034/j.1399-6576.1999.430714.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein SM, Bergh A, Steele SM, Georgiade GS, Greengrass RA. Thoracic paravertebral block for breast surgery. Anesth Analg. 2000;90:1402–5. doi: 10.1097/00000539-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Terheggen MK, Wille F, Borel Rinkes IH, Ionescu TI, Knape JT. Paravertebral blockade for minor breast surgery. Anesth Analg. 2002;94:355–9. doi: 10.1097/00000539-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Coveney E, Weltz CR, Greengrass R, Iglehart JD, Leight GS, Steele SM, et al. Use of paravertebral block anesthesia in the surgical management of breast cancer: experience in 156 cases. Ann Surg. 1998;227:496–501. doi: 10.1097/00000658-199804000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Ercole FJ, Scott D, Bell E, Klein SM, Greengrass RA. Paravertebral blockade for Modified Radical Mastectomy in a pregnant patient. Anesth Analg. 1999;88:1351. doi: 10.1097/00000539-199906000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Kroner K, Knudsen UB, Lundby L, Hvid H. Long term phantom breast syndrome after mastectomy. Clin J Pain. 1992;8:346–50. doi: 10.1097/00002508-199212000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Kroner K, Krebs B, Skov J, Jorgenson HS. Immediate and long term phantom breast syndrome after mastectomy: incidence, clinical characteristics and relation to pre-mastectomy breast pain. Pain. 1989;36:327–34. doi: 10.1016/0304-3959(89)90092-4. [DOI] [PubMed] [Google Scholar]
- 11.Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg. 2006;103:703–8. doi: 10.1213/01.ane.0000230603.92574.4e. [DOI] [PubMed] [Google Scholar]
- 12.Exadaktylos AK, Buggy DJ, Moriarity DC, Mascha E, Sessler DI. Can anaesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levack ID, Holmes JD, Robertson GS. Abdominal wound perfusion for relief of post operative pain. Br J Anaesth. 1986;58:615–913. doi: 10.1093/bja/58.6.615. [DOI] [PubMed] [Google Scholar]
- 14.Sidiropoulou T, Buonomo O, Fabbi E, Silvi MB, Kostopanagiotou G, Sabato AF. A prospective comparison of continuous wound infiltration with ropivacaine versus single-injection paravertebral block after modified radical mastectomy. Anesth Analg. 2008;106:997–1001. doi: 10.1213/ane.0b013e31816152da. [DOI] [PubMed] [Google Scholar]
- 15.Hallynck TH, Soep HH, Thomis JA, Boelaert J, Daneels R, Dettli L. Should clearance be normalized to body surface or to lean body mass? Br J Clin Pharmacol. 1981;11:523–6. doi: 10.1111/j.1365-2125.1981.tb01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlacu CL, Frizelle HP, Moriarty DC, Buggy DC. Fentanyl and Clonidine as adjunctive analgesics with levobupivacaine in Paravertebral analgesia for breast surgery. Anaesthesia. 2006;61:932–7. doi: 10.1111/j.1365-2044.2006.04793.x. [DOI] [PubMed] [Google Scholar]
- 17.Lönnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade. Failure rate and complications. Anaesthesia. 1995;50:813–5. doi: 10.1111/j.1365-2044.1995.tb06148.x. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch J. Impact of postoperative nausea and vomiting in the surgical setting. Anaesthesia. 1994;49:30–3. doi: 10.1111/j.1365-2044.1994.tb03580.x. [DOI] [PubMed] [Google Scholar]
- 19.Metter S, Kitz D, Yuong M. Nausea and vomiting after outpatient laparoscopy: incidence, impact on recovery room stay and cost. Anesth Analg. 1987;66:S116. [Google Scholar]
- 20.Miguel R, Rothschiller J, Majchrzak J. Breast surgery is a high risk procedure for development of nausea and vomiting. Anesthesiology. 1993;79:A1095. [Google Scholar]
- 21.Quinn A, Brown J, Wallace P, Asbury A. Studies in postoperative sequelae: Nausea and vomiting - still a problem. Anesthesia. 1994;49:62–5. doi: 10.1111/j.1365-2044.1994.tb03316.x. [DOI] [PubMed] [Google Scholar]


