Abstract
The Indian pharmaceutical industry, like any other industry, has undergone significant change in the last decade. The role of a Medical advisor has always been of paramount importance in the pharmaceutical companies in India. On account of the evolving medical science and the competitive environment, the medical advisor's role is also increasingly becoming critical. In India, with changes in regulatory rules, safety surveillance, and concept of medical liaisons, the role of the medical advisor is evolving continuously and is further likely to evolve in the coming years in important areas like health economics, public private partnerships, and strategic planning.
Keywords: Clinical research, medical advisor, product launch, pharmacovigilance
INTRODUCTION
The pharmaceutical industry in India traditionally focused on the sales of the products, and the role of the medical advisor was mainly limited to training sales representatives on the product and supporting the marketing claims of the product.
Worldwide the pharmaceutical industry is expanding and India is gaining in importance as a manufacturer of pharmaceuticals.[1] With changes in the regulations in marketing practices and regulatory policies, the role of a medical advisor in the pharmaceutical industry in India has evolved and expanded to responding to healthcare practitioners and patient queries related to products, in addition to performing the registration clinical trials for the launch of new products.
It is practically difficult to quantify the value of a medical advisor's role in the organization. Whenever there is budgetary pressure, it is seen that investments on the function of medical affairs are curtailed, but now organizations are realizing that it is short-sightedness and could be potentially hazardous over a long-term.
CURRENT ROLE OF MEDICAL ADVISOR
Currently the medical advisor uses his clinical expertise in the following functions.[2]
Medical information: Provide accurate, fair, and balanced product knowledge, mostly in response to medical queries: From consumers and healthcare professionals
Training: Product training to sales representatives and marketing colleagues
Create database of scientific information: Compile product-specific information
Review of promotional material: Evaluate the scientific content of promotional and training materials in line with the applicable regulations / guidelines in India
Provide scientific support for exhibits during national meetings
Competitive intelligence: Remain updated with journals and other scientific literature, to gain competitive intelligence
The medical advisor's function in pharmaceutical companies in India has undergone a transformational change. External changes in Indian pharmaceutical industry like regulatory conditions and safety surveillance have increased the visibility of the medical advisor's function.
In the last few years, with many more stringent regulations for pharmaceutical marketing in India, such as, the notification from the Medical Council of India, 2009, the role of the medical advisor has become significant for the Medical Affairs function. The medical advisor has become a credible link of the pharmaceutical industry with external stakeholders [Figure 1].
Figure 1.
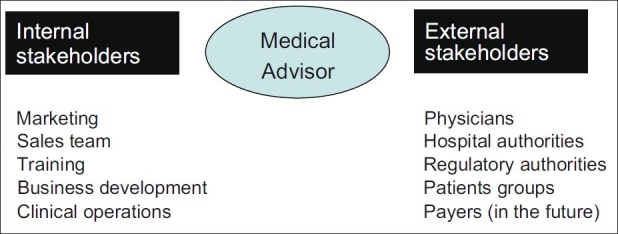
Internal and external stakeholders of Medical advisors
A critical success factor for the Medical Advisor's function is that he works closely with the marketing team for making strategies for product development, but at the same time, works at arms length from them in the medical evaluation, when evaluating the comparative risk benefit ratio of the drug.
In addition to above roles, the medical advisor is responsible for:
Scientific communication
The relationship between doctors and the pharmaceutical industry has gained increasing attention in recent years. Studies have proved that detailing activities by pharmaceutical representatives has influence on writing prescriptions.[3] It is important to provide updated, unbiased, accurate, and complete information to the healthcare practitioner. Every scientific promotional material distributed to physicians is reviewed for compliance with the Indian Drugs and Cosmetics Act, company guidelines, and Organization of Pharmaceutical Products of India guidelines.The activities in the medical affairs function are becoming critical for scientific communication with physicians.
A new concept of the regional medical advisor / regional medical liaison is emerging in the Indian pharmaceutical industry. They are the pharmaceutical physicians, often based in different Indian states. They interact with physicians on investigator-initiated studies, scientific communication, research activities, capacity building, and pipelines in the drug development. Even as sales representatives are required to restrict discussion to the approved prescribing information of a marketed product, medical liaisons can discuss and exchange scientific information related to other topics, including recent advances, research methodology, and so on.
In India, it is observed that physicians, because of their limited time, are sometimes unable to update themselves on the current developments in the medical field. Therefore, they look for medical liaisons to provide scientific information, which could really be of use to them in treating their patients better.
New product launch
Launching a new pharmaceutical product is a collaborative effort of regulatory, marketing, medical, and other functions in the pharmaceutical company. The process requires detailed planning, and also effective execution. Many critical activities take place well in advance of the actual launch of a new product. Each of these activities is time bound and has a set of objectives. The activities are divided into prelaunch, launch, and post launch activities.
Prelaunch
The role of a medical advisor includes highlighting the advantages of the new medicine, providing inputs on the exact medical positioning of the product, and discussing the possible high science / medical services that can be associated with the medicine. These discussions can be either one-on-one with practicing physicians or in form of advisory boards. The boards must include physicians of highest repute such as academicians, those having interest in original research and publications in their respective therapy area. Medical advisors often coordinate these programs and share new data available in different indications.
Launch
During launch, the medical advisor has a dual role. Internally, he is responsible for training of medical representatives, approving promotional material and medical inputs. Externally, he acts as the face of the organization by exposing much higher number of physicians to the product, addressing queries of physicians on usage of the medicine in patients and participating in physician meetings.
Post launch
In the post launch phase, it is important to update physicians on emerging information, answer queries on the drug's safety and efficacy profile. In addition, currently, medical advisors play an important role in partnering with physicians on investigator – initiated research and epidemiological studies which is the real need in India.
Interactions with stakeholders
Medical advisor is a critical link between a pharmaceutical company and external stakeholders [Figure 1]. External stakeholders include regulatory agencies, physicians, hospital administrators and patient groups.
Clinical research
Unfortunately in India, Medical affairs and Clinical research functions are often so separated in such a water tight manner that the interactions are often only at the strategic level. It is important for medical advisors to actively lead the research activities. These could include a protocol and case report form designing, selection of investigators, medical monitoring, development of the study report, and publication, in addition to coordinating with the Drugs Controller General of India during the submission and drug approval process. Medical advisors have a major role in early drug development especially phase IIa studies, basic research, and epidemiological research. This would include both the registration studies as well as the studies as part of global drug development.
Pharmacovigilance
In India, the pharmaceutical company holding the marketing license for the product has to ensure the responsibility of pharmacovigilance for their marketed products, as specified in Schedule Y.[4] In this regard, the role of a medical advisor in drug safety is of paramount importance. It not only includes screening of the available information on adverse events for its causality and expectedness, but also includes communication with the reporter or other physicians coordinating with the regulatory authorities, if required. Identifying signals and working to reduce the risk in specific populations, alerting the organization on possible risks and working on remedial actions are other areas of importance in pharmacovigilance.
Access to medicines
In India, this has come up as a new responsibility especially in diseases with unmet medical needs, and certain neglected or tropical diseases. The responsibility of a medical advisor is to provide access to specific or institutional requests in line with the norms outlined in the Drugs and Cosmetics Act, liaise with internal stakeholders [Figures 1 and 2]. on quantity and labeling details, and fulfill pharmacovigilance responsibilities. In addition, in the post launch period, a medical advisor has to play a role in designing an access program by which patients may not be deprived of medicines because of high cost alone. As we enter an era of biologics and other specialized treatments in chronic illness, the need for designing and developing such programs is going to increase further.
Figure 2.
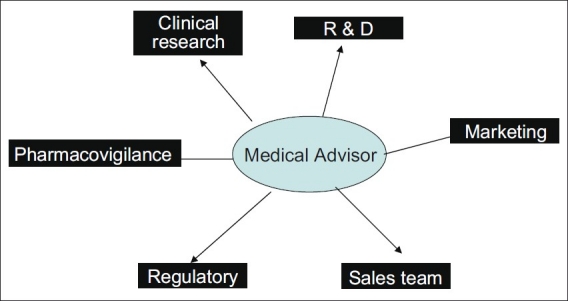
Schematic diagram of pharmaceutical physician's internal stakeholders
As discussed before, the Indian pharmaceutical industry has continued to evolve over the last few years from a product-centric approach to a disease-centric approach to a therapeutic area–centric approach to a patient-centric approach [Figure 3].
Figure 3.
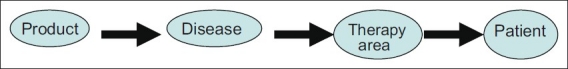
Evolution of focus of pharmaceutical industries in India
FUTURE ROLE
The main challenges that the global pharmaceutical industry is facing include shrinking and less productive pipelines, and longer drug development timelines in developed as well as emerging countries. This will have to bring in changes in the outlook of medical advisors working in the pharmaceutical industry in India. It may mean:
Focus on improving the efficiency in drug development
Involving payers and patient groups in strategic planning
Increasing presence in the emerging markets and addressing the needs of patients in these areas
Innovations in reaching to the physicians and helping them in the capacity building of healthcare
The pharmaceutical physician should take the lead in all these changes. In addition to this, the future role of pharmaceutical physicians would include:
Health economics
Currently, the role of pharmaceutical physicians in health economics, especially in India, is limited. There is an increased thought on the proactive role of the government in the supply of medicines in the public sector. In the long term, this could mean the requirement of India specific health economic studies. It is also possible that payers — private as well as public will become more vocal in their role in the selection of medicines. We envision that major efforts will be required in organized financing of healthcare in the future — the selection of the model (public or private or both) can be finalized later. The pharmaceutical physician could play an important role, be it in the development of pricing models, negotiating with payers on the ‘real value’ of medicines or capacity building.
Policy shaping
In India, there can be a gap between policy and implementation. This can be more acute in social sectors like health. Even as the government would continue to lead to shape the health policy, it would expect professionals, especially from the pharmaceutical industry, to help them draft these policies, provide expertise, and even assess the outcomes of their implementation. The pharmaceutical physician is expected to be more acceptable to other clinician groups as well as the government. The pharmaceutical physician would have a major role in surveillance and epidemiology studies, in the outcome assessment of health policy, and in employing management techniques in the planning stage.
Strategic planning
As the Indian industry has become global, there are avenues for collaboration between multinational companies, global Indian companies, and companies with a focus on biotechnology. The role of a medically qualified professional in the strategic planning of business and in looking for possible avenues for alliance and partnership is likely to become more pronounced. If there are not many innovative molecules in the pipeline for interested therapy area, companies may like to invest in acquisitions (of companies/ products) and alliances with smaller companies. In such cases, the medical advisors have a chance to utilize their expertise for giving critical and valuable medical inputs, which would help in shaping the company's future.
Public – Private partnership in healthcare
In future the role of public agencies and private players in delivering healthcare would be defined more clearly. It is possible that many private players would be more responsible for implementation of health programs. These would include capacity building of physicians and allied health workers, programs on preventive healthcare, and taking quality healthcare to the grassroot levels. Pharmaceutical physicians would be expected to forge such partnerships and work toward making it successful. Pharmaceutical physicians would often lead these programs and be responsible for its success.
Drug development
In the light of the limited drugs in the pipeline, medical advisors will have to play an important role in finding innovative and new drug delivery systems (NDDS) for the existing products and in developing a possible rationale for fixed-dose combinations (FDCs). This will need very close working with the industrial development as well as the regulatory department, to work in line with the regulations as well as to hasten the process of development.
Competencies of pharmaceutical physicians
To embrace a challenging future, pharmaceutical physicians would have to develop a robust knowledge base and skills [Table 1].
Table 1.
Competencies of a pharmaceutical physician

CONCLUSION
To summarize, pharmaceutical physicians have a long legacy in India. Going ahead, a medical advisor / pharmaceutical physician will be expected to play a much more prominent role in healthcare. Even as he is doing this, the pharmaceutical physician must not forget that their primary interest is the welfare of the patients. At the same time, he must also endeavor to align the company's interest to the patient's interest, that is, keep the patients at the center of all activities. To face a challenging, but interesting future, pharmaceutical physicians will have to strengthen their existing skills in pharmaceutical medicine and at the same time build new skills in healthcare management. This will help them to be in roles of leadership rather than in support functions. There is a need for a joint effort to develop these skills.
In last few years, the pharmaceutical industry in India has undergone significant changes. With this change, the competitive environment and evolving medical science, medical advisor's role is becoming critical. The role of medical advisor is evolving continuously and is further likely to evolve in areas like health economics, public private partnerships, and strategic planning.
ACKNOWLEDGMENT
The authors of this article wish to thank Dr. Amit Khare for his support in the literature review.
Footnotes
Source of Support: Nil
Conflict of Interest: Dr. Anant Patil and Dr. Viraj Rajadhyaksha are full time employees of Sanofi, Mumbai, India. All the opinions in this article are entirely those of the authors and not necessarily of Sanofi.
REFERENCES
- 1.Perlitz U. India's Pharmaceutical Industry on course for globalization . Deutsche Bank Research. 2008:1–12. [Google Scholar]
- 2.Espinosa MG, Pao MS, Park PJ, Sheth B, Barone JA, Alexander JG. The growth of pharmaceutical industry fellowship programs: Why all the excitement? Pharmacy Student. 2005:28–9. [Google Scholar]
- 3.Cohen GM. Pharmaceutical branding of resident physicians. J Am Med Assoc. 2001;286:1024–5. doi: 10.1001/jama.286.9.1024-a. [DOI] [PubMed] [Google Scholar]
- 4.Arora D. Pharmacovigilance obligations of the pharmaceutical companies in India. Indian J Pharmacol. 2008;40(Suppl 1):S13–6. [PMC free article] [PubMed] [Google Scholar]


