Abstract
Objective:
In childhood Guillain–Barré syndrome (GBS), the clinical profiles using intravenous immunoglobulin (IVIg) in addition to supportive care were studied.
Materials and Methods:
This was a retrospective analysis of 139 children with severe GBS admitted to our respiratory care unit managed with the IVIg as an adjunct intervention to conventional supportive and respiratory care.
Results:
In our case series of 139 cases, motor weakness was the most common presenting feature. Antecedent illness was found in 66.7% of cases in the preceding two weeks, which included nonspecific illness, acute respiratory infection, diarrhea, and chickenpox. At onset, sensory symptoms (pain and paresthesia) were noted in 59% of the cases and limb weakness in 77%. On admission, a majority (61.54%) were in Hughes neurological disability grading stage V; all had limb weakness at the peak deficit, autonomic disturbance was seen in 35.8%, and bulbar palsy in 52%. Duration of illness was less than three weeks in 67% of cases. The mean duration of ventilation was 21.5 days (range, 5-60 days).
Conclusions:
Male preponderance and motor weakness was the most common presenting illness and a majority achieved full recovery in our series. Although IVIg may be useful in the treatment of GBS, the key issue is excellent intensive care unit management.
Keywords: Guillain–Barré syndrome, intravenous immunoglobulin, supportive care
INTRODUCTION
Guillain–Barré syndrome (GBS) is the most common non-polio acute flaccid paralytic illness affecting children in the era of global eradication of poliomyelitis. GBS is an acute, monophasic, symmetrically progressive, peripheral ascending demyelinating polyneuropathy characterized by rapidly evolving symmetrical limb weakness, areflexia, absent or mild sensory signs, and variable autonomic disturbances. Although most patients have a favorable outcome, mortality is usually related to systemic problems or complications of hospitalization, rather than the actual disease.[1,2] GBS is the major cause of acute neuromuscular paralysis, with an annual incidence of 1.3-2 per 100,000 worldwide.[3,4] Electrophysiologically and pathologically, most patients suffering with GBS have motor axonal degeneration with minimal cellular inflammation, which is termed as ‘acute motor axonal neuropathy’.[5] Approximately two-thirds of all GBS cases are preceded by an infection such as mild respiratory infection or diarrhea.[6]
GBS is an acute inflammatory disorder of the peripheral nervous system thought to be due to autoimmunity for which immunotherapy is usually prescribed.[7]
The clinical criteria proposed by Asbury and Cornblath are generally accepted as the guideline for diagnosing GBS. Affected children usually recover in shorter time than adult, while the mortality rate was reported at 3-5%.[8,9]
We performed a retrospective study on the natural history in children with GBS to study their clinical profile using intravenous immunoglobulin (IVIg) in addition to supportive care.
MATERIALS AND METHODS
In this retrospective study of 139 children below the age of 12 years with GBS admitted to the respiratory care unit in the Postgraduate Medical Education Institute at Kolkata from July 2000 to June 2010 were included. Exclusion criteria was as follows: (a) those under 2 years of age, (b) children who did not attain independent walking premorbidly, (c) children with atypical form of GBS, (d) children who were not appropriately immunized with poliomyelitis vaccine as per the chronological ages, and (e) children with any other pre-existing disease. Asbury and Cornblath's clinical diagnostic criteria for GBS were used for clinical diagnosis.[10] The disability staging was done according to the Hughes neurological disability grading 7-point scale that was subsequently adapted by the Plasma Exchange/Sandoglobulin Guillain–Barré Syndrome Trial Group, 1997 (modifications in italics).[11,12]
All the patients were treated with IVIg in a dose of 2 g/kg body weight over 2-5 days as an adjunct intervention to conventional supportive and respiratory care. Duration of illness and the mean duration of ventilation were estimated. Time needed to improve one clinical grade (that is being weaned from ventilator or being able to walk) was estimated.
Statistical analysis
Medical case records of eligible patients were reviewed and noted with emphasis on outcome. Numerical parametric data were presented as percentages.
RESULTS
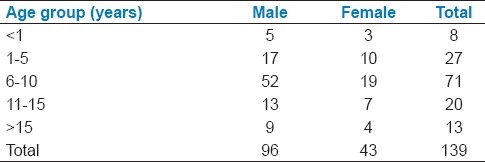
A total of 139 children with GBS were managed in our respiratory care during the study period, of which 96 (69.06%) were males. Slight preponderance (51.08%) were in the age group of 6-10 years [Table 1].
Table 1.
Age and sex distribution of GBS cases
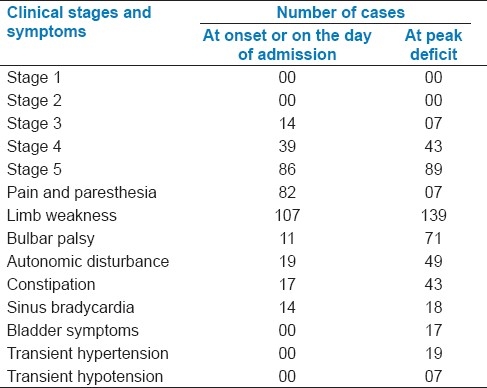
At onset, sensory symptoms (pain and paresthesia) were noted in 82 cases (58.99%), and limb weakness was seen in 107 cases (76.98%). On the day of admission, 86 cases were in stage V of illness, 39 patients in stage IV, and 14 patients in stage III. Clinical features at the peak deficit were analyzed. All the 139 cases had limb weakness, and 132 cases were in stages IV and V. Other features like bulbar palsy were present in 71 cases (51.08%), whereas on the day of admission, only 11 (7.91%) cases showed this feature. Autonomic disturbance was noted in 49 cases (35.25%) compared to 19 (13.67%) on the day of admission. We also observed constipation and sinus bradycardia. Apart from these, bladder symptoms were noted in 17 cases (12.23%), transient hypotension in 19 (13.67%), and transient hypertension in 7 cases (5.04%) [Table 2].
Table 2.
Clinical Stages and symptoms in the participants (n=139)
Areflexia was a characteristic feature and more proximal reflexes were elicited during the early phase of the disease that was consistent with acute features of GBS. In our series, history of antecedent illness was found in 91 cases (65.47%) in preceding two weeks. Of these antecedent illnesses, nonspecific illness was found in 53 cases (58.24%), respiratory tract infection in 23 cases (25.27%), diarrhea in 11 cases (12.09%), and chicken pox in 4 cases (4.40%). About 15 patients were put on mechanical ventilator, mostly in the second week of their management. Duration of illness was less than three weeks in 67% of cases. Mean duration of ventilation was 21.5 days (range, 5-60 days). Four patients died (two due to autonomic imbalance, one due to tracheal stenosis, and one by sudden extubation). In the electrophysiological studies, 124 cases showed the presence of acute inflammatory demyelinating polyneuropathy (AIDP). In the cerebrospinal fluid (CSF) study, 54 cases (38.85%) showed albumin-cytological dissociation. One case showed low CSF protein and polyneuropathy; the case was later confirmed by nerve conduction study. Average time needed to improve one clinical grade (that is being weaned from ventilator or being able to walk) was 20.4 days (range, 6-56 days).
DISCUSSION
In our series of 139 GBS cases, motor weakness was the most common presenting feature, with male children appearing to be at a greater risk for GBS in comparison with females. Antecedent illness was found in 66.7% of cases in the preceding two weeks, which included nonspecific illness, acute respiratory infection, diarrhea, and chicken pox. At onset, sensory symptoms (pain and paresthesia) were noted in 59% of the cases and limb weakness in 77%. On admission, majority (61.54%) were in Hughes neurological disability grading stage V; at the peak deficit, all had limb weakness, autonomic disturbance was in 35.8%, bulbar palsy in 52%. Duration of illness was less than three weeks in 67% cases. The mean duration of ventilation was 21.5 days (range, 5-60 days) and a vast majority achieved full recovery in our series.
In a retrospective multicentre study on the natural history and treatment effects in children with classical GBS, researchers collected reports of preceding five years from 92 pediatric hospitals in Germany and Switzerland. The age of patients ranged from 11 months to 17-7 years, median 6-3 years with bimodal peaks at 4 and 12 years. There was a slight preponderance of boys over girls. In 79% of the patients, a preceding illness was reported. Airway infections (37%) dominated over gastrointestinal infections (11%); in 23% of cases, no febrile illness was determined. In 79% of the patients, serological investigations was performed. The findings were negative in 60%. In the remainder, a broad spectrum of infectious agents was found. Mycoplasma pneumoniae, Cytomegalovirus, Epstein–Barr virus, Coxsackie virus, Varicella zoster virus, and Borrelia burgdorferi were the most frequent occurrences. Campylobacter jejuni was found in two patients. In four children, a vaccination had preceeded the onset of GBS. The first symptoms of GBS were weakness in 43%, ataxia in 27%, and paresthesia in 28%. Four patients presented with cranial nerve symptoms in the beginning. The children were admitted to the hospital at a median of four days after the first symptoms. Twenty percent of the patients were already unable to walk and 3% needed artificial ventilation. The disease progressed for a median of 10 days from the beginning. At the height of the disease, only 26% of the children were able to walk without support; 16% had to be artificially ventilated. The duration of intubation ranged between 4 and 94 days (median, 10 days). A lumbar puncture was performed in 169 patients 0-60 days (median 6 days) after the start of symptoms. Electrophysiological investigations were performed on day 10 in 103 patients. Nerve conduction velocity (NCV) findings were reported to be normal in 14% of cases. With the aim to delineate prognostic criteria at the height of the disease, there was a significant correlation of bladder dysfunction, cranial nerve palsies, and a sensory deficit with the degree of disability and the necessity of artificial ventilation. Regarding the effect of disease severity and the treatment modalities on the outcome variables, a positive effect of IVIg on the time to leave bed, and to walk unaided was demonstrated in children who were unable to walk, but not in those with tetraplegia or requiring artificial ventilation. Treatment with IVIg was shown to accelerate shorter time to recovery in the early phase, to leave bed, and walk unaided; but not later in enabling patients to leave hospital and to become free of the last symptoms.[13]
In the electrophysiological studies, 124 cases showed the presence of AIDP in our series. In a prospective study of 78 children from Mexico, AIDP was three times more common in male patients than in female patients.[14] This increased predilection for GBS has also been reported as a male-to-female ratio of 1.2:1 in a review of children with GBS. A similar ratio of 1.26:1 was found in a prospective study of 95 children with GBS in Western Europe.[15] In Pakistan, a combined adult and pediatric GBS study reported that 68% of all patients were male.[16] In a study of 52 Indian children with GBS, 75.4% were male.[17]
In the history of preceding antecedent illnesses, nonspecific illness was present in 53 cases (58.24%), upper respiratory tract infection (URTI) in 23 (25.27%), diarrhea in 11 (12.09%), and chicken pox in 4 cases (4.40%). In a review analysis, the researchers observed that two-thirds of GBS cases were associated with an antecedent infection two to three weeks before the onset of the symptoms, most commonly with C. jejuni or cytomegalovirus.[5] An Indian case-control study reports that 27.7% of childhood GBS cases were associated with C. jejuni infection.[18]
Paulson had reported an incidence of preceding febrile illness in 52%, whereas another study showed that 54% patients had preceding infection [most common being upper respiratory tract infection (URTI)].[19,20]
In this present study, bulbar involvement was seen in 52%. High incidence could be due to the majority of cases being in stages 4 and 5. Bulbar involvement was noted in 29-35% of cases in another series.[19]
The most common symptom at the onset was limb weakness, which was noted in a majority of children, ie, 107 (76.98%). The reported incidence of motor weakness as presenting symptom varied from 75-95%.[9,20]
Researchers noted varied results on the benefit of IVIg in shortening the course of GBS, though IVIg is easily administered and well tolerated and are currently the first-line immunotherapy in childhood GBS.[4] However, another group found no evidence of IVIg improving the outcome in childhood GBS when compared with supportive care.[20]
Hart et al. reported an incidence of 25% in their study group of children with GBS and also observed that 59% of the cases had sensory symptoms at onset.[20] Rantala et al. reported sensory symptoms in 20% of pediatric patients.[21]
Although we did not practice prophylactic ventilation, yet the percentage of patients who were treated with mechanical support was 38%. In our study, mean duration of ventilation was 21.5 days. In Paulson's series, 10.25% cases needed assisted ventilation.[20] In the published reports, the mean duration of ventilation in childhood GBS was in the range 21.5-25 days.[21,22] Rantala et al. reported in their series of 120 cases that only 20 children required assisted ventilation. None of the children without any of the following risk factors had respiratory failure. Cranial nerve involvement was more common in children who required assisted ventilation. The researchers concluded that preventive treatment with IVIg could be directed to children with any of the risk factors. For patients without the risk factors, clinical monitoring would be satisfactory.[21]
In the series of Kleyweg et al., 29% of the cases needed assisted ventilation, though the lowest (5%) incidence was shown in the series by Hart et al.[20,23] The highest incidence of ventilation (41.8%) was reported by Ramachandra and Kuruvilla, with the reason mentioned as practice of prophylactic ventilation, as well as severity of disease.[24]
In the CSF study, 54 cases (38.85%) showed albumin-cytological dissociation. In the series by Hart et al., this feature was seen in 64% of the cases, in Epstein and Sladky series it was 93%, and 89% in Paulson's study.[19,20,25]
A comparable study performed in India also reported definite benefits with the use of IVIg where comparison was done between IVIg use in 17 elective early admissions with that in eight children whose presentation to the hospital was delayed and who, in some cases, were already in respiratory distress. The researchers concurred with previously published reports, that early use of IVIg could reduce the mortality and the need for intubation and mechanical ventilation.[26] Limited evidence from three open trials in European children suggested that IVIg hastens recovery compared with supportive care alone.[7]
Duration of illness was less than three weeks in 67% of cases in our series. The researchers in different series concluded that the outcome of GBS is generally favorable in most patients. In childhood, is usually favorable, although prolonged and severe forms may develop.[27,28] Briscoe et al. reported a mean time of recovery after reaching the maximum disability of the disease as 19.3 days.[29]
Strength and limitation
Childhood GBS is an uncommon condition and ethical and logistic barriers prevent the study on a larger sample. In our resource-poor settings, we tried to study an issue that needs intensive medical supervision and follow-up. While the commonly accepted benefits of IVIg in adult GBS cannot be automatically extrapolated to the pediatric population, it was shown that IVIg has the potential to significantly reduce mortality and morbidity.
Future directions
The potential for progression from mild gait disturbance to the need for intubation for respiratory support is the true dilemma in the management of GBS. Numerous cases of childhood GBS do not need intubation, but one cannot ensure this without close observation and time. The role of immunoglobulin in the prognosis of childhood GBS is also doubtful. Moreover, early markers to stratify a child's risk of respiratory failure are also yet to find out. In childhood GBS, a large multicentric study is essential to reach a definite conclusion on natural history of the disease and the role of immunoglobulin in prognosis. Further research examining potentially more effective treatment with beta-interferon and immunosuppressive agents is required.
CONCLUSION
We studied the clinical presentations in children with GBS and tracked the treatment effects of IVIg in the prognosis of GBS as an adjunct intervention to conventional supportive and respiratory care. We observed a male preponderance and observable antecedent illness. Motor weakness was the most common presenting illness. GBS in children in our series had a more benign course and a majority made full spontaneous recovery. Although repeated IVIg may be useful in the treatment of GBS, the key issue is excellent intensive care unit management.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Aggarwal AN, Gupta D, Lal V, Behera D, Jindal SK, Prabhakar S. Ventilatory management of respiratory failure in patients with severe Guillain-Barré syndrome. Neurol India. 2003;51:203–5. [PubMed] [Google Scholar]
- 2.Swaiman KF, Iannaccone ST. Anterior horn cell and cranial motor neuron disease. In: Swaiman KF, Ashwal S, editors. Pediatric Neurology: Principles and Practice. 3rd ed. Vol. 70. St Louis: CV Mosby Company; 1999. pp. 1162–77. [Google Scholar]
- 3.Govoni V, Granieri E. Epidemiology of the Guillain-Barré syndrome. Curr Opin Neurol. 2001;14:605–13. doi: 10.1097/00019052-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Van Koningsveld R, Van Doorn PA, Schmitz PI, Ang CW, Van der Meché FG. Mild forms of Guillain-Barré syndrome in an epidemiologic survey in The Netherlands. Neurology. 2000;54:620–5. doi: 10.1212/wnl.54.3.620. [DOI] [PubMed] [Google Scholar]
- 5.van der Meché FG, Visser LH, Jacobs BC, Endtz HP, Meulstee J, van Doorn PA. Guillain-Barré syndrome: Multifactorial mechanisms versus defined subgroups. J Infect Dis. 1997;176:S99–102. doi: 10.1086/513779. [DOI] [PubMed] [Google Scholar]
- 6.Rees J. Guillain-Barre syndrome. Clinical manifestations and directions for treatment. Drugs. 1995;49:912–20. doi: 10.2165/00003495-199549060-00005. [DOI] [PubMed] [Google Scholar]
- 7.Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: A systematic review. Brain. 2007;130(9):2245–57. doi: 10.1093/brain/awm004. [DOI] [PubMed] [Google Scholar]
- 8.Markland LD, Riley HD., Jr The Guillain-Barré syndrome in childhood. Clin Pediatr (Phila) 1967;6:162–70. doi: 10.1177/000992286700600314. [DOI] [PubMed] [Google Scholar]
- 9.McLean S, Sheng F, Oon SF. Childhood Guillain-Barre Syndrome: Comparing Intravenous Immunoglobulin Treatment with Supportive Care. Trinity Student Medical Journal. 2005;6:60–7. [Google Scholar]
- 10.Asbury AK, Comblath DR. Assessment of current diagnostic criteria for Guillain-Barre syndrome. Ann Neurol. 1990;27:S21–4. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 11.Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial of prednisolone in acute polyneuropathy. Lancet. 1978;2:750–3. doi: 10.1016/s0140-6736(78)92644-2. [DOI] [PubMed] [Google Scholar]
- 12.Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Lancet. 1997;349:225–30. [PubMed] [Google Scholar]
- 13.Korinthenberg R, Schulte Monting J. Natural history and treatment effect in Guillain-Barre syndrome: A multicentre study. Arch Dis Child. 1996;74:281–7. doi: 10.1136/adc.74.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nachamkin I, Barbosa PA, Ung H, Lobato C, Rivera AG, Rodriguez P. Patterns of Guillain-Barre syndrome in children: Results from a Mexican population. Neurology. 2007;69:1665–71. doi: 10.1212/01.wnl.0000265396.87983.bd. [DOI] [PubMed] [Google Scholar]
- 15.Korinthenberg R, Schessl J, Kirschner J. Clinical presentation and course of childhood Guillain-Barré syndrome: A prospective multicentre study. Neuropediatrics. 2007;38:10–7. doi: 10.1055/s-2007-981686. [DOI] [PubMed] [Google Scholar]
- 16.Shafqat S, Khealani BA, Awan F, Abedin SE. Guillain-Barré syndrome in Pakistan: Similarity of demyelinating and axonal variants. Eur J Neurol. 2006;13:662–5. doi: 10.1111/j.1468-1331.2006.01071.x. [DOI] [PubMed] [Google Scholar]
- 17.Kalra V, Sankhyan N, Sharma S, Gulati S, Choudhry R, Dhawan B. Outcome in childhood Guillain-Barré syndrome. Indian J Pediatr. 2009;76:795–9. doi: 10.1007/s12098-009-0125-y. [DOI] [PubMed] [Google Scholar]
- 18.Kalra V, Chaudhry R, Dua T, Dhawan B, Sahu JK, Mridula B. Association of Campylobacter jejuni infection with childhood Guillain-Barré syndrome: A case-control study. J Child Neurol. 2009;24:664–8. doi: 10.1177/0883073808325649. [DOI] [PubMed] [Google Scholar]
- 19.Paulson GW. The Landry - Guillain - Barre - Strohi syndrome in childhood. Dev Med Child Neurol. 1970;12:604–7. doi: 10.1111/j.1469-8749.1970.tb01969.x. [DOI] [PubMed] [Google Scholar]
- 20.Hart DE, Rojas LA, Rosario JA, Recalde A, Roman GC. Childhood Guillain Barre Syndrome in Paraguay, 1990 to 1991. Ann Neurol. 1994;36:859–63. doi: 10.1002/ana.410360609. [DOI] [PubMed] [Google Scholar]
- 21.Rantala H, Uhari M, Cherry JD, Shields WD. Risk factors of respiratory failure in Children with Guillain Barre Syndrome. Pediatr Neurol. 1995;13:289–92. doi: 10.1016/0887-8994(95)00189-1. [DOI] [PubMed] [Google Scholar]
- 22.Graf WD, Katz JS, Eder DN, Smith AJ, Chun MR. Outcome in severe Guillain Barre Syndrome after immunotherapy or supportive care. Neurology. 1999;52:1494–7. doi: 10.1212/wnl.52.7.1494. [DOI] [PubMed] [Google Scholar]
- 23.Kleyweg RP, Van der mehe FG, Meulstee J. Treatment of Guillain Barre Syndrome with high dose gammaglobulin. Neurology. 1988;38:1639–41. doi: 10.1212/wnl.38.10.1639. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran R, Kuruvilla A. Guillain-Barre Syndrome in children and Adolescents- A Retrospective Analysis. J Indian Med Assoc. 2004;102:480–4. [PubMed] [Google Scholar]
- 25.Epstein MA, Sladky JT. The role of plasmapheresis in childhood Guillain Barre Syndrome. Ann Neurol. 1990;28:65–9. doi: 10.1002/ana.410280112. [DOI] [PubMed] [Google Scholar]
- 26.Shanbag P, Amirtharaj C, Fathak A. Intravenous immunoglobulins in severe Guillian-Barre syndrome in childhood. Ind J Ped. 2003;70:541–3. doi: 10.1007/BF02723152. [DOI] [PubMed] [Google Scholar]
- 27.Bella I, Chad DA. Neuromuscular disorders and acute respiratory failure. Neurol Clin. 1998;16:391–417. doi: 10.1016/s0733-8619(05)70070-0. [DOI] [PubMed] [Google Scholar]
- 28.Criado Molina A, Pérez Navero JL, Frías Pérez MA, Antón Gamero M, Ibarra de la Rosa I. [Prolonged Guillain-Barré syndrome] [Article in Spanish] An Pediatr (Barc) 2003;58:74–6. doi: 10.1016/s1695-4033(03)77997-1. [DOI] [PubMed] [Google Scholar]
- 29.Briscoe DM, McMenamin JB, O’Donohoe NV. Prognosis in Guillain-Barre syndrome. Arch Dis Child. 1987;62:733–5. doi: 10.1136/adc.62.7.733. [DOI] [PMC free article] [PubMed] [Google Scholar]


