Abstract
Aims:
To evaluate the role of sputum cytology in the diagnostic work-up of patients with suspected lung cancer
Settings and Design:
Spontaneously produced fresh sputum was analyzed in clinically suspected cases of lung cancer.
Materials and Methods:
Spontaneously produced fresh sputum was analyzed in 36 clinically suspected cases of lung cancer. It was carried out using the “fresh pick and smear” method, which employs examination of sputum for blood-tinged, discolored or solid particles and preparation of thin and even smears from these selected portions.
Statistical Analysis Used:
Average and means.
Results:
Sensitivity of sputum cytology was 60%, which increased with an increase in the number of samples examined.
Conclusions:
Sputum cytology in suspected cases of carcinoma of lung is a useful diagnostic tool. It may be called as a poor man's bronchoscopy.
KEYWORDS: Carcinoma of lung, exfoliative cytology, PAP stain, pick and smear method, sputum cytology
INTRODUCTION
“Sputum cytology” is an example of exfoliative cytology, which is based on spontaneous shedding of cells derived from the lining of an organ into a cavity from where they can be removed by noninvasive means.[1] It is a simple, accurate, reliable, cost-effective and noninvasive procedure for the assessment of respiratory diseases, including preinvasive and invasive pulmonary malignancies.[2,3]
In the present study, the efficacy of sputum cytology in the detection of clinically suspected lung carcinoma is evaluated.
The sputum is the collection of mucoid material that contains cells derived from the buccal cavity, the pharynx, the larynx and trachea: the bronchial tree and the pulmonary alveoli; as well as inflammatory cells, microorganisms, foreign material, etc.[1]
Sputum can be collected by one of the following two methods:[1]
Early morning spontaneously produced sputum.
Aerosol-induced sputum.
The simplest sputum specimen is one in which a fresh, early morning specimen produced by deep cough is collected and brought to the laboratory without any fixative.[4,5]
Sputum examined as multiple samples will detect the more central tumors, whereas bronchial washings and fine needle aspirations will detect the remaining ones, usually occurring as subpleural lesions.[5] Cytological examination of sputum has a dual purpose:[1]
To determine the presence of tumor
To classify the tumor as accurately as possible
MATERIALS AND METHODS
The present study included 36 clinically suspected cases of carcinoma of lung. Combined clinical, radiological, bacteriologic and/or histological examinations subsequently confirmed the diagnoses of these patients. One or more sputum examinations were carried out. Wherever feasible, bronchoalviolar lavage fluid and biopsy were obtained for comparison.
A fresh early morning sputum specimen, produced by deep cough, was collected in a shallow, wide-mouthed, sterile bottle made of transparent glass or a disposable plastic container. The container was properly labeled and sealed with a tight-fitting lid. To obtain satisfactory sputum samples, patients were explained about the procedure and given proper instructions.
The sputum was decanted into a Petri dish and examined against a black background. When present, blood-tinged, discolored or solid particles were selected for examination. In uniform-looking samples, six different portions were taken up. These portions of the sputum samples were utilized for detection of abnormal cells, fungal elements and acid fast bacilli. Stains like Papanicolaou, May-Grunwald, Ziehl-Neelsen and Gomori-Methenamine were used.
A small portion of sputum sample selected was transferred on a plain glass slide. With another clean glass slide, the particle on the glass slide was crushed with a rotary motion. Then, with overlapping horizontal strokes, the material was spread evenly over the slide so as to get a final preparation only slightly thicker than a blood smear. Few of the slides were fixed immediately in 95% ethyl alcohol fixative (for Papanicolaou stain) and the remaining slides were air-dried and fixed in 100% methanol (for May-Grunwald stain).
The results of the smears were reported as:
-
Normal/negative for malignancy[6]
Moderate number of alveolar macrophages, pigmented macrophages and neutrophils. Few ciliated columnar cells and mucous spirals.
-
Inflammatory lesions[1]
-
Acute inflammatory lesions.
- Papanicolaou-stained smears are predominantly cyanophilic.
- Plenty of damaged and intact polymorphonuclear leukocytes.
- Necrotic material forming threads and amorphous masses.
-
Chronic inflammatory lesions.
- Good number of neutrophils, alveolar macrophages, few lymphocytes, monocytes, occasional neutrophil and goblets cells.
- Spherical or ovoid papillary cell clusters (Creola bodies) of benign bronchial cells. Binuclated and trinucleated giant cell macrophages.
- Epithelioid cells, Langhan type of giant cell, necrosis and predominant lymphocytes (suggestive of tuberculosis) [Figure 1].
-
Plenty of eosinophils, elongated rhomboid-shaped Charcot-Leyden crystals and/or eosinophil granules (suggestive of allergic inflammation)
-
Squamous metaplasia[1,5] [Figure 2]
- Regular squamous metaplasia.
- Squamous metaplasia with mild atypia.
- Squamous metaplasia with moderate atypia.
- Squamous metaplasia with marked atypia.
-
Positive for malignancy[5]
Figure 1.
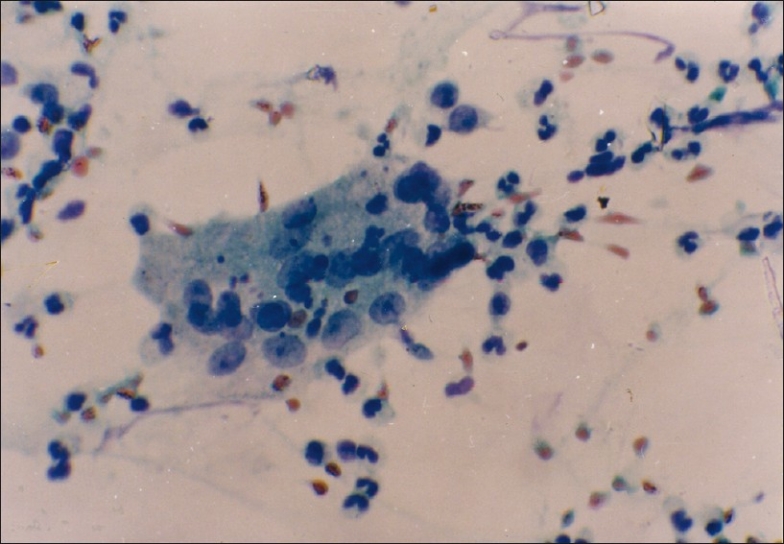
PAP stain of sputum showing Langhan's type of giant cell with peripherally placed nuclei (magnification, ×400)
Figure 2.
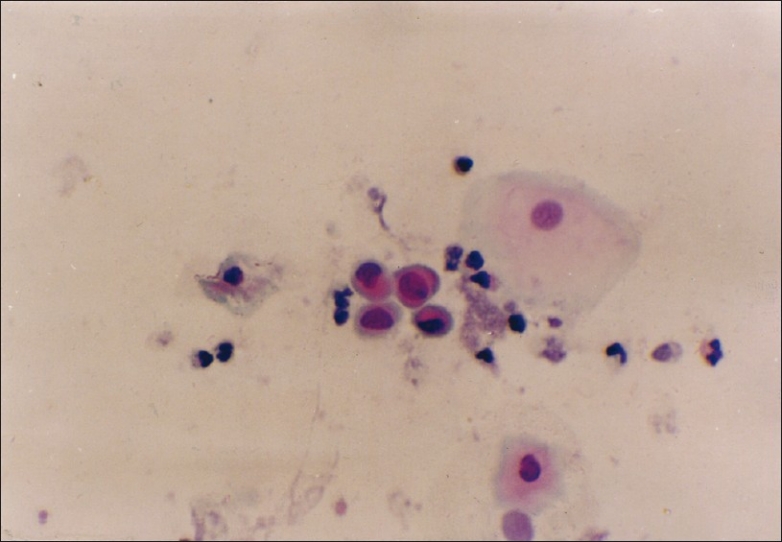
PAP stain of sputum showing squamous cell metaplasia with atypia (magnification, ×400)
Figure 3.
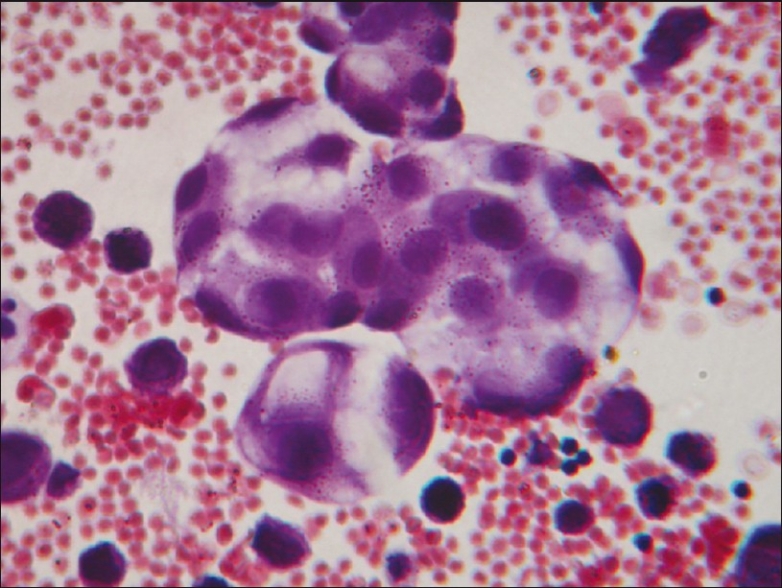
PAP stain of sputum showing cells of adenocarcinoma (magnification, ×400)
Figure 4.
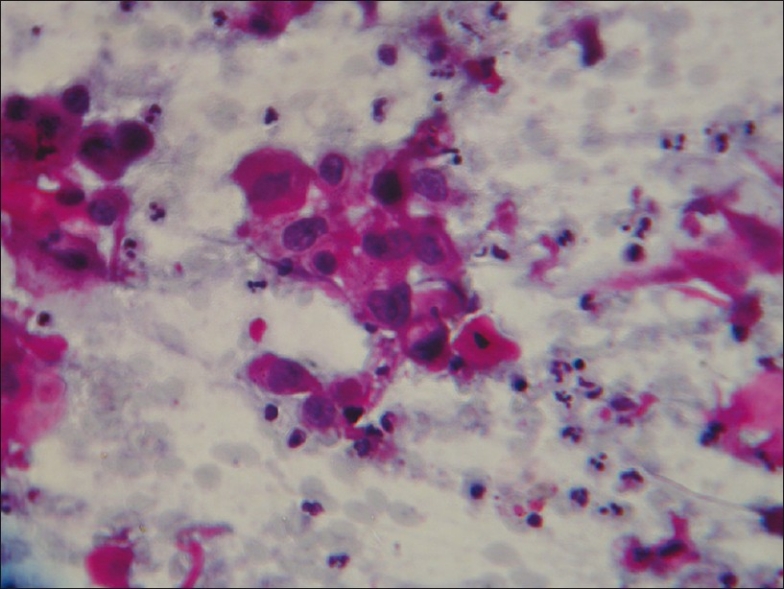
PAP stain of sputum showing squamous cell carcinoma (magnification, ×400)
RESULTS
The study included 36 patients with clinically suspected carcinoma of lung. However, of these, 20 cases were subsequently confirmed as lung malignancy by histopathologic and/or radiographic and clinical evidences.[7,8] Thus, we have a total of 20 confirmed cases of carcinoma of lung in the present study.
Of the 20 cases in which a definitive diagnosis of lung cancer was made, there was cytologic (sputum/ bronchoalveolar lavage /pleural fluid) and/or histopathologic evidence of squamous cell carcinoma in 13 (65%) cases, adenocarcinoma in five (25%) cases and small cell anaplastic carcinoma in one (5%) case. However, in one (5%) case, typing of tumor could not be performed.
The ages of the patients ranged from 36 to 75 years, with a mean of 62.6 years for males and 60.6 years for females. The patients included 15 (75%) males and five (25%) females. Thus, the male to female ratio was 3:1.
A history of smoking more than 15 cigarettes per day and for longer than 5 years was given by 14 cancer patients. We thus have a striking majority of 70% smokers in cancer patients.
Of the 20 confirmed cases of carcinoma of lung, sputum cytodiagnosis was positive for malignancy in 12 (60%) cases, inflammatory in three (15%), regular squamous metaplasia in one (5%), squamous metaplasia with mild atypia in one (5%), squamous metaplasia with moderate atypia in one (5%) and squamous metaplasia with marked atypia in one (5%) case. Thus, in our series, the pick up rate of cancer lung by sputum cytology was 60%. In 13squamous cell carcinoma patients, sputum cytology was positive for malignancy in 10 cases. Thus, the sensitivity of sputum cytology was 76.9% for squamous cell carcinoma compared with 40% for adenocarcinoma.
The first specimen of sputum was diagnostic of cancer in nine patients (45% of total), and the pick up rate increased to 55% by examination of second and to 60% by examination of third satisfactory sputum specimens.
Radiological and/or bronchoscopic studies revealed a central tumor in 11 cases, of which eight were positive for malignancy on sputum cytology, giving a pick up rate of 72.7% for central lesions compared with 44.4% (four cases) of nine peripheral lesions.
Bronchoalveolar lavage was done in 15 cancer cases. It was positive for malignancy in nine cases. Sputum cytology conducted in these cases showed malignancy in 11 cases. Sputum and BAL cytology together detected 13 of 15 cancer patients.
Thus, in these 15 cases, the following observations were made:
Sensitivity of BAL cytology – 60%.
Sensitivity of sputum cytology – 73.3%.
Sensitivity of BAL and sputum cytology – 86.7%.
Bronchoscopic biopsy could be conducted in 14 (70%) cancer cases, of which 10 (71.4%) biopsies revealed malignancy. Sputum examination in these 14 cases detected malignancy in nine (64.3%) cases.
DISCUSSION
Sputum represents a highly specialized product of the respiratory tract. In patients with sputum production, one can glean information pertinent to the abnormality through the intelligent examination of this material. Cytological examination of Papanicolaou stain of sputum is accepted as a useful diagnostic tool in carcinoma of lung.
In this study, sputum samples, spontaneously produced by deep cough, were brought without any fixative. The delay was avoided as degeneration takes place after 8-10 hours of collection.[5,7,9–11] However, Koss (2006) has suggested that specimens with high mucous content, like sputum, might be preserved for 12-24 hours, if refrigerated.[1] The other methods to induce sputum production are induction with a single dose of INS316 in patients with mild chronic bronchitis,[12] induction with hypertonic saline solution[13,14] and injection of neostigmineo.[15] However, it is suggested that the quality of spontaneously produced sputum and aerosol-induced sputum was comparable, with a better cell viability in the former.[5,16]
In the present study, sputum cytodiagnosis was carried out using the “fresh pick and smear” method, which employs examination of sputum for blood-tinged, discolored or solid particles and preparation of thin and even smears from these selected portions. This method enables effective concentration of diagnostic material by selection of suspicious areas. The pick and smear method is the most reliable method, and it has many advantages over other methods.[6]
The ages of the clinically confirmed lung cancer patients ranged from 38 to 75 years, with a mean of 62.1 years. A history of smoking more than 15 cigarettes per day and for more than 5 years was given by 70% of patients. When primary adenocarcinoma of lung, generally not related to smoking, is disregarded, we then had only one nonsmoker left in the primary lung cancer patients. The corrected incidence of smoking thus rose to 93.3%. Rossa, Joao and Eleonor noted 93% smokers in their study, which rose to 98% after correction.[17]
Sputum cytology detected 12 of 20 confirmed cases of carcinoma lung. Thus, the sensitivity (pick up rate) was 60%. It is comparable to 59.5% reported by Bocking Alfred, Stefan and Rene,[18] and 60.1% by Risse.[8] Sputum cytology in the early detection of malignant lung tumor is justifiably recognized as a time-honored method.[7,11,19] Hinston and Kuper have reported a 59.8% pick up rate.[20]
Sensitivity of sputum cytology increased with the number of satisfactory sputum samples examined, starting with 45% for one satisfactory sample examined to 55% for two and up to 60% for three. The average sensitivity of sputum cytology as reported by Bocking et al. is 40.5% for the first satisfactory sample examined and 59.5% for three samples examined.[18]
In our study, eight of the 11 patients with central lesions were positive for malignancy on sputum cytology thus giving a pick up rate of 72.7% for central tumors compared with 44.4% (four to nine cases) for peripheral lesions. These pick up rates are comparable to the 81% for central and 42% for peripheral tumors as reported by Duguid et al.[21]
In the present study, the sensitivity of sputum cytology was 76.9% for squamous cell carcinoma compared with 40% for adenocarcinoma; these pick up rates are comparable to the 67% for squamous cell carcinoma and 42% for adenocarcinoma as reported by Hinston and Kuper[20] and 70% and 25% by Payne et al.[22] This can be explained by the fact that squamous cell carcinomas are usually located centrally and readily exfoliate cells into the sputum.
Brochoscopic biopsy could be obtained in 14 lung cancer patients. In 10 cases, biopsy contained tumor (sensitivity = 71.4%); this pick up rate is comparable to 69% as reported by Payen et al.[22] It is important to know that in all the four cases with negative endoscopic biopsies, sputum cytology specimens showed malignant cells. The sputum cytology may establish the diagnosis of carcinoma of lung even when bronchoscopic biopsies are negative. This is particularly helpful when endoscopic biopsies suffer a low yield or can threat considerable danger to the patients (iatrogenic hemorrhage). Sputum cytology is also useful in places where facilities are not available. Mak et al. showed that for the maximum diagnostic yield in the diagnosis of lung cancer, biopsy should be combined with cytology using both washings and brushings.[23]
In the present study, squamous metaplasia with marked atypia was seen in three cases, of which two cases were diagnosed as squamous cell carcinoma on subsequent histopathologic study of bronchoscopic biopsies. Many authors, like Saccamanno[24] and Nasiell,[25] have suggested the possible role of squamous metaplasia as a precancerous lesion. However, it is advisable to investigate thoroughly and follow-up the cases showing squamous cell metaplasia with sever atypia.[7] Autofluorescence bronchoscopy (AFB) is now the gold standard for detecting preinvasive lesions. It is recommended that sputum cytology be followed by AFB as a screening regimen for high-risk groups with central lesions.[26] Sputum cytology examination followed by bronchoscopy is also a practical way of detecting early-stage lung cancer in these lesions.[27]
CONCLUSION
In conclusion, carcinoma of lung is more common in the seventh decade of life, with male patients predominating. Sensitivity of sputum cytology is 60%, and it increases with the number of samples examined. It is more for the central lesion compared with the peripheral lesion, and for squamous cell carcinoma compared with adenocarcinoma. Use of both sputum and BAL cytology increases the detection rate of lung cancer. Need for careful evaluation is patients with a sputum cytodiagnosis of sever atypia cannot be overemphasized.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Koss L, Melamed M. From Diagnostic cytology: Its origin and principles. In: Koss L, Melamed L, editors. Koss’ Diagnostic cytology and its histopathologic basis. Philadelphia: Lipincott Williams and Wilkins; 2006. pp. 3–20. 1036. [Google Scholar]
- 2.Roby TJ, Swan GE, Sorensen KW, Hubbard GA, Schumann GB. Discriminant analysis of lower respiratory tract components associated with cigarette smoking, based on quantitative sputum cytology. Acta Cytol. 1990;34:147–54. [PubMed] [Google Scholar]
- 3.Gupta KB, Garg S. Sputum induction - A useful tool in diagnosis of respiratory diseases. Lung India. 2006;23:82–6. [Google Scholar]
- 4.Kennedy TC, Proudfoot SP, Franklin WA, Merrick TA, Saccomanno G, Corkill ME, et al. Cytopathological analysis of sputum in patients with airflow obstruction and significant smoking histories. Cancer Res. 1996;56:4673–8. [PubMed] [Google Scholar]
- 5.Thunnissen FB. Sputum examination for early detection of lung cancer. J Clin Pathol. 2003;56:805–10. doi: 10.1136/jcp.56.11.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schumann GB, Roby TJ, Swan GE, Sorensen KW. Quantitative sputum cytologic findings in 109 nonsmokers. Am Rev Respir Dis. 1989;139:601–3. doi: 10.1164/ajrccm/139.3.601. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary M, Younus M, Rehman A, Zafar S, Bukhari S. The Importance of Sputum Cytology in the Diagnosis of Lung Cancer. Ann King Edward Med Uni. 2010;16:198–201. [Google Scholar]
- 8.Risse EK, van’t Hof MA, Vooijs GP. Relationship between patient charcteristics and the sputum cytologic diagnosis of lung cancer. Acta Cytol. 1987;31:159–65. [PubMed] [Google Scholar]
- 9.Tsang KW, Bentley AM, Mann JS, Belcher J, Pantin CF. Survey of outpatient sputum cytology: Influence of written instructions on sample quality and who benefits from investigation. Qual Health Care. 1992;1:48–50. doi: 10.1136/qshc.1.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann T, Meyer M, Patten FW, Johnson FL, Erozan YS, Frable WJ, et al. Premalignant and malignant cells in sputum from lung cancer patients. Cancer. 2009;117:473–81. doi: 10.1002/cncy.20052. [DOI] [PubMed] [Google Scholar]
- 11.Oswald NC, Hinson KF, Canti G, Husain OA, Girling DJ, Tall R, et al. Survey of the sputum cytology service in England and Wales. Thorax. 1975;30:489–96. doi: 10.1136/thx.30.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson FL, Donohue JF, Shaffer CL. Improved Sputum Expectoration Following a Single Dose of INS316 in Patients With Chronic Bronchitis. Chest. Chest. 2002;122:2021–9. doi: 10.1378/chest.122.6.2021. [DOI] [PubMed] [Google Scholar]
- 13.Gibson PG, Simpson JL, Hankin R, Powell H, Henryet RL. Relationship between induced sputum eosinophils and the clinical pattern of childhood asthma. Thorax. 2003;58:116–21. doi: 10.1136/thorax.58.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing S, Khanavkar B, Nakhosteen JA, Atay Z, Jöckel KH, Marek W RIDTELC Lung Study Group. Predictive value of image cytometry for diagnosis of lung cancer in heavy smokers. Eur Respir J. 2005;25:956–63. doi: 10.1183/09031936.05.00118903. [DOI] [PubMed] [Google Scholar]
- 15.Quin D. Use of neostigmin to increase the rate of lung cancer detection by sputum cytology. Acta Cytol. 1986;30:547–8. [PubMed] [Google Scholar]
- 16.Pizzichini MM, Popov TA, Efthimiadis A, Hussack P, Evans S, Pizzichini E, et al. Spontaneous and induced sputum to measure indices of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154:866–9. doi: 10.1164/ajrccm.154.4.8887576. [DOI] [PubMed] [Google Scholar]
- 17.Rosa UW, Joao CP, Eleonor SG. Cytology in diagnosis of cancer affecting the lung. Chest. 1973;63:203–7. doi: 10.1378/chest.63.2.203. [DOI] [PubMed] [Google Scholar]
- 18.Böcking A, Biesterfeld S, Chatelain R, Gien-Gerlach G, Esser E. Daignosis of bronchial carcinoma on sections of paraffin-embedded sputum sensitivity and specificity of an alternative to routine cytology. Acta Cytol. 1992;36:37–47. [PubMed] [Google Scholar]
- 19.Sumitani M, Takifuji N, Nanjyo S, Imahashi Y, Kiyota H, Takeda K, et al. Clinical relevance of sputum cytology and chest X-ray in patients with suspected lung tumors. Intern Med. 2008;47:1199–205. doi: 10.2169/internalmedicine.47.0777. [DOI] [PubMed] [Google Scholar]
- 20.Hinson KF, Kuper SW. The diagnosis of lung cancer by examination of sputum. Thorax. 1963;18:350–9. doi: 10.1136/thx.18.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duguid Helen LD, Huish DW. Clinical evaluation of cytodiagnosis in bronchial carcinoma. Br Med J. 1963;3:287–91. doi: 10.1136/bmj.2.5352.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne CR, Stovin PG, Barker V, McVittie S, Stark JE. Diagnostic accuracy of cytology and biopsy in primary bronchial carcinoma. Thorax. 1979;34:294–9. doi: 10.1136/thx.34.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mak VH, Johnston ID, Hetzel MR, Grubb C. Value of washings and brushings at fiberoptic bronchoscopy in the diagnosis of lung cancer. Thorax. 1990;45:373–6. doi: 10.1136/thx.45.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saccomanno G, Archer VE, Auerbach O, Saunders RP, Brennan LM. Development of carcinoma of the lung as reflected in exfoliated cells. Cancer. 1974;33:256–70. doi: 10.1002/1097-0142(197401)33:1<256::aid-cncr2820330139>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Nasiell M, Kato H, Auer G, Zetterberg A, Roger V, Karlen L. Cytomorphological grading and Feulgen DNA-analysis of metaplastic and neoplastic bronchial cells. Cancer. 1978;41:1511–21. doi: 10.1002/1097-0142(197804)41:4<1511::aid-cncr2820410440>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Mascaux C, Peled N, Garg K, Kato Y, Wynes MW, Hirsch FR. Early detection and screening of lung cancer. Expert Rev Mol Diagn. 2010;10:799–815. doi: 10.1586/erm.10.60. [DOI] [PubMed] [Google Scholar]
- 27.Lam B, Lam SY, Wong MP, Ooi CG, Fong DY, Lam DC, et al. Sputum cytology examination followed by autofluorescence bronchoscopy: A practical way of identifying early stage lung cancer in central airway. Lung Cancer. 2009;64:289–94. doi: 10.1016/j.lungcan.2008.09.016. [DOI] [PubMed] [Google Scholar]


