Abstract
Background and Objectives:
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease, mainly due to tobacco smoke. Pulmonary function tests (PFTs) are mandatory to diagnose COPD which shows irreversible airway obstruction. This study was aimed at understanding the behavior of biochemical parameters such as high sensitive C-reactive protein (hs-CRP) and lactate dehydrogenase (LDH) isoenzymes in COPD. Cytoplasmic cellular enzymes, such as LDH in the extracellular space, although of no further metabolic function in this space, are of benefit because they serve as indicators suggestive of disturbances of the cellular integrity induced by pathological conditions. The lung pattern is characterized by proportional increases in isoenzymes 3, 4, and 5. Hs-CRP indicates low grade of systemic inflammation.
Materials and Methods:
Total (n = 45) patients of COPD (diagnosed on PFTs) were included. We followed the guidelines laid by the institute ethical committee. Investigations performed on the serum were the serum for hs-CRP, LDH isoenzymes on agarose gel electrophoresis.
Results:
The results obtained showed that the value of hs-CRP was 4.6 ± 0.42 mg/L. The isoenzymes pattern was characterized by an increase in LDH-3 and LDH-4 fractions. This is evident even in those patients with normal LDH (n = 13) levels.
Interpretation and Conclusion:
This study states that there is a moderate positive correlation in between CRP and LDH-3 (r = 0.33; P = 0.01). Raised LDH-3 levels do not correlate with FEV1 % (forced expiratory volume in first second) predicted. Moreover, it associates positively with hs-CRP and smoking status and negatively with body mass index. This underlines the potential of these parameters to complement the present system of staging which is solely based upon FEV1 % predicted.
KEYWORDS: COPD, hs-CRP, LDH-3 isoenzyme
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a major and increasing global health problem. Global prevalence according to the study of global burden of diseases was estimated to be 9.34/1000 in men and 7.33/1000 in women. In India, the prevalence of COPD is shown to be 4.1% with a male-to-female ratio of 1.56:1 in the population of above 35 years of age.[1,2]
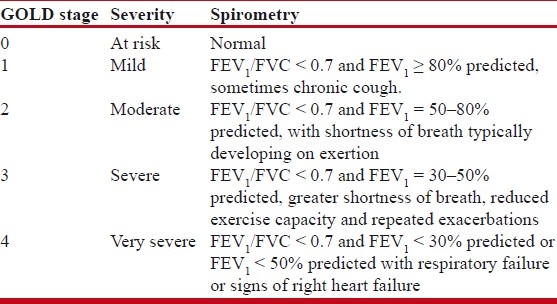
The diagnosis of COPD largely relies on a history of exposure to noxious stimuli (mainly tobacco smoke) and abnormal lung function tests. Persistent reduction in FEV1 (forced expiratory volume in first second) is the most typical finding in COPD. Another one is airflow obstruction, which is typically determined by spirometry. Key phenotypes obtained are FEV1 and FVC (forced vital capacity). Patients with airflow obstruction related to COPD have a chronically reduced ratio of FEV1/FVC. A classification of disease severity into four stages has been proposed by the GOLD guidelines based primarily on FEV1 [Table 1]. Although the pulmonary function test (PFT) is the mainstay for diagnosis of COPD, the staging on the basis of FEV1 alone as an index of severity for COPD has been debated. This includes only the functional respiratory impairment. There are studies which address clinical systemic impairment indicated by body mass index (BMI), MRC dyspnoea score, 6-min walk test and also the presence of systemic inflammation in COPD affecting other body systems beyond the lung compartment.[3–5]
Table 1.
GOLD Staging of COPD (updated in 2010)
Now there is a large body of research focusing on cellular and biochemical mechanisms of COPD. Moreover, GOLD has defined the COPD as “a disease state characterized by airflow limitation that is not fully reversible”, and the airflow limitation is usually both progressive and associated with an abnormal inflammatory response of the lungs to noxious particles and gases.[2,4]
However, presently there are no inflammatory markers to be included in the diagnosis of COPD. There are a few suggestions given by GOLD regarding inflammatory response to smoking and cellular and molecular mechanisms in stable COPD that are responsible for the persistence of the inflammatory response in COPD.[4] Components of inflammatory cascade such as inflammatory cells, cytokines, oxidative stress biomarkers, and inflammatory indicators like C-reactive protein (CRP) are the potential biomarkers that can be used in the diagnosis and prognosis of COPD. Presently CRP is recommended as an additional test for diagnosis and/or severity.[2]
Furthermore, chronic inflammation in the pulmonary tissue is also associated with systemic effects. A clear communication is present between the disease mechanisms in the pulmonary compartment and peripheral tissues leading to concept of COPD as a systemic inflammatory disease.[4,5] In addition to pulmonary alterations, other organ systems may be affected in COPD. Systemic effects of COPD include weight loss, nutritional abnormalities, and musculoskeletal dysfunction. CRP in the serum was proposed to measure as a marker of systemic inflammation. CRP is often used as a clinical marker of acute systemic inflammation. Since low-grade inflammation is evident in COPD, we studied high sensitive CRP (hs-CRP) levels in these patients.
Cytoplasmic cellular enzymes, like lactate dehydrogenase (LDH) (E.C.1.1.1.27; L-lactate: NAD oxidoreductase) in the extracellular space, although of no further metabolic function in this space, are of benefit because they serve as indicators suggestive of disturbances of the cellular integrity induced by pathological conditions. Since LDH is an enzyme present essentially in all major organ systems, serum LDH activity is abnormal in a large number of conditions. Although the increase in total serum LDH activity is rather nonspecific,[6] it is proposed that measurement of LDH activity levels and its isoenzymes pattern in the serum may provide additional information about the disease process of COPD.[7]
Finally, we proposed to correlate the LDH isoenzyme pattern and Hs-CRP with the clinical parameters of COPD and in between each other. Clinical parameters included were body mass index, GOLD staging, FEV1 % predicted.
Therefore with this perspective in the mind, we evaluated serum LDH-3 isoenzyme as an indicator suggestive of breach in the cellular integrity and CRP as a marker of systemic inflammation in COPD patients.
MATERIALS AND METHODS
In total 45 cases of COPD were studied. All were reporting to Medicine OPD of GHATI Hospital, Aurangabad. They were known cases of stable COPD without any exacerbation. They were identified on history and enrolled, and later called on one particular day and were evaluated for lung functions by spirometry and pulmonary functions were recorded. Patients were grouped into GOLD stages based upon FEV1 % predicted according to Table 1.
A sample size of 45 cases was thought to be enough to know the eligibility of these parameters for further studies. Blood samples were collected in plain bulbs and serum processed for following investigations.
Methods
Total LDH: Quantitative estimation of LDH: Principle of the method
LDH catalyzes the reduction of pyruvate by NADH, according the following reaction:[8]
Pyruvate + NADH + H + → l-lactate + NAD +
The rate of decrease in concentration of NADH, measured photometrically, is proportional to the catalytic concentration of LDH present in the sample.[9]
NAD: Nicotinamide adenine dinucleotide; NADH: Nicotinamide adenine dinucleotide hydrogen.
Separation of LDH isoenzymes by agarose gel electrophoresis
In this technique, agarose gel electrophoresis was used. The kit was provided with ready to use agarose gel, alkaline buffer, and staining solution. After the run of the electrophoresis, gels were stained with activity staining with the help of substrate solution provided. The stained gels are quantified with the densitometer scanning.[9,10]
C reactive protein
Quantitative estimation of low levels of CRP:
Principle of the method:
The method is a quantitative immune turbidimetric test for the measurement of low levels of CRP in plasma or serum.[11]
Observations, results, and statistical analysis
Statistical analysis was done using Graph pad and SPSS-17. Unpaired ‘t-tests’ and ANOVA tests were employed for the comparison in between stages and Pearson coefficient (r) and multivariate regression analysis was carried out to show the association.
When compared with the average reference value of the method used, almost 75% of patients showed an increase in the total LDH activity (Ref value = 361 ± 59).
The isoenzyme pattern was mainly characterized by an increase in LDH-3 and LDH-4 fractions at the cost of LDH-2 and LDH-1. This is evident in even those patients with normal LDH (n = 13) levels.
-
When we divide the patients into GOLD stages according to FEV1 % predicted, we got the following pattern.
- Total LDH increased in all the stages with no statistical difference in between the stages.
- Also, LDH-3 increased in all the stages over the reference range. But in between stages there was no difference (Ref value for LDH-3 = 18.6 ± 1.9).
Mean value of hs-CRP in the study group is 4.6 mg/L. Out of 45 patients, 35 patients have values more than 1.5 mg/L. The reference range for American males is 0.3-8.6 mg/L. For primary prevention of ischemic heart disease, values >3mg/L are considered high risk for ischemic cardiovascular diseases. In this sense, the hs-CRP value is elevated in 65% of the COPD patients.
In the GOLD stages, hs-CRP is higher in the earlier stages. As the stage advances, the hs-CRP value decreases. This decrease in stage 4 is very prominent and statistically significant [P = 0.03; Table 2].
Table 2.
Hs-CRP, LDH-3, and BMI in GOLD stages of COPD
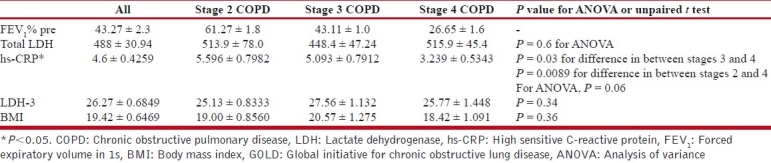
Relation of hs-CRP with LDH-3 and BMI
On multivariate regression analysis, hs-CRP is seen to be affected by LDH-3 (P = 0.04) and it is not confounded by BMI (P = 0.6). hs-CRP has a coefficient of regression of 0.06 with FEV1 % predicted. When the BMI value is not considered as an independent variable for hs-CRP, FEV1 % has a regression coefficient of 0.05 with hs-CRP. This shows that hs-CRP is the function of LDH-3; an indicator of lung inflammation, functional impairment shown by FEV1 % predicted in this cohort of COPD.
As a cohort, hs-CRP has positive correlation with LDH-3 (r = 0.33; P = 0.01).
In stages 2, 3, and 4, it has mild-to-moderate positive correlation with LDH-3. As a group, it has negative correlation with BMI which is very weak. LDH-3 has negative correlation with BMI (r = - 0.3; P = 0.05).
Parameters according to the smoking status
CRP, LDH-3 values are significantly higher in the ex-smoker COPD patients [Table 3].
Table 3.
Parameters according to the smoking status

DISCUSSION
To study serum LDH isoenzyme, hS-CRP in the patients of various stages of COPD at one point of time was the aim of our study. Also we aimed at correlating above parameters with BMI and FEV1 % predicted.
COPD is a chronic inflammatory disease. While identifying the risk factors for the COPD, cigarette smoking is of the foremost importance. Synthesis of existing data regarding inflammatory cell responses in human lungs following cigarette smoke exposure suggests the following sequence of events: (1) macrophages patrol the lower air space under normal conditions, and (2) cigarette smoke comes into contact with and activates lung epithelium cells and alveolar macrophages (AM) leading to cytokines/chemokines release followed by acute neutrophilic recruitment and subacute accumulation of macrophages in the respiratory bronchioles and alveolar spaces. T-cells (CD8+ > CD4+) and perhaps other inflammatory and immune cells are also recruited.[12] (3) An excess of oxidative products and the facilitation of colonization by microorganisms also interact in order to recruit more proinflammatory cells.[2,3]
Therefore, COPD inflammation as indicated by various studies is comprised of AM and neutrophils.[7,13] Surprisingly, in the end stage lung disease, long after smoking cessation, there remains an exuberant inflammatory response, suggesting that the mechanisms of cigarette smoke-induced inflammation that initiates the disease differs from mechanisms that sustain inflammation after smoking cessation. Thus, multiple interacting inflammatory cell types are present and contribute to disease pathogenesis.[12]
Several pulmonary disorders are associated with elevated serum LDH levels.[7,13–18] These disorders all have in common that cell damage or inflammation is both involved in pathogenesis.[7] Lung parenchymal cells, or local inflammatory cells including AM and polymorphonucleocytes (PMNs), may be a potential source of elevation of serum LDH associated with pulmonary diseases.[7] The study by NAM Cobben in ex-coal miner's pneumoconiosis showed that enzymatic markers such as LDH, ALP (alkaline phosphatase), and B-glucoronidase (BGD) are indicative of pulmonary inflammation and/or damage. The aim of this study was to investigate the serum LDH isoenzyme pattern after coal dust exposure and the possible relation to PFTs. Ex-coalminers (n = 201), with a history of coal dust exposure more than 20 years ago, were included in the study. The serum LDH activity was found to be elevated in 79.1% of the ex-coalminers (n = 159). Moreover, in 97.5% of the cases a high percentage of LDH-3 was demonstrated (n = 196).
In the same work, the authors hypothesized that AMs and PMNs release different LDH isoenzymes. Bronchoalveolar lavage fluid (BALF) samples obtained from patients with various pulmonary disorders were studied. Out of these samples, a group with mainly PMNs and another group with mainly AMs were selected. LDH activity and LDH isoenzymes were measured.
The cell-free fraction of BALF of the group with mainly AMs showed lower LDH activity compared to the group with mainly PMNs. The LDH isoenzyme pattern differed, with the LDH-3/LDH-5 ratios being lower in all BALF samples with predominantly PMNs compared to the BALF samples with predominantly AMs. The LDH isoenzyme pattern differed between BALF samples with mainly PMNs (high LDH-5) and BALF samples with mainly AMs (high LDH-3).
In conclusion this work showed measurement of LDH isoenzymes in a BALF sample reflected the cells present in that BALF sample. Therefore, determination of enzyme activity in BALF appears to be useful in monitoring pulmonary inflammation.[13,16,17]
This study shows that years after the exposure to tobacco smoke and disease initiation, there is cellular alteration in the lung tissue which is reflected in the raised LDH-3.This is parallel with the finding found in pneumoconiosis by NAM cobben long after the disease initiation.[13]
Therefore, raised serum LDH-3 levels in COPD may indirectly reflect the ongoing role of AMs. Raised serum LDH-3 in almost 85% of COPD patients underlines the continuation of inflammation, as these patients have stopped smoking at least 5 years back. Raised LDH-3 shows positive correlation with smoking history and hs-CRP and negative correlation with BMI. Although LDH-3 correlates negatively with FEV1 % predicted, there is no significant difference in LDH-3 in between the COPD stages [Tables 3–5]. However, LDH3 has significant positive correlation with smoking status and hs-CRP and negative correlation with BMI. Therefore, whether these parameters which envisage the inflammatory face of COPD are raised irrespective and before functional respiratory impairment? This needs to be ascertained in prospective and different models of COPD (such as rapid lung volume losers, chullah smokers, with more systemic impairment). Is this underlines the potential of these parameters to complement the present system of staging which is solely based upon FEV1 % predicted?
Table 5.
LDH, BMI, in relation with hs-CRP (cut off value as 3.0 mg/L)

Table 4.
LDH, hs-CRP, and FEV1% predicted according to BMI (cut-off for BMI taken is 19)

COPD also has systemic face through the emissary of systemic inflammation.[19–26] In the advanced stages of the disease, patients suffer from systemic complications such as cachexia and decreased muscle strength and increase the risk of cardiovascular disease two- to threefold. The link is of systemic inflammation present in COPD patients. Epidemiological analyses have shown a relationship between COPD and an increase in mortality and morbidity from cardiovascular diseases. Lung inflammation contributed by the AM and bronchial epithelial cells are important in secreting the mediators that elicit local and a systemic inflammatory response when exposed to cigarette smoke or particulate matter air pollution. The cytokines produced in the lung are capable of stimulating the marrow to produce a leukocytosis, increase platelet count, and stimulate the liver to produce acute phase proteins such as hs-CRP and fibrinogen.[5] hs-CRP is a marker of systemic inflammation. It is stable molecule with a half-life of 18–24 h. It is easy to measure, and it has been shown to provide prognostic information in the general population. In accordance with the previous studies, we get the higher hs-CRP values in almost 80% of patients (hs-CRP>1 mg/L). For the risk of ischemic heart disease, almost 65% of the patients fall in the high-risk group (hs-CRP>3 mg/L) [Table 5]. We conclude that there is a presence of systemic inflammation in COPD patients. The study states that there is moderate positive correlation between hs-CRP and LDH-3. Rise in LDH-3, which is lung specific, is hand in hand with hs-CRP, which is a systemic inflammatory marker.
However, the drop in the hs-CRP in stage 4 is conspicuously prominent. The reason for this could not be elucidated. More meticulous account of comorbidities and larger study population may clear this issue.
Limitations of the study
The study of LDH isoenzymes in BALF; immediate to the lung tissue, cellular nature of BALF, study of cytokines; major inducer of the CRP production by the hepatocytes, measurement of indicators of oxidative stress in BALF and serum would have highlighted more on the spillage of local inflammation of lung into the systemic circulation and their role in monitoring pulmonary inflammation in COPD.
SUMMARY AND CONCLUSION
This study was carried out on COPD patients. In total, 45 patients were studied. Out of 45, 12 were in stage 2, 15 were in stage 3, and 17 were stage 4 of GOLD staging COPD. This system is based upon the FEV1 % predicted which is the functional indicator of airflow obstruction. Further, this study estimated LDH-3 and CRP in the serum.
Release of cytosolic LDH into the serum is due to the increased cellular permeability. Isoenzyme determination localizes the tissue source of the enzyme. In this study, raised serum LDH-3 is found in the majority of COPD patients. This is the reflection of underlying lung inflammation as lung has LDH-3 and LDH-4 isoenzymes of LDH.
From this study, it is evident that there is the presence of systemic inflammation in COPD. Moreover, the rise in hs-CRP positively correlates with LDH-3.
These findings need to be ascertained in the larger and well systematized study with a prospective approach so as to test the utility of LDH-3 and hs-CRP in categorization of COPD patients.
ACKNOWLEDGMENTS
The authors solemnly acknowledge Mrs. Dr. Sane, Associate Professor, Department of Biochemistry, Sir J. J. Group of Hospitals, Mumbai, for helping in the separation of isoenzymes on agarose gel electrophoresis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jindal SK, Aggarwal AN, Chaudhry K, Chhabra SK, D’Souza GA, Gupta D, et al. Multicentric Study on epidemiology of Chronic Obstructive Pulmonary Disease and its relationship with tobacco smoking and environmental tobacco smoke exposure. Indian J Chest Dis Allied Sci. 2006;48:23–9. [PubMed] [Google Scholar]
- 2.Goldcopd.com (Internet) 2005 update: executive Summary, Global Strategy for the Diagnosis, Management, and prevention of COPD. 2005. Sep, [Last accessed on 2005]. Available from: http://www.goldcopd.com/
- 3.Groneberg DA, Chung KF. Models of chronic obstructive pulmonary disease. Respir Res. 2004;5:18. doi: 10.1186/1465-9921-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rufino R, Lapa e Silva JR. Cellular and biochemical bases of chronic obstructive pulmonary disease. J Bras Pneumol. 2006;32:241–8. [PubMed] [Google Scholar]
- 5.van Eeden SF, Yeung A, Quinlam K, Hogg JC. Systemic Response to Ambient Particulate Matter: Relevance to Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc. 2005;2:61–7. doi: 10.1513/pats.200406-035MS. [DOI] [PubMed] [Google Scholar]
- 6.Mauro P, Bais R, Wouter W. van Solinge. In: Burtis CA, Ashwood ER, Bruns De, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 4th ed. St.Louis,MO: Elsevier Saunders; [Google Scholar]
- 7.Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–42. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 8.Lott JA, Nemensanszky E. Lactate dehydrogenase. In: Lott JA, Wolf L, editors. Clinical enzymology, a case – oriented approach. New York: Field, rich and associates, Inc; 1987. pp. 213–44. [Google Scholar]
- 9.Kachmar JF, Moss DW. In Enzymes. In: Tietz NW, editor. Fundamentals of clinical chemistry. Philadelphia: Saunders; 1976. pp. 652–60. [Google Scholar]
- 10.Moses GC, Ross ML, Henderson AR. Ten electrophoretic methods compared with a selected method for quantifying lactate dehydrogenase isoenzymes in serum. Clin Chem. 1988;34:1885–90. [PubMed] [Google Scholar]
- 11.Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, et al. Evaluation of nine automated high-sensitivity C- reactive protein methods: Implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47:418–25. [PubMed] [Google Scholar]
- 12.Reilly JJ, Silverman EK, Shapiro SD. Chronic obstructive pulmonay disease. In: Kasper Dl, Braunwald E, Fauci AS, Hauser SL, Longo Dl, Jameson JL., editors. Harrison's Principles of internal medicine. 16th Ed. New York City: McGraw-Hill; 2005. pp. 1548–52. [Google Scholar]
- 13.Cobben NA, Drent M, Schols AM, Lamers RJ, Wouters EF, van Diejien-Visser MP. Serum lactate dehydrogenase and its isoenzyme pattern in ex-coalminers. Respir Med. 1997;91:616–23. doi: 10.1016/s0954-6111(97)90008-1. [DOI] [PubMed] [Google Scholar]
- 14.DeRemee RA. Serum lactic dehydrogenase activity and diffuse interstitial pneumonitis. JAMA. 1986;204:236–40. [PubMed] [Google Scholar]
- 15.Matusiewicz SP, Williamson IJ, Sime PJ, Brown PH, Wenham PR, Crompton GK, et al. Plasma lactate dehydrogenase: A marker of disease activity in cryptogenic fibrosing alveolitis and extrinsic allergic alveolitis. Eur Respir J. 1993;6:1282–6. [PubMed] [Google Scholar]
- 16.Cobben NA, Jacobs JA, van Dieijen-Visser MP, Mulder PG, Wouters EF, Drent M. Diagnostic value of BAL fluid cellular profile and enzymes in infectious pulmonary disorders. Eur Respir J. 1999;14:496–502. doi: 10.1034/j.1399-3003.1999.14c04.x. [DOI] [PubMed] [Google Scholar]
- 17.Drent M, Cobben NA, Henderson RF, Jacobs JA, Wouters EF, van Dieijen-Visser MP. BAL fluid LDH activity and LDH isoenzyme pattern in lipoid pneumonia caused by anintravenous injection of lamp oil. Eur Respir J. 1996;9:2416–8. doi: 10.1183/09031936.96.09112416. [DOI] [PubMed] [Google Scholar]
- 18.Chumbalkar VC. Lactate dehydrogenase enzyme - 6: Evaluations in clinical medicine and its partial characterization. [Dissertation] MD (Biochemistry) Pune University. 2000 [Google Scholar]
- 19.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C- reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–53. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto-Plata VM, Mullerova H, Toso JF, Feudjo-Tepie M, Soriano JB, vessey RS, et al. C-reactive protein in patients with COPD, control smokers and non- smokers. Thorax. 2006;61:23–28. doi: 10.1136/thx.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balasubramanian VP, Varkey B. Chronic obstructive pulmonary disease: effects beyond the lungs. Curr Opin Pulm Med. 2006;12:106–12. doi: 10.1097/01.mcp.0000208449.73101.ac. [DOI] [PubMed] [Google Scholar]
- 23.Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolicandfunctionalimpairment in advanced COPD. Thorax. 2006;61:1–3. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wouters EF. Local and Systemic Inflammation in Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc. 2005;2:26–33. doi: 10.1513/pats.200408-039MS. [DOI] [PubMed] [Google Scholar]
- 25.Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51:819–24. doi: 10.1136/thx.51.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasuda N, Goth K, Minatoguchi S, Asano K, Nishigaki K, Nomura M, et al. An increase of soluble Fas, an inhibitor of apoptosis, associated with progression of COPD. Respir Med. 1998;92:993–9. doi: 10.1016/s0954-6111(98)90343-2. [DOI] [PubMed] [Google Scholar]


