Sir,
A 67-years-old male was admitted in the chest ward with fever, sore throat, generalized pruritus, and extensive blisters over the skin for 2 days. He was receiving isoniazid (INH), rifampicin (RFN), ethambutol (EMB), and pyrazinamide (PZN) thrice weekly for 14 days for new sputum-positive pulmonary tuberculosis. He was not taking any other drug at the time of presentation. On examination his body weight was 50 kg, body mass index (BMI) - 23 kg/m2, axillary temperature - 103.1°C, pulse rate - 112/min, respiratory rate - 22/min, blood pressure (BP) - 106/72 mmHg, and SpO2 – 98%. Skin examination revealed presence of blisters on a dusky pruritic macules over front and back of chest and abdomen and both upper and lower limbs, involving 60% of the body surface area, as measured on the basis of Lund and Browder chart [Figure 1].[1] Nikolsky’ sign was positive, i.e., it was able to extend the area of superficial sloughing by gentle lateral pressure on the skin surface. He also had oropharyngeal, conjunctival, and nasal ulcerations. Examination of other systems revealed no abnormality. He was clinically diagnosed to have new sputum-positive pulmonary tuberculosis with drug-induced toxic epidermal necrolysis TEN, all anti – tuberculous drugs ATD were stopped and he was shifted to intensive care unit. Investigations revealed hemoglobin (Hb) - 10.8 g/dl, white blood cell (WBC) - 8.2 × 109/L, neutrophils - 70%, lymphocytes - 26%, eosinophils - 4%, reticulocyte index - 2, erythrocyte sedimentation rate ESR - 90 mm in first hour, fasting plasma glucose - 122 mg/dl, liver function test - normal, serum Na - 123 mg/dl, serum K - 4.5 mg/dl, blood urea nitrogen - 30 mg/dl, and serum creatinine - 1.2 mg/dl. Urine examination was normal. Human immunodeficiency virus HIV 1 and 2 serology was nonreactive. Sputum smear was positive for acid fast bacilli (AFB). Chest X-ray (posteroanterior view) revealed infiltrations in the left upper and mid zones with cavitation [Figure 2]. Bacterial culture from the skin lesions revealed no growth. He was given intravenous normal saline and nutritional support. Skin care was given with local application of povidone iodine and calamine lotion. His vital signs were closely monitored and intake output chart was maintained. His general condition improved, and skin and mucosal lesions healed completely by 2 weeks. As he was having sputum-positive pulmonary tuberculosis, he urgently needed restarting of ATD, but it was impossible to make a new regimen with exclusion of all the four drugs of the regimen. So we had no option other than going for drug challenge test with great caution. We decided to reintroduce ATDs one by one with gradually escalating dose. No adverse reaction was noted when INH and RFN were reintroduced one by one in staged fashion. He could be safely put on daily INH - 300 mg and RFN - 450 mg regimen. But he developed morbiliform rash with pruritus, fever, and arthralgia within 48 hours of introduction of EMB - 100 mg. EMB was immediately withdrawn. After normalization of skin rashes, PZA - 250 mg was added but he developed similar skin reaction on the next day and PZA was immediately withdrawn. After stabilization, streptomycin (SM) and levofloxacin (LFX) were added and he was continued with daily regimen containing INH - 300 mg, RFN - 450 mg, SM - 500 mg, and LFX - 750 mg. There was no reappearance of skin lesion. His sputum became negative for AFB at the end of 2 months. Then he was continued with INH 300 mg and RFN 450 mg for next 7 months. Subsequent sputum examinations at 4th, 6th, and 9th months were negative for AFB. He was declared cured after end of 9 months.
Figure 1.
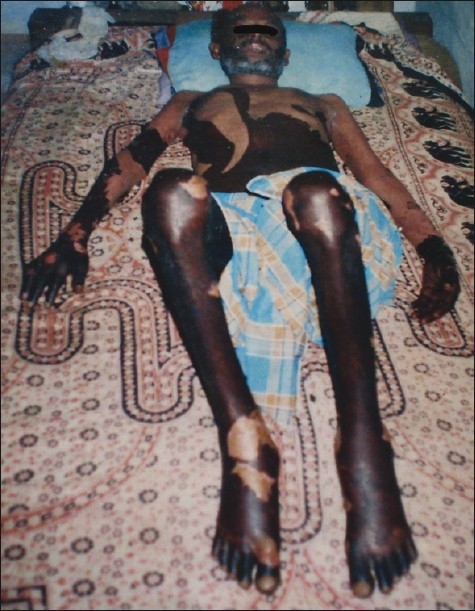
Full thickness necrosis of epidermis with detachment
Figure 2.
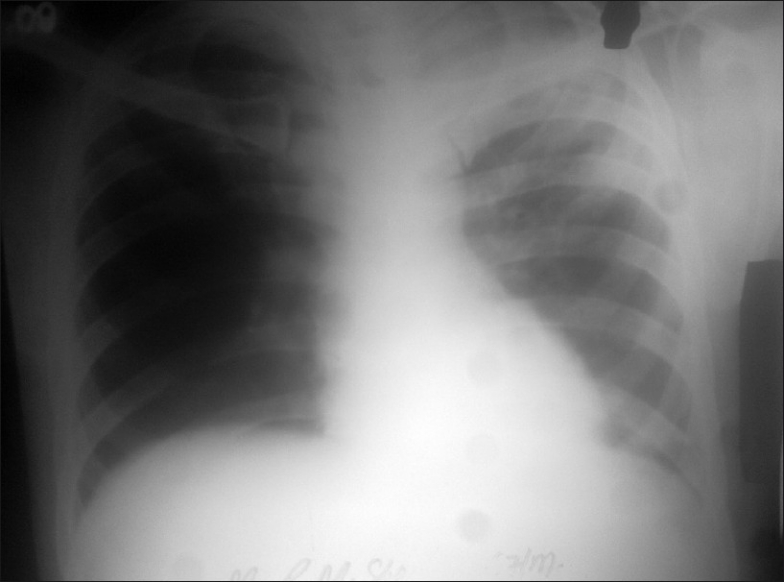
Chest X-ray (PA view) showing left upper zone infiltration with cavitation
TEN, also called Lyell's syndrome, was first described in 1956.[2] It is characterized by[3]
Full thickness necrosis of epidermis (with absence of substantial dermal inflammation) with blister formation, with detachment of >30% of body surface area;
Two or more sites of mucosal erosions (oropharynx, nose, eye, genitalia, intestinal tract, and respiratory tract);
Constitutional symptoms such as fever and arthralgia.
Cases with <10% detachment are called Stevens Johnson syndrome (SJS) and between 10% and 30% detachment are called SJS/TEN overlap.
Initial presentation of TEN is with flu-like symptom lasting for 2–3 days. The skin lesion begins with painful and burning morbiliform eruption on face and upper trunk spreading on the entire trunk and proximal limbs but often sparing the hairy portion of the scalp. The process tends to occur in waves over a period of 3–5 days. Two or more areas of mucosal involvement are seen in 85%–95% cases. Complete healing of skin lesion occurs within 3–4 weeks by re-epithelization but mucosal lesion heals by 8 weeks. Common systemic abnormalities include anemia, lymphopenia, hyperglycemia, and hyponatremia. Mortality in TEN is about 30%–40 %, mainly due to acute respiratory distress syndrome and multiorgan failure often precipitated by sepsis and septicemia.
TEN is induced by drugs in 80% cases and the common drugs triggering TEN include antiepileptics, sulfonamides, ampicillin, and nonsteroidal antiinflammatory drugs.[4] TEN induced by ATD is relatively rare. TEN is reported with the use of INH, RFN, EMB, PZA, SM, and thiacetazone, but quinolones have low risk association.[5]
TEN probably has an immunological basis mediated by type IV hypersensitivity reaction directed by drug-specific T cell. Identification of the responsible drug is often difficult because the patient often takes more than one drug. Reexposure to the drug for the challenge test is generally avoided because of fatal consequences. However, there is report of successful provocation test in TEN.[5] Helpful clues to identify the responsible drug without resorting to challenge test are as follows.[6]
Most drugs that cause TEN are often given 1–3 weeks previously.
Recurrence within 48–72 hours on administration of a drug previously recorded as causing TEN.
A drug is unlikely to be responsible for TEN if it was given < 24 hours ago or if the duration of treatment exceeds 3 weeks.
Our patient had onset of skin blister and mucosal erosion after 2 weeks following initial treatment but developed similar lesions within 2 days in the next episode. This patient had normocytic normochromic anemia and hyperglycemia but not lymphopenia. As he developed TEN with the first-line ATDs which are essential for successful treatment of tuberculosis, it was necessary to carry out the challenge test with great caution though there was risk of fatal outcome. Fortunately, the challenge test could be done safely and it was found that both E and Z were responsible for TEN in this patient. Therapy could be safely continued with a modified daily regimen containing INH - 300 mg, RFN - 450 mg, SM - 500 mg, LFX - 750 mg for 2 months followed by 7 months of INH - 300 mg and RFN - 450 mg. The patient was declared cured after 9 months of ATD.
REFERENCES
- 1.Ghosh S. Burn. In: Williams NS, Bulstrode CJK, O’Connele PR, editors. Bailey and Love's Short Practice of Surgery. 25th ed. London: Hodder Arnold; 2008. pp. 378–93. [Google Scholar]
- 2.Lyell A. Toxic epidermal necrolysis: An eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355–61. doi: 10.1111/j.1365-2133.1956.tb12766.x. [DOI] [PubMed] [Google Scholar]
- 3.Roujeau JC, Stern RS, Wintroub BU. Cutaneous drug reactions. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jamesom JL, et al., editors. Harrison's Principles of Internal Medicine. 17th ed. Vol. 1. New York: McGraw Hill Medical; 2008. pp. 343–9. [Google Scholar]
- 4.Sane SP, Bhatt AD. Stevens Johnsons syndrome and toxic epidermal necrolysis -challenges of recognition and management. J Assoc Physicians India. 2000;48:999–1003. [PubMed] [Google Scholar]
- 5.Breathnach SM. Erythema multiforme, Stevens Johnson syndrome and Toxic epidermal necrolysis. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Text Book of Dermatology. 7th ed. Vol. 4. Oxford: Blackwell Publishing; 2004. pp. 74.1–74.20. [Google Scholar]
- 6.Avakian R, Flowers FP, Araujo OE, Ramos-caro FA. Toxic Epidermal Necrolysis. A review. J Am Acad Dermatol. 1991;25:69–79. doi: 10.1016/0190-9622(91)70176-3. [DOI] [PubMed] [Google Scholar]


