Abstract
The following discussion of the esophagogastric junctions includes commentaries on the three component structures of the sphincteric segment between the stomach and the esophagus; the pressure contributions from the three sphincteric components in normal subjects and in gastroesophageal reflux (GERD) patients; the mechanism of action of endoscopic plication to determine the underlying pathophysiology of GERD; and in vitro muscle strip studies of defects within the gastroesophageal sphincteric segment potentially leading to GERD.
Keywords: clasp muscle fibers, sling muscle fibers, gastroesophageal junction, lower esophageal sphincter, upper gastric sphincter defects in GERD, missing sphincter, Endocinch, muscarinic receptors, in vitro muscle strips, stretch-induced tone, nicotine-induced relaxations
1. Structure of the gastroesophageal junction HPZ
Larry Miller, Anil Vegesna, and James G. Brasseur vivi@temple.edu
Background
Relaxation and phasic tone of the crural sphincter and its contribution in the gastroesophageal junction have been studied by Ingelfinger and Boyle et al.1,2 Code et al. were early reporters of an intraluminal HPZ in the segment separating the esophageal body from the gastric cardia, and suggested that intrinsic smooth muscles of the distal esophagus may be responsible for maintaining a high-pressure barrier to reflux.3 Liebermann-Meffert et al.4 measured, in cadavers a thickened wall of smooth muscle at the junction that coincides with the gastric sling-clasp muscle groups. Relative alignments and contributions of all the different sphincteric components have recently been quantified in the normal gastroesophageal junction HPZ.5
Aim
To quantitate skeletal and smooth-muscle contributions to the normal gastroesophageal junction HPZ. To identify the relative position of various components of the HPZ, and their changes with respect to respiration.
Methods
Intraluminal manometric pull-throughs were carried out concurrently with high-frequency ultrasound in 15 normal subjects during breath-hold at FI and FE. Pull-throughs were repeated after intravenous atropine to suppress smooth-muscle pressure. Postatropine pressures were subtracted from full pressure after referencing to the anatomical right crus (from ultrasound), which indirectly measures the smooth-muscle contribution to the gastroesophageal junction HPZ. In a separate study, seven patients undergoing general anesthesia for nonesophageal pathology were administered cisatrcurium to paralyze the crural sphincter and directly measure the smooth-muscle contribution to the gastroesophageal junction HPZ. Displacements of the anatomical versus physiological crural sphincter were determined from the initial position of the catheter at initiation of pull-through.
All subjects gave institutional review board approved informed consent to take part in this study. Exclusion criteria included subjects on any medication that could affect the gastroesophageal HPZ including prokinetic agents, erythromycin type antibiotics, and anticholinergics. Other exclusion criteria were patients with GERD, hiatal hernia, abdominal surgery involving the stomach or esophagus, diabetes, scleroderma, achalasia, hypertension, cardiac disease, and current pregnancy.
Results
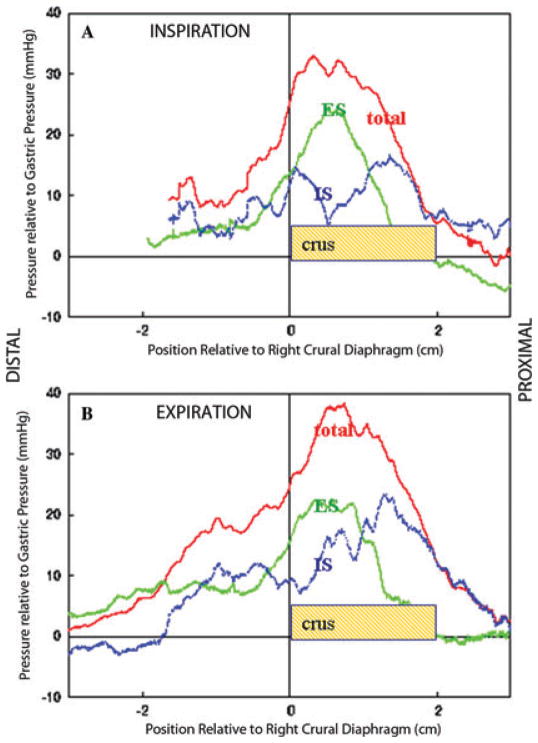
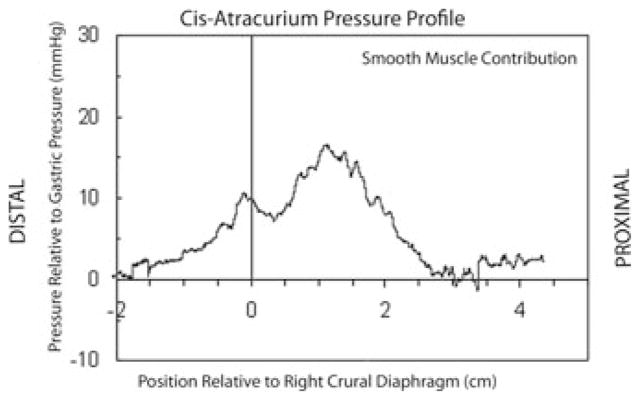
The HPZ was axially broader in FE. However, the atropine-suppressed (striated) pressure profile was independent of diaphragmatic position and was correlated with the location of the anatomic crus (R2 = 0.83) while displacing ~2 cm between FI-FE diaphragmatic positions. The subtracted (smooth muscle contribution) pressure profile was double peaked, with one peak proximal that moved in lockstep with the diaphragm, while the other pressure peak is distal to the diaphragm (Fig. 1). The FI-subtracted profile matched closely the direct smooth-muscle profile with cisatrcurium during breath holding in the inspiratory phase (Fig. 2). The proximal peak moved with the crus and the two peaks separated ~1 cm as the crural diaphragm moved superiorly from FI to FE.
Figure 1.
Panels A and B show average pressure curves from the same group of 15 normal subjects, referenced to distal margin of the right crus muscles Panel A is for full inspiration and Panel B for full expiration. The red curves are averaged pressure before administering atropine, the green curves are post atropine pressure and the blue curve is the average of the difference between pre and post atropine pressures (effectively the red curve minus the green curve). The vertical line at zero is the lower margin of the right crural diaphragm.
Figure 2.
Average pressure curve in full inspiration, referenced to the lower margin of the right crus muscles, from seven subjects undergoing surgery for nongastric or esophageal pathology after giving Cis-atracurium to paralyze the skeletal muscle.
Conclusions
The atropine-ablated and subtracted esophagogastric segment (EGS) pressure profiles approximated the striated and smooth-muscle sphincteric contributions, indicating that the smooth-muscle sphincter has two components. The proximal smooth-muscle component, the lower esophageal circular muscle, moves with the crural sphincter, implying rigid attachment by phrenoesophageal ligaments. The distal smooth-muscle component is distal to the diaphragm, approximately at the location of the sling-clasp muscle fibers at the esophagocardiac junction. We propose that this third sphincteric component is associated with the sling-clasp muscle groups, may be the first line of defense against the reflux of gastric contents into the esophagus, and may have a significant role in protecting against GERD. Grant support from NIH.
2. Defects in gastroesophageal junction HPZ leading to GERD
Anil Vegesna, Larry Miller, and James G. Brasseur vivi@temple.edu
We have summarized research5 showing that the tonic pressure contribution to the HPZ of the EGS contains contributions from a skeletal muscle crural diaphragmatic component1,2 and two distinct components of the smooth-muscle intrinsic sphincter, a proximal physiological component associated with the lower esophageal circular muscle,12 and a distal component associated with the sling-clasp muscle fibers.5
Aims
To report on research in which the pressure contributions from the three sphincteric components in normal subjects were compared with those in GERD patients.11
To define the mechanism of action of endoscopic plication and to determine the underlying pathophysiology of GERD.
Methods
A simultaneous endoluminal ultrasound (EUS) and manometry catheter was used to study 15 healthy volunteers (age 34 ± 8.5 years) and seven patients (age 45 ± 10.7 years) with symptomatic GERD. Endoscopic plication was performed in GERD patients using the Bard Endocinch device. The catheter was pulled through the EGS before and after administration of atropine.6 Preatropine (complete muscle tone), postatropine (nonmuscarinic muscle tone plus residual muscarinic tone), and subtracted (pure muscarinic muscle tone) pressure contributions to the sphincter were averaged after referencing spatially to the lower margin of the right crural diaphragm and the pull-through start position. In patients who had undergone endoscopic plication, evaluation with simultaneous ultrasound and manometry was performed before and one month after the Endocinch procedure. All subjects gave institutional review board approved informed consent to take part in this study. Exclusion criteria included subjects on any medication which could affect the gastroesophageal HPZ including prokinetic agents, erythromycin type antibiotics, and anticholinergics. Other exclusion criteria were abdominal surgery involving the stomach or esophagus, diabetes, scleroderma, achalasia, and current pregnancy.
Results
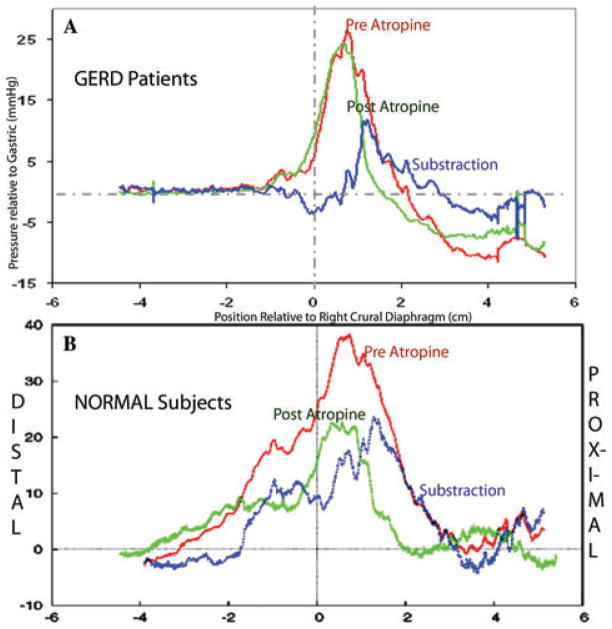
In the normal group the atropine-resistant and atropine-attenuated pressures identified the crural and smooth-muscle sphincteric components, respectively. The subtraction curve in normal subjects contained proximal and distal pressure components. The proximal component moved with the crural diaphragm between FI and FE and represents the lower esophageal circular sphincter. The distal component coincided with the gastric sling-clasp muscle fiber complex. The subtraction curve in the GERD patients contained only a single pressure component, representing the lower esophageal circular muscle, that moved with the crural diaphragm, while the distal pressure peak of the intrinsic smooth-muscle component, which was previously recognized in the normal subjects, was absent (Fig. 3B).
Figure 3.
Similarly to Figure 1, Panel A shows average pressure curves in full expiration from seven GERD patients, referenced to right crural diaphragm. Panel B shows the average pressure curves in full expiration from 15 normal control subjects from Figure 1. The red curve shows average pressure preatropine pressure and the green curve post atropine. The blue curve is the subtraction curve (red curve minus green curve). The vertical line at zero is the lower margin of the right crus muscles.
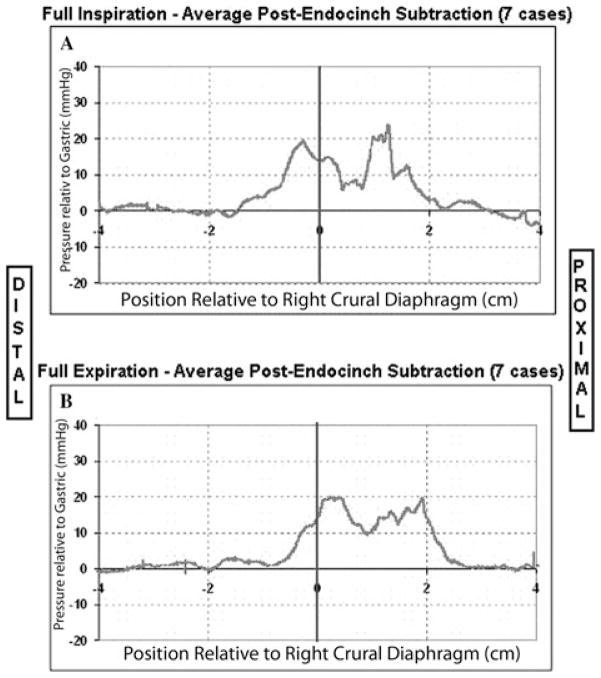
In GERD patients after the Endocinch procedure, the distal pressure peak, which was previously absent (Fig. 3A), was reestablished at the same axial location as the distal pressure peak in normal volunteers (Fig. 4). The newly established distal pressure in GERD patients was attenuated by the application of atropine. Using high-resolution ultrasound we localized all sutures within the plications to the submucosal space. Eighty percent of the patients in this study experienced either an improvement or elimination of their reflux symptoms in the short term.
Figure 4.
The average subtraction pressure curves from seven GERD patients after the Endocinch procedure in full inspiration (Panel A) and full expiration (Panel B) procedure.
Conclusions
We hypothesize that the lower muscarinic smooth-muscle pressure component is the gastric sling/clasp muscle fiber (upper gastric sphincter) component. The absence of this lower smooth-muscle pressure component suggests that the gastric sling/clasp muscle fiber component is defective in GERD patients. The distal muscarinic pressure peak normally constitutes one-third of the gastroesophageal junction HPZ pressure profile as measured by the area under the curve in normal subjects. This distal pressure profile may be important to the antireflux barrier, since it is the most distal component at the esophagogastric junction and therefore the first line of defense against reflux of gastric contents into the esophagus in the resting state.
Endoscopic plication in GERD patients reestablishes this distal pressure profile by correcting a structural anatomic abnormality of the gastric clasp and sling muscle fibers. The observation that atropine attenuates this newly established pressure profile implies that the neural innervations to this area remain intact and functioning. Hence the underlying abnormality might be due to an anatomic structural abnormality of the gastric clasp and sling muscle fibers. Endoscopic plication has fallen out of favor for the treatment of GERD, because of limited long-term efficacy, which might be due to the tension in the sutures pulling out through the flimsy submucosal tissue over time, leading to breakdown of the plications. [Grant support from NIH, BARD].
3. Defects within the gastroesophageal sphincteric segment potentially leading to GERD: in vitro muscle strip studies
Michael R. Ruggieri, Anil K. Vegesna, Alan S. Braverman, and Larry S. Miller rugg@temple.edu
The major neurotransmitter controlling most smooth-muscle contraction, including gastrointestinal smooth muscle, is acetylcholine. Cholinergic receptors are classified into two major classes: muscarinic and nicotinic. Muscarinic receptors are G-protein coupled receptors that consist of a single polypeptide chain that spans the plasma membrane seven times. There are five subtypes of muscarinic receptors. Nicotinic receptors are ligand-gated ion channel receptors that consist of five polypeptide subunits that surround the central ion channel. Because there are many different isoforms of these five subunits that can form functional receptors, the potential diversity of subunit composition for nicotinic receptors far exceeds the capacity of the currently available ligands to distinguish subtypes on the basis of selectivity of ligands.
It has been known for at least 50 years that administration of the muscarinic receptor antagonist atropine reduces the pressure of the lower esophageal HPZ in humans.7 A considerable amount of work has been carried out on stretch-induced tone in smooth-muscle strips from the lower esophageal HPZ that demonstrates that this tone is calcium sensitive but is not reduced by atropine.8,9
Therefore, stretch-induced tone of in vitro muscle strips from the lower esophageal HPZ is not the major mechanism of in vivo sphincteric tone because it is unaffected by atropine. In this investigation we used relaxation of muscarinic receptor precontracted muscle strips as an in vitro model of in vivo sphincteric relaxation. Whole human stomach and esophagus specimens were obtained by third-party organizations (National Disease Research Interchange and the International Institute for the advancement of Medicine) from brain dead patients on life support after harvesting organs to be used for organ transplant.
Very limited medical history is available for these patients and no medical diagnosis of GERD is available. Medical history is limited to interviews with the next of kin including reports of the use of acid suppressive medication and heartburn, which was classified as probable GERD. Definitive diagnosis of GERD is based on histologic diagnosis of Barrett’s esophagus by a board-certified histopathologist. Because our previous in vivo studies using ultrasonography combined with high-resolution manometry demonstrated that the sling-clasp fiber contribution to the pressure profile is missing in GERD patients, this study focused on muscle strips dissected from the clasp and sling fibers in these human specimens.
Cumulative concentration response curves to the mixed muscarinic and nicotinic receptor agonist carbachol produced greater tension in sling fibers than clasp fibers, confirming previous studies.10 Increasing intensity of contraction continued to be produced by carbachol concentrations up to 30 μM but higher concentrations (100 μM, 300 μM, and 1mM) produced abrupt relaxations. The muscarinic receptor selective agonist bethanechol (0.01 μM–1mM) also produced greater contractions in sling than clasp fibers and these muscarinic contractions were relaxed in a dose-dependent fashion by nicotine (30 μM–1mM). Both high-concentration carbachol-induced relaxations and nicotine-induced relaxations in bethanechol precontracted strips were greater in clasp than sling fibers. In both sling and clasp fibers, the nicotine-induced relaxations of bethanechol precontracted strips were prevented in a dose-dependent fashion by the nitric oxide synthase inhibitor L-nitro arginine methyl ester, and the β adrenergic receptor antagonist propranolol, whereas the glycine receptor antagonist ginkgolide B was inefective in both and the GABAA antagonist SR95531 inhibited relaxations in sling fibers only.13
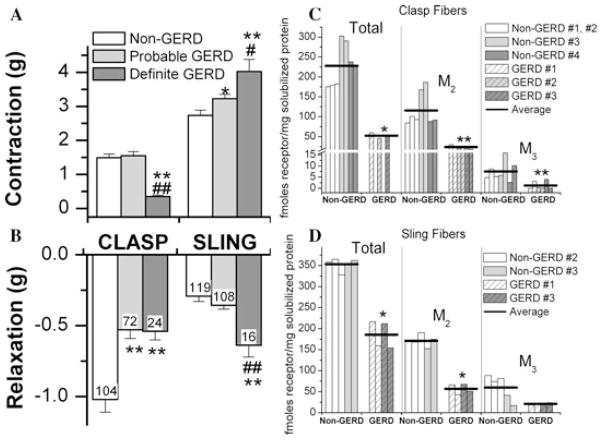
Figure 5A and 5B shows that clasp fibers from two definite GERD donors contracted less in response to maximally effective carbachol concentrations compared with 11 non-GERD and four probable GERD donors, whereas clasp fibers from both probable and definite GERD donors relaxed less than non-GERD donors. Sling fibers of both probable and definite GERD donors contracted greater than non-GERD donors and sling fibers from definite GERD donors relaxed more than non-GERD donors. The density of M2 and M3 muscarinic receptors, determined by immunoprecipitation, was lower in clasp fibers of GERD donors (Fig. 5C) and M2 density was lower in sling fibers of GERD donors (Fig. 5D).
Figure 5.
(A) and (B) show that clasp fibers from two definite GERD donors contracted less in response to maximally effective carbachol concentrations than 11 non-GERD and four probable GERD donors, whereas clasp fibers from both probable and definite GERD donors relaxed less than non-GERD donors. Sling fibers of both probable and definite GERD donors contracted greater than non-GERD donors, and sling fibers from definite GERD donors relaxed more than non-GERD donors. The density of M2 and M3 muscarinic receptors, determined by immunoprecipitation, was lower in clasp fibers of GERD donors (C) and M2 density was lower in sling fibers of GERD donors (D).
These results demonstrate that the in vitro contractility and muscarinic receptor density of the gastric sling-clasp muscle fibers is abnormal in GERD patients, which may help explain the missing sling-clasp fiber pressure contribution to the upper gastric sphincter HPZ that we observed in GERD patients.
Concise summaries.
The relative alignments and contributions of the different sphincteric components have recently been quantified in the normal gastroesophageal junction high-pressure zone (HPZ).5
By carrying out intraluminal manometric pull-throughs concurrently with high-frequency ultrasound in normal subjects during breath-hold at full inspiration (FI) and full expiration (FE), it has been shown that the HPZ is axially broader in FE. However, the atropine-suppressed pressure profile was independent of diaphragmatic position and was correlated with the anatomic crus.
The high pressure zone of the distal esophagus consists of three individual components. The first component is the skeletal muscle crural diaphragm. The smooth-muscle sphincter has two components. The proximal smooth-muscle component (lower esophageal circular muscle, LEC) moves with the crural sphincter. The distal smooth muscle appears to be associated with sling/clasp muscle fibers at the esophagocardiac junction (upper gastric sphincter). This third sphincteric component could be considered the first line of defense against the reflux of gastric contents and may have a significant role in preventing gastroesophageal reflux disease (GERD).
Comparison of the pressure contributions from the three sphincteric components in normal subjects to those in GERD patients has shown that the subtraction curve in the GERD patients contained only a single pressure peak that moved with the crural diaphragm, while the distal pressure peak of the intrinsic smooth-muscle component (the upper gastric sphincter), which was previously observed in normal subjects, was absent.
Endoscopic plication in GERD patients reestablishes this distal pressure profile, apparently by correcting a structural anatomic abnormality of the gastric clasp and sling muscle fibers. The observation that atropine attenuates this newly established pressure profile implies that the neural innervations to this area remain intact and functioning.
It has been known for at least 50 years that administration of the muscarinic receptor antagonist atropine reduces the pressure of the lower esophageal HPZ in humans. Several studies have shown that stretch-induced tone of in vitro muscle strips from the lower esophageal high-pressure zone is unaffected by atropine and thus is not the major mechanism of in vivo sphincteric tone.
In an investigation using relaxation of muscarinic receptor precontracted muscle strips as an in vitro model of in vivo sphincteric relaxation, cumulative concentration response curves to the mixed muscarinic and nicotinic receptor agonist carbachol produced greater tension in sling fibers than clasp fibers, confirming the results of previous studies.
The in vitro contractility and muscarinic receptor density of the gastric sling/clasp muscle fibers is abnormal in GERD patients, which may help explain the missing sling-clasp fiber contribution to the upper gastric sphincter HPZ that is observed in GERD patients.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ingelfinger FJ. Esophageal motility. Physiol Rev. 1958;38:533–584. doi: 10.1152/physrev.1958.38.4.533. [DOI] [PubMed] [Google Scholar]
- 2.Boyle JT, Altschuler SM, Nixon TE, et al. Role of the diaphragm in the genesis of the lower esophageal sphincter pressure in the cat. Gastroenterology. 1985;88:723–730. doi: 10.1016/0016-5085(85)90143-x. [DOI] [PubMed] [Google Scholar]
- 3.Code CF, Fyke FE, Jr, Schlegel JF, et al. The gastroesophageal sphincter in healthy human beings. Gastroenterologia. 1956;86:135–150. doi: 10.1159/000200544. [DOI] [PubMed] [Google Scholar]
- 4.Liebermann-Meffert D, AllgÖwer M, Schmid P, Blum AL. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31–38. [PubMed] [Google Scholar]
- 5.Brasseur JG, Ulerich R, Dai Q, et al. Pharmacological dissection of the human gastroesophageal segment into three sphincteric components. J Physiol. 2007;3:961–975. doi: 10.1113/jphysiol.2006.124032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodds WJ, Dent J, Hogan WJ, et al. Effect of atropine on esophageal motor function in humans. Am J Physiol. 1981;240:G290–G296. doi: 10.1152/ajpgi.1981.240.4.G290. [DOI] [PubMed] [Google Scholar]
- 7.Bettarello A, Tuttle SG, Grossman MI, et al. Effects of autonomic drugs on gastroesophageal reflux. Gastroenterology. 1960;39:340–346. [PubMed] [Google Scholar]
- 8.Farre R, et al. Mechanisms controlling function in the clasp and sling regions of porcine lower oesophageal sphincter. Br J Surg. 2007;94:1427–1436. doi: 10.1002/bjs.5831. [DOI] [PubMed] [Google Scholar]
- 9.Farre R, et al. Pharmacologic characterization of intrinsic mechanisms controlling tone and relaxation of porcine lower esophageal sphincter. J Pharmacol Exp Ther. 2006;316:1238–1248. doi: 10.1124/jpet.105.094482. [DOI] [PubMed] [Google Scholar]
- 10.Tian ZQ, et al. Responses of human clasp and sling fibers to neuromimetics. J Gastroenterol Hepatol. 2004;19:440–447. doi: 10.1111/j.1440-1746.2003.03307.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller LS, Dai Q, Vegesna A, Korimilli A, Ulerich R, Schiffner B, Brasseur JG. A Missing Sphincteric Component of the Gastro-Esophageal Junction in Patients with GERD. Neurogastroentero Motil. 2009;20:813–821. doi: 10.1111/j.1365-2982.2009.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahrilas PJ. Anatomy and physiology of the gastroesophageal junction. Gastroenterol Clin North Am. 1997;26:467–486. doi: 10.1016/s0889-8553(05)70307-1. [DOI] [PubMed] [Google Scholar]
- 13.Braverman AS, Vegesna AK, Miller LS, Barbe MF, Tiwana MI, Hussain K, Ruggieri MR., Sr Pharmacologic specificity of nicotinic receptor mediated relaxation of muscarinic receptor pre-contracted human gastric clasp and sling muscle fibers. J Pharmacol Exp Ther. 2011;338:39–46. doi: 10.1124/jpet.110.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]