Abstract
The family of fibroblast growth factor receptors (FGFRs) is encoded by four distinct genes. FGFR1 and FGFR4 are both expressed during myogenesis, but whereas the function of FGFR1 in myoblast proliferation has been documented, the role of FGFR4 remains unknown. Here we report on a new splice form of FGFR4 cloned from primary cultures of mouse satellite cells. This form, named FGFR4(−16), lacks the entire exon 16, resulting in a deletion within the FGFR kinase domain. Expression of FGFR4(−16) coincided with that of wildtype FGFR4 in all FGFR4-expressing tissues examined. Moreover, expression of both FGFR4 forms correlated with the onset of myogenic differentiation, as determined in mouse C2C12 cells and in the inducible myogenic system of 10T½-MyoD-ER cell line. Both endogenous and overexpressed forms of FGFR4 exhibited N-glycosylation. In contrast to FGFR1, induced homodimerization of FGFR4 proteins did not result in receptor tyrosine phosphorylation. Surprisingly, coexpression of FGFR4 forms and a chimeric FGFR1 protein resulted in FGFR4 tyrosine phosphorylation, raising the possibility that FGFR4 phosphorylation might be enabled by a heterologous tyrosine kinase activity. Collectively, the present study reveals novel characteristics of mouse FGFR4 gene products and delineates their expression pattern during myogenesis. Our findings suggest that FGFR4 functions in a distinctly different manner than the prototype FGFR during myogenic differentiation.
Keywords: Fibroblast growth factor receptor, FGFR4, alternative splicing, N-glycosylation, tyrosine phosphorylation, myogenesis, satellite cells, SU5402, AP20187
Introduction
Members of the fibroblast growth factor (FGF) family elicit a wide range of biological responses during embryogenesis and postnatal life. FGFs typically mediate their effects through a family of high affinity transmembrane tyrosine kinase receptors. FGF receptors are encoded by four distinct genes (FGFR1 through FGFR4), with further FGFR diversity generated by alternative splicing (Johnson and Williams, 1993). Cell surface heparan sulphate proteoglycans (HSPG) are also involved in binding of FGF to FGFR, contributing to the stabilization of the receptor-ligand complex (Mohammadi et al., 2005). Distinct and overlapping affinities between FGFs, FGFRs and HSPGs, combined with specific temporal and spatial expression patterns of these molecules, form a highly regulated signaling system (Eswarakumar et al., 2005; Zhang et al., 2006).
The extracellular ligand-binding domain of FGFR is connected to the cytoplasmic domain by a single transmembrane helix. The cytoplasmic domain contains a conserved protein tyrosine kinase core with additional regulatory sequences that are subjected to autophosphorylation and to phosphorylation by heterologous protein kinases. FGF binding to the extracellular domain of prototypic FGFR induces receptor dimerization and activation of the intracellular tyrosine kinase domain by autophosphorylation of conserved tyrosines, followed by activation of downstream signaling cascades that include Ras-MAPK, PLC-PKC, PI3K-Akt and p38 MAPK pathways (Raffioni et al., 1999; Boilly et al., 2000; Eswarakumar et al., 2005). The extracellular domain of FGFR was shown to undergo N-glycosylation and mutations that impaired glycosylation resulted in permanent activation of FGFR, as observed in several human diseases (Mangasarian et al., 1997; Winterpacht et al., 2000; Duchesne et al., 2006; Hatch et al., 2006).
FGFR4 is expressed in a tissue-specific manner during embryogenesis and displays a unique affinity to certain FGF ligands (Stark et al., 1991; Korhonen et al., 1992; Ornitz et al., 1996; Powell et al., 1998; Rappolee et al., 1998; Xie et al., 1999; Xu et al., 1999; Harmer et al., 2004). Nevertheless, FGFR4 function is not essential during embryogenesis and adult life (Weinstein et al., 1998), though it may appear to be involved in several metabolic pathways (Yu et al., 2000; Gutierrez et al., 2006). Earlier studies pointed to a reduced autophosphorylation and tyrosine kinase activity of FGFR4 compared to other FGFRs (Vainikka et al., 1994; Wang et al., 1994; Shaoul et al., 1995; Wang and Goldfarb, 1997; Raffioni et al., 1999). In the absence of a clear mitogenic effect of FGFR4, identification of potential FGFR4 ligands relied on an expressed chimeric receptor where the FGF binding domain was of FGFR4 origin and the intracellular domain was of FGFR1 origin (Ornitz et al., 1996). Furthermore, there is evidence that FGFR4 function may not necessarily require FGF ligand. First, heparin, in the absence of FGF, activated FGFR4 (Gao and Goldfarb, 1995; Cavallaro et al., 2001). Second, FGFR4 was found to function in a complex with N-CAM, independently of FGF (Cavallaro et al., 2001). Detection of N-linked glycosylation on an overexpressed extracellular domain of human FGFR4 (Tuominen et al., 2001) suggested that the function of this receptor might be regulated by glycosylation, similar to the other FGFRs. However, N-glycosylation of native FGFR4 has not been analyzed yet.
Both FGFR1 and FGFR4 are expressed during skeletal muscle development (Stark et al., 1991; Korhonen et al., 1992; Gonzalez et al., 1996; Marics et al., 2002) and during myogenesis of satellite cells, the myogenic progenitors involved in postnatal growth and repair of skeletal muscle (Johnson and Allen, 1995; Kastner et al., 2000). FGFR4 expression and function was also implicated in muscle regeneration in vivo (Zhao and Hoffman, 2004; Zhao et al., 2006). While FGFR1 seems to play a role in supporting myoblast proliferation, the specific role of FGFR4 during myogenesis has remained an enigma (Olwin et al., 1994; Itoh et al., 1996; Marics et al., 2002; Shefer and Yablonka-Reuveni, 2007).
This study provides new insights into FGFR4 expression and modifications during myogenesis in murine culture models. Here, we defined the expression patterns of the full-length (wildtype) FGFR4 and of a novel splice form during myogenic differentiation, and analyzed functional posttranslational modifications of these FGFR4 forms in native and overexpressed states. Furthermore, we identified a novel mechanism of FGFR4 tyrosine phosphorylation, which was not dependent on the classic process of receptor homodimerization, but was rather enabled by the presence of a heterologous FGFR. Taken together, our findings suggest that FGFR4 functions in a different manner than the prototype FGFR during myogenic differentiation.
Materials and Methods
Animals
C57BL/6 mice were the source of tissues used in this study. Muscle was harvested from 2–4 month old mice for primary myogenic cultures. For all other analyses of RNA and protein expression, tissues were harvested from 4-day old mice. Animal handling procedures were approved by the University of Washington Animal Care Committee.
Cell cultures
DMEM was supplemented with 50 U/ml penicillin and 50 mg/ml streptomycin. Primary myogenic cultures were prepared from hindlimb muscles and cultured using rich growth medium (DMEM containing 20% fetal bovine serum (FBS), 10% horse serum (HS) and 1% chicken embryo extract) (Shefer et al., 2006). The mouse myogenic line C2 (Yaffe and Saxel, 1977) (Yablonka-Reuveni and Rivera, 1997), and its subclone C2C12 (ATCC), were maintained in DMEM containing 20% FBS. For accelerated differentiation, cells were switched to DMEM containing 2% HS after reaching near confluence. The inducible myogenic cell line 10T½-MyoD-ER, derived from 10T½ mouse fibroblasts stably transfected with a chimeric MyoD-estrogen receptor binding domain (Hollenberg et al., 1993) (Bergstrom et al., 2002) provided by Stephen Tapscott, Fred Hutchinson Cancer Research Center) was maintained in DMEM containing 10% FBS. Myogenic differentiation of 10T½-MyoD-ER cells was induced by switching the growth medium to DMEM (phenol red free; Invitrogen) containing 2% horse serum and 10−7 M β-estradiol (Sigma-Aldrich). Additional cell lines used in this study included 293T and NIH3T3 (ATCC), cultured in DMEM containing 10% FBS.
When indicated, culture medium was supplemented with one of the following reagents: 40 μM SU5402 – an inhibitor of FGFR1 autophosphorylation/activation and subsequent downstream signaling (Mohammadi et al., 1997); Calbiochem); a stock solution of 10 mM was constituted in 100% DMSO. 100 nM AP20184 – a dimerizer of proteins containing an F36V domain, which is a modified FKBP-binding domain (Whitney et al., 2001); a stock solution of 1 mM was constituted in 100% ethanol (ARIAD Pharmaceuticals, Cambridge, MA; www.ariad.com/regulationkits). Exposure times to the different drugs are indicated in the Results section.
Transient transfection
The cell lines 293T, NIH3T3, C2, C2C12 cells were transfected with expression plasmids using PolyFect Transfection Reagent (Qiagen), following manufacturer manual. Transfected cells were maintained in their corresponding growth medium for 48 hours and harvested for Western blots or immunostaining.
Viral transduction
Ecotropic retroviral supernatants were generated by transient transfection of packaging 293T Phoenix eco cells (Kinsella and Nolan, 1996); ATCC) using calcium phosphate transfection method. Culture supernatant, containing packaged retroviral, was mixed with polybrene reagent (5 mg/ml) and used for infection of C2 and C2C12 cells. Cultures were exposed to viral supernatant for 16 hours and then received fresh growth medium for 48 hours. Cells were then harvested using trypsin, and GFP+ cells were collected by flow cytometry (Influx Cell Sorter; Cytopeia Incorporated), expanded in growth medium and stored in liquid nitrogen.
Isolation of total and poly(A)+ RNA, cDNA generation and PCR amplification
Total RNA was extracted from cultured cells and mouse tissues using the RNAeasy kit (Qiagen). Subsequent isolation of poly(A)+ mRNA was performed using the oligo(dT)-cellulose based QuickPrep micro mRNA Purification Kit (Amersham Biosciences). The quantity and purity of RNA preparations was tested by spectrophotometry at 260/280 nm. cDNA was generated using the Omniscript RT Kit (Qiagen) and oligo-dT primers (Amersham). PCR amplification for cloning purposes was performed using the Advantage 2 system (Clontech). The correctness of all regions cloned using PCR amplification was confirmed by sequencing. PCR amplification for gene expression analysis by semi quantitative RT-PCR was performed using the HotStartTaq Polymerase system (Qiagen); RT-PCR primer sequences are listed later in the Materials and Methods section.
Sequence analysis software
DNA and protein sequence analysis were performed using the Vector NTI software. The nucleic acid and protein sequence data library searches were performed using the Internet based BLAST (NCBI) and FASTA (EMBO) search engines. The Clone Manager 7, (Sci Ed Central) software was used for cloning design.
Cloning of mouse FGFR4 wildtype and (−16) cDNA
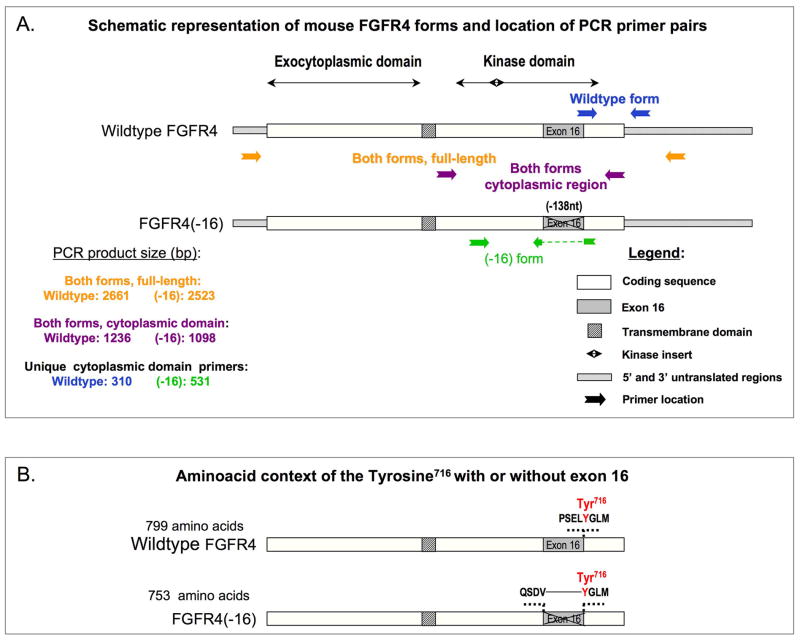
FGFR4 cDNA was cloned from a mouse primary myogenic culture. Poly(A)+ RNA used for the production of cDNA was isolated from a 1-week-old culture when both myoblasts and myotubes were present. The full coding sequence of FGFR4 was then retrieved by PCR using the paired forward / reverse primers: GTGGTCAGTGGGAAGTCTGG / TGCCATGTCTTCTGTCGTTC. cDNA products were cloned into pCRII-TOPO vector using the pCRII-TOPO cloning system (Invitrogen). The sequencing of the clones revealed two FGFR4 forms, a wildtype (referred to as FGFR4) and a form that lacks exon 16 (referred to as FGFR4(−16)). Schematics of both these forms are shown in Fig. 1. Subsequently, the full-length sequence of wildtype FGFR4 was transferred into the pCR3 vector (Invitrogne) by cleavage with HindIII and Not I followed by religation.
Fig. 1.
Schematic representations of: (A) Wild type and (−16) splice form of mouse FGFR4 cDNA; the positions of PCR primers used to identify different portions of the corresponding RNA transcripts are indicated with colored arrows and product sizes are listed; primer sequences are detailed in Materials and Methods. (B) Changed aminoacid context of the Tyr716 surroundings in FGFR4(−16) compared to the wild type protein form. The central shaded box represents the transmembrane domain in both schematics.
Additional vectors
a) Constructs provided by others
pCLXFv2E, contains cDNA sequences of the extracellular and transmembrane domains of human p75 low affinity nerve growth factor receptor (LNGFR) followed by two copies of a modified FKBP-binding protein (F36V) and hemagglutinin (HA) sequence ((Amara et al., 1999); provided by Roy Pollock, ARIAD). Specific binding of a dimerizer drug AP20187 (ARIAD) to F36V-containing vectors induces homodimerization of the corresponding proteins (Whitney et al., 2001). MGFIM, a MSCV-based retroviral vector containing enhanced green fluorescent protein-internal ribosomal entry site (GFP-IRES) cassette ((Jin et al., 2000); provided by C. Anthony Blau, University of Washington). pBJ-F36V, contains a 14-amino acid cytoplasmic membrane targeting myristylation domain upstream of the N-terminus of F36V followed by HA epitope tag downstream of the C-terminus of F36V ((Whitney et al., 2001); provided by Anthony Blau). MGFIM-F36Vfgfr-1, a retroviral bicistronic construct coding for GFP-IRES followed by FGFR1 dimerization inducible construct with cytoplasmic domain of FGFR1 inserted between the myristylation domain and HA epitope described in PBJ-F36V, and its control empty vector lacking FGFR1 sequence ((Whitney et al., 2001); provided by Charles Murry, University of Washington). The inducible chimeric FGFR1 construct in this retroviral cassette is referred to in the present study as iFGFR1. pCS2/FGFR31c-myc, coding a chimeric FGFR3-1-myc receptor containing the extracellular and transmembrane domains of FGFR3 linked to the intracellular domain of FGFR1, followed by a myc tag at the c-terminus of FGFR1 sequence ((Lin et al., 1998); provided by David Ornitz, Washington University). pCS2/FGFR3c-myc, coding for the entire sequence of FGFR3 receptor with a myc tag linked to the c-terminus of the receptor sequence (provided by David Ornitz).
b) FGFR4 constructs generated for this study
FGFR4-HA was cloned by inserting the cytoplasmic domain of FGFR4 (cR4) into the pCLXFv2E vector to generate pCLXFv2E-cR4, and subsequently replacing the 3′ end of pCR3-R4 with the corresponding part from the pCLXF-cR4-HA using the Xma I and Not I fragment of pCR3-FGFR4, and the Sal I and Xma I fragment of pCLXFv2E-cR4-HA. FGFR4(−16)-HA was cloned by inserting the cytoplasmic domain of FGFR4(−16) (cR4(−16)) into the pCLXFv2E vector to generate pCLXFv2E-cR4(−16)-HA, and later by replacing the 3′ end of pCR3-R4 with the corresponding part from the pCLXF-c4(16)-HA using the Xma I and Not I fragment of pCR3-FGFR4, and the Sal I and Xma I fragment of pCLXFv2E-cR4(−16)-HA. MGFIM-pCLXFv2Efgfr-4, a retroviral vector containing GFP-IRES followed by a chimeric FGFR4 receptor containing the cytoplasmic domain of FGFR4, was generated by cloning in-frame the cytoplasmic part of mouse FGFR4 between the F36V domain and HA tag in pCLXFv2E described above. MGFIM-pCLXFv2Efgfr-4(−16) was generated as MGFIM-pCLXFv2E–fgfr-4, but with the cytoplasmic domain of FGFR4(−16). MGFIM-F36Vfgfr-4 was generated by inserting in-frame the cytoplasmic part of mouse FGFR4 between the F36V and HA domains in pBJ followed by cloning the resulting chimeric FGFR4 into MGFIM downstream to the IRES sequence. The inducible chimeric FGFR1 construct in this retroviral cassette is referred to as iFGFR1. pCS2/FGFR3-4-myc was generated by replacing the FGFR1 cytoplasmic domain in the above mentioned pCS2/FGFR31c-myc with the FGFR4 cytoplasmic domain. pCS2/FGFR3-4(−16)-myc was generated by replacing the FGFR1 cytoplasmic domain in pCS2/FGFR31c-myc with the FGFR4(−16) cytoplasmic domain.
Semi-quantitative RT-PCR
The PCR program consisted of incubation at 95°C for 15 min, followed by 20 to 36 cycles of 94°C for 45 sec, 60°C for 1 min, 72°C for 1 min, and a final extensionstep was carried out for 10 min at 72°C. PCR products wereseparated on 1% agarose gels, containing 0.25 mg/ml ethidium bromide, or 1:10,000 dilution of SYBR Green I stain (Molecular Probes). Each analysis shown in the Results was repeated at least three times. The sequences of the paired primers, and their product size were:
FGFR1, GCCCTGGAAGAGAGACCAGC / GAACCCCAGAGTTCATGGATGC, 244bp;
FGFR2, CGGAGACAGGTAACAGTTTCGGC / CTATTCCCGGAGGTTGCCTTTC, 453bp;
FGFR3, CTGTATGTGCTGGTGGAGTACGC/ GAGAGGTCCAAGTACTCGTCGGT, 644bp;
FGFR4 wildtype form, TGGAGGAGCTCTTCTCACTGC / TCAGAGAAAGGGAAGGGGCT, 310bp;
FGFR4(−16) form, GTGGCTGTGAAGATGCTGAA / CATTAGCCCATACACGTCACTCTG, 532bp;
FGFR4 both forms, GTGGCTGTGAAGATGCTGAA / CAAAACAGGGCCAGAGAGAG, 1066bp and 928bp;
MyoD, GGAGGAGCACGCACACTTCT / CGCTGTAATCCATCATGCCA, 464bp;
Myf5, CAGCCAAGAGTAGCAGCCTTCG / GTTCTTTCGGGACCAGACAGGG, 440bp, (Kastner et al., 2000);
myogenin, CCATCCAGTACATTGAGCGCCTA / GGGGCTCTCTGGACTCCATCTT, 551bp;
sarcomeric myosin, CAGGACACCAGCGCCCA / TCCTCGGCCTCCTCCAGCTC, 310bp (Yoon et al., 1992);
c-met, TCCAGAGCTGGTCCAAGCAGT / TCTGGCAAGACCGAAATCAGC, 505bp;
PDGFRα, AGAGATCGCTGTACGATCGGC / AAGTGACACCGATGTACGCGTT, 705bp;
PDGFRβ, ATCTTTGTGCCAGATCCCACC / TAGTAGCCCGCTTCTGACACCTTC, 830bp;
GAPDH, CCTCTGGAAAGCTGTGGCGT / TTGGAGGCC ATGTAGGCCAT, 430bp;
rpS6, TGCTCTTGGTGAAGAGTGGAAGG / TCTTGGCAATCTGTTCCTGGC, 575bp.
Antibodies
A rabbit antiserum directed against FGFR4 was generated using the KLH-coupled peptide NH2-RPPGPDLSPDGPRSSEG-COOH (Biosynthesis, Inc.). The anti-FGFR4 IgG fraction was purified from the antiserum based on selective affinity to the FGFR4 immunogenic peptide using Ulatralink Immobilization Kit (Pierce Biotechnology). This antibody has been disclosed to the University of Washington technology transfer office (Reference No. 7371D).
Additional primary antibodies used in immunostaining and Western blot analysis: i) Antibodies generated in mouse: anti-tubulin (T-6074, Sigma-Aldrich), anti-PCNA (sc-56, Santa Cruz Biotechnology [SCBT]), anti-phosphotyrosine (PY20) (sc-508, SCBT), anti-HA (sc-7392, SCBT), anti-myc clone 9E10 (M-5546, Sigma-Aldrich). ii) Antibodies generated in rabbit: anti-MyoD (sc-304, SCBT), anti-HA (sc-805, SCBT), anti-Flg (FGFR1) (sc-121, SCBT), anti-phospho-FGF Receptor (Tyr653/654) (Cell Signaling).
Protein extraction, immunoprecipitation, and deglycosylation
Cultures and tissues were lysed in Triton X-100 lysis buffer (1% Triton X-100 in a solution of 50mM Tris pH 7.5, 150mM NaCl, 5mM EDTA, 2.5mM EGTA, supplemented with a Protease Inhibitor Cocktail from Roche Diagnostics; cat.# 1836170) for 15 minutes (at 4°C) with occasional vortexing. The lysates were then centrifuged for 3 minutes at 2000×g to harvest the supernatant fractions. For lysates prepared to analyze phosphoryalted proteins, the lysis buffer was also supplemented with the Phosphatase Inhibitor Cocktails 1 and 2 (Sigma-Aldrich). Final supernatants were subjected directly to Western blot analysis or first processed by immunoprecipitation and/or deglycosylation.
Immunoprecipitations was carried out using anti-rabbit IgG Agarose or Protein A Agarose (Sigma-Aldrich). The agarose beads were pre-coated with 1 mg/ml BSA in Triton X-100 lysis buffer for one hour at 4°C with constant rotation. The agarose beads with the complexes were then washed in the lysis buffer, resuspended in SDS-PAGE sample loading buffer, boiled for 5 minutes, cooled on ice, and resolved by the SDS-PAGE.
Deglycosylation of lysates or immunoprecipitated samples was carried out with PNGase-F enzyme (New England Biolabs) in the provided buffer. Samples containing 20–80 μg protein were incubated with 500–1500 U of the enzyme for 2 or 16 hours at 37°C followed by SDS-PAGE analysis.
Immunoblotting
Proteins resolved by SDS-PAGE (typically, 6% acrylamide) were transferred to polyvinylidene fluoride (PVDF) Immobilon-P Transfer Membrane (Millipore) and were detected with specific primary antibodies. The corresponding two secondary antibodies used were: anti-rabbit IgG and anti-mouse, each conjugated to horseradish peroxidase-conjugated (Sigma-Aldrich). Immunodetection was carried out by luminol oxidation reactions using the ECL+ Western blotting detection system (Amersham) and exposed to CL-XPosure Film (Kodak) for 5 seconds to 5 minutes. Images were scanned using the Epson Expression 1600 scanner and processed using Adobe Photoshop software. Each analysis shown in the Results is a representative of at least three independent repeats.
Immunofluorescence
Cultures were fixed with 4% paraformaldehyde, processed for single and double immunofluorescence, and stained with DAPI to localize nuclei. All details regarding immunostaining, microscopy, camera and cell imaging software are as in our previous publication (Shefer et al., 2006). Images were assembled using Adobe Photoshop software.
Results
Two forms of FGFR4 are expressed during myogenesis in satellite cell cultures
In order to characterize FGFR4 gene products that are expressed during myogenesis, we cloned FGFR4 cDNA from mouse primary myogenic cultures. We identified two forms, one corresponding to the previously published wildtype FGFR4 form (Stark et al., 1991), and the second is a novel FGFR4 variant that lacks the entire exon 16 (depicted in Fig. 1). We refer to these two forms as wildtype FGFR4 (or FGFR4) and FGFR4(−16), respectively. Sequences of both forms were deposited in GenBank under accession numbers DQ388428 and AY493377, respectively. Exon 16 skipping results in a deletion of 138 nucleotides within the tyrosine kinase coding domain of FGFR4. While all four conserved tyrosines within the tyrosine kinase domain of FGFR4 (Hart et al., 2001) are preserved in FGFR4(−16), tyrosine Y716 is placed in a new amino acid context in the form that lacks exon 16 (Fig. 1B). In the wildtype form, tyrosine Y716 codon is formed by the last base at the 3′ end of exon 16 and the two first bases in the 5′ end of the adjacent exon 17, resulting in a PSELYGLM sequence. In FGFR4(−16), tyrosine Y716 codon is preceded by codons contributed by the 3′ end of exon 15, resulting in a QSDVYGLM sequence (Fig. 1B).
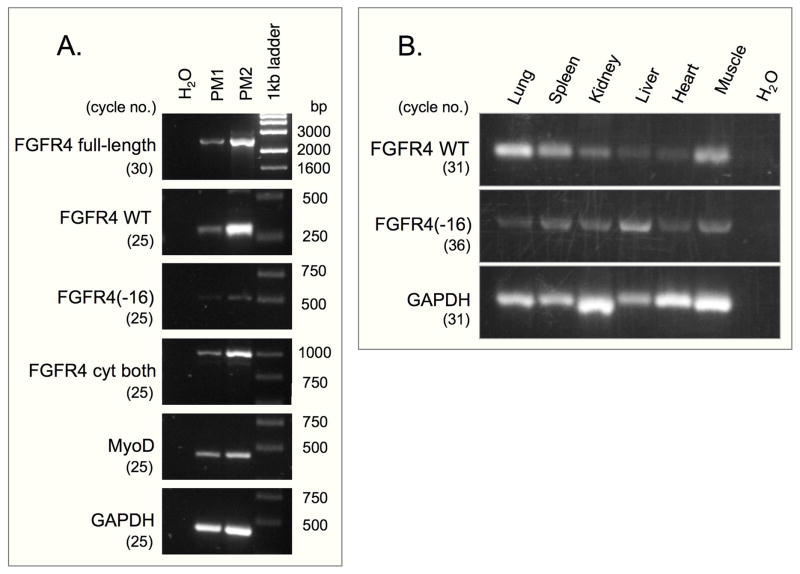
Specific PCR primers were designed for analyzing the expression of each FGFR4 form during myogenesis. The positions of these primers are depicted schematically in Fig. 1A and their sequences are detailed in the Materials and Methods section. Both FGFR4 forms were expressed in primary myogenic cells (Fig. 2A) and in several organs from neonatal mice (Fig. 2B). In all cases, detection of FGFR4(−16) required a higher number of amplification cycles than detection of FGFR4.
Fig. 2.
RT-PCR analysis of mouse FGFR4 forms expressed in: (A) primary myogenic cells cultured for 7 days; MyoD gene expression served as a reference for the myogenic characteristic of the cultures. (B) Organs from 4-day old mice. cDNA templates were made from (A) purified poly(A)+ RNA isolated from two independent cultures (PM1 and PM2) and (B) total RNA. The positions of the corresponding primers for FGFR4 forms are detailed in Fig. 1. GAPDH expression is provided as internal sample control. Number of PCR cycles applied per each primer set is indicated in parentheses.
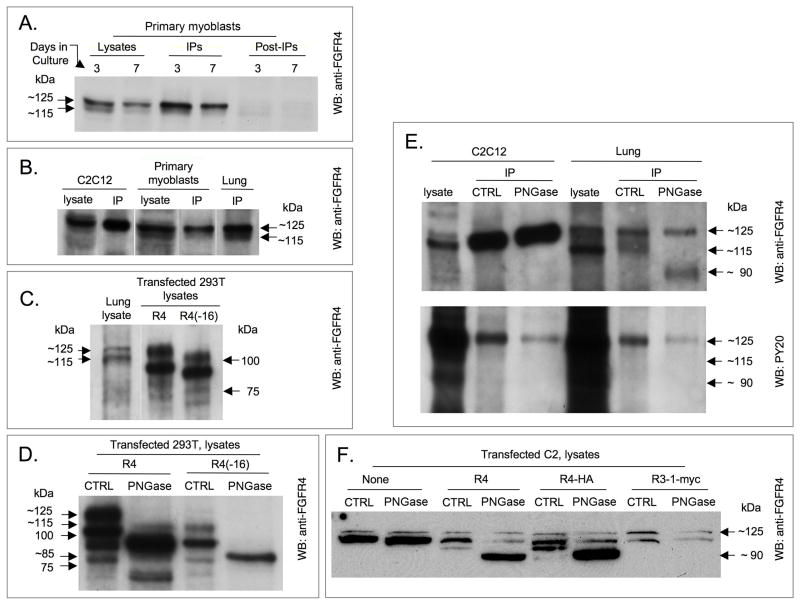
To facilitate detection of endogenous FGFR4 protein expression during myogenesis, we developed a rabbit polyclonal antibody against a peptide corresponding to amino acid positions 563–579 of mouse FGFR4. The immunogenic peptide corresponds to an epitope within exon 13 of mouse FGFR4, which covers the kinase-split insert domain of FGFR4 protein and is conserved in a wide-range of mammalian species, including human (B. Kwiatkowski and Z. Yablonka-Reuveni, unpublished). The antibody recognizes mouse endogenous and overexpressed FGFR4 forms as shown by Western blot and immunoprecipitation analyses (Fig. 3). Western blot detection of FGFR4 in lysates prepared from 3 and 7-day-old primary myogenic cultures is shown in Fig. 3A. Day 3 cultures are comprised mainly of proliferating myoblasts, while by day 7, cultures display differentiating myoblasts and myotubes in addition to proliferating myoblasts (Shefer et al., 2006). Two FGFR4-immunoreactive protein bands of 115 and 125 kDa were observed in cell lysates. Reactivity of the anti-FGFR4 antibody with endogenous FGFR4 in extracts and immunoprecipitates from the myogenic cell line C2C12, primary myogenic cultures and lung (all of mouse origin) is further presented in Figs. 3A–C. As shown in Fig. 3, immunoprecipitation with anti-FGFR4 resulted in a preferential enrichment of the higher molecular weight band. The relative reduction in the 115-kDa band following immunoprecipitation might be related to its susceptibility to deglycosylation (discussed in the next section), which could potentially result in further proteolysis. It is also possible that the 115-kDa band interacted with other partners, which reduced the efficiency of its immunoprecipitation. Extracts of C2 and C2C12 cultures demonstrated little of the 115-kDa FGFR4 band even in the original lysates, and this form was not detected after immunoprecipitation (Fig. 3). Western blot analyses of lysates of transfected 293T cells demonstrated that each overexpressed FGFR4-form produced four FGFR4-immunoreactive protein bands (Fig. 3C). Based on the migration position in SDS-PAGE, it is possible that of the two endogenously-expressed FGFR4 forms detected, the 125-kDa band corresponded to the slowest migrating band observed upon overexpressing FGFR4, and the 115-kDa band corresponded to the slowest migrating band observed when overexpressing FGFR4(−16).
Fig. 3.
Detection of endogenous and overexpressed forms of mouse FGFR4 by Western blotting. FGFR4 bands were detected directly in the initial lysates or following immunoprecipitation (IP) with the same anti-FGFR4 antibody used for developing the blots. (A) Primary myogenic cultures; lanes identified as post-IP depict the unbound material shown as control. (B) C2C12 cells, primary myoblasts, and lung. (C) Detection of overexpressed forms of FGFR4 in 293T cell lysates; parallel detection of endogenous FGFR4 in lung extracts is provided for size comparison. (D) Detection of native (CTRL) and deglycosylated (PNGase-treated) forms of FGFR4 and FGFR4(−16) overexpressed in 293T cell lysates. (E) Detection of native and PNGase treated forms of FGFR4 in anti-FGFR4 immunoprecipitates of mouse myoblast cell lysates and lung extracts; Western blots were reacted with anti-FGFR4 and anti-phoshpotyrosine (PY20) antibodies (upper and lower panels, respectively). (F) Detection of native and PNGase-treated forms of FGFR4 and FGFR4-HA overexpressed in C2 mouse myoblasts. Parallel controls of naive (nontransfected) C2 cells and of C2 cells transfected with FGFR3-1-myc are included.
Both FGFR4 forms display N-linked glycosylation
The cloned FGFR4 wildtype and FGFR4(−16) forms code for proteins with 799 and 753 amino acids, respectively, with predicted molecular masses of 88.6 and 83.3 kDa, respectively. The observed molecular weights of endogenous and overexpressed FGFR4 forms, as determined by Western blotting, were higher than anticipated from their predicted amino acid sequence. This finding prompted the consideration of posttranslational modifications associated with both FGFR4 forms. Specifically, we investigated the possible involvement of N-linked glycosylation, which can result in an increased molecular weight in the range observed in the present study. Lysates of 293T cells overexpressing FGFR4 forms were treated with the N-glycosidase enzyme PNGase-F. This treatment resulted in generation of FGFR4 molecules with a lower molecular weight compared to mock treated lysates, as shown by Western blot analysis (Fig. 3D). For each form of overexpressed FGFR4, the several FGFR4 bands detected prior to PNGase treatment were reduced to prominent single bands with molecular weights of ~ 90 and 85 kDa for the wildtype and (−16) form, respectively (Fig. 3D). These estimated values, corresponded well with the predicted molecular mass of each form. The 75-kDa band that was detected following deglycosylation of lysates overexpressing wildtype FGFR4 (Fig. 3D) might be a stable degradation product or a processed form of the receptor, since its molecular weight is lower than the predicted molecular mass.
Among the endogenously expressed FGFR4 forms, only the 115-kDa molecules were fully deglycosylated by PNGase, while a large portion of the 125-kDa band did not undergo deglycosylation (Fig. 3E). Notably, only the endogenously expressed 125-kDa FGFR4 protein, but not the endogenously expressed 115-kDa band and the 90-kDa bands generated by deglycosylation, were identified with the PY20 antibody, which specifically recognizes phosphorylated tyrosine residues (Fig. 3E). The difference in the outcome of deglycosylation of the endogenous 125-kDa FGFR4 band and the overexpressed 125-kDa form was also observed when FGFR4 constructs were overexpressed in C2 or C2C12 mouse myoblasts (Fig. 3F). Plasmid-driven protein expression in C2 or C2C12 cells, especially that of FGFR4(−16), was less efficient than in 293T cells and we thus limited the deglycosylation analysis to the wildtype form (Fig. 3F). Parallel overexpression of a control FGFR construct (chimeric FGFR3-1-myc) in mouse myoblasts and PNGase-F treatment did not cause a decline in FGFR4 molecular weight (Fig. 3F). This control also indicated that plasmid overexpression resulted in a reduced intensity of endogenous FGFR4 band, compared with non-transfected cells (Fig. 3, left lane). Altogether, our results indicate that the endogenously expressed 125-kDa FGFR4 form may be subjected to additional modifications that protect and stabilize the protein from undergoing deglycosylation with PNGase-F. It is also possible that the endogenously expressed 125-kDa band is not accessible to PNGase-F due to interactions with other protein partners or that the modification of the protein differs from N-glycosylation, predicated based on the identified modification of the overexpressed FGFR4 proteins.
Peak FGFR4 expression coincides with the onset of myogenic differentiation
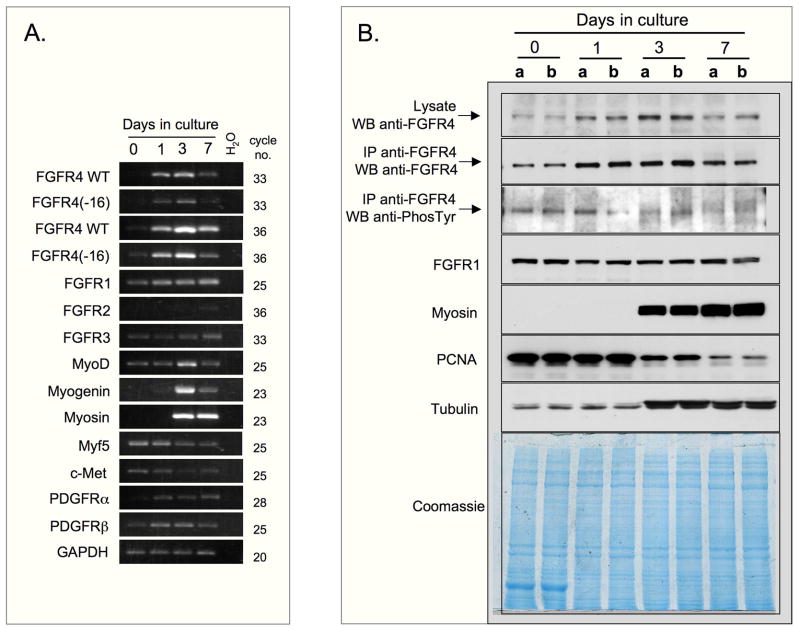
To determine if FGFR4 expression is modulated during myogenic differentiation, the temporal expression of FGFR4 mRNA and protein was analyzed in C2C12 cultures (Figs. 4A and B). Cells were initiated in serum-rich growth medium and switched to a low-serum medium to induce synchronous differentiation. Parallel cultures were harvested at different time points for total RNA isolation and for preparation of protein extracts, with day 0 indicating the time point of the switch from serum-rich to low-serum medium. Semi-quantitative RT-PCR (Fig. 4A) demonstrated that expression levels of both forms of FGFR4 were initially low, but then rose gradually over 3 days, with a subsequent decline by day 7. The temporal expression pattern of FGFR4 protein paralleled that of transcript expression and also peaked on culture day 3 (Fig. 4B; in agreement with data shown in Fig, 3, only one main FGFR4 protein band was observed in the present study of C2C12 cells). Peak FGFR4 expression coincided with cell cycle withdrawal and differentiation as implicated by the decline in PCNA expression (Fig. 4B) and increased expression of the myogenic differentiation genes MyoD, myogenin and sarcomeric myosin (Figs. 4A and B). In contrast, Myf5 maximal expression preceded the rise in FGFR4 expression. None of the other growth factor receptors examined demonstrated an expression pattern similar to that of the FGFR4 forms. Levels of FGFR1 and FGFR3 did not change appreciably throughout the days in culture, while FGFR2 was barely detectable at all times (Figs. 4A and B). Expression levels of c-met (HGF receptor) and the PDGF receptors, whose ligands were previously shown to influence myogenesis of C2 cells (Yablonka-Reuveni et al., 1990; Anastasi et al., 1997; Yablonka-Reuveni and Rivera, 1997), also did not change appreciably (Fig. 4A). In agreement with data shown in Fig. 3, the analysis of the temporal expression of FGFR4 protein, summarized in Fig. 4B, also revealed a low tyrosine phosphorylation level of the endogenously expressed FGFR4 (125-kDa band). This signal becomes more diffused during later time points.
Fig. 4.
FGFR4 expression during myogenic differentiation of C2C12 cells. Cells were switched to differentiation medium at time 0 and harvested at different days as indicated at the top of the panels. (A) RT-PCR analysis on templates made from total RNA; GAPDH expression is provided as internal sample control. (B) Western blot analysis; per each time point, lanes “a” and “b” depict results of two independent parallel cultures. The levels of FGFR4 were assayed directly in cell lysates or after immunoprecipitation with anti-FGFR4. Tyrosine phosphorylation of FGFR4 was assayed in immunoprecipitated samples with PY20 antibody. Tubulin levels (found to increase by day 3 in correlation with differentiation) and Coomassie staining are provided as gel loading control.
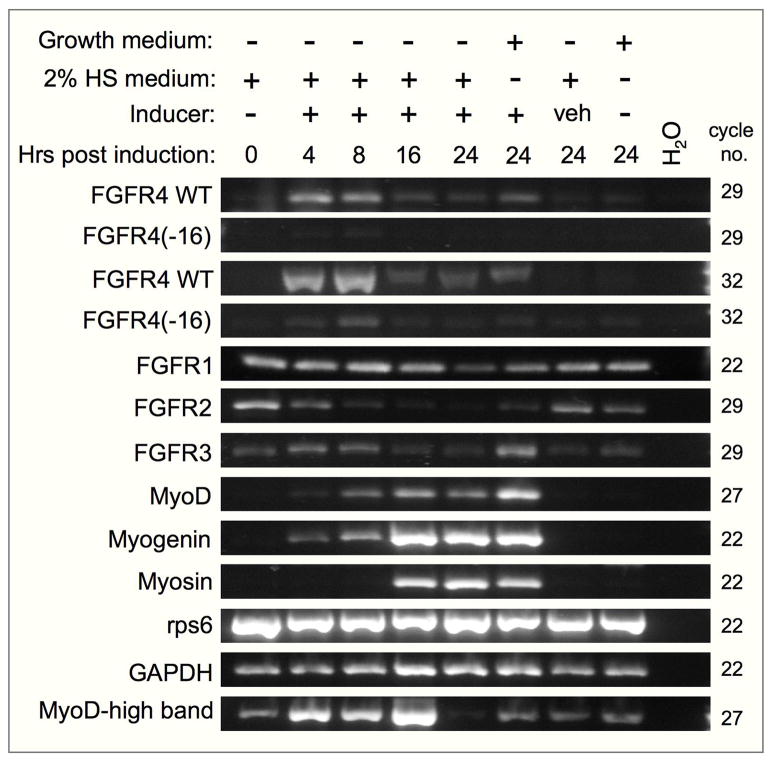
We further tested the expression of FGFR4 in the 10T½-MyoD-ER cell line, an inducible myogenic differentiation system. These cells express a chimeric MyoD-estrogen receptor protein that resides in the cytoplasm, but upon addition of β-estradiol the chimeric MyoD translocates to the nucleus and rapidly initiates endogenous MyoD expression and myogenic differentiation (Bergstrom et al., 2002). FGFR4 was rapidly upregulated in these cells upon estradiol induction. Peak FGFR4 transcript expression was detected within 4 hours following estradiol addition, coinciding with the initial expression of endogenous MyoD and myogenin (Fig. 5). However, while expression of myogenin and sarcomeric myosin rose robustly 16 hours following induction, FGFR4 and FGFR4(−16) expression levels already declined by that time. Expression levels of FGFR1 and FGFR3 did not change appreciably upon induction, while FGFR2 levels were highest in non-induced cells (Fig. 5). In addition to the PCR product of native MyoD (464 bp), we detected a second product with the MyoD primers (Fig. 5, MyoD-high band, ~ 1400 bp). This product corresponds to the predicted 1380 bp product from MyoD-ER mRNA. The MyoD-ER construct was generated by cloning the hormone binding domain of estrogen receptor into exon 1 of mouse MyoD cDNA (Hollenberg et al., 1993) and would be recognized by our MyoD primers, whose sequences are from exon 1 and exon 1–2 of native MyoD. The increased level of MyoD-ER band upon β-estradiol addition was unexpected.
Fig. 5.
RT-PCR analysis of FGFR4 and FGFR4(−16) expression during myogenic differentiation of the 10T½-MyoD-ER cell line. Cells were maintained in growth medium (10% FBS) or in 2% horse serum (HS) with or without the β-estradiol (10−7 M) inducer or the inducer vehicle (veh) as indicated at the top. Number of PCR cycles applied per each gene is indicated to the right. Expression of rps6 and GAPDH is provided as internal sample control. MyoD-high band represents a product of MyoD-ER mRNA.
Contrary to FGFR1, overexpressed FGFR4 does not exhibit tyrosine phosphorylation
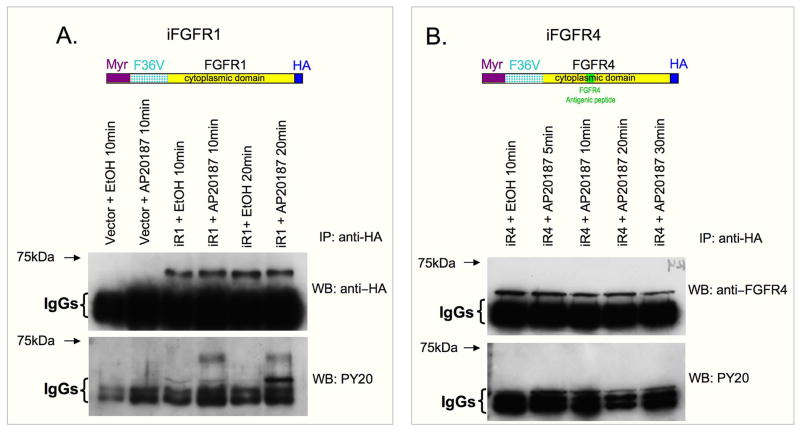
We were interested in establishing a system for investigating FGFR4 phosphorylation in order to compare the phosphorylative potential of wildtype and (−16) FGFR4 forms. Published reports on the reduced phosphorylative activity of FGFR4 (detailed in the Introduction) raised the possibility that FGFR4 activation might require a specific FGF ligand and/or cofactor that was absent in the experimental models that had been analyzed. Therefore, to investigate FGFR4 phophorylation, we used a regulated dimerization system based on a chimeric construct containing the cytoplasmic domain of the desired FGFR linked to a dimerizer-binding domain, and targeted to the cell membrane with a myristylation domain (referred below as iFGFR). This system does not require the presence of specific FGF ligands or cofactors. Based on published studies (Whitney et al., 2001; Welm et al., 2002), it was expected that the monomeric chimeric FGFRs expressed in target cells would dimerize upon addition of the ARIAD dimerizer drug AP20187, and that dimerization would lead to autophosphorylation and induction of downstream signaling cascades. When C2 (or C2C12) cells were transduced with retrovirally-based iFGFR constructs, the iFGFR4 did not exhibit phosphorylation of tyrosines, while control iFGFR1 chimeric protein was phosphorylated (Fig. 6). Similarly, activation of downstream signaling proteins such as ERK1/2 and Akt was not detected upon the addition of AP20187 to these myogenic cells transduced with iFGFR4 (data not shown), while the control iFGFR1 activated MAPK signaling as previously described (Whitney et al., 2001). A different inducible construct of FGFR4 with an extracellular domain of low affinity nerve growth factor receptor and two binding domains of ARIAD dimerizer (see Material and Methods for further details) also failed to undergo phosphorylation when expressed in C2 cells (data not shown). Taken together, these studies employing FGF independent dimerizable receptors, demonstrated a clear difference in the phosphorylative potential of FGFR4 and FGFR1.
Fig. 6.
Western blot analysis of tyrosine phosphorylation of FGFR in an inducible dimerization system expressed in C2 mouse myoblats. Schematics of the chimeric FGFR constructs are presented at the top of each corresponding panel. Dimerization was induced by the addition of AP20187 (100 nm) for the length of time indicated at the top of each lane. Negative controls included cells expressing the vector itself without the cytoplasmic FGFR domain, that were exposed to the dimerizer or the vehicle only (ethanol) (panel A) and cells expressing the construct but exposed to the vehicle only (panels A and B). iFGFR proteins were recovered from the cell lysates by immunoprecipitation with anti-HA. Receptor expression level was determined with anti-HA (panel A) or anti-FGFR4 (panel B). Tyrosine phosporylation was analyzed with PY20 antibody (bottom blot in each panel).
Next, based on similar studies concerning other FGFRs (Eswarakumar et al., 2005), we hypothesized that high-level expression of full-length FGFR4 transfected into 293T cells may result in receptor dimerization and autophosphorylation even if the native ligand was not available in the system. No FGFR4 phosphorylation was detected when cells were harvested 48 hours following transfection with wildtype FGFR4 while a control chimeric receptor FGFR3-1-myc was highly phosphorylated under the same conditions (Figs. 7B and C). Overexpressed FGFR3-myc also displayed phosphorylation, although to a lesser extend than the chimeric FGFR3-1-myc receptor (Figs. 7B). As shown in Fig. 7D, the phosphorylation of FGFR3-1-myc was fully abolished following 30-min administration of SU5402, a pharmacological inhibitor of FGFR1 autophosphorylation (Mohammadi et al., 1997). Interestingly, this analysis also demonstrated that the antibody against FGFR1 used in the present study recognized the FGFR1 domain in the FGFR3-1-myc chimera only when the protein was not phosphorylated; here, only the SU5402-treated lysate exhibited reactivity with the anti-FGFR1 antibody, which was generated against a c-terminal epitope in FGFR1 (Fig. 7D). This analysis confirmed that the FGFR3-1-myc chimeric receptor functions in the classic fashion of dimerization-driven autophosphorylation of tyrosines, and that 293T cells supported FGFR autophosphorylation. Nevertheless, the replacement of FGFR1 cytoplasmic domain with FGFR4 cytoplasmic domain in this chimera did not result in phosphorylation (Fig. 7C). Notably, FGFR4(−16) constructs, including iFGFR(−16), FGFR4(−16)-HA and chimeric FGFR3-4(−16)-myc also did not display tyrosine phosphorylation when tested in the models shown in Figs. 6 and 7 (data not shown).
Fig. 7.
Tyrosine phosphorylation analysis of different FGFR constructs overexpressed in 293T cells. Western blotting with PY20 antibody was used for detecting phosphorylation in immunoprecipitated FGFR proteins. (A) Schematics of constructs used in the studies summarized in Figs. 7 and 8; epitop tags at the c-terminus of each construct (myc or HA) were utilized for immunoprecipitation and Western blot analysis with corresponding anti-myc and anti-HA antibodies. (B, C) Western blot detections of immunoprecipitated FGFR4 proteins; overeexpressed FGFR4(−16) and FGFR3-4(−16), analyzed in parallel, also yielded no phosphorylation (data not shown). (D) Assessment of SU5402 (40 nM) effect on tyrosine phosphorylation of overexpressed FGFR3-1; drug added for 30 minutes prior to lysate harvesting impaired phosphorylation of the chimeric receptor. Plain control (untreated cells) and inhibitor vehicle control (DMSO) were analyzed in parallel. Also shown in the panel, only the non-phosphorylated FGFR3-1 form (i.e., SU5402-treated) reacted with the anti-FGFR1 antibody in Western blot analysis.
Coexpression of FGFR4 and FGFR3-1 results in phosphorylation of FGFR4
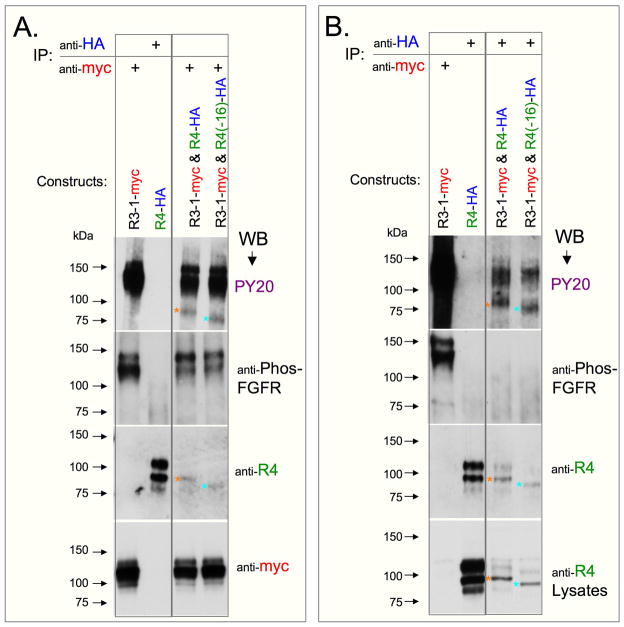
In view of the absence of FGFR4 phosphorylation in systems discussed above, we raised the possibility that FGFR4 phosphorylation may be executed differently from other FGFRs. We show here that coexpression of FGFR4-HA (or FGFR4(−16)-HA) with FGFR3-1-myc in 293T cells resulted in tyrosine phosphorylation of FGFR4 proteins, as determined by Western blotting following immunoprecipitation with anti-myc (Fig. 8A) or anti-HA (Fig. 8B). Each of the FGFR4 PY20-reactive bands (Fig. 8A and B, top panels) is identified with a different colored asterisk that is also shown in the corresponding blots that were stripped and re-probed with anti-FGFR4 antibody (Figs. 8A and 8B, 3rd blot from the top). The migration position of the PY20-reactive forms indicates that their molecular weights were lower than 100 kDa (Fig. 8), thus corresponding with deglycosylated FGFR4 forms (shown in Fig. 3D). Western blots of both immunoprecipitates and lysates (Fig. 8B, 3rd and 4th panels from the top, respectively) revealed enhanced levels of the lower molecular weight FGFR4 bands relative to the higher molecular weight bands of FGFR4 in co-transfected cells, compared with cells transfected with the FGFR4 expression vector only. Notably, immunoprecipitation with either anti-myc or anti-HA resulted in a similar pattern of the recovered FGFR4, with respect to the phosphorylation signals generated with the PY20 antibody and the total amounts of FGFR4 detected with anti-FGFR4 antibody. However, lysates of cotransfected cells that were immunoprecipitated with anti-myc displayed a higher level of recovered FGFR3-1-myc proteins than lysates immunoprecipitated with anti-HA (not shown), which relates to the apparent reduced PY20 signal of the FGFR3-1-myc band when immunoprecipitated with anti-HA (Figs. 8B versus 8A, top panels upper bands).
Fig. 8.
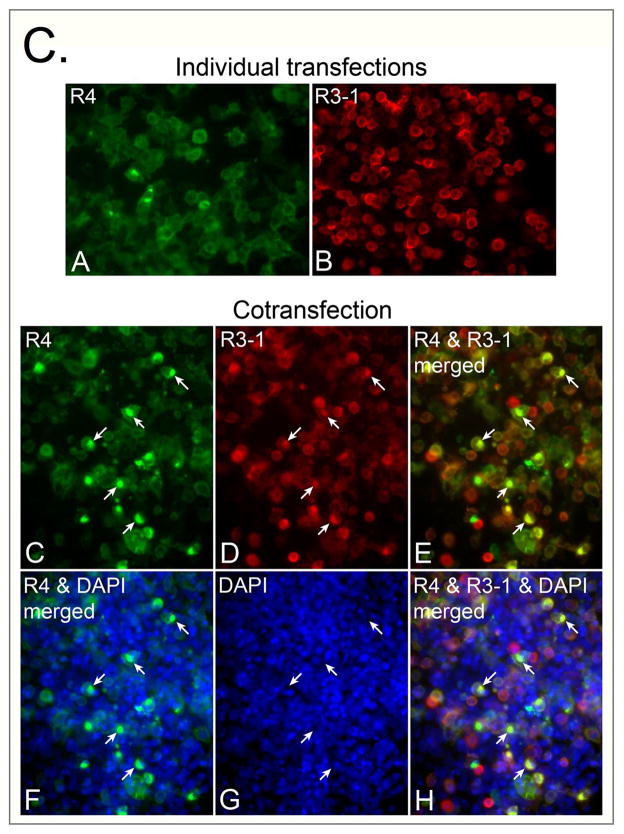
Outcome of co-expression of FGFR4 (or FGFR4(−16)) and FGFR3-1 in 293T cells. Schematics of expressed constructs are depicted in Fig. 7A. (A, B) Western blot analysis of tyrosine phosphorylation of FGFR4 and FGFR4(−16) HA-tagged constructs when co-expressed with FGFR3-1-myc. Expressed constructs and antibodies used for immunoprecipitation are indicated at the top of the panels. Antibodies used for parallel Western blot detections are listed on the right of each panel. (C) Immunofluorescence analysis, depicting expression patterns of the transfected constructs when expressed individually or coepxressed. In both individually and cotransfected cultures, detection of FGFR4 was achieved with rabbit antibody against HA (green signal) and detection of FGFR3-1 was achieved with a mouse anti-myc antibody (red signal). Nuclei were counter-stained with DAPI (shown only for the cotransfected culture). Arrows in parallel images of the cotransfected culture point to the positions of perinuclear structures with dense co-expression of both constructs; these regions are not labeled by DAPI.
In addition to the analyses of tyrosine phosphorylation of FGFR proteins with PY20 antibody, we also tested an antibody directed against phosphotyrosines 653/654, which reside within the ATP binding domain of FGFR1. While the same dual tyrosine context is present on FGFR4 (Hart et al., 2001), no obvious phosphorylation signal was detected in FGFR4-HA and FGFR4(−16)-HA bands when immunoprecipitates from cells co-overexpressing FGFR3-1 and FGFR4 constructs were developed with the anti-phospho-FGFR antibody (Fig. 8B). The Western blot signal produced by anti-phospho-FGFR was weaker than the signal detected with PY20 antibody, even when analyzing FGFR3-1-myc protein (Fig. 8A). It is possible therefore that the apparent lack of reactivity of FGFR4 forms with anti-phospho-FGFR may be related to the lower sensitivity of that antibody.
We also attempted to analyze the outcome of co-expression of FGFR3-myc and FGFR4 constructs in 293T cells. FGFR4 was not phosphorylated under this condition (data not shown). This result supports the view that the cytoplasmic domain of FGFR1 is required for the specific phosphorylation of FGFR4, as both FGFR3-1 and FGFR3 proteins contain the same extracellular and transmembrane domains. However, it is possible that the observed high level tyrosine phosphorylation in the FGFR3-1 chimera (compared to the weaker phosphorylation of FGFR3), rather than the presence of FGFR1 cytoplasmic domain, is the actual contributory factor for FGFR4 phosphorylation.
The observation that immunoprecipitation with either anti-HA and anti-myc yielded both FGFR4 and FGFR3-1 proteins (Figs. 8A and 8B) suggested that the two receptors could form a heterologous complex. To further investigate this aspect, the outcome of FGFR4 and FGFR3-1 co-expression in 293T cells was investigated at the single cell level (Fig. 8C; panel should be further enlarged for details). To monitor expression and localization of FGFR4-HA and FGFR3-1-myc, cultures were fixed 48 hours after transfection and immunolabeled with antibodies against HA and myc epitopes. Control cells, transfected with each vector alone, showed staining at the cell periphery and additional intracellular signal distribution of the overexpressed receptor, with infrequent cells presenting a more densely localized intracellular FGFR4 expression (Fig. 8C, panels A and B). Differently, when cells were cotransfected with both constructs, more cells displayed a densely localized pattern of receptor proteins, with frequent colocalization of both constructs to the same intracellular structure. The intracellular localization of the densely immunostained structures resembled that described in an earlier study, where proximity to the Golgi apparatus and FGFR sorting to the recycling compartment was reported (Haugsten et al., 2005).
Discussion
In this report we defined the patterns of FGFR4 transcript and protein expression during myogenic differentiation. We identified a new splice variant of FGFR4 that is expressed during myogenesis and demonstrated that FGFR4 gene expression peaks at the onset of differentiation. Additionally, our studies revealed intricate posttranslational modifications of the receptor. We uncovered a novel mechanism of FGFR4 tyrosine phosphorylation, which required the presence of a functional (phosphorylated) heterologous FGFR. We did not detect FGFR4 tyrosine phosphorylation when FGFR4 constructs were overexpressed alone, or upon the use of ARIAD inducible receptor homodimerization system. We therefore suggest that FGFR4 tyrosine phosphorylation is regulated in a different manner than the classic receptor homodimerization. It is reasonable to consider that in our experimental setting the chimeric FGFR1 receptor contributed directly to FGFR4 phosphorylation through its potent tyrosine kinase activity and not necessarily by means of FGFR heterodimerization. Alternatively, the chimeric FGFR1 might have activated other intermediaries that interacted with FGFR4. Collectively, the present study provides novel insights into the characteristics of mouse FGFR4 gene products at the transcript and protein levels, and their expression pattern during myogenesis.
We discovered a novel FGFR4 splice form, which we cloned from primary mouse myoblasts, progeny of satellite cells. Unlike other FGF receptors, for which multiple splice forms with different functions were previously described, FGFR4 splice forms were identified only in few studies. Two C-terminally truncated forms, FGFR4(−17a) and FGFR4(−17b), were reported in mouse, both generated by alternate splice sites in exon 17 (van Heumen et al., 1999). Additionally, a form that fails to splice intron 4, resulting in a premature stop codon that generates a soluble C-terminally truncated form was reported in human MCF-7 breast cancer cells (Ezzat et al., 2001). An alternative initiation site in intron 5 of FGFR4, resulting in an N-truncated cytoplasmic form, contributing to pituitary tumorigenesis, was also reported (Yu et al., 2003; Ezzat et al., 2004). A third FGFR4 soluble receptor, where exon 9 coding the single transmembrane domain is replaced with intron 9, was identified in human cell lines (Takaishi et al., 2000).
The new FGFR4 form we identified lacks the entire exon 16, resulting in a deletion of 138 nucleotides within the tyrosine kinase domain. We detected transcripts corresponding to the new form in mouse myogenic cultures as well as in several mouse tissues. We further determined that the expression level of FGFR4(−16) form was always lower than the full-length form, but paralleled its expression during myogenesis in all cell models analyzed herein. The deletion of exon 16 in the FGFR4(−16) variant puts the conservative tyrosine Y716 in a new amino acid context. Predictions based on known crystal structure of the FGFR1 receptor (Plotnikov et al., 1999) and the high similarity between the sequences of FGFR1 and FGFR4 led us to hypothesize that FGFR4(−16) splice form might be a kinase dead mutant, due to a significant deletion in the activation loop. This could potentially result in FGFR4(−16) acting as a dominant negative inhibitor of FGFR signaling. However, co-expression of FGFR4(−16) and FGFR3-1 constructs in 293T cells resulted in tyrosine phosphorylation at a similar level to that detected when wildtype FGFR4 was co-transfected with FGFR3-1. This finding indicates that the deletion of exon 16 does not influence tyrosine phosphorylation properties of FGFR4 in our heterologous FGFR expression system. It may be considered that FGFR4 tyrosine phosphorylation observed in the heterologous coexpression system does not necessarily relate to FGFR4 tyrosine kinase activity, and therefore, exon 16 deletion does not lead to an apparent effect on FGFR4 phosphorylation in this coexpression system. Nevertheless, the specificity of FGFR4-binding partners could still be affected by such a deletion in the context of the native (endogenously expressed) FGFR4(−16) form. It is also possible that the primary activity of FGFR4 does not rely on its tyrosine phosphorylation. Such a notion can be supported by the lack of FGFR4 phosphorylation when overexpressed in 293T cells, and when analyzed in the ARIAD inducible dimerization systems. FGFR1 constructs were highly phosphorylated when examined in these systems. The minimal tyrosine phosphorylation of endogenous or overexpressed wildtype FGFR4 observed in the present study is in agreement with reports of FGFR4 involvement in FGF trafficking, a process shown not to require the receptor’s intracellular kinase domain (Citores et al., 2001). FGFR1-mediated translocation of FGF to the cytosol and nucleus was also not impaired when the kinase domain was deleted (Sorensen et al., 2006). Thus, it is possible that FGFR4 (as well as other FGFRs) can function in a tyrosine kinase-independent fashion, apart from the widely accepted homodimerization and tyrosine autophosphorylation mode of function.
It is noteworthy that our pilot RT-PCR analysis of RNA isolated from a cell culture model of human myoblasts (kindly provided by S. Tapscott) suggests the presence of wildtype and (−16) FGFR4 forms in that model as well. As in mouse, exon 16 in human is comprised of 138 nucleotides with 88% homology between mouse and human. Furthermore, at the amino acid level, exon 16 is highly conserved (97% homology) between mouse and human. A somatic mutation in FGFR4 exon 16 was identified in human lung adenocarcinoma (Marks et al., 2007) in a region that is 100% conserved across human and mouse. Hence, further studies on the functional distinctions between wildtype and (−16) FGFR4 forms during normal and pathological states are warranted.
In this report we also provide novel insights on the glycosylation of FGFR4. The endogenous FGFR4 forms of molecular weights 125 and 115 kDa corresponded to the glycosylated wildtype and FGFR4(−16) forms found in overexpression experiments. However, among the endogenously expressed FGFR4 forms, only the 115-kDa form seemed to undergo complete deglycosylation, while all FGFR4 protein bands, detected upon overexpressing wildtype and (−16) forms, underwent deglycosylation. This observation suggests that some, or possibly all, of the 125-kDa endogenous molecules were protected from deglycosylation. Additionally, endogenous and overexpressed FGFR4 forms displayed diverse phosphorylation patterns. Only low levels of tyrosine phosphorylation of the endogenous 125 kDa-FGFR4 form were observed, while the phosphorylation of the endogenously expressed 115-kDa FGFR4 protein was below detection levels. Differently, the overexpressed FGFR4 forms were not phosphorylated, but when coexpressed with chimeric FGFR3-1, the lower molecular weight (presumably deglycosylated) forms of FGFR4 underwent tyrosine phosphorylation. Taken together, these observations may imply that both endogenous and overexpressed FGFR4 forms can undergo low-level tyrosine phosphorylation, but the specific environment and the presence of another active FGFR determine if FGFR4 phosphorylation takes place. The observed interplay between glycosylation/deglycosylation and the phosphorylation levels of overexpressed FGFR4 may modulate FGFR4 function. A similar modulation was shown for other FGFRs, where mutations that abrogate the capacity of the receptor to be glycosylated lead to a receptor that was constitutively active and to consequent pathological outcomes (Mangasarian et al., 1997; Winterpacht et al., 2000; Hatch et al., 2006). Glycosylation of FGFR4 may thus, prevent premature or disadvantageous tyrosine phosphorylation. Such protection from activation may stabilize the receptor during its internalization and transport to the recycling compartment in the cytoplasm (Haugsten et al., 2005). Additionally, N-glycans associated with FGFR1 were shown to interfere with interactions of the receptor with FGF and/or with heparan sulfate (Duchesne et al., 1996). Hence, the low phosphorylation and high glycosylation levels of FGFR4 observed in the current study may reflect an influence of N-glycsoylation on ligand interaction and activity of the FGFR4 forms. Collectively, our study establishes involvement of N-glycosylation in regulating FGFR4 function. Future studies on the expression of different FGFR4 forms and their phosphorylation and glycosylation in a range of developmental/pathological states might provide information about the different functions of the array of FGFR4 forms identified here.
This study further defined the expression pattern of FGFR4 and its novel splice form during myogenesis. FGFR4 expression was upregulated during early days of differentiation, followed by their decline in fully developed myotubes. Furthermore, activation of MyoD in the inducible cell culture system 10T½-MyoD-ER led to a rapid increase in the levels of FGFR4, followed by the expression of differentiation-linked genes. In the latter inducible system, FGFR4 upregulation was noticeably more synchronous compared with C2 cells or primary myoblasts. The temporal expression of wildtype FGFR4 transcripts during myogenesis in culture and in vivo was previously reported (Graves and Yablonka-Reuveni, 2000; Kastner et al., 2000; Zhao and Hoffman, 2004), as well as the role of MyoD in regulating FGFR4 expression (Zhao et al., 2006). However, here we demonstrate for the first time a tight interplay between the induction of myogenic differentiation and FGFR4 upregulation, establishing FGFR4 upregulation as an early step in the differentiation program, preceding upregulation of myogenin. Myogenin expression has been proposed to mark the phase of myogenic differentiation decision, preceding terminal cell cycle withdrawal (Andres and Walsh, 1996), but the events preceding myogenin upregulation have not been resolved. Hence, based on its pattern of expression, FGFR4 may play a role in the transition into the myogenin-expressing state. Clearly, the question “what functional role might the wildtype and (−16) FGFR4 forms play during myogenesis?” cannot be avoided. We attempted to investigate the role of FGFR4 during myogenic differentiation by several approaches. First, we analyzed myogenic differentiation in myofiber and primary cultures from muscles of FGFR4 null mice (Weinstein et al., 1998), but were unable to identify obvious impairments in myogenic differentiation compared with wildtype mice, apart from a subtle delay in the transition from proliferation to differentiation (unpublished; studies reviewed in Shefer and Yablonka-Reuveni, 2007). Second, we investigated the outcome of transient overexpression of FGFR4 forms in C2C12 cells. However, this approach typically results in high-level expression of the introduced protein, well above physiological levels. Our pilot studies did not reveal any obvious affect of wildtype FGFR4 on proliferation and MyoD expression compared to a control construct directing eGFP expression. Differently, transient overexpression of FGFR4(−16) persistently resulted in a very small number of transfected C2C12 cells, suggesting that high-level expression of this form might impair cell survival or lead to proliferative disadvantage in a myogenic cell context. To further investigate the role of FGFR4 during myogenesis, we are developing viral vectors for efficient expression of FGFR4 fusion proteins tagged with fluorescent epitopes. Such an approach will enable tracing FGFR4 intracellular localization in conjunction with other regulatory and structural proteins in live cells undergoing myogenesis.
Collectively, the characteristic expression of FGFR4 during myogenic differentiation and the unique mode of FGFR4 phosphorylation discovered in this study prompted us to suggest that FGFR4 functions during myogenesis in a distinctly different manner than FGFR1. We propose that the two FGFR4 forms contribute to functions other than typical tyrosine kinase signaling cascades. Based on the interaction between FGFR4 and chimeric FGFR1 observed in the present study, we further hypothesize that FGFR4 may engage molecules required for FGFR1 function, or interact with FGFR1 directly, thereby preparing myogenic cells for initiating differentiation decision. A proper balance between the different forms of FGFR4 identified in the present study may provide means for fine tuning FGFR4 role in myogenic differentiation.
Acknowledgments
We thank Heather Fullerton and Daniel Van de Mark for excellent technical support and Gabi Shefer (Tel Aviv University) and Ricardo Almuly (University of Washington) for helpful comments on the manuscript. We are also grateful to Mossa Mohammadi (New York University), Yossi Schlessinger (Yale University) and Sjur Olsnes (Institute for Cancer Research, Norwegian Radium Hospital) for their important input on FGFR molecular structure and tyrosine kinase activity. We also thank Stephen Tapscott (Fred Hutchinson Cancer Center), Charles Murry and C. Anthony Blau (University of Washington), David Ornitz (Washington University), and Roy Pollock (ARIAD) for kindly providing cells/constructs, as listed in the Material and Methods. We express gratitude to Thong Pham and Peter Rabinovitch for their assistance with cell sorting, performed at the core facility of the University of Washington Nathan Shock Center of Excellence. This work was supported by a grant to Z.Y.R. from the National Institute on Aging (AG013798). Z.Y.R. acknowledges additional support during the course of this study from the National Institute on Aging (AG021566) and the USDA Cooperative State Research, Education and Extension Service (NRI, 2003-35206-12843).
Literature Cited
- Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara JF, Courage NL, Gilman M. Cell surface tagging and a suicide mechanism in a single chimeric human protein. Hum Gene Ther. 1999;10:2651–2655. doi: 10.1089/10430349950016681. [DOI] [PubMed] [Google Scholar]
- Anastasi S, Giordano S, Sthandier O, Gambarotta G, Maione R, Comoglio P, Amati P. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J Cell Biol. 1997;137:1057–1068. doi: 10.1083/jcb.137.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom DA, Penn BH, Strand A, Perry RL, Rudnicki MA, Tapscott SJ. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol Cell. 2002;9:587–600. doi: 10.1016/s1097-2765(02)00481-1. [DOI] [PubMed] [Google Scholar]
- Boilly B, Vercoutter-Edouart AS, Hondermarck H, Nurcombe V, Le Bourhis X. FGF signals for cell proliferation and migration through different pathways. Cytokine Growth Factor Rev. 2000;11:295–302. doi: 10.1016/s1359-6101(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol. 2001;3:650–657. doi: 10.1038/35083041. [DOI] [PubMed] [Google Scholar]
- Citores L, Khnykin D, Sorensen V, Wesche J, Klingenberg O, Wiedlocha A, Olsnes S. Modulation of intracellular transport of acidic fibroblast growth factor by mutations in the cytoplasmic receptor domain. J Cell Sci. 2001;114:1677–1689. doi: 10.1242/jcs.114.9.1677. [DOI] [PubMed] [Google Scholar]
- Duchesne L, Tissot B, Rudd TR, Dell A, Fernig DG. N-glycosylation of fibroblast growth factor receptor 1 regulates ligand and heparan sulfate co-receptor binding. J Biol Chem. 2006;281:27178–27189. doi: 10.1074/jbc.M601248200. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Ezzat S, Zheng L, Asa SL. Pituitary tumor-derived fibroblast growth factor receptor 4 isoform disrupts neural cell-adhesion molecule/N-cadherin signaling to diminish cell adhesiveness: a mechanism underlying pituitary neoplasia. Mol Endocrinol. 2004;18:2543–2552. doi: 10.1210/me.2004-0182. [DOI] [PubMed] [Google Scholar]
- Ezzat S, Zheng L, Yu S, Asa SL. A soluble dominant negative fibroblast growth factor receptor 4 isoform in human MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2001;287:60–65. doi: 10.1006/bbrc.2001.5546. [DOI] [PubMed] [Google Scholar]
- Gao G, Goldfarb M. Heparin can activate a receptor tyrosine kinase. Embo J. 1995;14:2183–2190. doi: 10.1002/j.1460-2075.1995.tb07212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AM, Hill DJ, Logan A, Maher PA, Baird A. Distribution of fibroblast growth factor (FGF)-2 and FGF receptor-1 messenger RNA expression and protein presence in the mid-trimester human fetus. Pediatr Res. 1996;39:375–385. doi: 10.1203/00006450-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Graves DC, Yablonka-Reuveni Z. Vascular smooth muscle cells spontaneously adopt a skeletal muscle phenotype: a unique Myf5(−)/MyoD(+) myogenic program. J Histochem Cytochem. 2000;48:1173–1193. doi: 10.1177/002215540004800902. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Ratliff EP, Andres AM, Huang X, McKeehan WL, Davis RA. Bile acids decrease hepatic paraoxonase 1 expression and plasma high-density lipoprotein levels via FXR-mediated signaling of FGFR4. Arterioscler Thromb Vasc Biol. 2006;26:301–306. doi: 10.1161/01.ATV.0000195793.73118.b4. [DOI] [PubMed] [Google Scholar]
- Harmer NJ, Pellegrini L, Chirgadze D, Fernandez-Recio J, Blundell TL. The crystal structure of fibroblast growth factor (FGF) 19 reveals novel features of the FGF family and offers a structural basis for its unusual receptor affinity. Biochemistry. 2004;43:629–640. doi: 10.1021/bi035320k. [DOI] [PubMed] [Google Scholar]
- Hart KC, Robertson SC, Donoghue DJ. Identification of tyrosine residues in constitutively activated fibroblast growth factor receptor 3 involved in mitogenesis, Stat activation, and phosphatidylinositol 3-kinase activation. Mol Biol Cell. 2001;12:931–942. doi: 10.1091/mbc.12.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch NE, Hudson M, Seto ML, Cunningham ML, Bothwell M. Intracellular retention, degradation, and signaling of glycosylation-deficient FGFR2 and craniosynostosis syndrome-associated FGFR2C278F. J Biol Chem. 2006;281:27292–27305. doi: 10.1074/jbc.M600448200. [DOI] [PubMed] [Google Scholar]
- Haugsten EM, Sorensen V, Brech A, Olsnes S, Wesche J. Different intracellular trafficking of FGF1 endocytosed by the four homologous FGF receptors. J Cell Sci. 2005;118:3869–3881. doi: 10.1242/jcs.02509. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Cheng PF, Weintraub H. Use of a conditional MyoD transcription factor in studies of MyoD trans-activation and muscle determination. Proc Natl Acad Sci U S A. 1993;90:8028–8032. doi: 10.1073/pnas.90.17.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Mima T, Mikawa T. Loss of fibroblast growth factor receptors is necessary for terminal differentiation of embryonic limb muscle. Development. 1996;122:291–300. doi: 10.1242/dev.122.1.291. [DOI] [PubMed] [Google Scholar]
- Jin L, Zeng H, Chien S, Otto KG, Richard RE, Emery DW, Blau CA. In vivo selection using a cell-growth switch. Nat Genet. 2000;26:64–66. doi: 10.1038/79194. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Johnson SE, Allen RE. Activation of skeletal muscle satellite cells and the role of fibroblast growth factor receptors. Exp Cell Res. 1995;219:449–453. doi: 10.1006/excr.1995.1251. [DOI] [PubMed] [Google Scholar]
- Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem. 2000;48:1079–1096. doi: 10.1177/002215540004800805. [DOI] [PubMed] [Google Scholar]
- Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- Korhonen J, Partanen J, Alitalo K. Expression of FGFR-4 mRNA in developing mouse tissues. Int J Dev Biol. 1992;36:323–329. [PubMed] [Google Scholar]
- Lin HY, Xu J, Ischenko I, Ornitz DM, Halegoua S, Hayman MJ. Identification of the cytoplasmic regions of fibroblast growth factor (FGF) receptor 1 which play important roles in induction of neurite outgrowth in PC12 cells by FGF-1. Mol Cell Biol. 1998;18:3762–3770. doi: 10.1128/mcb.18.7.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangasarian K, Li Y, Mansukhani A, Basilico C. Mutation associated with Crouzon syndrome causes ligand-independent dimerization and activation of FGF receptor-2. J Cell Physiol. 1997;172:117–125. doi: 10.1002/(SICI)1097-4652(199707)172:1<117::AID-JCP13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Marics I, Padilla F, Guillemot JF, Scaal M, Marcelle C. FGFR4 signaling is a necessary step in limb muscle differentiation. Development. 2002;129:4559–4569. doi: 10.1242/dev.129.19.4559. [DOI] [PubMed] [Google Scholar]
- Marks JL, McLellan MD, Zakowski MF, Lash AE, Kasai Y, Broderick S, Sarkaria IS, Pham D, Singh B, Miner TL, Fewell GA, Fulton LL, Mardis ER, Wilson RK, Kris MG, Rusch VW, Varmus H, Pao W. Mutational Analysis of EGFR and Related Signaling Pathway Genes in Lung Adenocarcinomas Identifies a Novel Somatic Kinase Domain Mutation in FGFR4. PLoS ONE. 2007;2:e426. doi: 10.1371/journal.pone.0000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Goetz R. A protein canyon in the FGF-FGF receptor dimer selects from an a la carte menu of heparan sulfate motifs. Curr Opin Struct Biol. 2005;15:506–516. doi: 10.1016/j.sbi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Olwin BB, Arthur K, Hannon K, Hein P, McFall A, Riley B, Szebenyi G, Zhou Z, Zuber ME, Rapraeger AC, et al. Role of FGFs in skeletal muscle and limb development. Mol Reprod Dev. 1994;39:90–100. doi: 10.1002/mrd.1080390114. discussion 100–101. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Plotnikov AN, Schlessinger J, Hubbard SR, Mohammadi M. Structural basis for FGF receptor dimerization and activation. Cell. 1999;98:641–650. doi: 10.1016/s0092-8674(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Powell PP, Wang CC, Horinouchi H, Shepherd K, Jacobson M, Lipson M, Jones R. Differential expression of fibroblast growth factor receptors 1 to 4 and ligand genes in late fetal and early postnatal rat lung. Am J Respir Cell Mol Biol. 1998;19:563–572. doi: 10.1165/ajrcmb.19.4.2994. [DOI] [PubMed] [Google Scholar]
- Raffioni S, Thomas D, Foehr ED, Thompson LM, Bradshaw RA. Comparison of the intracellular signaling responses by three chimeric fibroblast growth factor receptors in PC12 cells. Proc Natl Acad Sci U S A. 1999;96:7178–7183. doi: 10.1073/pnas.96.13.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee DA, Patel Y, Jacobson K. Expression of fibroblast growth factor receptors in peri-implantation mouse embryos. Mol Reprod Dev. 1998;51:254–264. doi: 10.1002/(SICI)1098-2795(199811)51:3<254::AID-MRD4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Shaoul E, Reich-Slotky R, Berman B, Ron D. Fibroblast growth factor receptors display both common and distinct signaling pathways. Oncogene. 1995;10:1553–1561. [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Yablonka-Reuveni Z. The ins and outs of satellite cell myogenesis: the role of the ruling growth factors. In: Schiaffino S, Partridge T, editors. Skeletal Muscle Repair and Regeneration. Springer; 2007. pp. 107–143. in press. [Google Scholar]
- Sorensen V, Wiedlocha A, Haugsten EM, Khnykin D, Wesche J, Olsnes S. Different abilities of the four FGFRs to mediate FGF-1 translocation are linked to differences in the receptor C-terminal tail. J Cell Sci. 2006;119:4332–4341. doi: 10.1242/jcs.03209. [DOI] [PubMed] [Google Scholar]
- Stark KL, McMahon JA, McMahon AP. FGFR-4, a new member of the fibroblast growth factor receptor family, expressed in the definitive endoderm and skeletal muscle lineages of the mouse. Development. 1991;113:641–651. doi: 10.1242/dev.113.2.641. [DOI] [PubMed] [Google Scholar]
- Takaishi S, Sawada M, Morita Y, Seno H, Fukuzawa H, Chiba T. Identification of a novel alternative splicing of human FGF receptor 4: soluble-form splice variant expressed in human gastrointestinal epithelial cells. Biochem Biophys Res Commun. 2000;267:658–662. doi: 10.1006/bbrc.1999.2010. [DOI] [PubMed] [Google Scholar]
- Tuominen H, Heikinheimo P, Loo BM, Kataja K, Oker-Blom C, Uutela M, Jalkanen M, Goldman A. Expression and glycosylation studies of human FGF receptor 4. Protein Expr Purif. 2001;21:275–285. doi: 10.1006/prep.2000.1375. [DOI] [PubMed] [Google Scholar]
- Vainikka S, Joukov V, Wennstrom S, Bergman M, Pelicci PG, Alitalo K. Signal transduction by fibroblast growth factor receptor-4 (FGFR-4). Comparison with FGFR-1. J Biol Chem. 1994;269:18320–18326. [PubMed] [Google Scholar]
- van Heumen WR, Claxton C, Pickles JO. Fibroblast growth factor receptor-4 splice variants cause deletion of a critical tyrosine. IUBMB Life. 1999;48:73–78. doi: 10.1080/713803466. [DOI] [PubMed] [Google Scholar]
- Wang JK, Gao G, Goldfarb M. Fibroblast growth factor receptors have different signaling and mitogenic potentials. Mol Cell Biol. 1994;14:181–188. doi: 10.1128/mcb.14.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JK, Goldfarb M. Amino acid residues which distinguish the mitogenic potentials of two FGF receptors. Oncogene. 1997;14:1767–1778. doi: 10.1038/sj.onc.1201021. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–3623. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- Welm BE, Freeman KW, Chen M, Contreras A, Spencer DM, Rosen JM. Inducible dimerization of FGFR1: development of a mouse model to analyze progressive transformation of the mammary gland. J Cell Biol. 2002;157:703–714. doi: 10.1083/jcb.200107119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney ML, Otto KG, Blau CA, Reinecke H, Murry CE. Control of myoblast proliferation with a synthetic ligand. J Biol Chem. 2001;276:41191–41196. doi: 10.1074/jbc.M103191200. [DOI] [PubMed] [Google Scholar]
- Winterpacht A, Hilbert K, Stelzer C, Schweikardt T, Decker H, Segerer H, Spranger J, Zabel B. A novel mutation in FGFR-3 disrupts a putative N-glycosylation site and results in hypochondroplasia. Physiol Genomics. 2000;2:9–12. doi: 10.1152/physiolgenomics.2000.2.1.9. [DOI] [PubMed] [Google Scholar]
- Xie MH, Holcomb I, Deuel B, Dowd P, Huang A, Vagts A, Foster J, Liang J, Brush J, Gu Q, Hillan K, Goddard A, Gurney AL. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine. 1999;11:729–735. doi: 10.1006/cyto.1999.0485. [DOI] [PubMed] [Google Scholar]
- Xu X, Weinstein M, Li C, Deng C. Fibroblast growth factor receptors (FGFRs) and their roles in limb development. Cell Tissue Res. 1999;296:33–43. doi: 10.1007/s004410051264. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J Cell Biol. 1990;111:1623–1629. doi: 10.1083/jcb.111.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Influence of PDGF-BB on proliferation and transition through the MyoD-myogenin-MEF2A expression program during myogenesis in mouse C2 myoblasts. Growth Factors. 1997;15:1–27. doi: 10.3109/08977199709002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Seiler SH, Kucherlapati R, Leinwand L. Organization of the human skeletal myosin heavy chain gene cluster. Proc Natl Acad Sci U S A. 1992;89:12078–12082. doi: 10.1073/pnas.89.24.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Wang F, Kan M, Jin C, Jones RB, Weinstein M, Deng CX, McKeehan WL. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J Biol Chem. 2000;275:15482–15489. doi: 10.1074/jbc.275.20.15482. [DOI] [PubMed] [Google Scholar]
- Yu S, Asa SL, Weigel RJ, Ezzat S. Pituitary tumor AP-2alpha recognizes a cryptic promoter in intron 4 of fibroblast growth factor receptor 4. J Biol Chem. 2003;278:19597–19602. doi: 10.1074/jbc.M212432200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Caretti G, Mitchell S, McKeehan WL, Boskey AL, Pachman LM, Sartorelli V, Hoffman EP. Fgfr4 is required for effective muscle regeneration in vivo. Delineation of a MyoD-Tead2-Fgfr4 transcriptional pathway. J Biol Chem. 2006;281:429–438. doi: 10.1074/jbc.M507440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Hoffman EP. Embryonic myogenesis pathways in muscle regeneration. Dev Dyn. 2004;229:380–392. doi: 10.1002/dvdy.10457. [DOI] [PubMed] [Google Scholar]