Abstract
ACA8 is a plasma membrane-localized isoform of calmodulin (CaM)-regulated Ca2+-ATPase of Arabidopsis thaliana. Several phosphopeptides corresponding to portions of the regulatory N-terminus of ACA8 have been identified in phospho-proteomic studies. To mimic phosphorylation of the ACA8 N-terminus, each of the serines found to be phosphorylated in those studies (Ser19, Ser22, Ser27, Ser29, Ser57, and Ser99) has been mutated to aspartate. Mutants have been expressed in Saccharomyces cerevisiae and characterized: mutants S19D and S57D—and to a lesser extent also mutants S22D and S27D—are deregulated, as shown by their low activation by CaM and by tryptic cleavage of the N-terminus. The His-tagged N-termini of wild-type and mutant ACA8 (6His-1M-I116) were expressed in Escherichia coli, affinity-purified, and used to analyse the kinetics of CaM binding by surface plasmon resonance. All the analysed mutations affect the kinetics of interaction with CaM to some extent: in most cases, the altered kinetics result in marginal changes in affinity, with the exception of mutants S57D (KD ∼10-fold higher than wild-type ACA8) and S99D (KD about half that of wild-type ACA8). The ACA8 N-terminus is phosphorylated in vitro by two isoforms of A. thaliana calcium-dependent protein kinase (CPK1 and CPK16); phosphorylation of mutant 6His-1M-I116 peptides shows that CPK16 is able to phosphorylate the ACA8 N-terminus at Ser19 and at Ser22. The possible physiological implications of the subtle modulation of ACA8 activity by phosphorylation of its N-terminus are discussed.
Keywords: Arabidopsis thaliana, Ca2+-ATPase, calcium-dependent protein kinase, calmodulin, plasma membrane, phosphorylation
Introduction
Cytosolic calcium is a key element in the transduction of a variety of endogenous and environmental signals in plant cells. An increasing amount of evidence indicates that signal specificity is encoded by the amplitude, frequency, and time extension of cytosolic Ca2+ waves, which in turn depend on the activity of Ca2+ channels—which when open flood the cytosol with Ca2+ from the apoplast and/or intracellular stores—and of active Ca2+ transporters—which extrude Ca2+ to the apoplast or sequester it in intracellular stores. Fine-tuning of the Ca2+ transport systems in response to different signals is thus a crucial feature of Ca2+-mediated signal transduction (Sanders et al., 2002; Boursiac and Harper, 2007; McAinsh and Pittman, 2009; Das and Pandey, 2010; Dodd et al., 2010; Bonza and De Michelis, 2011; Pittman et al., 2011).
In plant cells, Ca2+ extrusion from the cytoplasm is accomplished either through tonoplast-localized Ca2+–H+ antiporters powered by a proton-motive force, or through Ca2+ pumps powered by ATP hydrolysis, localized both at the plasma membrane (PM) and at intracellular membranes. PM Ca2+ pumps are likely to play a crucial role in re-establishing the low basal Ca2+ concentration especially after its increase due to opening of PM Ca2+ channels. Indeed, the available evidence, albeit fragmentary, demonstrates their involvement in fundamental processes such as development, hormonal regulation, and response to biotic and abiotic stresses; however, their physiological role and the mechanisms underlying their regulation in response to specific signals have not been ascertained yet (Boursiac and Harper, 2007; Bonza and De Michelis, 2011; Pittman et al., 2011).
PM Ca2+ pumps are calmodulin (CaM)-regulated Ca2+-ATPases, belonging to the P-type ATPase superfamily: three isoforms of CaM-regulated Ca2+-ATPase, all belonging to the same cluster, have been identified as PM-localized pumps in Arabidopsis thaliana: among these, the best characterized at the biochemical level is ACA8, a widely expressed isoform found in all plant organs (Bonza and De Michelis, 2011; Pittman et al., 2011).
ACA8, like other plant isoforms of CaM-regulated Ca2+-ATPases, has an extended cytosolic N-terminal domain containing an autoinhibitory domain partially overlapping the CaM-binding site: CaM binding suppresses the autoinhibitory action of the N-terminal domain and determines both the increase of Vmax and the decrease of the K0.5 for free Ca2+ (Bonza and De Michelis, 2011; Pittman et al., 2011).
ACA8 is also regulated by acidic phospholipids such as phosphatidylserine or phosphatidylinositol-4P, which activate the pump via two distinct mechanisms, involving their binding to different sites: acidic phospholipids binding to a site in the protein N-terminus, overlapping the autoinhibitory and CaM-binding domain, stimulates ACA8 activity similar to CaM or to cleavage of the N-terminus, while their binding to a second, as yet unidentified, site further stimulates ACA8 activity by lowering its K0.5 for free Ca2+ (Meneghelli et al., 2008).
CaM-regulated Ca2+-ATPases can also be modulated by phosphorylation. In the pumps of animal cells, which have the regulatory domain localized at the extended C-terminus, the C-terminal portion is the target of phosphorylation by different protein kinases that phosphorylate different amino acids in different isoforms: each phosphorylation event has a peculiar effect on the pump activity (Enyedi et al., 1996, 1997; Penniston and Enyedi, 1998; Verma et al., 1999). In plants, it has been shown that in vitro phosphorylation of a serine residue just downstream the CaM-binding site of ACA2—an A. thaliana isoform of the endoplasmic reticulum—by a calcium-dependent protein kinase (CDPK) severely inhibits CaM-stimulated enzyme activity, without disrupting CaM binding (Hwang et al., 2000). Also the N-terminus of BCA1—an isoform of CaM-regulated Ca2+-ATPase of the tonoplast of Brassica oleracea—can be phosphorylated in vitro by protein kinase C at two serine residues, one within the CaM-binding domain, but the effect of phosphorylation on pump activity was not determined (Malmström et al., 2000).
Data from large-scale phospho-proteomic studies have identified several phosphopeptides corresponding to portions of the N-terminus of ACA8. In particular (Table 1), four serine residues that are phosphorylated (S19, S22, S27, and S29) are localized ∼20 amino acids upstream of the CaM-binding and autoinhibitory domain, one (S57) is within the CaM-binding site, and one (S99) is 30 amino acids downstream (Nühse et al., 2003, 2004, 2007; Benschop et al., 2007; Niittylä et al., 2007; Sugiyama et al., 2008; Whiteman et al., 2008; Jones et al., 2009; Reiland et al., 2009; Chen et al., 2010; Nakagami et al., 2010). For some of these residues, evidence has also been provided that phosphorylation is up-regulated by hormones such as abscisic acid and gibberellins (S27 and S29; Chen et al., 2010) or by elicitors such as flagellin (S27 and S99; Nühse et al., 2003, 2004, 2007; Benschop et al., 2007), or down-regulated in response to sucrose administration to cultured cells (S22; Niittylä et al., 2007). Since these serine residues are not conserved in most isoforms of A. thaliana ACA, phosphorylation of any of them could represent an isoform-specific mechanism of regulation of pump activity.
Table 1.
Serine residues in the ACA8 N-terminus that have been found to be phosphorylated in vivo
| Phosphorylated residue | Plant material | Effectors | Reference |
| Ser19 | Cultured cells | Nühse et al. (2003, 2004) | |
| Ser22 | Cultured cells | Sucrose (–) | Nühse et al. (2003, 2004); Niittyla et al. (2007);Sugiyama et al. (2008); Nakagami et al. (2010) |
| Ser27 | Cultured cells, seedlings, shoots, leaves | Abscisic acid (+), flagellin 22 (+) | Nühse et al. (2003, 2004, 2007); Benschop et al. (2007); Sugiyama et al. (2008); Whiteman et al. (2008); Jones et al. (2009); Reiland et al. (2009); Chen et al. (2010); Nakagami et al. (2010) |
| Ser29 | Cultured cells | Abscisic acid (+), gibberellins (+) | Nühse et al. (2003, 2004); Sugiyama et al. (2008); Chen et al. (2010); Nakagami et al. (2010) |
| Ser57 | Cultured cells | Sugiyama et al. (2008); Nakagami et al. (2010) | |
| Ser99 | Cultured cells | Flagellin 22 (+) | Benschop et al. (2007) |
Here it is shown that substitution of any of the above-mentioned serine residues with aspartate, which mimics the effect of phosphorylation, affects the regulatory properties of ACA8, generating partially deregulated pumps (mutants S19D, S57D, and, to a lesser extent, mutants S22D and S27D), modifying the kinetics of interaction with CaM (all tested mutations), and/or changing the affinity for CaM (S57D and S99D mutants). It is also shown that the ACA8 N-terminus is phosphorylated in vitro by CDPK (Harper et al., 2004; Das and Pandey, 2010) at Ser19 and Ser22.
Materials and methods
Plasmid constructs
Site-directed mutagenesis of ACA8 was conducted using the Quickchange site-directed mutagenesis kit (Stratagene, Santa Clara, CA, USA, catalogue no. 200518) according to the manufacturer’s protocol using wild-type (WT) ACA8 full-length cDNA inserted in the pYES2 vector (Invitrogen, Carlsbad, CA, USA, catalogue no. V825-20v) as a template; primers are listed in Supplementary Table S1 available at JXB online. Introduction of the correct mutations and absence of errors were confirmed by sequencing.
Standard PCRs performed with GoTaq® polymerase (Promega, Madison, WI, USA, catalogue no. M3175) were used to amplify the first 116 amino acids (1M-I116) at the N-terminus of ACA8 mutants, using mutated ACA8 full-length cDNA as templates and the following specific oligonucleotides: (S) 5′ CTTGGTCATATGACGAGTCTCTTGAAGTC; and (AS) 5′ GCTCGGGATCCTCAAATTCCAAAATCACCAGCC. The S primer contains an NdeI restriction site and the AS primer contains a BamHI restriction site (underlined). To produce recombinant WT and mutated N-terminal domains that could later be purified by NTA affinity chromatography, the coding sequences for the N-termini of WT and mutants of ACA8, obtained using the restriction enzymes reported above, were inserted into Escherichia coli expression vector pET15b (Merck KGaA, Darmstadt, Germany, catalogue no. 69661), in this way fusing a 6His tag to the N-terminus of the peptide. Introduction of the correct mutations and absence of errors were confirmed by sequencing.
Ps 658 G-CPK1ci and Ps 652 G-CPK16-F399A plasmids encoding, respectively, calcium-independent mutants of isoforms CPK1 and CPK16 of A. thaliana CDPK, sandwiched between N-terminal glutathione S-transferase (GST) and a C-terminal 6His tag, were kindly provided by Professor J. F. Harper (University of Nevada, Reno, NV, USA).
Yeast transformation and growth media
The DNA coding for WT and mutant ACA8 proteins is inserted in the pYES2 vector (Invitrogen), under the control of a galactose-inducible promoter. Those plasmids were used for transformation of Saccharomyces cerevisiae strain K616 (MATα pmr1::HIS3 pmc1::TRP1 cnb1::LEU2, ade2, ura3; Cunningham and Fink, 1994) using a lithium acetate/polyethylene glycol method (Bækgaards et al., 2006). Transformants were selected for uracil prototrophy on synthetic complete medium lacking uracil (SC-URA) as described (Bonza et al., 2004). Plant pumps were expressed in yeast grown in SC-URA medium containing 2% (w/v) galactose, 1% (w/v) raffinose, 50 mM succinic acid/TRIS (pH 5.5), 0.7% (w/v) yeast nitrogen base, and 10 mM CaCl2, for 24 h at 30 °C.
Isolation of yeast microsomes
Yeast cells were homogenized and microsomes were harvested as previously reported (Bonza et al., 2004). Protein concentration was determined using the Bio-Rad assay (Bio-Rad, Hercules, CA, USA, catalogue no. 500-001).
Electrophoresis and immunoblotting analysis
SDS–PAGE, western blotting, and immunodecoration with polyclonal antibody against the ACA8 small cytoplasmic loop region were performed as described (Luoni et al., 2004); the antibody does not recognize any protein band in microsomes extracted from K616 yeast transformed with the empty vector (data not shown). Signal quantification was performed using the Fluor-Chem™SP Imaging System and AlphaEaseFC software by Alpha Innotech (MMedical, Cornaredo, MI, Italy).
Trypsin treatment
The microsomal fraction (1 mg protein ml−1) was incubated for 10 min at 25 °C in 0.1 mM EDTA, 0.5 mM ITP, 80 mM BTP (BIS TRIS propane)-HEPES pH 7.0, in the presence or absence of 150 μg ml−1 trypsin. The reaction was stopped by addition of a 100-fold excess of soybean trypsin inhibitor. Proteins were precipitated by centrifugation at 20 000 g for 1 h at 4 °C. Pellets were resuspended in 25 mM MOPS-KOH pH 7.0, 10% (w/w) glycerol, 5 μg ml−1 leupeptin, 10 mM benzamidine, 1 μg ml−1 chymostatin, 1 μg ml−1 pepstatin. Quantitative and reproducible recovery of proteins was tested using the Bio-Rad assay and western blot signal quantification.
Assays of ACA8 activity
ACA8 activity in yeast microsomes (∼2–4 μg of protein per sample) was measured as eosin-sensitive MgITP hydrolysis, taking advantage of the high sensitivity of plant PM Ca2+-ATPase to this inhibitor (De Michelis et al., 1993; Bonza et al., 2004; Fusca et al., 2009). The assay medium contained 80 mM BTP-HEPES pH 7.0, 5 mM (NH4)2SO4, 50 mM KNO3, 1 μM A23187, 0.1 mg ml−1 Brij58, 1 μg ml−1 oligomycin, 2 mM phosphoenolpyruvate, 10 U ml−1 pyruvate kinase, and MgSO4 and ITP at a final concentration of 3 mM and 1 mM respectively. The free Ca2+ concentration was buffered at 10 μM with 1 mM EGTA. Unless otherwise specified, bovine testes CaM (Sigma, St. Louis, MO, USA, catalogue no. P1431) was supplied at 1 μM. Eosin-sensitive ITPase activity was evaluated as the difference between activity measured in the absence of inhibitor and that measured in the presence of 0.2 μM eosin Y in the assay medium. Samples were incubated at 25 °C for 60 min, during which the reaction proceeds linearly. All the assays were performed at least three times, with three replicates.
Expression and purification of the WT and mutated His-tagged ACA8 N-terminus
Vectors coding for WT and mutated His-tagged ACA8 N-terminus (6His-1M-I116) were used to transform E. coli strain BL21(DE3)pLysE (Merck KGaA, Darmstadt, Germany, catalogue no. 69389-3) by standard procedures. Purification of fusion proteins was performed as described (Luoni et al., 2004).
Surface plasmon resonance
Surface plasmon resonance spectroscopy analysis was performed with a BIAcoreX optical biosensor instrument (Biacore AB, Uppsala, Sweden) as described (Bækgaard et al., 2006; Luoni et al., 2006), but using a free Ca2+ concentration of 3.5 μM in the eluent buffer. The His-tagged ACA8 N-termini were injected into the measure flow cell of an NTA sensor chip (Biacore, AB, catalogue no. BR-1004-07) until a resonance response of 300–850 units was obtained. After changing the immobilization buffer with eluent buffer, bovine testes CaM, 50–350 nM in eluent buffer, was injected over the two flow cells. After the dissociation phase, the NTA chip was completely regenerated by injection of regeneration buffer. Results are presented as a reference cell-subtracting sensorgram, a plot of resonance signal changes as a function of time. The data were analysed using BIA evaluation 3.0 software (Biacore AB), and kinetics analyses of primary sensorgrams were carried out by global fitting using a 1:1 Langmuir binding model.
Expression and purification of CPK1 and CPK16
Vectors coding for CPK1 and CPK16 were used to transform E. coli strain DH5α by standard procedures. E. coli harbouring recombinant plasmids were grown in Luria–Bertani complete medium (GENESPIN, Milan, Italy, catalogue no. STS-LB1000) under ampicillin selection. Overnight cultures grown at 37 °C were diluted 10-fold and grown for 2 h at 28 °C (∼0.6 OD600) before 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) addition, and growth was continued for 2 h more. All the following steps were carried out at 4 °C. A 400 ml aliquot of culture was centrifuged for 15 min at 3000 g and the pellet was suspended in 20 ml of lysis buffer containing 20 mM TRIS-HCl pH 7.8, 500 mM NaCl, and 1 mM phenylmethylsulphonyl fluoride (PMSF). Cells were lysed by the addition of 1 mg ml−1 lysozyme, incubation on ice for 15 min, and addition of 0.4% Triton X-100 followed by sonication. Cellular debris and unlysed cells were removed by centrifugation at 12 000 g for 10 min. The supernatant was incubated for 30 min with ∼1 ml of nickel-NTA–agarose (Qiagen GmbH, Germany, catalogue no. 1018244) on a rocking platform. Resin was pelleted by centrifugation at 3000 g for 10 min, washed extensively with 20 mM TRIS-HCl pH 7.8 plus 500 mM NaCl, and eluted with 300 mM imidazole in 20 mM TRIS-HCl pH 6.0 plus 500 mM NaCl. The eluate was diluted 5-fold with GST binding buffer containing 50 mM TRIS-HCl pH 7.4, 150 mM NaCl, 10 mM EDTA, 1 mM dithiothreitol (DTT), and 0.4 % Triton X-100, and incubated for 30 min with 2 ml of glutathione–Sepharose 4B (GE Healthcare Bio-Science AB, Sweden, catalogue no. 20182003-2 ). Resin was pelleted by centrifugation at 3000 g for 10 min and washed extensively with binding buffer, followed by one wash with 50 mM TRIS-HCl pH 7.5. Protein was eluted with 10 mM glutathione in 50 mM TRIS-HCl pH 8.0 and concentrated by centrifugation in 30 000 Da cut-off VIVASPIN6 concentrators (SartoriusStedim Biotech GmbH, Germany, catalogue no. VS0621). Purified enzyme was stored at –80 °C in 50% glycerol, 20 mM TRIS-HCl pH 7.5, 100 mM NaCl, 1 mM DTT. Typically, a purification starting from 400 ml of culture yielded ∼0.5–1.5 mg of pure protein, capable of phosphorylating the synthetic substrate Syntide 2 (data not shown).
Kinase assay
The kinase assay was performed in 20 mM TRIS-HCl pH 7.5, 6 mM MgC12, 0.5 mg ml−1 bovine serum albumin (BSA), 1 mg ml−1 phosphatidylcholine suspended in buffer by sonication, and 2.8 mM ATP labelled with 0.95 kBq nmol−1 [γ-32P]ATP (PerkinHelmer ITALIA S.p.A., catalogue no. NEG502A250UC), using 2.5 μg of purified enzyme in a 25 μl reaction. Assays were initiated by the addition of 20 μM substrate and transferred from ice to 22 °C controlled temperature for 3 h. Reactions were terminated by solubilization in Laemmli buffer (Laemmli, 1970). For autoradiography, aliquots corresponding to 2–3 μg of purified substrate protein were loaded on to 18% polyacrylamide gel and subjected to SDS–PAGE and blotting as described in Luoni et al. (2004). Blots were exposed to Kodak Biomax MS film at 80 °C for 2–4 d. For quantification of phosphate incorporation, 5 μl of each solubilized sample were spotted on a 0.2 μm nitrocellulose filter paper square (GE Healthcare Bio-Science AB, catalogue no. RPN3032D). Filters were immersed in 75 mM phosphoric acid, washed three times (7 min) with the same solution, allowed to dry, and dissolved in 10 ml of Filter Count (Packard, Meriden, CT, USA, catalogue no. 6013149). Radioactivity was measured by liquid scintillation counting (Tri-carb LSC 1500, Packard). Radioactivity associated with samples incubated in the absence of substrate protein was subtracted from the reported data.
Results
Each of the serine residues of ACA8 which have been found to be phosphorylated in vivo (Nühse et al., 2003, 2004, 2007; Benschop et al., 2007; Niittylä et al., 2007; Sugiyama et al., 2008; Whiteman et al., 2008; Jones et al., 2009; Reiland et al., 2009; Chen et al., 2010; Nakagami et al., 2010) has been mutated to aspartate whose negative charge mimics phosphorylation, or to alanine to make it non-phosphorylatable. Mutant proteins have been expressed in S. cerevisiae strain K616, which is devoid of endogenous Ca2+-ATPases (Cunningham and Fink, 1994), and characterized in the isolated microsomal fraction.
Western blot of the microsomal proteins with an antiserum against a sequence in the small cytoplasmic loop of ACA8 (Luoni et al., 2004) shows that all the proteins were substantially intact (Fig. 1, top panel) and functional (Fig. 1, line a). The expression level of the mutants was evaluated by quantification of signal intensity in western blot of microsomes isolated from at least two yeast inductions (Fig. 1, line b). Expression of most mutants was similar to that of WT ACA8: only the expression level of mutants S57D and S99D was significantly different (60% and 140%, respectively, P <0.05) from that of WT ACA8. Molecular activities (Fig. 1, line c) were computed from the ratio between activity in the presence of CaM (Fig. 1, line a) and signal intensity in western blot (Fig. 1, line b). Molecular activities of the mutants were not significantly different from that of WT ACA8 (values ranged between 50±10% and 163±31% of the WT), indicating that the introduced mutations had no major effect on ACA8 activity.
Fig. 1.
Expression of single point S/D or S/A ACA8 mutants in yeast strain K616. Top: after SDS–PAGE and blotting, yeast microsomal proteins (4 μg per lane) were immunodecorated with an antiserum against the ACA8 small cytoplasmic loop; the blot shown in the figure is one representative of three or more. Bottom: aACA8 activity (nmol Pi min−1 mg−1 protein) was measured in the presence of 1 μM CaM. Results are the mean of 3–10 experiments performed on at least two different microsomal membrane preparations, ±SEM. bQuantification of ACA8 protein was performed by densitometric scanning analysis of western blots immunodecorated with an antiserum against the ACA8 small cytoplasmic loop, setting the WT value at 1 arbitrary unit; values reported are the mean of at least three quantification analyses performed on at least two different microsomal membrane preparations, ±SEM; variability of WT expression was evaluated by loading six independent microsomal preparations on the same gel. cMolecular activities, evaluated as the ratio between ACA8 activity in the presence of CaM and the ACA8 protein level, are expressed as a percentage of that of WT ACA8 ±SEM.
Effect of S/D mutations on ACA8 autoinhibition
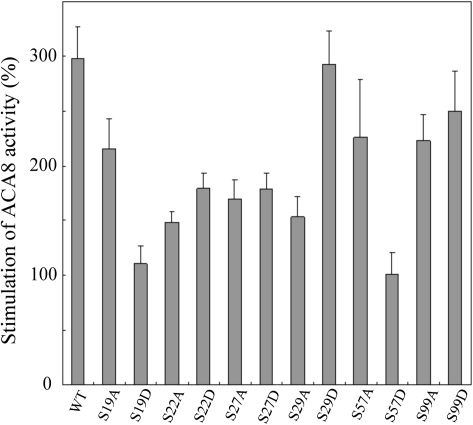
To test the degree of autoinhibition of ACA8 mutants, the effects of CaM on pump activity were evaluated. Figure 2 shows that under the applied experimental conditions, CaM stimulated the activity of WT ACA8 by ∼300%. The response to CaM was drastically reduced (P < 0.01) in two of the mutants, S19D and S57D, which were stimulated by ∼100%; S/A mutation of the same residues only marginally affected CaM stimulation. Mutations of Ser22 and Ser27 generated proteins somewhat less stimulated by CaM (150–180%, P < 0.05) than the WT, but in these cases the effect was independent of the substitution made. S/D mutation of residues Ser29 and S99 did not affect ACA8 response to CaM; strangely, the S29A mutant was less stimulated by CaM than the WT and the S29D mutant. This result could suggest that ACA8 was phosphorylated at Ser29 in vivo by some yeast kinase. However, mass spectrometric analysis of WT ACA8 purified from yeast microsomes by CaM affinity chromatography (Fusca et al., 2009; Bonza and Luoni, 2010) showed that the protein had not been phosphorylated in vivo under the applied yeast growth conditions, with the possible exception of Ser19, which was not identified in any tryptic peptide (data not shown). Thus, the low response to CaM of ACA8 mutants S22A, S27A, and S29A points to the relevance of these serine residues per se in determining the amplitude of the response of ACA8 to CaM.
Fig. 2.
Stimulation of WT and mutant ACA8 by CaM. ACA8 activity was measured in the presence or absence of 1 μM CaM. Results are shown as percentage stimulation over the activity measured in the absence of added CaM. Values reported are the mean of 3–10 experiments ±SEM.
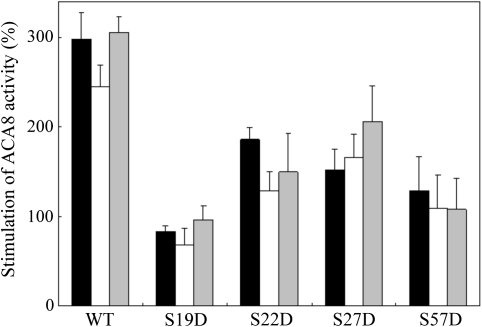
Altogether, the results reported above suggest that the introduction of a negative charge at Ser19 or at Ser57 of ACA8—and, to a lesser extent, also at Ser22 or at Ser27—hampers the autoinhibitory action of the N-terminal domain, generating partially deregulated mutants. Alternative explanations of the low degree of CaM activation in these mutants would be a dramatic loss of affinity for CaM or the inability to shift to the active conformation upon CaM binding. The finding that the molecular activities in the presence of CaM of the S19D, S22D, S27D, and S57D mutants are similar to that of WT ACA8 (see Fig. 1) makes the latter explanation unlikely. However, if this was the case, these mutants should be as responsive as the WT to tryptic cleavage of the N-terminus (Rasi-Caldogno et al., 1993; Luoni et al., 2004; Fusca et al., 2009; Bonza and De Michelis, 2011). Figure 3 shows that, in agreement with previously reported results (Luoni et al., 2004; Fusca et al., 2009), tryptic cleavage of the N-terminus stimulated the activity of WT ACA8 similarly to CaM and that the two effects were not additive. The same was true for all of the tested mutants, which were equally less stimulated than the WT by CaM and by tryptic cleavage of the N-terminus. Saccharomyces cerevisiae strain K616 is unable to grow in Ca2+-deprived media (Cunningham et al., 1994; Bonza et al., 2004; Bækgaards et al., 2006; Fusca et al., 2009). None of the produced mutants was able to complement the phenotype of the K616 yeast strain (data not shown). This result confirms previous observations (Bonza et al., 2004; Bækgaards et al., 2006; Fusca et al., 2009) that only largely deregulated ACA8 mutants allow growth of K616 in the presence of very low Ca2+ concentrations.
Fig. 3.
Effect of controlled proteolysis on the activity of WT and mutant ACA8. The microsomal fraction purified from yeast expressing WT or mutant ACA8 was treated with (white and grey bars) or without (black bars) trypsin as detailed in the Materials and methods; ACA8 activity was measured in the presence (black and grey bars) or absence (white bars) of 1 μM CaM. Results are shown as percentage stimulation over the activity measured in control membranes in the absence of added CaM. Values reported are the mean of 3–5 experiments ±SEM.
Effect of S/D mutations on ACA8 affinity for CaM
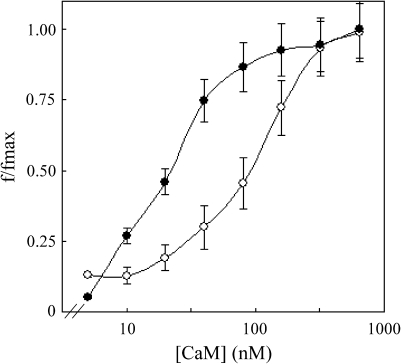
Preliminary experiments were performed by measuring the effect of increasing concentrations of CaM on the activity of WT and mutant ACA8. Figure 4 shows the results of such an experiment conducted on the S57D ACA8 mutant. The S57D activation curve was shifted to higher CaM concentrations than that of WT ACA8, indicating a lower apparent affinity for CaM of the mutant protein. The activation curves of all the other mutants were roughly similar to that of WT ACA8 (data not shown).
Fig. 4.
Stimulation of WT and S57D ACA8 as a function of CaM concentration. Eosin-sensitive ITPase activity of microsomal fractions (2–4 μg of total proteins) from yeast expressing WT (filled circles) and S57D (open circles) ACA8 mutant was measured in the presence of increasing concentrations of exogenous CaM. Activation of WT and S57D ACA8 (f/fmax) is expressed as the ratio between stimulation by CaM at the indicated CaM concentration (f) and maximal stimulation (fmax). Fmax values were 417±23% for WT ACA8 and 93±1% for the S57D mutant. Values reported are the mean of three experiments ±SEM.
It has previously been shown that mutations which weaken the autoinhibitory interaction of the N-terminus with the catalytic head of the pump can diminish steric hindrance to CaM binding, distorting evaluation of the effect of mutation on ACA8 affinity for CaM as measured by concentration dependence of activation (Fusca et al., 2009). Thus, to determine the effect of the S/D mutations on ACA8 affinity for CaM, the first 116 amino acids of WT or mutant ACA8 fused to a 6His-tag were expressed in E. coli, purified, and used for CaM binding measurements by surface plasmon resonance (Bækgaard et al., 2006; Luoni et al., 2006), a technique which allows one to measure not only the affinity of the partners but also the kinetics of interaction.
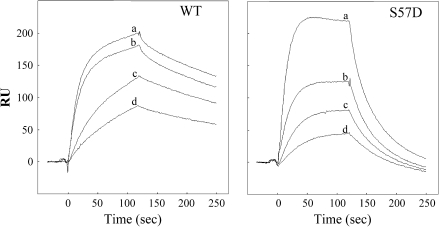
Figure 5 shows the results of a representative experiment performed on the N-terminus of WT and S57D ACA8: the S57D mutation had no major effect on the rate of complex formation, but drastically increased the rate of complex dissociation, resulting in an increase of the dissociation constant (KD) from 19 nM to 165 nM (Table 2). All of the other analysed mutations had less dramatic effects on the interaction of the ACA8 N-terminus with CaM (Table 2). The association rate constant (ka) values of the mutants ranged between half (S22D) and about twice (S19D) that of the N-terminus of WT ACA8. Values of the dissociation rate constant (kd) of the mutants ranged between about half (S22D and S99D) and about twice (S27D) that of the N-terminus of WT ACA8. For most mutants, the changes of the kinetic parameters brought about only minor changes of the kd values (e.g. in mutant S22D, the decrease in ka was largely compensated by the decrease in kd): only in mutant S99D did the decreased rate of dissociation determine a decrease of the KD value to about half that of the WT.
Fig. 5.
Kinetics of CaM binding to the N-terminus of WT and S57D ACA8. Phases of the interaction between CaM and peptides 6His-1M-I116 derived from WT and S57D ACA8 were registered by surface plasmon resonance spectroscopy, using different concentrations of bovine testes CaM (a=350 nM; b=200 nM; c=100 nM; d=50 nM) as the flowing analyte. The reported signal (RU) is the difference in resonance units between the signal recorded in the measuring cell, with immobilized peptide, and the signal recorded in the peptide-free reference cell.
Table 2.
Kinetics of binding of bovine testes CaM to the N-terminus of WT and mutant ACA8 measured by surface plasmon resonance Each value with the corresponding SEM is the average of constants derived from the analysis of at least five binding curves.
| ka (×105 M−1s−1) | kd (×10−3 s−1) | KD (nM) | |
| WT | 1.27±0.02 | 2.44±0.01 | 19.3±0.3 |
| S19D | 2.24±0.06 | 3.25±0.02 | 14.5±0.4 |
| S22D | 0.58±0.02 | 1.39±0.01 | 23.9±0.6 |
| S27D | 1.96±0.03 | 5.20±0.03 | 26.5±0.4 |
| S29D | 1.71±0.02 | 3.82±0.02 | 22.3±0.2 |
| S57D | 1.54±0.01 | 25.5±0.22 | 165±1.8 |
| S99D | 1.21±0.04 | 1.37±0.01 | 11.3±0.4 |
Phosphorylation of the ACA8 N-terminus by CDPK
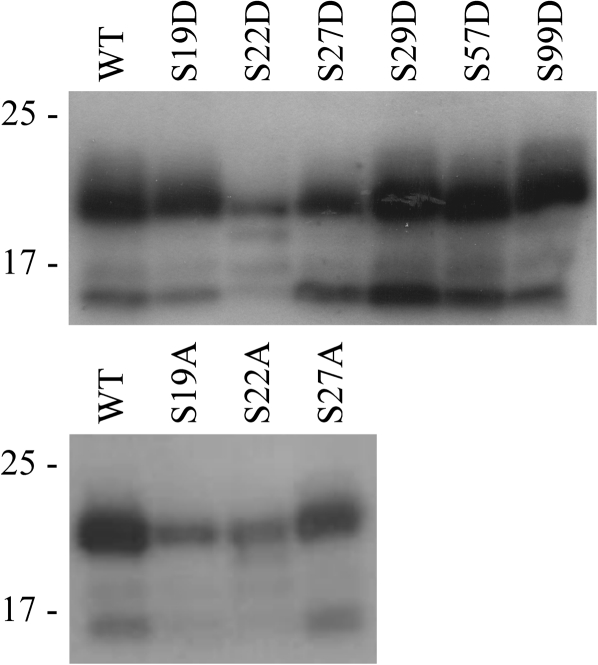
Evidence has been presented that CPK1, an A. thaliana isoform of CDPK, phosphorylates a serine residue in the N-terminus of ACA2, an isoform of A. thaliana CaM-regulated Ca2+-ATPase localized at the endoplasmic reticulum (Hwang et al., 2000). It was checked whether CPK1 was also able to phosphorylate the N-terminus of ACA8; since CPK1 is localized at peroxisomes, CPK16, a PM-localized A. thaliana isoform, was also tested (Dammann et al., 2003). Ca2+-independent mutants (Harper et al., 1994; Vitart et al., 2000) of CPK1 and CPK16 were expressed in E. coli sandwiched between N-terminal GST and a C-terminal 6His-tag and purified by two-step affinity chromatography (Harper et al., 1994). Figure 6 shows that both kinases were able to phosphorylate the His-tagged ACA8 N-terminus: upon phosphorylation with CPK16 under the applied conditions, phosphate incorporation was 0.60±0.08 nmol nmol−1 of ACA8 N-terminus. To determine which residue(s) of the ACA8 N-terminus are phosphorylated by CPK16, phosphorylation assays were performed on the His-tagged N-termini of ACA8 S/D mutants (Fig. 7, top panel). While mutants S29D, S57D, and S99D were phosphorylated similarly to WT ACA8, the phosphorylation level was drastically reduced in mutant S22D and lower than that of the WT also in mutants S19D and S27D. The fact that none of the mutations abolished ACA8 phosphorylation by CPK16 indicates that the enzyme is able to phosphorylate the ACA8 N-terminus at more than one residue. Since the introduction of the negative charge of the aspartate residue may affect phosphorylation of the neighbouring serine residues, phosphorylation assays were performed on the His-tagged N-termini of ACA8 S/A mutants. The bottom panel of Fig. 7 shows that phosphorylation of ACA8 by CPK16 was strongly reduced by mutations S19A and S22A, while mutant S27A was phosphorylated similarly to the WT.
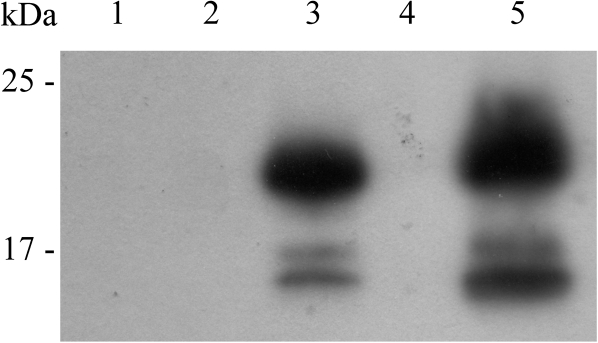
Fig. 6.
Phosphorylation of the ACA8 N-terminus by different isoforms of CDPK. The N-terminus of WT ACA8 (6His-1M-I116) was phosphorylated with Ca2+-independent mutants of A. thaliana CPK1 or CPK16 as described in the Materials and methods. Samples were solubilized and aliquots corresponding to 3 μg of 6His-1M-I116 were subjected to SDS–PAGE, blotting, and autoradiography (lane 1, 6His-1M-I116; lane 2, CPK1; lane 3, 6His-1M-I116 plus CPK1; lane 4, CPK16; lane 5, 6His-1M-I116 plus CPK16). Results are from one experiment representative of four.
Fig. 7.
Phosphorylation of WT and mutant ACA8 N-termini by CPK16. WT or mutant ACA8 N-termini were phosphorylated with a Ca2+-independent mutant of A. thaliana CPK16 as described in the Materials and methods. Samples were solubilized and aliquots corresponding to 3 μg (top panel) or 2 μg (bottom panel) of 6His-1M-I116 were subjected to SDS–PAGE, blotting, and autoradiography. Results are from one experiment representative of three.
Discussion
The finding that ACA8, a widely expressed isoform of PM Ca2+-ATPase, is phosphorylated in vivo at serine residues localized within or close to the autoinhibitory and CaM-binding domain, and that phosphorylation of at least some of these residues is responsive to nutritional, hormonal, and pathogenic signals (Nühse et al., 2003, 2004, 2007; Benschop et al., 2007; Niittylä et al., 2007; Sugiyama et al., 2008; Whiteman et al., 2008; Jones et al., 2009; Reiland et al., 2009; Chen et al., 2010; Nakagami et al., 2010) opens up a new avenue for fine-tuning of its activity. To investigate the possible effect of phosphorylation on ACA8 activity, each of these residues was mutated to aspartate, which introduces a negative charge mimicking the effect of phosphorylation. The results obtained by biochemical analysis of these mutants indicate that phosphorylation of serine residues can affect ACA8 activity both by hampering the autoinhibitory action of the N-terminus and by changing the kinetics of activation by CaM and de-activation by CaM release, and, thus, at least in some instances, ACA8 affinity for CaM.
Mutation to aspartate of several phosphorylatable serine residues in the N-terminus of ACA8 generates partially deregulated proteins, with higher basal activity, less responsive to activation by CaM, and less stimulated by tryptic cleavage of the N-terminus. The effect is strongest in mutants S19D and S57D, but significant (P < 0.05) also in mutants S22D and S27D; only ACA8 mutants S29D and S99D are autoinhibited similarly to the WT protein. The ACA8 N-terminal autoinhibitory domain, which is enriched in basic residues (Bækgaard et al., 2006), interacts with a sequence in the small cytoplasmic loop rich in acidic residues (Luoni et al., 2004). Alanine scanning mutagenesis of these acidic residues generates partially deregulated ACA8 mutants, indicating that the electrostatic interaction between the positively charged N-terminal autoinhibitory domain and the negatively charged domain in the small cytoplasmic loop plays a role in ACA8 autoinhibition (Fusca et al., 2009). Thus, the negative charge introduced by the S/D mutations—or by serine phosphorylation—in the N-terminus would hamper its autoinhibitory interaction with the small cytosolic loop. However, in the case of Ser22 and Ser27, the phenotype of the S/A ACA8 mutants is similar to that of the S/D mutants, suggesting that the -OH group of serine is also important per se in the autoinhibitory mechanism.
All the analysed mutations affect the kinetics of interaction with CaM to some extent. The strongest phenotype is that of mutant S57D, which has a kd value ∼10-fold higher than that of WT ACA8, but 2-fold changes in one or both of the kinetic constants of the interaction are also evident in mutants S19D, S22D, S27D, and S99D. Despite the different kinetics, the affinity for CaM of most mutants is fairly similar to that of WT ACA8 (KD values ranging between 15 nM and 26 nM for the mutants, versus 19 nM for the WT), with two exceptions: mutant S99D which has a KD value of 11 nM and mutant S57D which has a KD value of 165 nM, nearly 10-fold higher than that of WT ACA8, largely due to its higher kd value. The strongest phenotype of mutant S57D can be easily explained by the localization of this serine residue within the sequence defining the ACA8 CaM-binding site (Bonza et al., 2000; Bækgaard et al., 2006); interestingly, mutation of ACA8 Ser57 to alanine determines a decrease in the kd, resulting in a KD value about half that of the WT (Bækgaard et al., 2006). Thus residue Ser57 in ACA8 CaM-binding domain plays a crucial role in determining the stability of its interaction with CaM.
Based on these results, a major effect of phosphorylation of serine residues in the ACA8 N-terminus would be to modify the rate of pump activation following an increase of cytoplasmic Ca2+ concentration which increases the concentration of the active Ca2+–CaM complex or of its de-activation when the return of the cytosolic free Ca2+ concentration toward basal levels drastically lowers the concentration of Ca2+–CaM. The rate of activation would be halved upon phosphorylation of Ser22 and nearly doubled upon phosphorylation of Ser19 or Ser27; the rate of de-activation would by halved in the case of phosphorylation of Ser99 and would increase from 2- to 10-fold following phosphorylation of Ser27 or of Ser57 (see Table 2).
Phosphorylation of some of these serine residues affects both the kinetics of CaM activation and de-activation and the autoinhibitory action of the ACA8 N-terminus. The two effects may exert a similar or contrasting effect on Ca2+ extrusion, depending on the phosphorylated residue and on the values of the relevant cytoplasmic parameters. For example, phosphorylation of Ser19 would favour ACA8 activation following an increase of cytosolic Ca2+, both by inhibiting the autoinhibitory action of the N-terminus and by accelerating binding of the Ca2+–CaM complex; activation would probably last longer, despite the slightly increased rate of CaM release, unless de-phosphorylation intervenes. Similarly, in the case of Ser57, the destabilizing effect of phosphorylation on ACA8 interaction with CaM might be largely counteracted by the inhibition of the autoinhibitory action of the N-terminus. Upon phosphorylation of Ser22, the rate and extent of ACA8 activation following an increase of cytosolic Ca2+ would be the result of its opposite effects on the autoinhibitory action of the N-terminus and on the rate of CaM binding; conversely, the moderate decrease of autoinhibition and the decrease of the CaM dissociation rate would both contribute to keep the pump active longer.
Altogether, the subtle effects of phosphorylation of one or more serine residues in the N-terminus on ACA8 activity may have important consequences on the spatio-temporal characteristics of cytoplasmic Ca2+ waves, and thus participate in deciphering the Ca2+ signal. This makes identification of protein kinase(s) and phosphatase(s) controlling the phosphorylation state of ACA8 in response to different signals an important goal for future research. Here it has been shown that two isoforms of A. thaliana CDPK—CPK1 and CPK16—are able to phosphorylate the ACA8 N-terminus in vitro. The effect of single point mutations on phosphorylation indicates that CPK16, the more efficient of the two isoforms tested, phosphorylates the ACA8 N-terminus at two different serine residues: Ser19—which is part of a consensus motif recognized by CDPKs (Cheng et al., 2002)—and Ser22. Further work is needed to determine which isoform(s) of CDPK phosphorylate ACA8 in vivo and under which conditions phosphorylation occurs.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Pairs of primers used for site-directed mutagenesis of ACA8.
Acknowledgments
This work was supported in part by the Italian Ministry for Instruction, University and Research in the PRIN 2007 framework. The authors are grateful to Professor J. F. Harper, University of Nevada, Reno, NV, USA for kindly providing plasmids encoding different isoforms of A. thaliana CDPK and for helpful suggestions regarding their expression, purification, and assay.
Glossary
Abbreviations
- BSA
bovine serum albumin
- Brij 58
polyoxyethylene 20 cethyl ether
- BTP
BIS TRIS propane {1-3-bis[tris(hydroxymethyl)methylamino]propane}
- CaM
calmodulin
- IPTG
isopropyl-β-D-thiogalactopyranoside
- NTA
nitrilotriacetic acid
- PM
plasma membrane
- PMSF
phenylmethylsulphonyl fluoride
- WT
wild type
References
- Bækgaards L, Luoni L, De Michelis MI, Palmgren MG. The plant plasma membrane Ca2+ pump ACA8 contains overlapping as well as physically separated autoinhibitory and calmodulin-binding domains. Journal of Biological Chemistry. 2006;281:1058–1065. doi: 10.1074/jbc.M508299200. [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Mohammed S, O’Flaherty M, Heck AJR, Slijper M, Menke FLH. Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Molecular and Cellular Proteomics. 2007;6:1198–1214. doi: 10.1074/mcp.M600429-MCP200. [DOI] [PubMed] [Google Scholar]
- Bonza MC, De Michelis MI. The plant Ca2+-ATPases repertoire: biochemical features and physiological functions. Plant Biology. 2011;13:421–430. doi: 10.1111/j.1438-8677.2010.00405.x. [DOI] [PubMed] [Google Scholar]
- Bonza MC, Luoni L. Plant and animal type 2B Ca2+-ATPases: evidence for a common auto-inhibitory mechanism. FEBS Letters. 2010;584:4783–4788. doi: 10.1016/j.febslet.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Bonza MC, Luoni L, De Michelis MI. Functional expression in yeast of an N-deleted form of At-ACA8, a plasma membrane Ca2+-ATPase of Arabidopsis thaliana, and characterization of a hyperactive mutant. Planta. 2004;218:814–823. doi: 10.1007/s00425-003-1160-y. [DOI] [PubMed] [Google Scholar]
- Bonza MC, Morandini P, Luoni L, Geisler M, Palmgren MG, De Michelis MI. At-ACA8 encodes a plasma membrane-localized calcium-ATPase of Arabidopsis with a calmodulin-binding domain at the N terminus. Plant Physiology. 2000;123:1495–1505. doi: 10.1104/pp.123.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Harper JF. The origin and function of calmodulin regulated Ca2+ pumps in plants. Journal of Bioenergetic and Biomembranes. 2007;39:409–414. doi: 10.1007/s10863-007-9104-z. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hoehenwarter W, Weckwerth W. Comparative analysis of phytohormone-responsive phosphoproteins in Arabidopsis thaliana using TiO2-phosphopeptide enrichment and mass accuracy precursor alignment. The Plant Journal. 2010;63:1–17. doi: 10.1111/j.1365-313X.2010.04218.x. [DOI] [PubMed] [Google Scholar]
- Cheng S-H, Willmann MR, Chen H-C, Sheen J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiology. 2002;129:469–485. doi: 10.1104/pp.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+-ATPases. Journal of Cell Biology. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann C, Ichida A, Hong B, Romanowsky SM, Hrabak EM, Harmon AC, Pickard BG, Harper JF. Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiology. 2003;132:1840–1848. doi: 10.1104/pp.103.020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Pandey GK. Expressional analysis and role of calcium regulated kinases in abiotic stress signalling. Current Genomics. 2010;11:2–13. doi: 10.2174/138920210790217981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michelis MI, Carnelli A, Rasi-Caldogno F. The Ca2+ pump of the plasma membrane of Arabidopsis thaliana—characteristics and sensitivity to fluorescein derivatives. Botanica Acta. 1993;106:20–25. [Google Scholar]
- Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annual Review of Plant Biology. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- Enyedi A, Elwess NL, Filoteo AG, Verma AK, Paszty K, Penniston JT. Protein kinase C phosphorylates the ‘a’ forms of plasma membrane Ca2+ pump isoform 2 and 3 and prevents binding of calmodulin. Journal of Biological Chemistry. 1997;272:27525–27528. doi: 10.1074/jbc.272.44.27525. [DOI] [PubMed] [Google Scholar]
- Enyedi A, Verma AK, Filoteo AG, Penniston JT. Protein kinase C activates the plasma membrane Ca2+ pump isoform 4b by phosphorylation of an inhibitory region downstream of the calmodulin-binding domain. Journal of Biological Chemistry. 1996;50:32461–32467. doi: 10.1074/jbc.271.50.32461. [DOI] [PubMed] [Google Scholar]
- Fusca T, Bonza MC, Luoni L, Meneghelli S, Marrano CA, De Michelis MI. Single point mutations in the small cytoplasmic loop of ACA8, a plasma membrane Ca2+-ATPase of Arabidopsis thaliana, generate partially deregulated pumps. Journal of Biological Chemistry. 2009;284:30881–30888. doi: 10.1074/jbc.M109.006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Breton G, Harmon A. Decoding Ca2+ signals through plant protein kinases. Annual Review of Plant Biology. 2004;55:263–288. doi: 10.1146/annurev.arplant.55.031903.141627. [DOI] [PubMed] [Google Scholar]
- Harper JF, Huang J-F, Lloyd SJ. Genetic identification of an autoinhibitor in CDPK, a protein kinase with a calmodulin-like domain. Biochemistry. 1994;33:7267–7277. doi: 10.1021/bi00189a031. [DOI] [PubMed] [Google Scholar]
- Hwang I, Sze H, Harper JF. A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2000;97:6224–6229. doi: 10.1073/pnas.97.11.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AME, MacLean D, Studholme DJ, Sanz A, Andreasson E, Rathjen JP, Peck SC. Phosphoproteomic analysis of nuclei-enriched fractions from Arabidopsis thaliana. Journal of Proteomics. 2009;72:439–451. doi: 10.1016/j.jprot.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luoni L, Bonza MC, De Michelis MI. Calmodulin/Ca2+-ATPase interaction at the Arabidopsis thaliana plasma membrane is dependent on calmodulin isoform showing isoform-specific Ca2+ dependencies. Physiologia Plantarum. 2006;126:175–186. [Google Scholar]
- Luoni L, Meneghelli S, Bonza MC, De Michelis MI. Auto-inhibition of Arabidopsis thaliana plasma membrane Ca2+-ATPase involves an interaction of the N-terminus with the small cytoplasmic loop. FEBS Letters. 2004;574:20–24. doi: 10.1016/j.febslet.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Malmström S, Åkerlund HE, Askerlund P. Regulatory role of the N terminus of the vacuolar calcium-ATPase in cauliflower. Plant Physiology. 2000;122:517–526. doi: 10.1104/pp.122.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Pittman JK. Shaping the calcium signature. New Phytologist. 2009;181:275–294. doi: 10.1111/j.1469-8137.2008.02682.x. [DOI] [PubMed] [Google Scholar]
- Meneghelli S, Fusca T, Luoni L, De Michelis MI. Dual mechanism of activation of plant plasma membrane Ca2+-ATPase by acidic phospholipids: evidence for a phospholipid binding site which overlaps the calmodulin-binding site. Molecular Membrane Biology. 2008;25:539–546. doi: 10.1080/09687680802508747. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Sugiyama N, Mochida K, Daudi A, Yoshida Y, Toyoda T, Tomita M, Ishihama Y, Shirasu K. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiology. 2010;153:1161–1174. doi: 10.1104/pp.110.157347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittylä T, Fuglsang AT, Palmgren MG, Frommer WB, Schulze WX. Temporal analysis of sucrose-induced phosphorylation changes in plasma membrane proteins of Arabidopsis. Molecular and Cellular Proteomics. 2007;6:1711–1726. doi: 10.1074/mcp.M700164-MCP200. [DOI] [PubMed] [Google Scholar]
- Nühse TS, Bottrill A, Jones AME, Peck SC. Quantitative phosphoproteomics analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. The Plant Journal. 2007;51:931–940. doi: 10.1111/j.1365-313X.2007.03192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Stensballe A, Jensen ON, Peck SC. Phosphoproteomics of the Arabidopsis plasma membrane and a new phosphorylation site database. The Plant Cell. 2004;16:2394–2405. doi: 10.1105/tpc.104.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Stensballe A, Jensen ON, Peck SC. Large-scale analysis of in vivo phosphorylated membrane proteins by immobilized metal ion affinity chromatography and mass spectrometry. Molecular and Cellular Proteomics. 2003;2:1234–1243. doi: 10.1074/mcp.T300006-MCP200. [DOI] [PubMed] [Google Scholar]
- Penniston JT, Enyedi A. Modulation of the plasma membrane Ca2+ pump. Journal of Membrane Biology. 1998;165:101–109. doi: 10.1007/s002329900424. [DOI] [PubMed] [Google Scholar]
- Pittman JK, Bonza MC, De Michelis MI. Ca2+ pumps and Ca2+ antiporters in plant development. In: Geisler M, Venema K, editors. Transporters and pumps in plant signalling. Berlin: Springer-Verlag; 2011. pp. 133–161. [Google Scholar]
- Rasi-Caldogno F, Carnelli A, De Michelis MI. Controlled proteolysis activates the plasma membrane Ca2+ pump of higher plants. Plant Physiology. 1993;103:385–390. doi: 10.1104/pp.103.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiland S, Messerli G, Baerenfaller K, Gerrits B, Endler A, Grossman J, Gruissem W, Baginsky S. Large scale Arabidopsis phosphoproteomic profiling reveals novel choloroplast kinase substrates and phosphorylation networks. Plant Physiology. 2009;150:889–903. doi: 10.1104/pp.109.138677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. The Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama N, Nakagami H, Mochida K, Daudi A, Tomita M, Shirasu K, Ishihama Y. Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in Arabidopsis. Molecular Systems Biology. 2008 doi: 10.1038/msb.2008.32. 4, article 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma AK, Paszty K, Filoteo AG, Penniston JT, Enyedi A. Protein kinase C phosphorylates plasma membrane Ca2+ pump isoform 4a at its calmodulin binding domain. Journal of Biological Chemistry. 1999;1:527–531. doi: 10.1074/jbc.274.1.527. [DOI] [PubMed] [Google Scholar]
- Vitart V, Christodoulou J, Huang J-F, Chazin WJ, Harper JF. Intramolecular activation of a Ca2+-dependent protein kinase is disrupted by insertions in the tether that connects the calmodulin-like domain to the kinase. Biochemistry. 2000;39:4004–4011. doi: 10.1021/bi992373m. [DOI] [PubMed] [Google Scholar]
- Whiteman S, Serazetdinova L, Jones AME, Sanders D, Rathjen J, Peck SC, Maathuis FJM. Identification of novel proteins and phosphorylation sites in a tonoplast enriched membrane fraction of Arabidopsis thaliana. Proteomics. 2008;8:3536–3547. doi: 10.1002/pmic.200701104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.