Abstract
Long-term effects of light quality on leaf hydraulic conductance (Kleaf) and stomatal conductance (gs) were studied in cucumber, and their joint impact on leaf photosynthesis in response to osmotic-induced water stress was assessed. Plants were grown under low intensity monochromatic red (R, 640 nm), blue (B, 420 nm) or combined red and blue (R:B, 70:30) light. Kleaf and gs were much lower in leaves that developed without blue light. Differences in gs were caused by differences in stomatal aperture and stomatal density, of which the latter was largely due to differences in epidermal cell size and hardly due to stomatal development. Net photosynthesis (AN) was lowest in R-, intermediate in B-, and highest in RB- grown leaves. The low AN in R-grown leaves correlated with a low leaf internal CO2 concentration and reduced PSII operating efficiency. In response to osmotic stress, all leaves showed similar degrees of stomatal closure, but the reduction in AN was larger in R- than in B- and RB-grown leaves. This was probably due to damage of the photosynthetic apparatus, which only occurred in R-grown leaves. The present study shows the co-ordination of Kleaf and gs across different light qualities, while the presence of blue in the light spectrum seems to drive both Kleaf and gs towards high, sun-type leaf values, as was previously reported for maximal photosynthetic capacity and leaf morphology. The present results suggest the involvement of blue light receptors in the usually harmonized development of leaf characteristics related to water relations and photosynthesis under different light environments.
Keywords: Amphistomatous, Cucumis sativus, leaf development, leaf hydraulic conductance, light quality, osmotic stress, photosynthesis, stomatal conductance, stomatal density, stomatal opening
Introduction
Leaves are a major bottleneck in the plant water transport capacity (Sack and Holbrook, 2006). At least 25% of the whole-plant resistance can be attributed to the leaf hydraulic resistance (i.e. the reciprocal of leaf conductance; 1/Kleaf), which is disproportional to the relatively short water-transport distance in the leaves (Sack et al., 2003). Efficient water delivery (i.e. high Kleaf) maintains leaves well-hydrated. Decreases in Kleaf usually cause leaves to become less hydrated, which is often associated with a reduction in stomatal conductance (gs) and, consequently, in carbon gain (Sperry, 2000; Sack and Holbrook, 2006; Johnson et al., 2009). Such a stomatal response is necessary for leaves to avoid damaging water potentials and runaway cavitation events (Sperry et al., 2002). It is thought that, in the long-term, the economics of gas exchange will set limits on the hydraulic capacity of leaves. Since the synthesis of xylem tissue is costly, the construction costs for the plant would be such that do not exceed the benefits of photosynthetic performance (Brodribb et al., 2010). It can therefore be expected that there is co-ordination between the capacity for liquid water transport through the leaf, the capacity for gas transport between the leaf and the surrounding air (water and CO2), and the leaf photosynthetic capacity.
Several studies have shown that liquid phase conductance (i.e. Kleaf) and vapour phase conductance (i.e. gs) are strongly co-ordinated both in short- (Sperry et al., 1993; Brodribb and Jordan, 2008) and the long-term (Brodribb et al., 2005; Sack et al., 2005; Lo Gullo et al., 2010). An increase in water supply capacity, by a higher Kleaf, would only benefit photosynthesis in the presence of sufficiently high intercellular CO2. gs is determined by the cross-sectional area available for gas fluxes, i.e. the product of pore area per stoma and stomatal density (Parlange and Waggoner, 1970). Short-term tuning of gs takes place by adjustments in the stomatal pore aperture (stomatal opening), while long-term adaptations of gs are mostly due to changes in the stomatal density and size (determining maximum gs; Franks and Beerling, 2009).
Besides density and size of stomata, their distribution among the two leaf surfaces might also influence gas exchange (Foster and Smith, 1986). Amphistomatous leaves (i.e. stomata at both leaf sides) have both higher water vapour (due to higher boundary layer conductance; Parkhurst, 1978; Mott et al., 1982) and intercellular carbon dioxide (due to thicker mesophyll; Parkhurst and Mott, 1990) conductances. However, amphistomatous leaves can also behave as functionally hypostomatous, by closing or nearly closing the adaxial stomata (Reich, 1984).
Light intensity has been shown to affect the efficiency of water delivery and the photosynthetic performance of leaves. Traits such as high photosynthetic capacity, high Kleaf, and high gs were co-found in sunny environments compared with shaded habitats within and across species (reviewed in Sack and Holbrook, 2006). Recent studies show that light intensity can also influence xylem hydraulic conductances in the rest of the water transport path between roots and leaves (Nardini et al., 2010; Sellin et al., 2010). In natural environments, within plant canopies, differences in light intensity often coexist with differences in spectral quality, as, for example in the case of shade by neighbours (Smith, 1982; Combes et al., 2000). It has been shown in cucumber plants, which were grown under different combinations of red and blue light supplied by light-emitting diodes (LEDs), that light quality by itself can induce photosynthetic and morphological properties in leaves that normally occur at high light intensity, although the plants were grown under low light intensity (Hogewoning et al., 2010b). Considering the often observed coordination between Kleaf, gs, and photosynthetic capacity, one could expect that light quality is also involved in determining the hydraulic transport capacity in leaves, and that light quality might even play a key role in establishing this co-ordination. To the best of our knowledge, the long-term effects of light quality on Kleaf in relation to gs and leaf photosynthetic performance were not previously investigated. Also, no in-depth study has been published on the impact of light quality during leaf development on the ability of leaves to cope and recover from a shortage of water supply with respect to their photosynthetic performance. This information will provide new insights into the ability of plants to optimally tune their multifactorial function across light qualities.
The aims of this study were: (i) to assess if the capacities of the aqueous and gaseous leaf water transport phases and leaf photosynthesis change and remain co-ordinated when plants are grown under different combinations of red and blue light at low light intensity; (ii) to relate gs with underlying leaf stomatal traits as being influenced by light quality and the leaf amphistomatous character; and (iii) to investigate the plasticity of leaves with different, light quality-induced Kleaf/gs combinations, to short-term water stress.
Cucumber was used as a model plant, since it is an amphistomatous species, has large petiolated simple leaves (facilitating the Kleaf measurements), and has already shown differences in photosynthetic capacity and leaf morphology due to the spectral composition of light during growth under low light intensity.
Materials and methods
Plant material and growth conditions
Cucumber seeds (Cucumis sativus L. cv. Hoffmann giganta) were sown, germinated, and seedlings were grown up to the cotyledon stage (i.e. fully open cotyledons and before the appearance of the first leaf) under 100 μmol m−2 s−1 photosynthetic photon flux density (PPFD; determined using LI-190/LI-1400, Li-Cor, Lincoln, NE) and 16/8 h day/night cycle by white fluorescent tubes (Philips TLD 58W/84, Eindhoven, The Netherlands). This period was about one week. Subsequently, four seedlings were transplanted in each of the three growth-units (l×w×h=0.8×0.8×1.6 m; Homebox S, EastSide- Impex., Berlin, Germany) under the same PPFD-level but three different light spectral qualities, using red LEDs (R; Red, LXK2-PD12-S00, Philips), blue LEDs (B; Royal-Blue, LXK2-PR14-R00, Philips), and a combination of red and blue LEDs (RB; R:B, 70:30). Light spectra (Fig. 1) were determined with a calibrated spectroradiometer (USB2000 spectrometer, Ocean Optics, Duiven, The Netherlands). The growth-units had a constant day and night temperature (21±1 °C), and relative air humidity (75%) resulting in a vapour pressure deficit of 0.62±0.04 kPa. The plants were grown under ∼400 μmol mol−1 CO2 concentration. Leaf temperature did not differ significantly between plants grown in different light qualities [determined by using an infrared thermometer (Raynger™ ST, Raytec Corporation, Santa Cruz, CA)]. Plants were watered automatically by half-strength Hoagland solution (pH=5.4±0.2, EC=1 mS cm−1) circulating in a well-aerated root dipping hydroponic system. The solution was replenished weekly to sustain the pH and EC at constant levels. Three weeks after transplanting the measurements started on the first fully expanded leaf.
Fig. 1.
Light spectra of the red (dotted line), blue (dashed line), and red and blue (70:30; solid line) light environments measured in the growth-units at plant level.
Leaf gas exchange
The measurements of leaf gas exchange [stomatal conductance (gs), net photosynthetic rate (AN)], and operating efficiency of photosystem II (ΦPSII= ; Baker, 2008) were performed using two portable gas exchange systems Li-Cor 6400 (Li-Cor Biosciences, Lincoln, NE) combined with two leaf chamber fluorometers 6400-40 (Li-Cor Biosciences, Lincoln, NE). Microclimatic conditions were adjusted in the leaf chamber to be identical with those of the growth environment. Light intensity in the leaf chamber was set at 100 μmol m−2 s−1 in all cases, while spectral quality was set according to the spectral quality in the growth-unit (treatment). Leaf chamber temperature was stable at 21 °C and the CO2 reference concentration was 400 μmol mol−1. Relative air humidity was controlled at 75%. All measurements were carried out inside the growth-units. In total, eight plants were used for gas exchange measurements per light quality treatment (2×4 plants per treatment).
; Baker, 2008) were performed using two portable gas exchange systems Li-Cor 6400 (Li-Cor Biosciences, Lincoln, NE) combined with two leaf chamber fluorometers 6400-40 (Li-Cor Biosciences, Lincoln, NE). Microclimatic conditions were adjusted in the leaf chamber to be identical with those of the growth environment. Light intensity in the leaf chamber was set at 100 μmol m−2 s−1 in all cases, while spectral quality was set according to the spectral quality in the growth-unit (treatment). Leaf chamber temperature was stable at 21 °C and the CO2 reference concentration was 400 μmol mol−1. Relative air humidity was controlled at 75%. All measurements were carried out inside the growth-units. In total, eight plants were used for gas exchange measurements per light quality treatment (2×4 plants per treatment).
Leaf hydraulic conductance (Kleaf)
The night before the Kleaf measurement, polyurethane glue (Kompi power, BISON, Goes, The Netherlands) was applied on the attached petioles of five leaves per light quality treatment and, subsequently, it was surrounded by a silicon tube used as a matrix. This aimed to provide a hard sheath to the fragile petiole, which would withstand compression without damaging the petiole (facilitating connection with the measurement system). The following day, the silicon tube was removed from the petiole and the leaf was excised from the mother plant. All manipulations in the laboratory were done under water to prevent the entrance of air into the vessels that had been opened by cutting. The petiole was adjusted with a razor blade to 2 cm length. A new razor blade was used for each cut. A silicone tube was pushed over the petiole cut end and attached to the measurement system. During measurements, flow was always in the natural direction, from the petiole to the inner leaf compartments. The time between collection from the plant and the start of the measurement was approximately 10 min.
Kleaf was measured using the vacuum pump method (Sack et al., 2002). The leaf was sealed inside a glass desiccator with a moist paper towel to maintain a saturated humidity environment, with the tube of solution attached to the petiole running out through the centre of a rubber stopper to a balance. The water was pulled through the leaf using a vacuum pump (7550-62, Barnant, Barrington, IL), while measuring flow rates of solution into the petiole with a balance. Weight was measured at a per second sample rate and averaged over 30 s. A degassed aqueous solution of potassium chloride (10 mM) and calcium chloride (1 mM) at room temperature (20±2 °C) was used throughout the measurements (van Ieperen and van Gelder, 2006). For each leaf, five levels of partial vacuum were applied, 0.04, 0.05, 0.06, 0.07 and 0.08 MPa, in increasing order of absolute air pressure. When the first vacuum level was applied, the flow typically increased for 20±10 min before stabilizing, while after each subsequent vacuum level change, values stabilized typically after 10±5 min. All measurements were made with the desiccator being under the same light source (fluorescent tubes, PPFD: 5±2 μmol m−2 s−1).
The absolute (i.e. non-normalized) hydraulic conductance was calculated by the slope of the regression of flow rate into the leaf versus pressure (equation 1; Kolb et al., 1996; Sack et al., 2002). Kleaf was then determined by dividing the absolute conductance of the leaf by its area (determined by ImageJ open access software, version 1.44p). The method used was independent of the stomatal component. During the measurement, water flow through the lamina was driven by both the suction produced by the pump and the transpiration from the leaf inside the desiccator. The latter was minimized by increasing the humidity in the desiccator with wet tissue paper. Nonetheless, transpiration into the flask created a consistent driving force at different vacuum levels, i.e. it affected only the y-intercept, and not the slope of the flow versus vacuum level relationship. The reason is that transpiration rate is a function of the mole fraction driving force and gs, neither of which is affected by ambient pressure (Nobel, 1991).
| (1) |
Stomatal traits
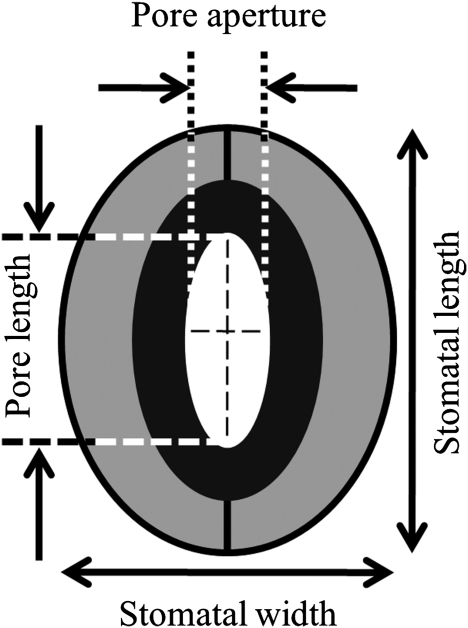
All morphological stomatal features were determined using the silicon rubber impression technique (Weyers and Meidner, 1990). Details of the impression method and image analysis are described by Fanourakis et al. (2011). The measurements were made on four leaves (one leaf per plant) per light quality treatment on both leaf surfaces. Stomatal features were assessed at both adaxial (i.e. upper) and abaxial (i.e. lower) leaf epidermes, using a part of the leaf that was midway between the tip and the base and away from the edge. Leaf veins were avoided as they support no stomata, and all cell densities quoted are therefore interveinal values. For determining the stomatal and epidermal cell densities (SD and ECS, respectively) a magnification of ×250 was used, and five fields of view were counted in each sampling area. Rectangular fields of view (Poole and Kurschner, 1999) were used and an unbiased decision was made concerning the inclusion of stomata and non-stomatal epidermal cells on the edge of a field of view (Kubinova, 1993). Stomatal index (SI) was calculated according to Salisbury (1928). ECD was estimated as the guard cell density (2× SD) plus the non-stomatal epidermal cell density. For the individual stomatal parameters, a magnification of ×1000 was used, and 20 randomly selected stomata per sampling area were measured. The stomata length, stomata width, pore length, and pore aperture were measured (Fig. 2). Stoma width was chosen instead of guard cell width, since the latter undergoes changes up to 50% as stomata close (Shope and Mott, 2006). To calculate pore area, it was assumed that the pore was elliptical (major axis pore length; minor axis pore aperture). The same procedure was used for estimating stomatal area (major axis stomatal length, minor axis stomatal width). Pore area per leaf area was calculated as the sum of the pore area per stoma×stomatal density of the two leaf surfaces.
Fig. 2.
Schematic representation of the stomatal and pore dimensions as measured by image analysis. The grey area represents the guard cells, the black area the pore walls, and the white area (internal ellipse) depicts the stomatal pore area.
Effect of short-term osmotic stress on leaf gas exchange and dynamics of recovery
To estimate the effect of short-term osmotic stress applied to the roots on the photosynthetic performance of fully developed leaves, as well as their recovery when stress application ceases, two levels of osmotic potential were realized in the growth medium (0.10 and 0.15 MPa) of four plants per light quality treatment. The osmotic potential of the nutrient solution was initially 0.05 MPa (control), and was adjusted by adding PEG-6000 (Michel and Kaufmann, 1973). The osmolality of the solutions was measured (using VAPRO 5520, Wescor inc., Logan, UT), and was then converted to osmotic potential (Ψπ) using the van't Hoff equation (Nobel, 1991). The gs, AN, intercellular CO2 concentration (Ci), and ΦPSII were measured on leaves of plants developed under the different light qualities, as described in gas exchange measurements. Following the measurements, the control medium was replenished by another medium with an osmotic potential of 0.10 MPa. The plants were left in this medium for the next 24 h. Subsequently, the above-mentioned parameters were recorded. The same procedure was followed for the second level of osmotic stress, where the nutrient solution was replaced by the one having the highest osmotic potential (0.15 MPa). Plants were left for 24 h and then were measured. Finally, the plants were returned to the control medium (0.05 MPa) and left to recover. Similar measurements were performed during the next 24 and 48 h.
Data analysis
The data were analysed using the statistical analysis software package IBM SPSS Statistics 19 (IBM Corporation, NY). The parameters investigated were statistically tested by one-way analysis of variance (ANOVA) and the means were compared by the least significant difference (LSD) multiple comparison test (P ≤0.05). The effects of light quality on leaf stomatal traits were tested on both leaf sides (abaxial and adaxial) and statistically tested by two-way ANOVA. Linear regression analysis (P ≤0.05) was used to test the significance of the correlation between Kleaf and gs, between Kleaf and ΦPSII and between Kleaf and AN of leaves developed under the three different light qualities investigated in this study.
Results
Leaf gas exchange and hydraulic conductance (Kleaf)
Light quality during leaf development exerted a considerable influence on both gas exchange parameters and Kleaf (Fig. 3). The absence of blue light (R) resulted in very low gs and Kleaf values. gs and Kleaf of leaves developed under B and RB light were at least 3-fold higher compared with leaves developed under R light (Fig. 3A, B). AN was the highest in leaves which developed under RB light, and the lowest in R-grown leaves (Fig. 3C). ΦPSII was significantly lower (15%) in R-grown than in RB- and B-grown leaves, which had approximately similar ΦPSII (about 0.74; Fig. 3D). gs showed a strong and statistically significant correlation with Kleaf across the applied light qualities (r2=1.0; P <0.05; Fig. 4), although it has to be considered that the regression analysis is done on the averages obtained from only three light quality treatments. No statistically significant correlations were observed between ΦPSII and Kleaf, and between AN and Kleaf across the applied light qualities.
Fig. 3.
Stomatal conductance (gs, A), leaf hydraulic conductance (Kleaf, B), net photosynthetic rate (AN, C), and operating efficiency of PSII (ΦPSII, D) of cucumber leaves, grown at different light qualities [R, red; RB, red and blue (red:blue, 70:30); and B, blue]. Different letters indicate significant differences (P ≤0.05; n=5 for Kleaf, n=8 for the other parameters). Error bars indicate the SEM.
Fig. 4.
Relationship of mean stomatal conductance (gs, n=8) to mean leaf hydraulic conductance (Kleaf, n=5) across the three investigated light qualities [R, red; RB, red and blue (red:blue, 70:30); and B, blue]. A significant linear regression (r2=1, P <0.05) suggests a proportional relation between gs and Kleaf. Error bars indicate the SEM.
Stomatal traits
Light quality significantly affected almost all the examined stomatal anatomical features that can contribute to gs. Leaves grown in the presence of blue light (B and RB) had significantly higher adaxial, abaxial, and total (adaxial + abaxial) SDs compared with R-grown leaves (Table 1). In all treatments, adaxial SD was lower than abaxial SD. The fraction adaxial over the total SD was similar (0.39) on leaves developed in the presence of blue light and slightly reduced (0.32) on leaves of R-grown plants. Differences in SI directly reflect differences in the percentage of epidermal cells that undertook differentiation to stomatal cells during leaf development. SI was significantly lower on the adaxial leaf surface than on the abaxial leaf surface at all light qualities. Light quality influenced SI only on the adaxial leaf surface, where the SI was slightly lower on R-grown leaves than on B- and RB-grown leaves (Table 1). ECD was not different between the adaxial and abaxial leaf surfaces, while R-grown leaves had a significantly lower ECD on both leaf surfaces than leaves that were grown in the presence of blue light (Table 1). The combination of larger epidermal cells (i.e. lower ECD) and similar (abaxial) or slightly reduced (adaxial) differentiation percentage into stomata (i.e. similar or lower SI) explains the reduced SD on R-grown leaves compared with RB- and B-grown leaves (Table 1). Stomatal size, represented by stomatal area, was not different between the adaxial and abaxial leaf surfaces and between the light qualities, despite a small but statistically significant effect of both factors on stomatal width (Table 1). Pore aperture of individual stomata was significantly higher on the abaxial than on the adaxial leaf surface, and higher in B-grown leaves than in R-grown leaves (Table 1). As expected, the pore area per leaf area (i.e. sum of pore area per stoma ×SD of the two leaf surfaces) was not evenly distributed between the adaxial and abaxial leaf surfaces, and was mostly situated (∼70%) at the abaxial leaf surface in all light qualities investigated (Table 1). Leaves, which developed in the presence of blue light had a significantly higher pore area per leaf area than R-grown leaves, with B-grown leaves having more than double the pore area per leaf area than R-grown leaves (Table 1). The differences observed were due to both differences in stomatal density and pore aperture.
Table 1.
Stomatal traits on the adaxial and abaxial surfaces of cucumber leaves grown at different light qualities [R, red; B, blue; RB, red and blue (red:blue, 70:30)].
| Stomatal trait | Light quality | Mean | ||
| R | RB | B | ||
| SD (no. mm−2) | ||||
| Adaxial | 153 | 285 | 273 | 237 b |
| Abaxial | 322 | 438 | 417 | 393 a |
| Mean | 238 b | 361 a | 345 a | |
| Total | 475 b | 723 a | 690 a | |
| SI (–) | ||||
| Adaxial | 0.10 c | 0.14 b | 0.14 b | 0.13 |
| Abaxial | 0.24 a | 0.26 a | 0.24 a | 0.25 |
| Mean | 0.17 | 0.20 | 0.19 | |
| ECD (no. mm−2) | ||||
| Adaxial | 1744 | 2216 | 2145 | 2035 |
| Abaxial | 1686 | 2152 | 2122 | 1987 |
| Mean | 1715 b | 2184 a | 2133 a | |
| Stomatal length (μm) | ||||
| Adaxial | 21.0 | 21.6 | 22.8 | 21.8 |
| Abaxial | 22.1 | 21.0 | 21.0 | 21.3 |
| Mean | 21.5 | 21.3 | 21.9 | |
| Stomatal width (μm) | ||||
| Adaxial | 11.8 | 11.9 | 12.9 | 12.2 b |
| Abaxial | 12.6 | 13.2 | 13.6 | 13.1 a |
| Mean | 12.2 b | 12.6 b | 13.2 a | |
| Stomatal area (μm2) | ||||
| Adaxial | 194 | 202 | 231 | 209 |
| Abaxial | 218 | 218 | 224 | 220 |
| Mean | 206 | 210 | 228 | |
| Pore length (μm) | ||||
| Adaxial | 12.1 bc | 13.6 a | 12.7 ab | 12.8 |
| Abaxial | 12.7 ab | 11.0 c | 11.2 c | 11.6 |
| Mean | 12.4 | 12.3 | 12.0 | |
| Pore aperture (μm) | ||||
| Adaxial | 0.81 | 0.61 | 0.90 | 0.78 b |
| Abaxial | 0.82 | 1.24 | 1.55 | 1.20 a |
| Mean | 0.82 b | 0.93 ab | 1.23 a | |
| Pore area per leaf area (μm2 mm−2) | ||||
| Adaxial | 1210 | 1849 | 2448 | 1836 b |
| Abaxial | 2642 | 4644 | 5579 | 4288 a |
| Mean | 1926 b | 3247 a | 4013 a | |
| Total | 3852 b | 6493 a | 8027 a |
Different letters indicate significant differences (P ≤0.05; n=4). SD, stomatal density; ECD, epidermal cell density; SI, stomatal index.
Effect of short-term osmotic stress on leaf gas exchange and dynamics of recovery
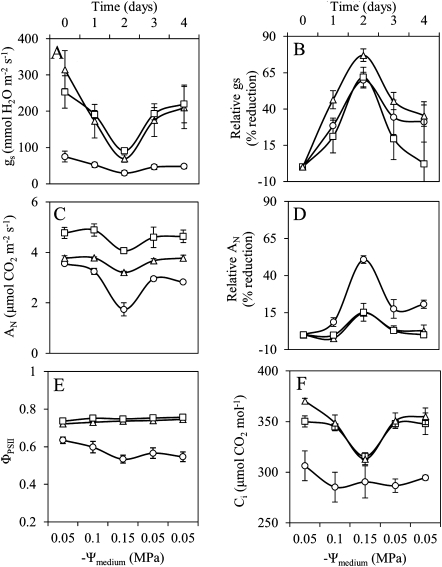
The induction of osmotic water stress in the root environment, in two steps on consecutive days from –0.05 through –0.10 to –0.15 MPa, increasingly reduced gs in leaves grown at all light qualities (Fig. 5A). Despite the large differences in absolute reductions of gs among the light quality treatments, relative reductions in gs were comparable among the leaves grown at the different light qualities, and similarly increased with increasing osmotic stress (Fig. 5B). During the first 48 h after release from the osmotic stress, similar recovery patterns of relative gs were observed among all leaves, irrespective of light quality (Fig. 5B). Before applying osmotic stress, Ci was already significantly lower in R-grown leaves than in leaves grown in the presence of blue light (Fig. 5F). Neither the application of osmotic stress, nor the release from osmotic stress induced a statistically significant change in Ci in the R-grown leaves (Fig. 5F). In the leaves grown in the presence of blue light, a very clear reduction and recovery of Ci was observed, but only at the highest osmotic stress (Fig. 5F). In all leaves, AN was reduced in response to the highest level of osmotic stress, but this reduction was more severe in R-grown leaves than in the RB- and B-grown leaves. In RB- and B-grown leaves, full recovery of AN appeared within 24 h after the release from osmotic stress, but not in R-grown leaves, which still suffered some reduction in AN at the end of the experiment (48 h after release of the stress). ΦPSII only decreased in R-grown plants after the application of osmotic stress and did not recover after alleviating osmotic stress (Fig. 5E).
Fig. 5.
Stomatal conductance (gs, A), net photosynthetic rate (AN, C), operating efficiency of PSII (ΦPSII, E), and intercellular CO2 concentration (Ci, F) of cucumber leaves, grown at the three investigated light qualities (circles, R-grown; triangles, B-grown; squares, RB-grown leaves) and at constant growth medium osmotic potential (Ψπ, 0.05 MPa), which were further transferred to different growth medium Ψπ values. Time 0 corresponds to measurements at the control medium prior to transfer. All measurements were conducted 1 d after each respective change of the growth medium Ψπ. (B) and (D) depict the relative reduction of the gs and AN, respectively. Values are the means of measurements in four leaves ±SEM.
Discussion
Co-ordination of Kleaf and gs under monochromatic red, blue, and combined red and blue light
In this study, it has been shown for the first time that both Kleaf and gs are largely influenced by light quality during leaf development, but that the co-ordination between Kleaf and gs remains conserved (Fig. 4). Differences in both Kleaf and gs due to monochromatic red, blue or combined red and blue light during leaf development were large (up to 3-fold), and revealed that both gs and Kleaf were largely enhanced by the presence of blue light (Fig. 3A, B). Increased gs and Kleaf were also observed in response to a high light intensity (Sellin et al., 2008) and were related to optimal functioning under high light conditions. Supplying light at an intensity of only 100 μmol m−2 s−1 for 16 h d−1 is sufficient to grow normal cucumber plants, as was previously shown under different light spectra, including the solar spectrum (Hogewoning et al., 2010a) and combinations of only red and blue light (Hogewoning et al., 2010b). The latter research showed that adding and increasing the proportion of blue in a red/blue light spectrum at low light intensity increased maximal photosynthetic capacity and leaf mass per area (Hogewoning et al., 2010b), a parameter reflecting leaf morphological changes in response to light intensity towards ‘sun-type’ leaves (Poorter et al., 2009). It is shown here that Kleaf and gs increase in a co-ordinated way in response to the presence of blue light in the light spectrum (Fig. 3A, B). This provides indirect evidence for the involvement of blue light receptors (cryptochromes, phototropins) in the long-term regulation of Kleaf and gs and further strengthens the idea that blue light stimulates ‘sun-type’ characteristics in leaves (Hogewoning et al., 2010b).
The effect of light quality on Kleaf
Light intensity influences leaf hydraulics via effects on Kleaf, as has been shown in shade- and sun-adapted leaves of many angiosperms (Nardini et al., 2005; Sellin and Kupper, 2007). So far, the role of light quality during leaf development on Kleaf has received less attention. Leaf hydraulic conductance is composed of an intra- and an extra-xylem component connected in series, with the latter possibly having the lowest conductivity (i.e. highest resistance) in amphistomatous leaves (Mott, 2007). A higher conductance of leaf xylem is mainly related to wider xylem conduits and higher venation densities, while the conductance of the extra-xylem pathway is influenced by a palisade/spongy mesophyll ratio and leaf thickness (Sack and Holbrook, 2006), as well as by environmental factors, which can fluctuate in the short-term (i.e. light intensity and temperature) via the regulation of plasma membrane aquaporins (Cochard et al., 2007). It has been observed in Capsicum annuum that, besides secondary xylem thickness in stems, the thickness of palisade and spongy mesophyll in leaves was enhanced in light spectra containing blue rather than monochromatic red light (Schuerger et al., 1997). Recent studies also suggested that short-term changes in light intensity (Scoffoni et al., 2008) and quality (Voicu et al., 2008; Sellin et al., 2011) affect Kleaf through changes in the conductance of the extra-xylem component. For instance, a short-term (≤5 h) light treatment of Betula pendula shoots resulted in the lowest Kleaf values under red light, intermediate under white, and highest under blue light (Sellin et al., 2011). However, it is most likely that, in the present study, the observed differences in Kleaf due to light quality can be mainly attributed to structural changes that occurred during leaf development because all measurements of Kleaf were done at the same light spectrum and intensity (fluorescent tubes, PPFD: 5±2 μmol m−2 s−1). The latter strengthens the notion that light quality during leaf development modulates Kleaf through structural changes, but supplementary research is necessary to find out if this is caused by structural changes in either the intra- or extra-xylem water transport pathway in the leaf.
The effect of light quality on gs and underlying stomatal traits
In the present study, it is shown in cucumber that monochromatic red light during leaf development strongly reduced gs compared with monochromatic blue or a combination of blue and red light. An analysis of the explanatory factors of gs reveals that differences in both pore aperture and SD play a role and together cause a strong effect on pore area per leaf area (Table 1). The relative contribution of the abaxial surface to leaf gs was similar for the three light qualities investigated (∼70%; Table 1), demonstrating that the amphistomatous character of the cucumber leaves was unaffected by light quality. SD was significantly higher on leaves grown in the presence of blue light (Table 1; Wang et al., 2009; Hogewoning et al., 2010b). This higher SD was not due to an increased production of stomata, as SI was not substantially affected by the presence of blue light (Table 1). Instead, this higher SD was due to a higher ECD (i.e. a lower epidermal cell size) on leaves that developed in the presence of blue light (Table 1). The present results show that stomatal pore aperture increases with an increasing percentage of blue light, but it remains unclear whether this is caused by structurally larger stomata (Table 1), or by a direct effect of blue light on stomatal opening. Many previous studies demonstrated that blue light significantly enhances stomatal conductance via a rapid and reversible regulation of stomatal aperture (Meidner, 1968), probably mediated by the blue-light-absorbing carotenoid zeaxanthin (Zeiger et al., 2002), but also phototropins and cryptochromes (Kinoshita et al., 2001; Mao et al., 2005) might play a role. Almost no studies have been devoted on the specific role of blue light on stomatal development. Schoch et al. (1984) demonstrated in Vigna sinensis that supplying blue light during the night decreased SI, whereas red light applied at the same period increased SI. In Dendranthema grandiflorum an increasing fraction of blue slightly increased SI (Rajapakse and Kelly, 1993) and in Gossypium barbadense an increasing blue/red ratio increased adaxial SD (Lu et al., 1993). Recently, Kang et al. (2009) showed, in Arabidopsis thaliana, that blue light via cryptochrome (CRY) inhibits the production of COP1, a key repressor of photomorphogenesis, which resulted in a stimulation of stomatal development. Similar effects were shown for phytochrome (PHY) B (Lake et al., 2001), while in Arabidopsis thaliana the effects of CRY, phyA, and phyB were shown to be additive (Kang et al., 2009). The effects of osmotic stress in the root environment on gs demonstrated that functional stomata developed under all the applied light qualities (Fig. 5A, B). The almost similar relative reduction in gs, caused by osmotic stress in all the applied light qualities, indicates similar degrees of stomatal closure. The large differences in absolute gs between light quality treatments were most likely due to different SDs, thus mostly a result of structural rather than functional differences. Previously, it has been reported that gs of cucumber leaves, which developed under monochromatic red light, was unresponsive to both light intensity and quality (Hogewoning et al., 2010b). In this study, it has been demonstrated that the stomata on R-grown leaves kept normal responsiveness to the induction and release from osmotic stress (Fig. 5). This indicates at least partial functioning of the stomata in response to hormonal (e.g. abscisic acid) and hydraulic signals (Comstock, 2002). It also suggests that light and drought-related signals might influence gs in an independent manner (Harada and Shimazaki, 2009).
Photosynthetic plasticity under osmotic stress of leaves that differ in Kleaf due to light quality
Based on an extensive study of 16 forest species, which yielded a strong correlation between Kleaf and ΦPSII, it has been suggested that the maximum photosynthetic rate of the leaves is constrained by their vascular supply (Brodribb and Feild, 2000). It is shown here that the absence of blue light causes a substantial reduction in both Kleaf and ΦPSII (Fig. 3B, D). However, the presence of blue and red light enhances net photosynthesis when compared with the monochromatic light qualities (Fig. 3C). The latter was probably caused by specific effects of the light spectrum on photosynthetic performance. Hogewoning et al. (2010b) indicated that the lower AN in B-grown leaves is related to a lower chlorophyll content compared with RB-grown leaves, while the lower quantum efficiency of photosynthesis of blue compared with red light (McCree, 1972) and a suboptimal distribution of photons over PSII and PSI due to light quality (Hogewoning et al., 2010b) could also have contributed to the observed differences in photosynthetic rates. The lower AN in R-grown leaves compared with B- and RB-grown leaves, which is paralleled by reductions in both ΦPSII and Ci (Fig. 5E, F), seems to suggest stomatal limitation on photosynthesis. However, variations in mesophyll conductance for CO2 transport in the leaf, caused by differences in light quality, also cannot be excluded. It has previously been suggested that blue light might influence mesophyll conductance in the short-term (Loreto and Tsonev, 2009). However, Hogewoning et al. (2010b) had already shown that, most likely, the low photosynthetic rates and ΦPSII observed in R-grown leaves were due to disorders in the development and functioning of the photosynthetic machinery, as sustained by reduced values of dark-adapted Fv/Fm and gas exchange measurements under low O2. The additional reduction of ΦPSII during osmotic stress, which was only observed in R-grown leaves and remained after the release from the stress, indicates further damage of the photosynthetic machinery. These results demonstrate that the leaves, which developed under monochromatic red light, were more vulnerable to leaf damage induced by osmotic stress than the B- and RB-grown leaves. Although this reduction in photosynthetic performance of R-grown leaves correlates well with a low Kleaf and a low Ci compared with the other light quality treatments, it provides no evidence for a causal relationship between a limitation in leaf water supply and the reduced ability to deal with osmotic induced stress.
Conclusions
In cucumber, light quality during leaf development strongly influences Kleaf as well as gs, but Kleaf and gs remain co-ordinated across growth-light qualities. Light quality structurally influences leaf gs via an effect of epidermal cell size on stomatal density. The absence of blue light causes a severe reduction in both the water supply/demand capacities and leaf photosynthetic performance, while the photosynthetic performance of R-grown leaves was shown to be highly vulnerable to water stress. Based on our results, it seems likely that the presence of blue light during growth plays a critical role in optimally tuning the multifactorial function of leaves.
Acknowledgments
We are very grateful to Jeremy Harbinson, Sander Hogewoning, and Govert Trouwborst for their helpful discussions and suggestions and thank Arjen van de Peppel for his support during the experiments.
Glossary
Abbreviations
- AN
net leaf photosynthesis
- B
blue light
- Ci
intercellular CO2 concentration
- ECD
epidermal cell density
- gs
stomatal conductance
- Kleaf
leaf hydraulic conductance
- LED
light emitting diode
- PPFD
photosynthetic photon flux density
- R
red light
- RB
red and blue light (R:B, 70:30)
- SD
stomatal density
- SI
stomatal index
- ΦPSII
photosystem II operating efficiency
- Ψπ
osmotic potential
References
- Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS. Stem hydraulic supply is linked to leaf photosynthetic capacity: evidence from New Caledonian and Tasmanian rainforests. Plant, Cell and Environment. 2000;23:1381–1388. [Google Scholar]
- Brodribb TJ, Feild TS, Sack L. Viewing leaf structure and evolution from a hydraulic perspective. Functional Plant Biology. 2010;37:488–498. [Google Scholar]
- Brodribb TJ, Holbrook NM, Zwieniecki MA, Palma B. Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima. New Phytologist. 2005;165:839–846. doi: 10.1111/j.1469-8137.2004.01259.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Jordan GJ. Internal coordination between hydraulics and stomatal control in leaves. Plant, Cell and Environment. 2008;31:1557–1564. doi: 10.1111/j.1365-3040.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- Cochard H, Venisse J-S, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiology. 2007;143:122–133. doi: 10.1104/pp.106.090092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes D, Sinoquet H, Varlet-Grancher C. Preliminary measurement and simulation of the spatial distribution of the morphogenetically active radiation (MAR) within an isolated tree canopy. Annals of Forest Science. 2000;57:497–511. [Google Scholar]
- Comstock JP. Hydraulic and chemical signalling in the control of stomatal conductance and transpiration. Journal of Experimental Botany. 2002;53:195–200. doi: 10.1093/jexbot/53.367.195. [DOI] [PubMed] [Google Scholar]
- Fanourakis D, Carvalho SMP, Almeida DPF, Heuvelink E. Avoiding high relative air humidity during critical stages of leaf ontogeny is decisive for stomatal functioning. Physiologia Plantarum. 2011;142:274–286. doi: 10.1111/j.1399-3054.2011.01475.x. [DOI] [PubMed] [Google Scholar]
- Foster JR, Smith WK. Influence of stomatal distribution on transpiration in low-wind environments. Plant, Cell and Environment. 1986;9:751–759. [Google Scholar]
- Franks PJ, Beerling DJ. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences, USA. 2009;106:10343–10347. doi: 10.1073/pnas.0904209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A. Shimazaki K-i. 2009. Measurement of changes in cytosolic Ca2+ in Arabidopsis guard cells and mesophyll cells in response to blue light. Plant and Cell Physiology. 50:360–373. doi: 10.1093/pcp/pcn203. [DOI] [PubMed] [Google Scholar]
- Hogewoning SW, Douwstra P, Trouwborst G, van Ieperen W, Harbinson J. 2010a. An artificial solar spectrum substantially alters plant development compared with usual climate room irradiance spectra. Journal of Experimental Botany. 61:1267–1276. doi: 10.1093/jxb/erq005. [DOI] [PubMed] [Google Scholar]
- Hogewoning SW, Trouwborst G, Maljaars H, Poorter H, van Ieperen W, Harbinson J. 2010b. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. Journal of Experimental Botany. 61:3107–3117. doi: 10.1093/jxb/erq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Woodruff DR, McCulloh KA, Meinzer FC. Leaf hydraulic conductance, measured in situ, declines and recovers daily: leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiology. 2009;29:879–887. doi: 10.1093/treephys/tpp031. [DOI] [PubMed] [Google Scholar]
- Kang C-Y, Lian H-L, Wang F-F, Huang J-R, Yang H- Q. Cryptochromes, phytochromes, and COP1 regulate light-controlled stomatal development in Arabidopsis. The Plant Cell. 2009;21:2624–2641. doi: 10.1105/tpc.109.069765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M. Shimazaki K-i 2001. phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 414:656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- Kolb KJ, Sperry JS, Lamont BB. A method for measuring xylem hydraulic conductance and embolism in entire root and shoot systems. Journal of Experimental Botany. 1996;47:1805–1810. [Google Scholar]
- Kubinova L. Recent stereological methods for the measurement of leaf anatomical characteristics: estimation of volume density, volume and surface area. Journal of Experimental Botany. 1993;44:165–173. [Google Scholar]
- Lake JA, Quick WP, Beerling DJ, Woodward FI. Plant development: signals from mature to new leaves. Nature. 2001;411:154–154. doi: 10.1038/35075660. [DOI] [PubMed] [Google Scholar]
- Lo Gullo MA, Raimondo F, Crisafulli A, Salleo S, Nardini A. Leaf hydraulic architecture and water relations of three ferns from contrasting light habitats. Functional Plant Biology. 2010;37:566–574. [Google Scholar]
- Loreto F, Tsonev T. The impact of blue light on leaf mesophyll conductance. Journal of Experimental Botany. 2009;60:2283–2290. doi: 10.1093/jxb/erp112. [DOI] [PubMed] [Google Scholar]
- Lu Z, Quiñones MA, Zeiger E. Abaxial and adaxial stomata from pima cotton (Gossypium barbadense L.) differ in their pigment content and sensitivity to light quality. Plant, Cell and Environment. 1993;16:851–858. [Google Scholar]
- Mao J, Zhang Y-C, Sang Y, Li Q-H, Yang H- Q. A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proceedings of the National Academy of Sciences, USA. 2005;102:12270–12275. doi: 10.1073/pnas.0501011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCree KJ. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agricultural Meteorology. 1972;9:191–216. [Google Scholar]
- Meidner H. The comparative effects of blue and red light on the stomata of Allium cepa L. and Xanthium pennsylvanicum. Journal of Experimental Botany. 1968;19:146–151. [Google Scholar]
- Michel BE, Kaufmann MR. The osmotic potential of polyethylene glycol 6000. Plant Physiology. 1973;51:914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA. Leaf hydraulic conductivity and stomatal responses to humidity in amphistomatous leaves. Plant, Cell and Environment. 2007;30:1444–1449. doi: 10.1111/j.1365-3040.2007.01720.x. [DOI] [PubMed] [Google Scholar]
- Mott KA, Gibson AC, O'Leary JW. The adaptive significance of amphistomatic leaves. Plant, Cell and Environment. 1982;5:455–460. [Google Scholar]
- Nardini A, Gortan E, Salleo S. Hydraulic efficiency of the leaf venation system in sun- and shade-adapted species. Functional Plant Biology. 2005;32:953–961. doi: 10.1071/FP05100. [DOI] [PubMed] [Google Scholar]
- Nardini A, Grego F, Trifilò P, Salleo S. Changes of xylem sap ionic content and stem hydraulics in response to irradiance in. Laurus nobilis. Tree Physiology. 2010;30:628–635. doi: 10.1093/treephys/tpq017. [DOI] [PubMed] [Google Scholar]
- Nobel PS. Physicochemical and environmental plant physiology. San Diego: Academic Press; 1991. [Google Scholar]
- Parkhurst DF. The adaptive significance of stomatal occurrence on one or both surfaces of leaves. Journal of Ecology. 1978;66:367–383. [Google Scholar]
- Parkhurst DF, Mott KA. Intercellular diffusion limits to CO2 uptake in leaves: studies in air and helox. Plant Physiology. 1990;94:1024–1032. doi: 10.1104/pp.94.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlange J-Y, Waggoner PE. Stomatal dimensions and resistance to diffusion. Plant Physiology. 1970;46:337–342. doi: 10.1104/pp.46.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole I, Kurschner W. Stomatal density and index. The practice. In: Jones TP, Rowe NP, editors. Fossil plants and spores: modern techniques. London: The Geological Society; 1999. pp. 257–260. [Google Scholar]
- Poorter H, Niinemets Ü Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Rajapakse NC, Kelly JW. Spectral filters influence transpirational water loss in chrysanthemum. HortScience. 1993;28:999–1001. [Google Scholar]
- Reich PB. Relationship between leaf age, irradiance, leaf conductance, CO2 exchange, and water use efficiency in hybrid poplar. Photosynthetica. 1984;18:445–453. [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant, Cell and Environment. 2003;26:1343–1356. [Google Scholar]
- Sack L, Holbrook NM. Leaf hydraulics. Annual Review of Plant Biology. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- Sack L, Melcher PJ, Zwieniecki MA, Holbrook NM. The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. Journal of Experimental Botany. 2002;53:2177–2184. doi: 10.1093/jxb/erf069. [DOI] [PubMed] [Google Scholar]
- Sack L, Tyree MT, Holbrook NM. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytologist. 2005;167:403–413. doi: 10.1111/j.1469-8137.2005.01432.x. [DOI] [PubMed] [Google Scholar]
- Salisbury EJ. On the causes and ecological significance of stomatal frequency, with special reference to the woodland flora. Philosophical Transactions of the Royal Society of London. Series B, Containing Papers of a Biological Character. 1928;216:1–65. [Google Scholar]
- Schoch PG, Jacques R, Lecharny A, Sibi M. Dependence of the stomatal index on environmental factors during stomatal differentiation in leaves of Vigna sinensis L.: II. Effect of different light quality. Journal of Experimental Botany. 1984;35:1405–1409. [Google Scholar]
- Schuerger AC, Brown CS, Stryjewski EC. Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Annals of Botany. 1997;79:273–282. doi: 10.1006/anbo.1996.0341. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Pou A, Aasamaa K, Sack L. The rapid light response of leaf hydraulic conductance: New evidence from two experimental methods. Plant, Cell and Environment. 2008;31:1803–1812. doi: 10.1111/j.1365-3040.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- Sellin A, Kupper P. Temperature, light and leaf hydraulic conductance of little-leaf linden (Tilia cordata) in a mixed forest canopy. Tree Physiology. 2007;27:679–688. doi: 10.1093/treephys/27.5.679. [DOI] [PubMed] [Google Scholar]
- Sellin A, Õunapuu E, Kupper P. Effects of light intensity and duration on leaf hydraulic conductance and distribution of resistance in shoots of silver birch (Betula pendula) Physiologia Plantarum. 2008;134:412–420. doi: 10.1111/j.1399-3054.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- Sellin A, Õunapuu E, Karusion A. Experimental evidence supporting the concept of light-mediated modulation of stem hydraulic conductance. Tree Physiology. 2010;30:1528–1535. doi: 10.1093/treephys/tpq091. [DOI] [PubMed] [Google Scholar]
- Sellin A, Sack L, Õunapuu E, Karusion A. Impact of light quality on leaf and shoot hydraulic properties: a case study in silver birch (Betula pendula) Plant, Cell and Environment. 2011;34:1079–1087. doi: 10.1111/j.1365-3040.2011.02306.x. [DOI] [PubMed] [Google Scholar]
- Shope JC, Mott KA. Membrane trafficking and osmotically induced volume changes in guard cells. Journal of Experimental Botany. 2006;57:4123–4131. doi: 10.1093/jxb/erl187. [DOI] [PubMed] [Google Scholar]
- Smith H. Light quality, photoperception, and plant strategy. Annual Review of Plant Physiology. 1982;33:481–518. [Google Scholar]
- Sperry JS. Hydraulic constraints on plant gas exchange. Agricultural and Forest Meteorology. 2000;104:13–23. [Google Scholar]
- Sperry JS, Alder NN, Eastlack SE. The effect of reduced hydraulic conductance on stomatal conductance and xylem cavitation. Journal of Experimental Botany. 1993;44:1075–1082. [Google Scholar]
- Sperry JS, Hacke UG, Oren R, Comstock JP. Water deficits and hydraulic limits to leaf water supply. Plant, Cell & Environment. 2002;25:251–263. doi: 10.1046/j.0016-8025.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- Van Ieperen W, van Gelder A. Ion-mediated flow changes suppressed by minimal calcium presence in xylem sap in Chrysanthemum and Prunus laurocerasus. Journal of Experimental Botany. 2006;57:2743–2750. doi: 10.1093/jxb/erl039. [DOI] [PubMed] [Google Scholar]
- Voicu MC, Zwiazek JJ, Tyree MT. Light response of hydraulic conductance in bur oak (Quercus macrocarpa) leaves. Tree Physiology. 2008;28:1007–1015. doi: 10.1093/treephys/28.7.1007. [DOI] [PubMed] [Google Scholar]
- Wang H, Gu M, Cui J, Shi K, Zhou Y, Yu J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. Journal of Photochemistry and Photobiology B: Biology. 2009;96:30–37. doi: 10.1016/j.jphotobiol.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Weyers JDB, Meidner H. Methods in stomatal research. Harlow, England: Longman Scientific and Technical; 1990. [Google Scholar]
- Zeiger E, Talbott LD, Frechilla S, Srivastava A, Zhu J. The guard cell chloroplast: a perspective for the twenty-first century. New Phytologist. 2002;153:415–424. doi: 10.1046/j.0028-646X.2001.NPH328.doc.x. [DOI] [PubMed] [Google Scholar]