Abstract
Pyrabactin, a synthetic agonist of abscisic acid (ABA), inhibits seed germination and hypocotyl growth and stimulates gene expression in a very similar way to ABA, implying the possible modulation of stomatal function by pyrabactin as well. The effect of pyrabactin on stomatal closure and secondary messengers was therefore studied in guard cells of Pisum sativum abaxial epidermis. Pyrabactin caused marked stomatal closure in a pattern similar to ABA. In addition, pyrabactin elevated the levels of reactive oxygen species (ROS), nitric oxide (NO), and cytoplasmic pH levels in guard cells, as indicated by the respective fluorophores. However, apyrabactin, an inactive analogue of ABA, did not affect either stomatal closure or the signalling components of guard cells. The effects of pyrabactin-induced changes were reversed by pharmalogical compounds that modulate ROS, NO or cytoplasmic pH levels, quite similar to ABA effects. Fusicoccin, a fungal toxin, could reverse the stomatal closure caused by pyrabactin, as well as that caused by ABA. Experiments on stomatal closure by varying concentrations of ABA, in the presence of fixed concentration of pyrabactin, and vice versa, revealed that the actions of ABA and pyrabactin were additive. Further kinetic analysis of data revealed that the apparent KD of ABA was increased almost 4-fold in the presence of ABA, suggesting that pyrabactin and ABA were competing with each other either at the same site or close to the active site. It is proposed that pyrabactin could be used to examine the ABA-related signal-transduction components in stomatal guard cells as well as in other plant tissues. It is also suggested that pyrabactin can be used as an antitranspirant or as a priming agent for improving the drought tolerance of crop plants.
Keywords: Abscisic acid, cytoplasmic pH, fusicoccin, guard cell, nitric oxide, Pisum sativum, pyrabactin, reactive oxygen species, stomatal closure
Introduction
Stomatal closure is an adaptation to conserve water loss during drought/water stress conditions. During the stress conditions, the synthesis and mobilization of abscisic acid (ABA) form key physiological events, facilitating stomatal closure by ABA (Seo and Koshiba, 2002; Christmann et al., 2006). In view of the powerful effects of ABA, the signalling components during ABA-induced stomatal closure have been examined extensively (Hetherington, 2001; Wasilewska et al., 2008; Acharya and Assmann, 2009; Kim et al., 2010). Protein phosphatases such as ABI1, ABI2, and HAB1 are negative regulators during ABA-induced stomatal closure, while protein kinases such as SnRK2s (including OST1) are positive regulators (Mustilli et al., 2002; Li et al., 2006; Hubbard et al., 2010; Kim et al., 2010). Other protein kinases such as CBLs and CIPKs also play crucial roles in ABA-induced stomatal closure. In addition, ABA promotes the activity of anion channels (e.g. SLAK1, AtALMT12) and down-regulates the activity of inward K+ channels (KAT1and KAT2) in stomatal guard cells (Geiger et al., 2009; Lee et al., 2009; Sirichandra et al., 2009; Kim et al., 2010). Besides the above signalling components, participation of several small molecules like reactive oxygen species (ROS), nitric oxide (NO), and ions like Ca2+, besides a rise in guard cell pH are all essential during ABA-mediated stomatal closure (Neill et al., 2002; Suhita et al., 2004; Gonugunta et al., 2008, 2009).
Despite repeated attempts, the identity of ABA putative receptors was not established for a long time. In 2009, two independent groups identified and established that PYR/PYL/RCAR proteins, that belong to the cyclase subfamily of the START/Bet v I protein superfamily, acted as ABA receptors in Arabidopsis (Ma et al., 2009; Park et al., 2009). Soon after, the crystallization, molecular modelling, and simulation of the structure of PYR/PYL/RCAR proteins unravelled the novel mechanisms of their function (Nishimura et al., 2009; Yin et al., 2009; Melcher et al., 2010a). In the absence of ABA, PP2Cs keep the pool of SnRK2s dephosphorylated and limit the phosphorylation of transcription factors involved in ABA-induced gene expression. When present, ABA binds to PYR/PYL and then to PP2C making a functional complex, and blocks the normal function of PP2C. As a result, the SnRK2s stay in a phosphorylated state and activate the transcription factors and induce ABA-activated gene expression (Cutler et al., 2010; Melcher et al., 2010b; Raghavendra et al., 2010).
The identification of PYR/PYL proteins as ABA receptors was made possible by the discovery of pyrabactin (4-bromo-N-(pyridine-2-yl methyl) naphthalene-1-sulfonamide), a synthetic compound. Pyrabactin was found to suppress markedly seed germination and hypocotyl growth, besides the promotion of gene expression, very similar to the pattern with ABA (Park et al., 2009). Pyrabactin was considered as a potential anti-transpirant/stress adaptor, with possible uses in agriculture. It became clear that pyrabactin was acting as an agonist during ABA action. The expression of pyr/pyl mRNA was quite high not only in the seeds, but also in guard cells. In addition, the Arabidopsis quadruple mutants lacking pyr1pyl1pyl2pyl4 were impaired in ABA-induced stomatal closure and the ABA-inhibition of stomatal opening (Nishimura et al., 2010). All these studies imply that pyrabactin must affect guard cell function and stomatal closure. However, there have been no direct detailed experiments on stomatal closure in response to pyrabactin. In this report, the response of Pisum sativum guard cells to pyrabactin during stomatal closure was studied and the effects with ABA were compared. The effect of ABA on stomatal closure was examined in detail as well as changes in the signalling components, including pH, ROS, and NO. The influence of pyrabactin on stomata was then examined in the absence/presence of ABA and vice versa. Attempts were made to determine the apparent KD for pyrabactin and ABA.
Materials and methods
Chemicals
Pyrabactin and apyrabactin were from Sigma-Aldrich and Chembridge Corporation (San Diego, CA), respectively. DAF-2DA was from Calbiochem (Rockland, MA). BCECF-AM was from Invitrogen (Molecular Probes). The remaining chemicals were from Sigma-Aldrich. The stock solutions of ABA and fusicoccin were prepared in ethanol and methanol, respectively. The stocks of pyrabactin, apyrabactin, fluorescent probes, and DPI were in DMSO and all the remaining chemicals were in milli Q water. Stock solutions were prepared in such a way that the final concentration of solvent was <0.2% in the final medium.
Plant materials and growth conditions
Plants of pea (Pisum sativum L., cv. Arkel) were raised from seeds, procured from Pocha Seeds, Pune, India. The plants were grown outdoors under a natural photoperiod of approximately 12 h and an average temperature of 30/20 °C day/night. The second pair of fully unfolded leaves was picked at about 09.00 h from 9–15-d-old plants for subsequent use.
Stomatal closure in epidermal strips
The abaxial epidermis was peeled from the leaves and cut into pieces of c. 0.4 cm2. Twenty-five epidermal strips were transferred to 3 cm diameter Petri dishes containing 3 ml of opening medium (10 mM MES-KOH, pH 7.0, and 50 mM KCl). The epidermal strips were exposed to a bank of tungsten lamps, whose light was filtered through water jacket white light of 200–-250 μmol m−2 s−1, for 150 min, to get maximum stomatal opening. Photon flux was measured with a Li-Cor quantum sensor (Li-Cor Instruments Ltd, Lincoln, NE, USA). The temperature was maintained at 25±1 °C. After 150 min of illumination, three epidermal strips were transferred to each of 24 well plates, containing medium and the required concentrations of ABA, pyrabactin or test compounds (inhibitors or scavengers). Illumination was continued for the next 120 min, before measuring stomatal apertures. When used together, the test compounds were added 15 min prior to the addition of ABA or pyrabactin.
The width of the stomatal apertures was measured under a research microscope with the help of a precalibrated ocular micrometer. 10–-15 apertures were monitored at random in each of three different epidermal strips, from each treatment. The experiments were repeated on at least three different days, making each measurement of stomatal aperture an average of a minimum of 90 stomata.
Fluorescent probes to monitor ROS, NO or cytoplasmic pH changes
Changes in ROS, NO or cytoplasmic pH levels in guard cells were monitored by using respective fluorescent probes, 2′,7′-dichlorofluorescien diacetate (H2DCF-DA); 4, 5-diaminofluorescein diacetate (DAF-2DA); or 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein), acetoxymethyl ester (BCECF-AM) (Murata et al., 2001; Neill et al., 2002; Gonugunta et al., 2008). Epidermal peels were mounted on a microscope slide with medical adhesive Telesis V (Premiere Products Inc., Pacaima, California, USA). Stomata were allowed to open by incubating epidermal tissues under 200–250 μmol m−2 s−1 white light for 150 min, in a medium of 10 mM MES-KOH, pH 7.0, and 50 mM KCl. After 150 min, the epidermal tissues were loaded with 30 μM H2DCF-DA, 10 μM DAF-2DA or 5 μM BCECF-AM (30 min in dark), respectively, at 25±1 °C. The strips were rinsed with incubation buffer, to wash off excessive fluorophore. For studying time-course changes in ROS/NO/pH levels, the epidermal tissues were treated with 20 μM ABA or 20 μM pyrabactin, at zero-time and changes in fluorescence levels were measured at 3 min intervals. In the control sets, an equal and appropriate volume of ethanol or DMSO was added. Modulators were added 10 min prior to the addition of 20 μM ABA or 20 μM pyrabactin. The data are representative of the averages ±SE of three independent experiments, with measurements on a minimum of 60 individual stomata.
For time-course measurements, guard cells were observed under an inverted fluorescence microscope (Optiphot-2, Nikon, Japan) fitted with a monochrome high-resolution digital cooled CD camera (Cool snap FX) that enabled the quick capture of images, for further analysis later on. The captured images and the relative fluorescence emission of guard cells were analysed by using NIH Image for Windows (Murata et al., 2001; Suhita et al., 2004). Fluorescence intensity was measured in pixels in a scale of 0 (darkest) to 250 (brightest). The fluorescence intensity in the guard cells, without ABA, pyrabactin or any effectors (at the beginning of the experiment), was taken as 100% (Suhita et al., 2004; Gonugunta et al., 2008). In some of the experiments (as indicated in the figure legends), a confocal microscope (TCSSP-2, AOBS 4 channel UV and visible; Leica, Heidelberg, Germany) was used to observe the changes in fluorescence indicating ROS, NO or cytoplasmic pH.
Results
Pyrabactin induced stomatal closure and changes in ROS, NO, and cytoplasmic pH levels in guard cells
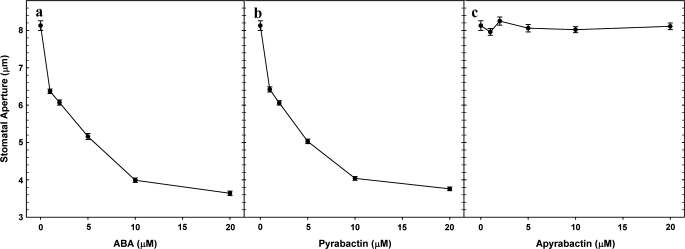
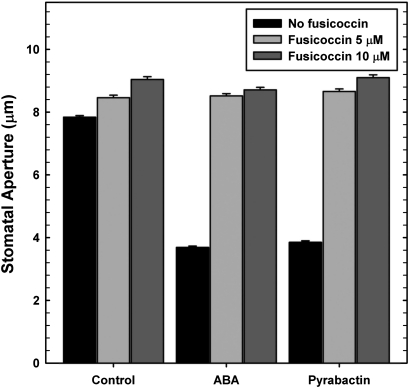
ABA or its analogue pyrabactin caused stomatal closure in a concentration-dependent manner. The concentrations of ABA or pyrabactin, required for maximal stomatal closure, were quite similar (Fig. 1a, b). By contrast, apyrabactin, an inactive analogue of ABA, did not have any significant effect on stomata (Fig. 1c). The presence of fusicoccin (FC, a fungal toxin) prevented the stomatal closure caused by either ABA or pyrabactin (Fig. 2).
Fig. 1.
Effect of ABA, pyrabactin or apyrabactin concentrations on stomata in abaxial epidermis of Pisum sativum. ABA or pyrabactin caused marked stomatal closure in a similar pattern, while apyrabactin did not have any significant effect. The data are averages of three independent experiments ±SE.
Fig. 2.
Effect of fungal toxin, fusicoccin (FC), on stomatal closure caused by ABA or pyrabactin. Fusicoccin completely relieved stomatal closure by ABA or pyrabactin. The data are means of three experiments ±SE.
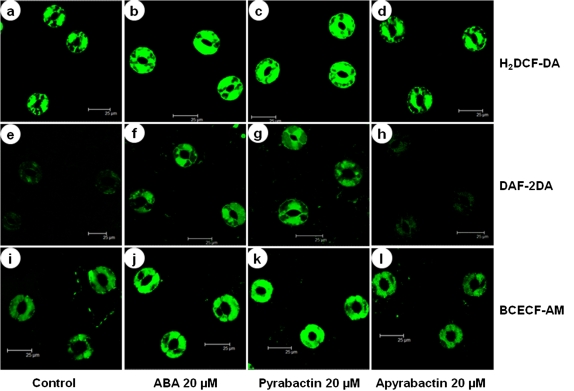
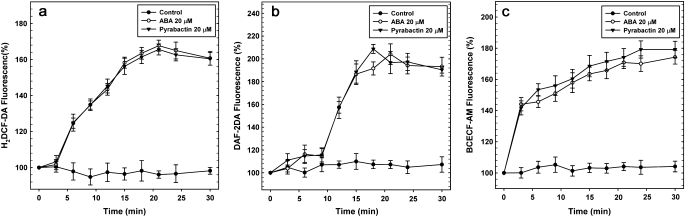
Pyrabactin increased ROS, NO, and cytoplasmic pH levels in guard cells within a few minutes after treatment, when compared with their respective controls (Fig. 3). Apyrabactin, an inactive analogue of ABA, did not cause any noticeable changes in ROS/NO/pH of guard cells (Fig. 3d, h, l). The initial rise of H2DCF-DA fluorescence (indicating ROS levels) was seen at 6 min after treatment and fluorescence peaked between 18–24 min (Fig. 4a). Similarly, NO-specific DAF-2DA fluorescence (indicating NO) showed an initial rise at 9 min after treatment and fluorescence peaked after 18 min of treatment (Fig. 4b). The BCECF-AM fluorescence (reflecting the pH) initially increased within 3 min and peaked after 24 min (Fig. 4c). A similar pattern of changes were observed with ABA.
Fig. 3.
Representative confocal images showing the changes in ROS, NO, and cytoplasmic pH changes in Pisum sativum guard cells in the presence or absence of ABA, pyrabactin or apyrabactin. (a–d) Changes in ROS levels as indicated by H2DCF-DA fluorescence. (e–h) Changes in NO levels as indicated by DAF2-DA fluorescence. (i–l) Changes in cytoplasmic pH levels as indicated by BCECF-AM fluorescence. ABA or pyrabactin increased the levels of ROS, NO, and cytoplasmic pH, compared to respective controls. (This figure is available in colour at JXB online.)
Fig. 4.
Changes with time in fluorescence of guard cells, loaded with fluorescent probes specific for ROS, NO or pH. The fluorescence was monitored at different times after exposure to pyrabactin or ABA, using an inverted fluorescence microscope. The details are described in the Materials and methods. ABA or pyrabactin increased with time in the fluorescence intensities of H2DCF-DA, DAF2-DA, and BCECF-AM reflecting the rise in ROS, NO, and pH of guard cells. The effects of pyrabactin and ABA were quite similar. The data are averages ±SE of three independent experiments, each representing a minimum of 60 individual stomata.
Modulators of ROS/NO/pH can relieve pyrabactin-induced stomatal closure and dampen the rise in ROS, NO or pH levels of guard cells
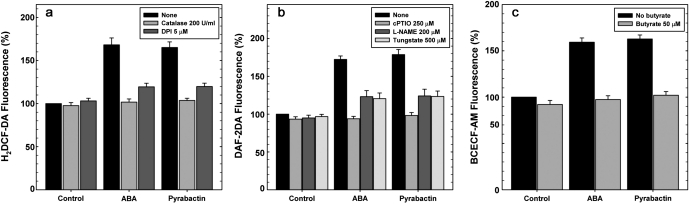
ROS modulators: DPI (NADPH oxidase inhibitor) or catalase (H2O2 scavenging enzyme), partially relieved stomatal closure by ABA or pyrabactin. Similarly, stomatal closure by ABA or pyrabactin was compromised in the presence of either cPTIO (NO scavenger), or L-NAME (nitric oxide synthase inhibitor) or tungstate (nitrate reductase inhibitor). Butyrate (a weak acid), relieved stomatal closure induced by ABA or pyrabactin (Fig. 5).
Fig. 5.
The effect of ROS, NO or pH modulators on stomatal closure caused by ABA or pyrabactin. The decrease in stomatal aperture by ABA or pyrabactin was relieved by ROS modulators, catalase or DPI (a), NO modulators cPTIO and L-NAME or tungstate (b), and pH modulator, butyrate (c). The data are means ±SE of three independent experiments, each representing a minimum of 90 individual stomata.
Catalase completely relieved the increase in H2DCF-DA fluorescence by pyrabactin or ABA, while DPI had a partial effect, conforming the increase in fluorescence due to ROS (Fig. 6). DAF-2DA fluorescence increase by ABA or pyrabactin was abolished by cPTIO (NO scavenger). Similarly, L-NAME or tungstate (inhibitors of NO synthase or nitrate reductase) restricted the DAF-2DA fluorescence increase by ABA or pyrabactin. Butyrate restricted the rise in BCECF-AM fluorescence by ABA or pyrabactin.
Fig. 6.
Effect of ROS, NO or pH modulators on H2DCF-DA, DAF2-DA, or BCECF-AM fluorescence levels respectively. Changes in fluorescence levels were monitored by using an inverted fluorescence microscope. (a) ROS modulators, catalase or DPI, prevented ABA or pyrabactin-induced increase of H2DCF-DA fluorescence. (b) NO modulators, cPTIO and L-NAME or tungstate, prevented ABA- or pyrabactin-induced increase of DAF2-DA fluorescence. (c) Butyrate, a pH modulator, restricted the ABA- or pyrabactin-induced increase in BCECF-AM fluorescence. The data are averages ±SE of three independent experiments, each with a minimum of 60 individual stomata.
Pyrabactin competes with ABA during stomatal closure
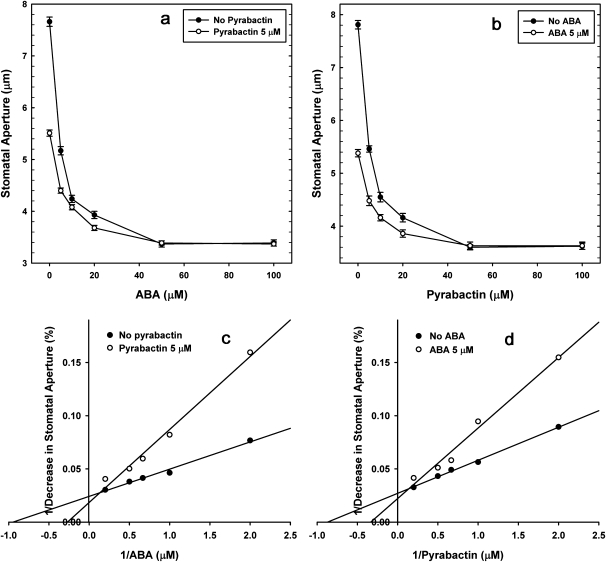
Experiments using varied concentrations of pyrabactin (0–100 μM), in the absence or presence of 5 μM ABA and vice-versa (varied concentrations of ABA in the absence or presence of 5 μM pyrabactin), revealed that the effects of ABA and pyrabactin were additive (Fig. 7). Kinetic analyses of these data indicated that the apparent IC50 of pyrabactin or ABA did not change much in the presence of ABA or pyrabactin (Fig. 7a, b). By contrast, the double reciprocal plots using low concentrations of varied concentrations of ABA or pyrabactin (0.5–5 μM) in the absence or presence of 5 μM pyrabactin or ABA demonstrated that the KD of ABA increased by almost 4-fold in the presence of pyrabactin and the KD of pyrabactin increased by nearly 3-fold in the presence of ABA (Fig. 7c, d).
Fig. 7.
Additive effect of ABA and pyrabactin during stomatal closure. (a) The apparent IC50 of ABA changed from 4.26 μM to 4.88 μM in the presence of 5 μM pyrabactin, when the change in stomatal closure was plotted as a function of concentration of ABA. (b) The apparent IC50 of pyrabactin changed from 4.47 μM to 5.05 μM in the presence of 5 μM ABA, when the change in stomatal closure was plotted as a function of concentration of pyrabactin. (c) The double reciprocal plot showing the increase of the apparent KD of ABA, almost 4-fold, from 1.08 μM to 3.82 μM in the presence of 5 μM pyrabactin. (d) The double reciprocal plot demonstrated that the apparent KD of pyrabactin increased by almost 3-fold from 1.17 μM to 3.05 μM in the presence of 5 μM ABA. The marked increase in KD of ABA/pyrabactin in the presence of pyrabactin/ABA suggested their competition at or near their binding site during stomatal closure. The data are the average means of three independent experiments.
Discussion
Pyrabactin, a synthetic ABA analogue, is considered to be a potential tool in future agriculture. Initially identified as a seed germination inhibitor, pyrabactin led the way for the identification, purification, and characterization of ABA receptors. Most of the earlier experiments with pyrabactin were done on either germinating seeds or in vitro reconstituted systems. Our results present an unequivocal and direct demonstration that pyrabactin is as powerful as ABA in promoting stomatal closure in abaxial epidermis.
Pyrabactin is as powerful as ABA in inducing stomatal closure
Pyrabactin caused a marked reduction in stomatal aperture. The effective concentrations, as well as the effect of pyrabactin on stomatal closure, were quite similar to ABA (Fig. 1). In contrast, apyrabactin did not induce closure of stomata suggesting that only pyrabactin can mimic ABA during stomatal closure. Park et al. (2009) have reported that pyrabactin caused the inhibition of seed germination like ABA, and facilitated the binding of PP2C with PYR1 during seed germination, while apyrabactin did not. These observations demonstrate that pyrabactin is an agonist of ABA, while apyrabactin is not an agonist. Several effects of ABA, such as the inhibition of seed germination or the promotion of stomatal closure are reversed by FC (She et al., 2010; Zeng et al., 2010). The stomatal closure caused by pyrabactin was also reversed completely by FC (Fig. 2), reconfirming our observations that pyrabactin is a strong mimic of ABA, in its effect on stomata.
Signalling components during pyrabactin-induced stomatal closure
The signalling components involved during ABA-induced stomatal closure have been extensively studied. After the initial recognition of ABA signal, through the ABA-PYR/PYL/RCAR-PP2C complex, the ABA-responsive kinases are activated. Subsequently, the guard cell pH becomes alkaline, the membrane-bound NADPH oxidase becomes active, the ROS levels are elevated, followed by a rise in NO levels (Suhita et al., 2004; Bright et al., 2006; Gonugunta et al., 2008; Kim et al., 2010). On exposure to pyrabactin too, there were marked increases in pH, ROS, and NO levels (Fig. 3). Again, apyrabactin, an inactive analogue, did not cause any significant changes in the signalling components of guard cells (Fig. 3). It was therefore concluded that pyrabactin is an active analogue of ABA, in relation to its influence on stomatal function and signal transduction in guard cells. The present study illustrated that pyrabactin could successfully induce stomatal closure and generate small intracellular components, ROS, and NO during stomatal closure besides increasing the cytoplasmic pH. However, the exact mechanism of the induction of these signalling events is not yet known. It is quite possible that pyrabactin induces ROS and NO production in guard cells by the mediation of PYR/PYL/RCARs, PP2Cs, and OST1/SnRK2.6.
The kinetics of the rise in pH/ROS/NO as indicated by the respective fluorophores (Fig. 4) suggested that there was marked similarity in the sequence of changes due to pyrabactin or ABA. The ability of DPI and L-NAME to dampen the pyrabactin-induced rise in ROS/NO indicates that NADPH oxidase and putative NOS play an important role during pyrabactin effects. That the action of either pyrabactin or ABA required the alkalinization of guard cells was evident by the ability of butyrate to prevent the rise in pH as well as closure (Figs 5, 6). The effect of pyrabactin on stomatal closure and its dependence on rise in pH/ROS/NO of guard cells strikes a strong similarity with the action of ABA as well as methyl jasmonate (Suhita et al., 2004; Gonugunta et al., 2008, 2009).
ABA and pyrabactin compete during stomatal closure
Further experiments on stomatal closure in response to varying concentrations of pyrabactin, in the presence of fixed concentration of ABA and vice versa, revealed interesting information on the apparent IC50 and KD values of pyrabactin in relation to ABA (Fig. 7). The method followed here is similar to that used for examining the ethylene effects on bud and flower drop of Begonia in the presence of the gaseous ethylene-binding inhibitor, silver thiosulphate (Serek et al., 1994). The apparent IC50 for pyrabactin did not change much in the presence of ABA or vice versa (Fig. 7a, b). By contrast, the KD of pyrabactin or ABA (about 4–5 mM) was elevated in the presence of ABA or pyrabactin (Fig. 7c, d). These results suggested that pyrabactin was competing with ABA during the induction of stomatal closure, either at the active site or very close to the active site on ABA receptors. These values of IC50 or KD values for pyrabactin or ABA (4–5 μM) appear high compared with the IC50 values reported for ABA (60–125 μM) during interaction with PP2C in vivo using a reconstituted system (Ma et al., 2009; Park et al., 2009). However, it has already been noted that the IC50 values of ABA to interact in vivo with PP2C (60 nM) can vary with that for suppressing root growth (3 μM), as observed by Ma et al. (2009). Similarly, IC50 values of 2–4 μM were reported for pyrabactin during the inhibition of seed germination and hypocotyl elongation (Park et al., 2009).
The limitations of our experiments are acknowledged. For example, only the amount of pyrabactin or ABA in the external medium is known. The actual concentrations of pyrabactin or ABA within the cells (at the ABA receptor level) would be much less. Further, the rate of movement of pyrabactin or ABA across the guard cell could also vary. These factors can explain the differences in the observed KD for pyrabactin/ABA in our experiments (done in vivo) and the values obtained during reconstitution attempts (in vitro) by Park et al. (2009) and Hao et al. (2010). In Arabidopsis seed germination assays, the pyrabactin concentration (100 μM) required to get an effect similar to ABA (10 μM) was almost 10 times higher (Park et al., 2009; Melcher et al., 2010b). However, our major point, that pyrabactin and ABA are competiting with each other, seems to be certain.
Concluding remarks
The ability of pyrabactin, an analogue of ABA, to induce stomatal closure in Pisum sativum leaf abaxial epidermis was as powerful as ABA. This observation opens up an exciting possibility of using pyrabactin as an anti-transpirant. However, it is necessary to explore the possibility of synthesizing pyrabactin and/or analogues at an affordable price for suitable application in agriculture. Since the pattern of signalling components in response to pyrabactin and the reversal of pyrabactin effects by modulators was quite similar to that of ABA, it is suggested that pyrabactin and similar synthetic compounds could be quite useful in studying the signal transduction mechanisms in guard cells as well as in other plant tissues. Being quite similar to ABA in its mode of action, pyrabactin offers a promising potential for use in improving the plant adaptation to drought or other stress conditions.
Acknowledgments
This work was supported by grants (to ASR) from the Department of Biotechnology (DBT, No. BT/PR9227/PBD/16/748/2007), the Council of Scientific and Industrial Research (CSIR, No. 38(1195)/08/EMR-II), the Department of Science and Technology (DST, No. SR/S2/JCB-06/2006), all from New Delhi, India. MR Puli was supported by a Research Fellowship from CSIR, New Delhi. We thank Nalini, Technical Assistant, Central Instrumentation Laboratory, for her help in using the confocal microscope. The departmental facilities were supported by grants from DST-FIST, UGC-SAP-CAS, and DBT-CREBB, all from New Delhi, India.
References
- Acharya BR, Assmann SM. Hormone interactions in stomatal function. Plant Molecular Biology. 2009;69:451–462. doi: 10.1007/s11103-008-9427-0. [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. The Plant Journal. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Christmann A, Moes D, Himmelbach A, Yang Y, Tang Y, Grill E. Integration of abscisic acid signaling in to plant responses. Plant Biology. 2006;8:314–325. doi: 10.1055/s-2006-924120. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annual Review of Plant Biology. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphate pair. Proceedings of the National Academy of Sciences, USA. 2009;106:21425–21430. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonugunta VK, Srivastava N, Puli MR, Raghavendra AS. Nitric oxide production occurs after cytosolic alkalinization during stomatal closure induced by abscisic acid. Plant, Cell and Environment. 2008;31:1717–1724. doi: 10.1111/j.1365-3040.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- Gonugunta VK, Srivastava N, Raghavendra AS. Cytosolic alkalinization is a common and early messenger preceding the production of ROS and NO during stomatal closure by variable signals, including abscisic acid, methyl jasmonate and chitosan. Plant Signaling and Behavior. 2009;4:561–564. doi: 10.4161/psb.4.6.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q, Yin P, Yan C, Yuan X, Li W, Zhang Z, Liu L, Wang J, Yan N. Functional mechanism of the abscisic acid agonist pyrabactin. Journal of Biological Chemistry. 2010;285:28946–28952. doi: 10.1074/jbc.M110.149005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM. Guard cell signaling. Cell. 2001;107:711–714. doi: 10.1016/s0092-8674(01)00606-7. [DOI] [PubMed] [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. Early signal transduction mechanisms: newly discovered components and newly emerging questions. Genes and Development. 2010;24:1695–1708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. A protein kinase-phosphatase pair interacts with an anion channel to regulate ABA signaling. Proceedings of the National Academy of Sciences, USA. 2009;106:21419–21424. doi: 10.1073/pnas.0910601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Assmann SM, Albert R. Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling. PLoS Biology. 2006;4 doi: 10.1371/journal.pbio.0040312. e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- Melcher K, Xu Y, Ng LM, et al. Identification and mechanism of ABA receptor antagonism. Nature Structural and Molecular Biology. 2010a;17:1102–1109. doi: 10.1038/nsmb.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher K, Zhou XE, Xu HE. Thirsty plants and beyond: structural mechanism of abscisic acid perception and signaling. Current Opinion in Structural Biology. 2010b;20:722–729. doi: 10.1016/j.sbi.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J. Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. The Plant Cell. 2001;13:2513–2523. doi: 10.1105/tpc.010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. The Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hancock JT. Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiology. 2002;128:13–16. [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Hitomi K, Arvai AS, Rambo RP, Hitomi C, Cutler SR, Schroeder JI, Getzoff ED. Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science. 2009;326:1373–1379. doi: 10.1126/science.1181829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Sarkeshik A, Nito K, et al. PYR/PYL/RCAR family members are major in vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. The Plant Journal. 2010;61:290–299. doi: 10.1111/j.1365-313X.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, et al. Abscisic acid inhibits type 2c protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA perception and signaling. Trends in Plant Science. 2010;15:395–401. doi: 10.1016/j.tplants.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Seo M, Koshiba T. Complex regulation of ABA biosynthesis in plants. Trends in Plant Science. 2002;7:41–48. doi: 10.1016/s1360-1385(01)02187-2. [DOI] [PubMed] [Google Scholar]
- Serek M, Sisler EC, Reid MS. Novel gaseous ethylene binding inhibitor prevents ethylene effects in potted flowering plants. Journal of the American Society for Horticultural Sciences. 1994;119:1230–1233. [Google Scholar]
- She XP, Huang AX, Li J, Han XZ. Inhibition of dark-induced stomatal closure by fusicoccin involves a removal of hydrogen peroxide in guard cells of Vicia faba. Physiologia Plantarum. 2010;140:258–268. doi: 10.1111/j.1399-3054.2010.01400.x. [DOI] [PubMed] [Google Scholar]
- Sirichandra C, Wasilewska Vlad F, Valon C, Leung J. The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action. Journal of Experimental Botany. 2009;60:1439–1463. doi: 10.1093/jxb/ern340. [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology. 2004;134:1536–1545. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C, Frey NFD, Leung J. An update on abscisic acid signaling in plants and more. Molecular Plant. 2008;1:198–217. doi: 10.1093/mp/ssm022. [DOI] [PubMed] [Google Scholar]
- Yin P, Fan H, Hao Q, Yuan X, Wu D, Pang Y, Yan C, Li W, Wang J, Yan N. Structural insights into the mechanism of abscisic acid signaling by PYL proteins. Nature Structural and Molecular Biology. 2009;16:1230–1237. doi: 10.1038/nsmb.1730. [DOI] [PubMed] [Google Scholar]
- Zeng W, Melotto M, He SY. Plant stomata: a checkpoint of host immunity and pathogen virulence. Current Opinion in Biotechnology. 2010;21:599–603. doi: 10.1016/j.copbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]