Abstract
During seed development and maturation, large amounts of storage proteins are synthesized and deposited in protein storage vacuoles (PSVs). Multiple mechanisms have been proposed to be responsible for transporting storage proteins to PSVs in developing seeds. In this study, a specific antibody was raised against the mung bean (Vigna radiata) seed storage protein 8S globulin and its deposition was followed via immunogold electron microscopy in developing mung bean cotyledons. It is demonstrated that non-aggregated 8S globulins are present in multivesicular bodies (MVBs) in early stages of cotyledon development where neither dense vesicles (DVs) nor a PSV were recognizable. However, at later stages of cotyledon development, condensed globulins were visible in both DVs and distinct MVBs with a novel form of partitioning, with the internal vesicles being pushed to one sector of this organelle. These distinct MVBs were no longer sensitive to wortmannin. This study thus indicates a possible role for MVBs in transporting storage proteins to PSVs during the early stage of seed development prior to the involvement of DVs. In addition, wortmannin treatment is shown to induce DVs to form aggregates and to fuse with the plasma membrane.
Keywords: Dense vesicle, endosome, multivesicular body, protein storage vacuole, storage pre-vacuolar compartment, storage protein, wortmannin
Introduction
Seeds accumulate large amounts of storage proteins during seed development, which will later be utilized to provide nutrients during seed germination and seedling growth (Bewley and Black, 1994). The precursors of these storage proteins are synthesized at the endoplasmic reticulum (ER), and their mature forms are deposited in specialized compartments termed protein storage vacuoles (PSVs; Müntz, 1998; Robinson and Hinz, 1999). Within the seed, they are present in the embryo as well as in the aleurone layer (Bethke et al., 2007). The PSV is unique to plants, and no equivalent organelle is present in animals and yeast (Jiang et al., 2000, 2001; Wang et al., 2009b). PSVs have a nearly pH-neutral lumen suitable for protein storage and are surrounded by limiting membrane marked by the presence of α-tonoplast intrinsic protein (TIP) (Jauh et al., 1999; Jiang et al., 2001). Interestingly, PSVs are not restricted to developing seed tissue, but also occur in cells at the tips of primary roots in young germinating seedlings (Olbrich et al., 2007; Zheng and Staehelin, 2011).
In legumes, the PSV appears to form de novo and replaces the pre-existing lytic-type vacuoles in an autophagic-like manner (Hoh et al., 1995). The major storage proteins in legumes are 7/8S and 11S globulins (Bewley and Black, 1994), and these are transported as trimers from the ER to the Golgi apparatus where they begin to aggregate at the periphery of the cis-cisternae (Müntz, 1998; Hillmer et al., 2001; Vitale and Hinz, 2005). This condensation-based sorting leads to the formation of so-called ‘dense vesicles’ (DVs) which are released from the trans-Golgi network (TGN) after being carried through the stack as a consequence of cisternal maturation (Hohl et al., 1996; Hillmer et al., 2001). Established for developing rice (Oryza sativa) endosperm (Krishnan et al., 1986), faba bean (Vicia faba) (zur Nieden et al., 1984), and pea (Pisum sativum) cotyledons (Hohl et al., 1996; Hinz et al., 1999), the DV pathway has in the meantime been seen to operate in seeds, such as soybean (Glycine max) (Mori et al., 2004), as well as in Arabidopsis thaliana (Otegui et al., 2006; Hinz et al., 2007). It describes a cis-Golgi-based protein sorting mechanism that is unique to plants (Robinson et al., 1998; 2005; Hillmer et al., 2001; Hinz et al., 2007), since it appears to involve a cis-driven condensation of cargo proteins, an event normally occurring at the TGN in mammalian cells exhibiting regulated secretion (Dartsch et al., 1998). Although these morphology-based results have now been supported by differential mutational analysis of the function of post-Golgi SNAREs and coat proteins (Sanmartin et al., 2007; Ebine et al., 2008; Zouhar and Rojo, 2009; Feraru et al., 2010), the mechanism underlying the sorting of globulins into DVs remains obscure, as is the function of the two vacuolar sorting receptor families in plants for DV formation (Castelli and Vitale, 2005; Wenzel et al., 2005; Craddock et al., 2008; von Lüpke et al., 2008; Zouhar and Rojo, 2009; Pompa et al., 2010; H. Wang et al., 2010; Zouhar et al., 2010).
There is genetic and biochemical evidence that both BP-80-type receptors (Shimada et al., 2002, 2003; Jolliffe et al., 2004; Miao et al., 2008; Zouhar et al., 2010) and RMR-type receptors (receptor homology transmembrane-RING H2 domain) (Park et al., 2005, 2007; Wang et al., 2011a) may interact with storage proteins. Hinz et al. (2007) who investigated the relative distribution of these two types of vacuolar sorting receptors in relation to the major storage globulin cruciferin in the Golgi apparatus of developing Arabidopsis embryos demonstrated that formation of DVs precedes the recruitment of vacuolar sorting receptors (VSRs) to the DV. In contrast, the RMR receptor and cruciferin co-localized to the cis-cisternae where DVs are formed.
Not only DV formation but also post-Golgi trafficking of storage globulins to the PSV remains somewhat unclear. In pea (Robinson et al., 1998) as well as in Arabidopsis (Otegui et al., 2006; Hinz et al., 2007). DVs appear to fuse to form a pre-vacuolar compartment (PVC), which may also receive non-storage proteins such as proteases via a second, clathrin-coated vesicle-mediated pathway (Otegui et al., 2006). The question therefore arises of whether these PVCs, although morphologically different, might resemble multivesicular bodies (MVBs) which are the equivalent of the late endosome in plants (Mo et al., 2006; Robinson et al., 2008; Tse et al., 2009; Cai et al., 2011). Recently, Otegui and co-workers (Reyes et al., 2011) have identified two distinct populations of MVBs in aleurone cells of developing maize (Zea mays) seeds, with one population receiving proteins directly from the ER, presumably via an autophagy-related pathway.
The situation has now been reinvestigated during the development of the mung bean (Vigna radiata) seed, using a highly specific antibody generated against an 8S globulin. It has been possible to detect non-aggregated 8S globulins in MVBs at early stages [8 days after flowering (DAF)] of cotyledon development, where neither DVs nor a PSV were recognizable. At later stages (16 DAF) of cotyledon development condensed globulins were visible in both DVs and MVBs. However, the MVBs revealed a novel form of partitioning, with the internal vesicles being pushed to one sector of this organelle. MVBs at this stage of development were no longer sensitive to wortmannin. Nevertheless, it is shown that wortmannin induces DVs to aggregate and subsequently to fuse with the plasma membrane (PM).
Materials and methods
Plant materials
Mung bean seeds were planted in pots in the greenhouse of the Department of Biology, CUHK. Cotyledons of developing mung bean were used for protein extraction and sample preparation for laser confocal microscopy or transmission electron microscopy (TEM).
Isolation and purification of 8S globulin
Total proteins were isolated from 16 DAF developing mung bean cotyledons as described previously (Wang et al., 2007, 2009a, b), followed by separation via 10% SDS–PAGE. Protein bands were visualized via Coomassie Bright Blue staining. The gel slice corresponding to 8S globulin was cut out and further ground to fine powder in liquid nitrogen, followed by the addition of protein extraction solution (50 mM TRIS-HCl, pH 7.4 containing 150 mM NaCl, 1 mM EDTA, 0.1 mM phenylmethylsulphonyl fluoride, and 5 mg ml−1 leupeptin) to release proteins. The supernatant containing 8S globulin was then recovered from the gel mixtures by centrifugation. Furthermore, the purity and yield of isolated 8S globulin was examined via SDS–PAGE.
Antibodies
Generation of 8S globulin antibody was as follows: purified 8S globulin was injected into rabbits and serum was collected by centrifugation. Affinity column-purified 8S globulin antibodies were obtained by passing serum through a 8S globulin column. Polyclonal VSRat-1 and Rha1 antibodies have been previously described (Lee et al., 2004; Tse et al., 2004). Immunoblotting analysis was performed with various antibodies at 4 mg ml−1 as described previously (Tse et al., 2004; J. Wang et al., 2010; Shen et al., 2011; Wang et al., 2011b).
Confocal immunofluorescence studies
Fixation and preparation of tissues from cotyledons of developing mung bean for paraffin-embedded sections, and their labelling and analysis by confocal immunofluorescence have been described previously (Jiang and Rogers, 1998; Jiang et al., 2000; Li et al., 2002). Cotyledons were cut into small cubes, followed by incubation in fixation solution containing 10% (v/v) formaldehyde, 50% (v/v) ethanol, and 5% (v/v) acetic acid for 24 h. The dehydration and infiltration of samples were performed with the Enclosed Tissue Processor Leica TP-1050 (Leica Microsystem Ltd), followed by sample embedding in paraffin blocks. Thin paraffin-embedded sections were sequentially de-paraffinized in 100% (v/v) xylene, 100% (v/v) ethanol, 90% (v/v) ethanol, 80% (v/v) ethanol, 70% (v/v) ethanol, 50% (v/v) ethanol, 30% (v/v) ethanol, and ddH2O for 2 min each step. The de-paraffinized sections were used for immunolabelling with anti-8S globulin antibodies at 2 μg ml−1. For single labelling, de-paraffinized sections were blocked in 5% (w/v) milk overnight, followed by washing with PBST (phosphate-buffered saline–Tween-20) for 2× 10 min. Samples on slides were circled using a pad pen before washing with PBST containing 1% (w/v) bovine serum albumin (BSA). Sections were incubated with primary antibody for 3–4 h, followed by washing with PBST for 3× 10 min. Sections were then incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:100) for 1 h, followed by washing with PBST for 1 h. The slide was blotted dry before Mowiol was added to the samples. After the slide was covered by a cover slip, samples were then examined with a confocal microscope (Bio-Rad Radiance 2100 system, Hemel Hempstead, UK). Images were processed using Adobe PhotoShop software (San Jose, CA, USA).
Electron microscopy studies
The general procedures for conventional ultrathin sectioning of chemically fixed samples of mung bean were performed essentially as described previously (Ritzenthaler et al., 2002; Wang et al., 2007). Preparation of high-pressure freezing/frozen substituted samples of mung bean has been described previously (Wang et al., 2007, 2009a, b). For the preparation of brefeldin A- (BFA) and wortmannin-treated samples of mung bean, mung bean seeds at 8 DAF and 16 DAF were cut across into two halves with a sharp blade, followed by their cut surfaces being immersed in MS (Murashige and Skoog) medium containing BFA (10 μg ml−1) or wortmannin (33 μM) for 2 h at room temperature. Subsequently, thin sections cut with a razor blade from the surfaces of the mung bean cotyledons were frozen in a high-pressure freezing apparatus (EMP2, Leica, Bensheim, Germany). Substitution was carried out in dry acetone containing 0.1% uranyl acetate at –85 °C in an AFS freeze-substitution unit (Leica, Wetzlar, Germany). Infiltration with HM20, embedding, and UV polymerization were performed stepwise at –35 °C. The single immunogold labelling on ultrathin sections with anti-VSRAt-1 (40 μg ml−1), anti-Rha1 (40 μg ml−1), or anti-8S globulin (10 μg ml−1) was performed using standard procedures (Tse et al., 2004, 2006; Lam et al., 2008).
For double immunogold labelling, sections were first blocked by floating on 3% (w/v) BSA for 20 min on solution before being incubated with the first primary antibody for 2 h. Sections were washed with 1% (w/v) BSA for 10 min three times, followed by incubation with the first secondary antibody conjugated to immunogold for 1 h. Sections were then washed with 1% (w/v) BSA and ddH2O for 5 min twice each before post-fixation with 1% (v/v) glutaraldehyde in 1× PBS for 10 min. Sections were washed with 1× PBS, followed by blocking with 0.02% (w/v) glycine in 1× PBS for 10 min. Sections were washed with 1× PBS for 10 min three times before incubation with the second primary antibody for 2 h. Sections were washed with 1% (w/v) BSA for 10 min three times, followed by incubation with the second secondary antibody conjugated to immunogold for 1 h. Sections were washed with 1% (w/v) BSA and ddH2O for 5 min twice each, followed by post-staining with aqueous uranyl acetate/lead citrate, prior to TEM examination using a Hitachi H-7650 transmission electron microscope with a CCD camera (Hitachi High-Technologies Corporation, Japan) operating at 80 kV.
Results
Characterization of storage protein synthesis in developing mung bean
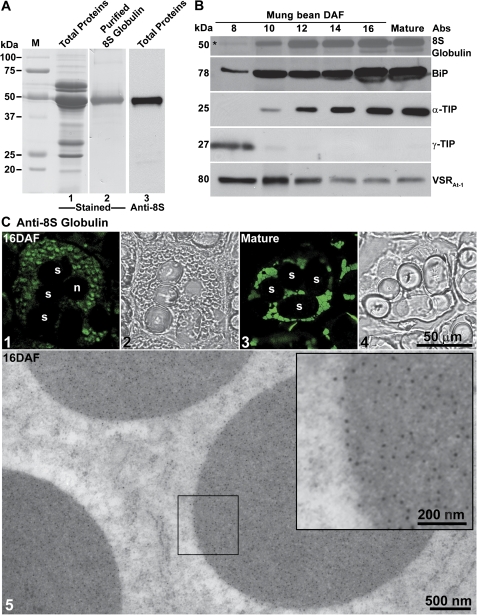
In mature mung bean seeds, the major storage protein is an 8S globulin (Bernardo et al., 2004). In order to detect and visualize this storage protein during seed development, a rabbit polyclonal antiserum was raised against purified 8S globulins (Fig. 1A, lanes 1 and 2). The specificity of the 8S globulin antibodies was then analysed by western blotting of the total proteins extracted from mung bean seeds. A single band was detected in the western blot, indicating the high specificity of the 8S globulin antibodies (Fig. 1A, lane 3). In order to chart the time course of storage protein synthesis during cotyledon development, seeds were collected at different DAF and processed for western blotting using different antibodies, including the 8S globulin antiserum.
Fig. 1.
Generation and characterization of 8S globulin antibodies in developing mung bean seeds. (A) Generation of 8S globulin antibodies. 8S globulin proteins (lane 2) were purified from the total protein extracts of developing mung bean cotyledons (lane 1) and used as antigens for rabbit injection. 8S globulin antibodies were purified by an affinity column coupled with the 8S globulin proteins for western blot analysis that recognized 8S globulin specifically (lane 3). (B) Protein profiles during mung bean seed development and maturation. Total proteins were extracted from mung bean cotyledons at different developmental stages (days after flowering, DAF), followed by protein separation via SDS–PAGE and western blot analysis using various antibodies as indicated. The detected protein bands with their expected sizes in kDa are shown. An asterisk indicates the weak protein band at 8 DAF as detected by anti-8S glubulin. (C) Subcellular localization of 8S globulin. (Panels 1–4) Developing and mature mung bean cotyledons were fixed and labelled with anti-8S globulin antibodies. Images were collected with a confocal microscope. (Panel 5) Ultrathin sections prepared from high-pressure frozen/freeze-substituted mung bean cotyledons (at 16 DAF) were labelled with 8S globulin antibodies. The inserted image in panel 5 is an enlarged image of the indicated area showing the gold particle labelling. n, nucleus; PSV, protein storage vacuole; s, starch. Scale bars are included as indicated. (This figure is available in colour at JXB online.)
As shown in Fig. 1B, 8S globulins were first detected in western blots with a weak signal at ∼8 DAF, followed by a steep increase at ∼10–12 DAF and further accumulated with seed development (Fig. 1B). Concomitant with this huge level of expression of luminal cargo protein, the levels of the ER-located chaperone protein, BiP, increased accordingly to ensure correct protein folding (Fig. 1B). A similar increase in the amounts of the characteristic marker protein for PSVs, α-TIP, was also observed. Conversely, the levels of γ-TIP, a marker of lytic vacuoles, declined rapidly and was no longer detectable after 10 DAF, indicating that the lytic vacuoles were gradually disappearing and being replaced by the PSVs to accommodate the storage proteins (Fig. 1B). VSR proteins were present throughout cotyledon development, proportionately more being detectable in the earlier stages of development (Fig. 1B).
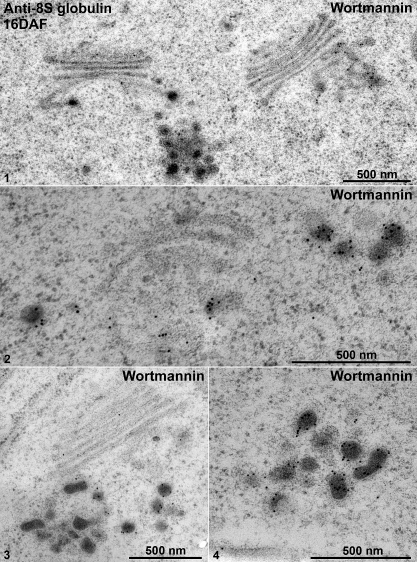
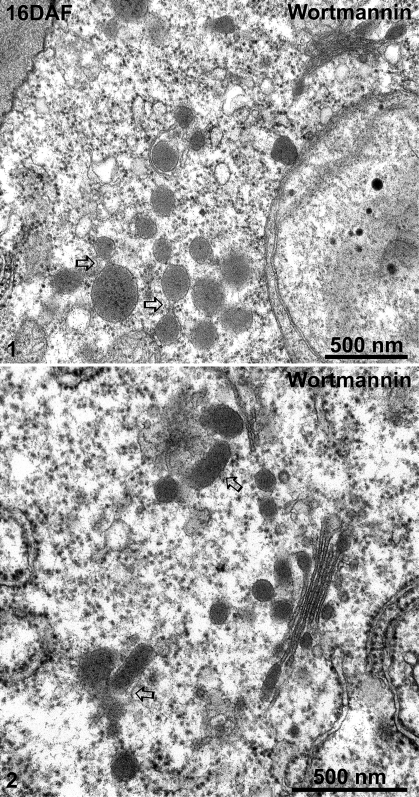
Fig. 8.
IEM analysis of Golgi and dense vesicles (DVs) in wortmannin-treated 16 DAF developing mung bean seeds. Developing mung bean cotyledons at 16 DAF were first treated with wortmannin, followed by high-pressure frozen/freeze-substitution (HPF) and IEM labelling using 8S globulin antibodies as indicated. Shown are representative examples of the Golgi and DVs in these wortmannin-treated cells. Scale bars=500 nm.
Confocal immunofluorescence microscopy was performed on sections cut from different stages in cotyledon development in order to monitor for storage protein deposition at the organelle level. Small 8S globulin-positive punctae were visible at intermediate stages of cotyledon development (16 DAF) (Fig. 1C, panel 1). These became larger and more frequent at later stages (Fig. 1C, panel 3). Such accumulations of 8S globulin in PSVs were also confirmed by immunogold electron microscopy (IEM) using anti-8S globulin antibodies and 16 DAF cotyledons in which dense gold labelling over the electron-opaque contents of PSVs was observed (Fig. 1C, panel 5).
Multivesicular pre-vacuolar compartments alter in morphology during seed development
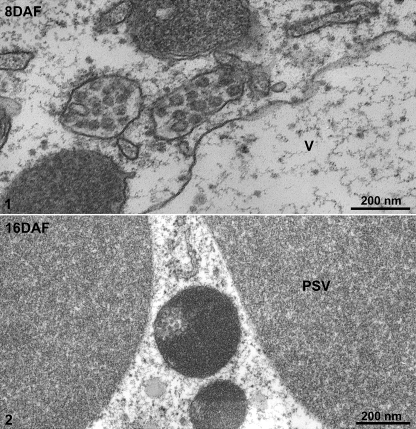
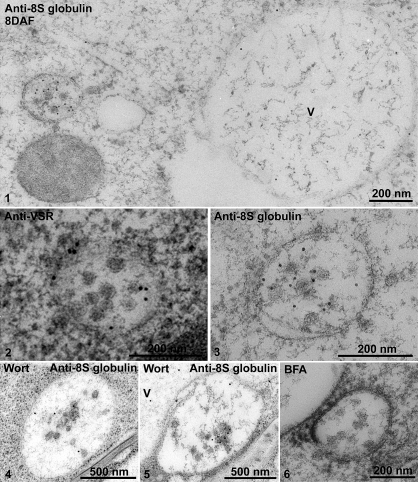
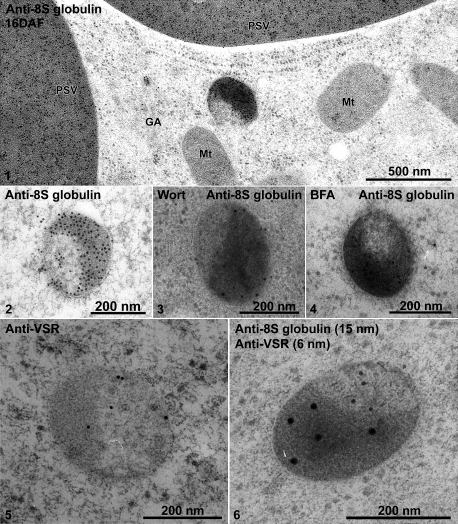
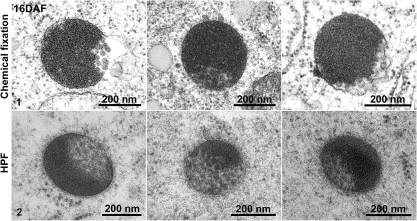
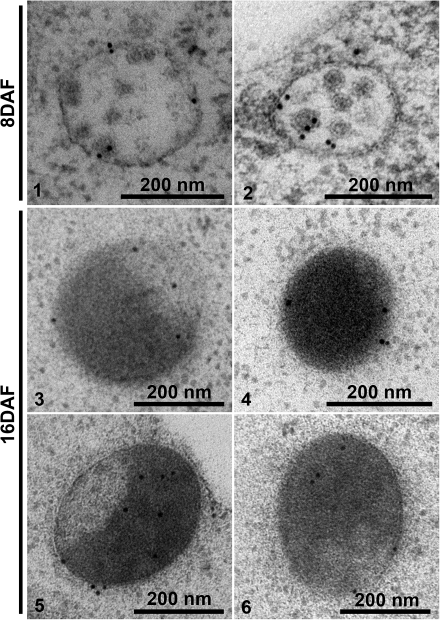
At the stages of cotyledon development when storage globulin synthesis was just beginning (8 DAF) typical MVBs were present (Fig. 2, panel 1). During the stage of maximal storage globulin deposition, similarly sized structures were observed which were partially filled with aggregates of storage globulins (Fig. 2, panel 2). Interestingly, the MVBs in 8 DAF cotyledons were labelled strongly with both the 8S globulin antibody and VSR antibodies (Fig. 3, panels 1–3). At 16 DAF, the PVCs also labelled positively with both antibodies, but a stratified distribution was visible (Fig. 4, panels 1 and 2). The electron-opaque sector labelled intensely with the 8S globulin antibody, but was nearly absent over the lighter, less-dense sector, which upon closer inspection contained small vesicles. This was better seen in chemically fixed samples than in cryofixed specimens (Fig. 5, panel 1). Conversely, labelling with the VSR antibody was conspicuously restricted to the sector with the internal vesicles (Table 1; Fig. 4, panels 5 and 6).
Fig. 2.
Ultrastructural analysis of the developing mung bean seeds. Developing mung bean cotyledons at 8 DAF and 16 DAF were collected, osmium fixed, and embedded in Spurr’s resin. Panel 1 shows MVBs and the vacuole (V) at 8 DAF, while panel 2 shows a PSV and distinct MVBs at 16 DAF. DAF, days after flowering; V, vacuole; PSV, protein storage vacuole. Scale bars are included as indicated.
Fig. 3.
IEM analysis of MVBs of 8 DAF developing mung bean seeds. Developing mung bean cotyledons at 8 DAF were collected, high-pressure frozen/freeze-substituted, and embedded in HM20. Ultrathin sections were then prepared, followed by IEM analysis using 8S globulin and VSR antibodies as indicated. Panels 1–3, MVBs labelled with anti-8S globulin and anti-VSR in untreated cells. Panels 4 and 5, samples treated with wortmannin. Panel 6, sample treated with BFA. VSR, vacuolar sorting receptor; V, vacuole; Wort, wortmannin; BFA, brefeldin A. Scale bars are included as indicated.
Fig. 4.
IEM analysis of distinct MVBs of 16 DAF developing mung bean seeds. Developing mung bean cotyledons at 16 DAF were collected, high-pressure frozen/freeze-substituted, and embedded in HM20. Ultrathin sections were then prepared, followed by IEM analysis using 8S globulin and VSR antibodies as indicated. Panel 1, an overview of labelling of anti-8S globulin on various organelles including the Golgi (GA), PSV, and the distinct MVB (also shown in panel 2). Panels 3 and 4, the 8S globulin-positive distinct MVBs in samples treated with wortmannin and BFA, respectively. Panels 5 and 6, distinct MVBs labelled with anti-VSR (panel 5) and double-labelled with anti-8S globulin and anti-VSR (panel 6). VSR, vacuolar sorting receptor; GA, Golgi apparatus; Mt, mitochondrion; PSV, protein storage vacuole; Wort, wortmannin; BFA, brefeldin A. Scale bars are included as indicated.
Fig. 5.
Ultrastructural analysis of the distinct MVBs in 16 DAF developing mung bean seeds. Developing mung bean cotyledons at 16 DAF were collected, followed by either chemical fixation (panel 1) or high-pressure frozen/freeze-substitution (HPF) (panel 2) for structural EM analysis. Scale bars are included as indicated.
Table 1.
Distribution of gold particles (GPs) on novel storage PVCs
| Antibody | No. (%) of GPs in protein aggregate area | No. (%) of GPs in internal vesicle area | Total organelles | Total GPs |
| Anti-8S globulin | 1320 (95%) | 70 (5%) | 20 | 1390 |
| Anti-VSR | 26 (24.7%) | 79 (75.3%) | 20 | 105 |
A Rha1 antibody was used to characterize the MVBs/PVCs further in developing mung bean cotyledons. Rha1 was previously reported to localize to PVCs in Arabidopsis cells and plays a crucial role in mediating the transport of soluble cargo proteins to the vacuole (Sohn et al., 2003; Lee et al., 2004). In this study, Rha1 was found to localize to these two types of MVBs/PVCs in both 8 DAF and 16 DAF developing mung bean cotyledon using IEM (Fig. 6).
Fig. 6.
IEM analysis of MVBs in developing mung bean seeds using anti-Rha1 antibody. Developing mung bean cotyledons at 8 DAF and 16 DAF were collected, high-pressure frozen/freeze-substituted, and embedded in HM20. Ultrathin sections were then prepared, followed by IEM analysis using anti-Rha1 antibodies as indicated. Panels 1 and 2, Rha1-positive MVBs in 8 DAF mung bean cotyledons. Panels 3–6, distinct MVBs labelled with anti-Rha1 antibody in 16 DAF mung bean cotyledons. Scale bars are included as indicated.
The two types of multivesicular PVCs differed in their response to wortmannin. This phosphatidylinositol 3-kinase (PI 3-kinase) inhibitor is well known to cause the enlargement of MVBs/PVCs (Tse et al., 2004; Miao et al., 2006; Lam et al., 2009), probably through homotypic fusion (Wang et al., 2009a), and does so for the MVBs in 8 DAF cotyledons (Table 2; Fig. 3, panels 4 and 5; Supplementary Fig. S1 available at JXB online). In contrast, MVBs containing the storage globulin in 16 DAF cotyledons showed no alteration in morphology after wortmannin treatment (Table 2; Fig. 4, panel 3). This seeming insensitivity to wortmannin was not due to a loss of wortmannin activity through inactivation or insufficient uptake, since there was indeed a wortmanin effect visible. In addition, control experiments with BFA treatment did not alter the morphology of these two types of multivesicular PVCs (Fig. 3, panel 6; Fig. 4, panel 4).
Table 2.
Effect of wortmannin treatment on the size of MVBs/PVCs in 8 DAF and16 DAF developing mung bean cotyledons
| Organelle | Wortmannin | Average size (diameter, nm) | Standard deviation of organelle size | No. of organelles counted |
| MVBs/PVCs in 8 DAF | Untreated | 350** | 71 | 30 |
| Treated | 1101** | 294 | 30 | |
| MVBs/PVCs in 16 DAF | Untreated | 349NS | 40 | 32 |
| Treated | 344NS | 45 | 32 |
Significant differences between wortmannin-untreated and treated MVBs/PVCs were analysed using two-tailed paired t-test (**P < 0.001; NS, non-significant). Data were collected and analysed from three independent experiments.
Strikingly, the distribution of a VSR homologue across the MVB/PVC differs with respect to the developmental stage: Whereas in 8 DAF, the VSR1 is localized at the outer membrane, in 16 DAF the VSR1 was mainly found on internal vesicles (found both at the outer membrane and on the internal vesicles) (Table 3). In contrast, in the developing Arabidopsis embryo the VSR1 was still mainly localized on the outer membrane of storage PVCs (Supplementary Fig. S2 at JXB online).
Table 3.
Distribution of immunogold VSRs across MVBs/PVCs in developing mung bean
| Samples | No. (%) of GPs inside MVBs/PVCs | No. (%) of GPs on MVB/PVC membrane | Total organelles | Total GPs |
| 8 DAF | 20 (18.5%) | 88 (81.5%) | 20 | 108 |
| 16 DAF | 77 (73.3%) | 28 (26.7%) | 20 | 105 |
DVs are first recognizable after 10 DAF, but 8S globulins are detectable in the Golgi apparatus earlier in development
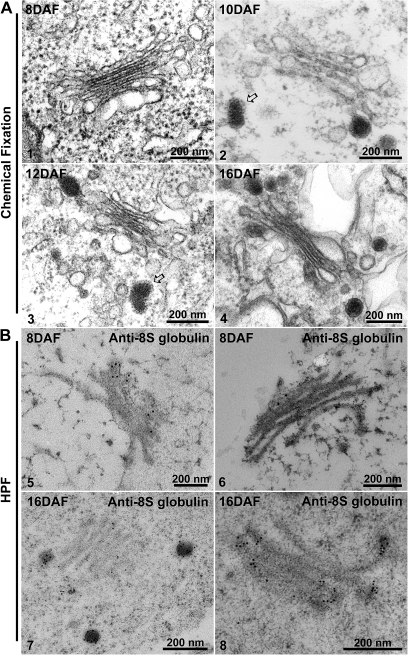
Because of their osmophilicity, DVs are best recognizable in chemically fixed samples. In such specimens, DVs were first identified in 10 DAF cotyledons (Fig. 7 compare panels 1 and 2), and were easily found in cotyledonary cells at later stages (Fig. 7, panels 3 and 4). As previously recorded for DVs in pea (Hohl et al., 1996) and Arabidopsis cotyledons (Hinz et al., 2007), the DVs in bean were frequently observed to have a clathrin-type coat at the level of the TGN/early endosome (see arrows in Fig. 7, panels 2 and 3). IEM was performed with 8S globulin antibodies on sections from 8 DAF and 16 DAF cryofixed cotyledons, and a positive labelling for Golgi stacks was obtained in both samples. As in the western blots (Fig. 1B), the labelling density across the stack sharply increased from 8 DAF with, on average, 2.5 gold dots per Golgi stack to up to 11.3 gold dots per stack at 16 DAF. The majority of the gold label in the 16 DAF samples was associated with DVs (Fig. 7, panels 7 and 8). The labelling displayed a gradient across the stack, with the strongest label in the cis-half and the rim of the stack (Table 4). In 8 DAF stacks, in contrast, the gradient of 8S globulin labelling was less steep (Table 4). No DVs were visible at 8 DAF. Thus, DV formation seems to be correlated with the strong increase in expression of 8S globulin. Furthermore, the sorting of 8S globulin in 8 DAF cotyledons seems to occur independently of DV formation.
Fig. 7.
Ultrastructural and IEM analysis of Golgi and dense vesicles (DVs) in developing mung bean seeds. Structural EM analysis. Developing mung bean cotyledons at various stages (8–16 DAF) as indicated were collected, followed by chemical fixation for structural EM analysis. (Panels 1–4) The arrows in panels 2 and 3 point to clathrin caps at the DV (see the Results). IEM analysis. Developing mung bean cotyledons at various stages (8 DAF and 16 DAF) as indicated were collected, followed by high-pressure frozen/freeze-substitution (HPF) and IEM labelling using 8S globulin antibodies as indicated. Scale bars=200 nm.
Table 4.
Distribution of 8S globulin across Golgi stacks of cotyledons of 8 DAF and 16 DAF developing mung bean
| Development stage |
cis to trans |
Rim–Cisternae |
|||
| cis | Medium | trans | Rim | Cisternae | |
| 8 DAF | 50 (40.7%) | 38 (30.1%) | 35 (29.2%) | 81 (65.9%) | 42 (34.1%) |
| 16DAF | 409 (62.6%) | 187 (28.6%) | 57 (8.8%) | 510 (78.1%) | 143 (21.9%) |
A total numbers of 50 and 58 Golgi stacks from 8 DAF and 16 DAF, respectively were counted for the presence of gold particles.
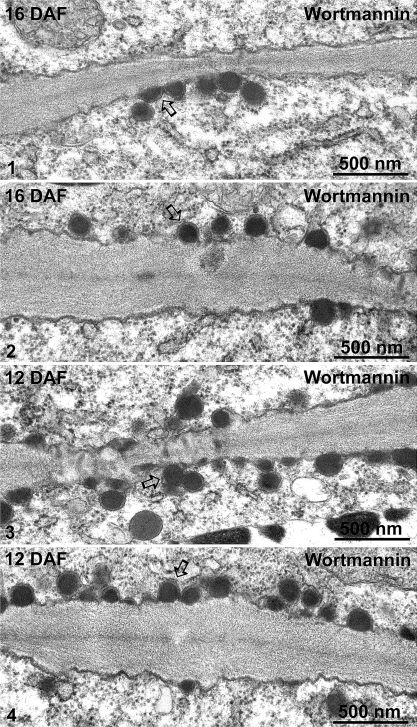
The 8S globulin-positive DVs of wortmannin-treated cotyledons at 16 DAF in both high-pressure frozen (Fig. 8, panels 1–4) and chemically fixed samples (Fig. 9, panels 1 and 2) were seen to accumulate as large clusters adjacent to or occasionally at some distance from the Golgi stack. Sometimes, DVs within these clusters even seem to fuse. Interestingly, wortmannin also seems to induce a massive fusion of electron-opaque vesicles with the plasma membrane (PM) in both 16 DAF and 12 DAF cotyledons (Fig. 10, panels 1–4).
Fig. 9.
Ultrastructural analysis of dense vesicles (DVs) in wortmannin-treated 16 DAF developing mung bean seeds. Developing mung bean cotyledons at 16 DAF were collected and treated with wortmannin then chemically fixed and embedded in Spurr’s resin for EM observations. Panels 1 and 2 show examples of aggregation/clustering of DVs. Arrows indicate examples of possible fusion of DVs. DAF, days after flowering. Scale bars=500 nm.
Fig. 10.
Ultrastructural analysis of the fusion profiles between dense vesicles (DVs) and plasma membrane (PM) in wortmannin-treated developing mung bean seeds. Developing mung bean cotyledons at 16 DAF and 12 DAF were treated with wortmannin then chemically fixed and embedded in Spurr’s resin for EM observations. Panels 1–4 show examples of possible fusion profiles between DVs and the PM. Arrows indicate examples of possible DV–PM fusions. DAF, days after flowering. Scale bars=500 nm.
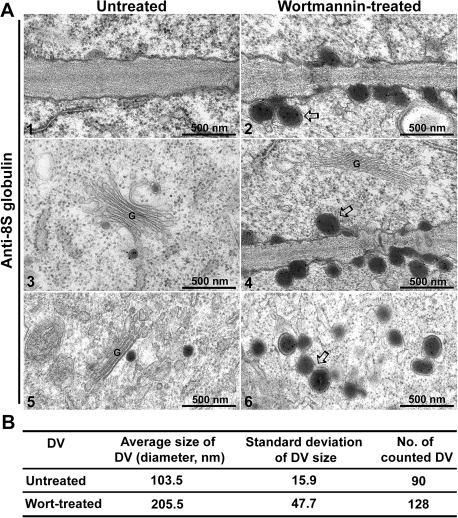
An IEM study with 8S globulin antibodies was carried out in both wortmannin-treated and untreated developing mung bean cotyledons, to characterize further the DVs and the secretory vesicles. As shown in Fig. 11, similar to previous observations (Fig. 10), in wortmannin-treated samples the 8S globulin was secreted via electron-opaque vesicles fused with the PM into the cell wall (Fig. 11A, panels 2, 4 and 6). Such structures were never seen in untreated samples (Fig. 11A, panels 1, 3, and 5). With an average diameter of 205 nm these vesicles in wortmannin-treated samples are clearly larger than the DVs in untreated samples which have a diameter of ∼103 nm (Fig. 11A, panels 1–6, 11B).
Fig. 11.
ImmunoEM study of DV profiles in wortmannin-treated versus untreated developing mung bean cotyledons. Developing mung bean cotyledons were treated or untreated with wortmannin as indicated, followed by sample preparation for immunoEM labelling with anti-8S globulin antibodies and TEM observations. (A) Ultrathin sections were labelled with anti-8S globulin for immunogold study. Panels 1, 3, and 5 show examples of PM and Golgi/DVs in untreated control samples, whereas panels 2, 4, and 6 show examples of PM–DV fusion and DV aggregation/fusion in wortmannin-treated samples. Arrows indicate examples of possible DV–PM fusions (panels 2 and 4) or DV fusion/aggregation (panel 6) in wortmannin-treated samples. Scale bar=500 nm. (B) Analysis of average diameter size of DVs (in nm) in wortmannin-treated versus untreated developing mung bean cotyledons.
Discussion
The DV pathway for sorting and transport of storage proteins
Several reports have stressed the role of aggregation for the formation of DVs. Pompa et al. (2010) have demonstrated that homotypic aggregation of phaseolin may already occur in the lumen of the ER. von Lüpke et al. (2008) have shown that binding of storage proteins to endomembranes from pea cotyledons requires the presence of a second, still unknown, soluble protein. Shortly afterwards or even perhaps concomittant with their binding to endomembranes, the storage proteins form larger heterotypic aggregates, which appear to serve as aggregation nuclei for the capture of globulins. These complexes are then somehow transported laterally into nascent DVs where further aggregation takes place. Binding and aggregation are pH dependent, being optimal at slightly acidic pH (as discussed in von Lüpke et al., 2008). Presumably due to the sequential expression of the different globulins, in pea the cargo of DVs shows a stratified appearance (Wenzel et al., 2005).
The results presented in this report indicate that in early stages of embryo development 8S globulins are sorted into the PSV independently of the formation of DVs, and that formation of DVs correlates with an increased amount of storage proteins present in the Golgi apparatus.
A similar effect has been discussed for the sorting of endogenous aleurain in developing cotyledons of A. thaliana (Hinz et al., 2007). Aleurain, a vacuolar protease bearing a sequence-specfic vacuolar sorting determinant (ssVSD), and thus normally being used as a classical non-DV transport marker, becomes accidentally trapped in DVs by the bulk flow of the storage proteins in later stages of seed development.
These results strongly suggest that the formation of DVs seems to be a passive, aggregation-driven process with low specificity. Furthermore, these results also indicate that the formation of DVs may not be a prerequisite for the sorting of storage proteins into the emerging PSV. In the light of these results, the function of receptors for the formation of DVs might also be called into question, as already discussed by von Lüpke et al. (2008). Although the formation of DVs at the cis-half of the stack clearly precedes the recruitment of VSRs into DVs at the trans-Golgi and early endosome, storage proteins do show a partial missorting into the PM in VSR deletion mutants (Shimada et al., 2003; Hinz et al., 2007; Hunter et al., 2008, Zouhar et al., 2010). This apparent discrepancy may now be explained by the observation that the sorting of storage proteins may switch from a more receptor-mediated to a more aggregation-driven mode in later stages of seed development. The signal in the cell wall, which was detected during the analysis of the mutants, resembles the total amount of accumulated protein being secreted over the whole period of seed development without any resolution in time. The effect observed, therefore, may be largely due to a mistargeting of storage proteins in early stages of seed development in which there are no DVs present.
The presence of two alternative, functionally distinct populations of late endosomes
The assumption that in later stages of seed development a second, aggregation-driven sorting mechanism takes over the transport of vacuolar proteins is further supported by the effect of wortmannin. This drug is a specific inhibitor of the PI 3-kinase, an enzyme involved in several essential steps of vacuole biogenesis: autophagy, retromer-mediated cargo receptor recycling from endosomes, and the formation of the internal vesicles of the MVB (Lam et al., 2007a, b, 2009). The inhibition of PI 3-kinase by wortmannin leads to a swelling of the MVB: in mammalian cells and in yeast, it has been discussed that this effect may be due to an inhibition of retrograde transport leading to an accumulation of surplus membrane from the anterograde vesicles. In plants, a swelling of the PVC also occurs but apparently due to a wortmannin-induced fusion of MVBs, in combination with a block on the internalization of membranes into the MVB (Wang et al., 2009a).
In this report, a differential effect of the drug wortmannin on the organelles of the post-Golgi secretory pathway of developing mung bean cotyledons is described. Whereas in 8 DAF cotyledons the MVBs swell after wortmannin treatment, the MVBs in 16 DAF cotyledons did not.
It was not possible to test for a possible inhibitory effect of wortmannin on the transport of the 8S globulin into the vacuole in the mung bean cotyledons. A heterologous transgenic transport assay was not carried out because the observed wortmannin effect has not been observed in any other tissue, and the results obtained would not necessarily reflect the in situ physiological situation (as discussed in Robinson et al., 2005). Nevertheless, the observed large accumulations of DVs, together with the observation of slightly larger electron-opaque vesicles fusing with the PM, might indicate that there may indeed be an additional and different effect of wortmannin on the transport of storage proteins into the PSV.
Vacuolar storage proteins may bear one or several of at least three different vacuolar sorting determinants (VSDs): a C-terminal VSD (ctVSD), a sequence-specific VSD (ssVSD), and a protein structure-dependent VSD (psVSD) (Vitale and Hinz, 2005). The VSD of mung bean 8S globulin is not known. There are several reports about differential effects of wortmannin on the sorting of proteins with distinct VSDs. Matsuoka et al. (1995) showed that wortmannin leads to a secretion of barley (Hordeum vulgare) lectin, a protein with a ctVSD, whereas sporamin, a protein bearing a ssVSD, was not secreted. This was later confirmed by Koide et al. (1999) who showed that whereas the sorting of a molecular transport marker bearing a ctVSD was inhibited, the sorting of the same molecular transport marker bearing a ssVSD was not. In contrast, daSilva et al. (2005) clearly demonstrated the wortmannin-induced secretion of a protein bearing an ssVSD, although they did not follow the targeting of a ctVSD protein in their investigation. Finally, Utsumi and co-workers (Maruyama et al., 2006) investigated the sorting of soybean glycinin, a protein bearing a distinct VSD. Although they did not show secretion of the storage proteins, sorting of the proteins into the PSV was blocked and the proteins appeared to be trapped in ill-defined post-Golgi compartments. These punctate compartments could be the DV agglomerations, as reported here.
Because wortmannin inhibits PI 3-kinase, Maruyama speculated that there must exist some lipid PI 3-kinase-dependent phosphorylation reaction prior to the fusion of the DV with the MVB which is unique to the DV-mediated transport of storage proteins (Maruyama et al., 2006). According to the present results, this reaction may be involved directly in the fusion of DVs with MVBs and, because anterograde transport is blocked before DV fusion, this pre-MVB block may in turn explain why the MVBs in later stages do not swell; that is, there is simply not enough membrane available to do so.
The inhibitory effect of wortmannin on the transport into the lytic vacuole is often explained to be due to a depletion of receptors at the TGN (daSilva et al., 2005). This causes the proteins to be redirected into the bulk flow pathway and to become secreted. In the case of seeds, however, the storage proteins continue to exit the Golgi but are not secreted, instead they accumulate within the cell in some kind of wortmannin-induced DV compartment.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. IEM analysis of wortmannin-induced enlarged MVBs/PVCs of 8 DAF developing mung bean cotyledons using anti-VSR and anti-8S globulin as indicated.
Figure S2. IEM analysis of MVBs/PVCs in developing mung bean (8 DAF and 16 DAF) and Arabidopsis (12 DAF) seeds using anti-VSRat-1 antibody as indicated.
Acknowledgments
This work was supported by grants from the Research Grants Council of Hong Kong (CUHK465708, CUHK466309, CUHK466610, CUHK466011, and HKUST6/CRF/08) and CUHK Schemes B/C to LJ, as well as from the German Research Council (Ro 440/14-1) to DGR.
References
- Bernardo AE, Garcia RN, Adachi M, Angeles JG, Kaga A, Ishimoto M, Utsumi S, Tecson-Mendoza EM. 8S globulin of mungbean [Vigna radiata (L.) Wilczek]: cloning and characterization of its cDNA isoforms, expression in Escherichia coli, purification, and crystallization of the major recombinant 8S isoform. Journal of Agricultural and Food Chemistry. 2004;52:2552–2560. doi: 10.1021/jf0305938. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Libourel IG, Aoyama N, Chung YY, Still DW, Jones RL. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiology. 2007;143:1173–1188. doi: 10.1104/pp.106.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. 2nd edn. New York: Plenum Press; 1994. [Google Scholar]
- Cai Y, Jia T, Lam SK, Ding Y, Gao C, San MW, Pimpl P, Jiang L. Multiple cytosolic and transmembrane determinants are required for the trafficking of SCAMP1 via an ER–Golgi–TGN–PM pathway. The Plant Journal. 2011;65:882–896. doi: 10.1111/j.1365-313X.2010.04469.x. [DOI] [PubMed] [Google Scholar]
- Castelli S, Vitale A. The phaseolin vacuolar sorting signal promotes transient, strong membrane association and aggregation of the bean storage protein in transgenic tobacco. Journal of Experimental Botany. 2005;56:1379–1387. doi: 10.1093/jxb/eri139. [DOI] [PubMed] [Google Scholar]
- Craddock CP, Hunter PR, Szakacs E, Hinz G, Robinson DG, Frigerio L. Lack of a vacuolar sorting receptor leads to non-specific missorting of soluble vacuolar proteins in Arabidopsis seeds. Traffic. 2008;9:408–416. doi: 10.1111/j.1600-0854.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- Dartsch H, Kleene R, Kern HF. In vitro condensation-sorting of enzyme proteins isolated from rat pancreatic acinar cells. European Journal of Cell Biology. 1998;75:211–222. doi: 10.1016/S0171-9335(98)80115-5. [DOI] [PubMed] [Google Scholar]
- daSilva LL, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F, Denecke J. Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. The Plant Cell. 2005;17:132–148. doi: 10.1105/tpc.104.026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebine K, Okatani Y, Uemura T, et al. A SNARE complex unique to seed plants is required for protein storage vacuole biogenesis and seed development of Arabidopsis thaliana. The Plant Cell. 2008;20:3006–3021. doi: 10.1105/tpc.107.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E, Paciorek T, Feraru MI, Zwiewka M, De Groodt R, De Rycke R, Kleine-Vehn J, Friml J. The AP-3 beta adaptin mediates the biogenesis and function of lytic vacuoles in Arabidopsis. The Plant Cell. 2010;22:2812–2824. doi: 10.1105/tpc.110.075424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer S, Movafeghi A, Robinson DG, Hinz G. Vacuolar storage proteins are sorted in the cis-cisternae of the pea cotyledon Golgi apparatus. Journal of Cell Biology. 2001;152:41–50. doi: 10.1083/jcb.152.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz G, Colanesi S, Hillmer S, Rogers JC, Robinson DG. Localization of vacuolar transport receptors and cargo proteins in the Golgi apparatus of developing Arabidopsis embryos. Traffic. 2007;8:1452–1464. doi: 10.1111/j.1600-0854.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- Hinz G, Hillmer S, Baumer M, Hohl Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the golgi apparatus of developing pea cotyledons in different transport vesicles. The Plant Cell. 1999;11:1509–1524. doi: 10.1105/tpc.11.8.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh B, Hinz G, Jeong BK, Robinson DG. Protein storage vacuoles form de novo during pea cotyledon development. Journal of Cell Science. 1995;108:299–310. doi: 10.1242/jcs.108.1.299. [DOI] [PubMed] [Google Scholar]
- Hohl I, Robinson DG, Chrispeels MJ, Hinz G. Transport of storage proteins to the vacuole is mediated by vesicles without a clathrin coat. Journal of Cell Science. 1996;109:2539–2550. doi: 10.1242/jcs.109.10.2539. [DOI] [PubMed] [Google Scholar]
- Jauh GY, Phillips TE, Rogers JC. Tonoplast intrinsic protein isoforms as markers for vacuolar functions. The Plant Cell. 1999;11:1867–1882. doi: 10.1105/tpc.11.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Phillips TE, Hamm CA, Drozdowicz YM, Rea PA, Maeshima M, Rogers SW, Rogers JC. The protein storage vacuole: a unique compound organelle. Journal of Cell Biology. 2001;155:991–1002. doi: 10.1083/jcb.200107012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Phillips TE, Rogers SW, Rogers JC. Biogenesis of the protein storage vacuole crystalloid. Journal of Cell Biology. 2000;150:755–770. doi: 10.1083/jcb.150.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Rogers JC. Integral membrane protein sorting to vacuoles in plant cells: evidence for two pathways. Journal of Cell Biology. 1998;143:1183–1199. doi: 10.1083/jcb.143.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe NA, Brown JC, Neumann U, Vicre M, Bachi A, Hawes C, Ceriotti A, Roberts LM, Frigerio L. Transport of ricin and 2S albumin precursors to the storage vacuoles of Ricinus communis endosperm involves the Golgi and VSR-like receptors. The Plant Journal. 2004;39:821–833. doi: 10.1111/j.1365-313X.2004.02167.x. [DOI] [PubMed] [Google Scholar]
- Koide Y, Matsuoka K, Ohto M, Nakamura K. The N-terminal propeptide and the C terminus of the precursor to 20-kilo-Dalton potato tuber protein can function as different types of vacuolar sorting signals. Plant and Cell Physiology. 1999;40:1152–1159. doi: 10.1093/oxfordjournals.pcp.a029500. [DOI] [PubMed] [Google Scholar]
- Krishnan HB, Franceschi VR, Okita TA. Immunochemical studies on the role of the Golgi complex in protein body formation in rice seeds. Planta. 1986;169:471–480. doi: 10.1007/BF00392095. [DOI] [PubMed] [Google Scholar]
- Lam SK, Cai Y, Tse YC, Wang J, Law AH, Pimpl P, Chan HY, Xia J, Jiang L. BFA-induced compartments from the Golgi apparatus and trans-Golgi network/early endosome are distinct in plant cells. The Plant Journal. 2009;60:865–881. doi: 10.1111/j.1365-313X.2009.04007.x. [DOI] [PubMed] [Google Scholar]
- Lam SK, Cai Y, Hillmer S, Robinson DG, Jiang L. SCAMPs highlight the developing cell plate during cytokinesis in tobacco BY-2 cells. Plant Physiology. 2008;147:1637–1645. doi: 10.1104/pp.108.119925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Siu CL, Hillmer S, Jang S, An G, Robinson DG, Jiang L. Rice SCAMP1 defines clathrin-coated, trans-golgi-located tubular–vesicular structures as an early endosome in tobacco BY-2 cells. The Plant Cell. 2007a;19:296–319. doi: 10.1105/tpc.106.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Tse YC, Robinson DG, Jiang L. Tracking down the elusive early endosome. Trends in Plant Science. 2007b;12:497–505. doi: 10.1016/j.tplants.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Sohn EJ, Lee MH, Hwang I. The Arabidopsis Rab5 homologs Rha1 and Ara7 localize to the prevacuolar compartment. Plant and Cell Physiology. 2004;45:1211–1220. doi: 10.1093/pcp/pch142. [DOI] [PubMed] [Google Scholar]
- Li YB, Rogers SW, Tse YC, Lo SW, Sun SS, Jauh GY, Jiang L. BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments. Plant and Cell Physiology. 2002;43:726–742. doi: 10.1093/pcp/pcf085. [DOI] [PubMed] [Google Scholar]
- Maruyama N, Mun LC, Tatsuhara M, Sawada M, Ishimoto M, Utsumi S. Multiple vacuolar sorting determinants exist in soybean 11S globulin. The Plant Cell. 2006;18:1253–1273. doi: 10.1105/tpc.105.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Bassham DC, Raikhel NV, Nakamura K. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. Journal of Cell Biology. 1995;130:1307–1318. doi: 10.1083/jcb.130.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Li KY, Li HY, Yao X, Jiang L. The vacuolar transport of aleurain–GFP and 2S albumin–GFP fusions is mediated by the same pre-vacuolar compartments in tobacco BY-2 and Arabidopsis suspension cultured cells. The Plant Journal. 2008;56:824–839. doi: 10.1111/j.1365-313X.2008.03645.x. [DOI] [PubMed] [Google Scholar]
- Miao Y, Yan PK, Kim H, Hwang I, Jiang L. Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. Plant Physiology. 2006;142:945–962. doi: 10.1104/pp.106.083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo B, Tse YC, Jiang L. Plant prevacuolar/endosomal compartments. International Review of Cytology. 2006;253:95–129. doi: 10.1016/S0074-7696(06)53003-7. [DOI] [PubMed] [Google Scholar]
- Mori T, Maruyama N, Nishizawa K, Higasa T, Yagasaki K, Ishimoto M, Utsumi S. The composition of newly synthesized proteins in the endoplasmic reticulum determines the transport pathways of soybean seed storage proteins. The Plant Journal. 2004;40:238–249. doi: 10.1111/j.1365-313X.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- Müntz K. Deposition of storage proteins. Plant Molecular Biology. 1998;38:77–99. [PubMed] [Google Scholar]
- Olbrich A, Hillmer S, Hinz G, Oliviusson P, Robinson DG. Newly formed vacuoles in root meristems of barley and pea seedlings have characteristics of both protein storage and lytic vacuoles. Plant Physiology. 2007;145:1383–1394. doi: 10.1104/pp.107.108985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS, Herder R, Schulze J, Jung R, Staehelin LA. The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies. The Plant Cell. 2006;18:2567–2581. doi: 10.1105/tpc.106.040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Oufattole M, Rogers JC. Golgi-mediated vacuolar sorting in plant cells: RMR proteins are sorting receptors for the protein aggregation/membrane internalization pathway. Plant Science. 2007;172:728–745. [Google Scholar]
- Park M, Lee D, Lee GJ, Hwang I. AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. Journal of Cell Biology. 2005;170:757–767. doi: 10.1083/jcb.200504112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompa A, De Marchis F, Vitale A, Arcioni S, Bellucci M. An engineered C-terminal disulfide bond can partially replace the phaseolin vacuolar sorting signal. The Plant Journal. 2010;61:782–791. doi: 10.1111/j.1365-313X.2009.04113.x. [DOI] [PubMed] [Google Scholar]
- Reyes FC, Chung T, Holding D, Jung R, Vierstra R, Otegui MS. Delivery of prolamins to the protein storage vacuole in maize aleurone cells. The Plant Cell. 2011;23:769–784. doi: 10.1105/tpc.110.082156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler C, Nebenfuhr A, Movafeghi A, Stussi-Garaud C, Behnia L, Pimpl P, Staehelin LA, Robinson DG. Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. The Plant Cell. 2002;14:237–261. doi: 10.1105/tpc.010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Baumer M, Hinz G, Hohl I. Vesicle transfer of storage proteins to the vacuole: the role of the Golgi apparatus and multivesicular bodies. Journal of Plant Physiology. 1998;152:650–667. [Google Scholar]
- Robinson DG, Hinz G. Golgi-mediated transport of seed storage proteins. Seed Science Research. 1999;9:267–283. [Google Scholar]
- Robinson DG, Jiang L, Schumacher K. The endosomal system of plants: charting new and familiar territories. Plant Physiology. 2008;147:1482–1492. doi: 10.1104/pp.108.120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Oliviusson P, Hinz G. Protein sorting to the storage vacuoles of plants: a critical appraisal. Traffic. 2005;6:615–625. doi: 10.1111/j.1600-0854.2005.00303.x. [DOI] [PubMed] [Google Scholar]
- Sanmartin M, Ordonez A, Sohn EJ, Robert S, Sanchez-Serrano JJ, Surpin MA, Raikhel NV, Rojo E. Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2007;104:3645–3650. doi: 10.1073/pnas.0611147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Wang J, Ding Y, Lo SW, Gouzerh G, Neuhaus JM, Jiang L. The rice RMR1 associates with a distinct prevacuolar compartment for the protein storage vacuole pathway. Molecular Plant. 2011;4:854–868. doi: 10.1093/mp/ssr025. [DOI] [PubMed] [Google Scholar]
- Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I. Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2003;100:16095–16100. doi: 10.1073/pnas.2530568100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Watanabe E, Tamura K, Hayashi Y, Nishimura M, Hara-Nishimura I. A vacuolar sorting receptor PV72 on the membrane of vesicles that accumulate precursors of seed storage proteins (PAC vesicles) Plant and Cell Physiology. 2002;43:1086–1095. doi: 10.1093/pcp/pcf152. [DOI] [PubMed] [Google Scholar]
- Sohn EJ, Kim ES, Zhao M, Kim SJ, Kim H, Kim YW, Lee YJ, Hillmer S, Sohn U, Jiang L, Hwang I. Rha1, an Arabidopsis Rab5 homolog, plays a critical role in the vacuolar trafficking of soluble cargo proteins. The Plant Cell. 2003;15:1057–1070. doi: 10.1105/tpc.009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse YC, Lam SK, Jiang L. Organelle identification and characterization in plant cells: using a combinational approach of confocal immunofluorescence and electron microscopy. Journal of Plant Biology. 2009;52:1–9. [Google Scholar]
- Tse YC, Lo SW, Hillmer S, Dupree P, Jiang L. Dynamic response of prevacuolar compartments to brefeldin A in plant cells. Plant Physiology. 2006;142:1442–1459. doi: 10.1104/pp.106.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. The Plant Cell. 2004;16:672–693. doi: 10.1105/tpc.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Hinz G. Sorting of proteins to storage vacuoles: how many mechanisms? Trends in Plant Science. 2005;10:316–323. doi: 10.1016/j.tplants.2005.05.001. [DOI] [PubMed] [Google Scholar]
- von Lüpke A, Schauermann G, Feussner I, Hinz G. Peripheral membrane proteins mediate binding of vacuolar storage proteins to membranes of the secretory pathway of developing pea cotyledons. Journal of Experimental Botany. 2008;59:1327–1340. doi: 10.1093/jxb/ern039. [DOI] [PubMed] [Google Scholar]
- Wang H, Rogers JC, Jiang L. Plant RMR proteins: unique vacuolar sorting receptors that couple ligand sorting with membrane internalization. FEBS Journal. 2011a;278:59–68. doi: 10.1111/j.1742-4658.2010.07923.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Tse YC, Law AH, Sun SS, Sun YB, Xu ZF, Hillmer S, Robinson DG, Jiang L. Vacuolar sorting receptors (VSRs) and secretory carrier membrane proteins (SCAMPs) are essential for pollen tube growth. The Plant Journal. 2010;61:826–838. doi: 10.1111/j.1365-313X.2009.04111.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhuang X, Hillmer S, Robinson DG, Jiang L. Vacuolar sorting receptor (VSR) proteins reach the plasma membrane in germinating pollen tubes. Molecular Plant. 2011b;4:845–853. doi: 10.1093/mp/ssr011. [DOI] [PubMed] [Google Scholar]
- Wang J, Cai Y, Miao Y, Lam SK, Jiang L. Wortmannin induces homotypic fusion of plant prevacuolar compartments. Journal of Experimental Botany. 2009a;60:3075–3083. doi: 10.1093/jxb/erp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ding Y, Wang J, Hillmer S, Miao Y, Lo SW, Wang X, Robinson DG, Jiang L. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. The Plant Cell. 2010;22:4009–4030. doi: 10.1105/tpc.110.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li Y, Lo SW, Hillmer S, Sun SS, Robinson DG, Jiang L. Protein mobilization in germinating mung bean seeds involves vacuolar sorting receptors and multivesicular bodies. Plant Physiology. 2007;143:1628–1639. doi: 10.1104/pp.107.096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Suen PK, Xu ZF, Jiang L. A 64 kDa sucrose binding protein is membrane-associated and tonoplast-localized in developing mung bean seeds. Journal of Experimental Botany. 2009b;60:629–639. doi: 10.1093/jxb/ern308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel D, Schauermann G, von Lupke A, Hinz G. The cargo in vacuolar storage protein transport vesicles is stratified. Traffic. 2005;6:45–55. doi: 10.1111/j.1600-0854.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- Zheng HQ, Staehelin A. Protein storage vacuoles are transformed into lytic vacuoles in root meristematic cells of germinating seedlings by multiple, cell type-specific mechanisms. Plant Physiology. 2011;155:2023–2035. doi: 10.1104/pp.110.170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouhar J, Munoz A, Rojo E. Functional specialization within the vacuolar sorting receptor family: VSR1, VSR3 and VSR4 sort vacuolar storage cargo in seeds and vegetative tissues. The Plant Journal. 2010;64:577–588. doi: 10.1111/j.1365-313X.2010.04349.x. [DOI] [PubMed] [Google Scholar]
- Zouhar J, Rojo E. Plant vacuoles: where did they come from and where are they heading? Current Opinion in Plant Biology. 2009;12:677–684. doi: 10.1016/j.pbi.2009.08.004. [DOI] [PubMed] [Google Scholar]
- zur Nieden U, Manteuffel R, Weber E, Neumann D. Dictyosomes participate in the intracellular pathway of storage proteins in developing Vicia faba cotyledons. European Journal of Cell Biology. 1984;34:9–17. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.