Abstract
The photosynthetic thylakoid has the highest level of lipid unsaturation of any membrane. In Arabidopsis thaliana plants grown at 22°C, approximately 70% of the thylakoid fatty acids are trienoic – they have three double bonds. In Arabidopsis, and other species, the levels of trienoic fatty acids decline substantially at higher temperatures. Several genetic studies indicate that reduced unsaturation improves photosynthetic function and plant survival at high temperatures. Here, these studies are extended using the Arabidopsis triple mutant, fad3-2 fad7-2 fad8 that contains no detectable trienoic fatty acids. In the short-term, fluorescence analyses and electron-transport assays indicated that photosynthetic functions in this mutant are more thermotolerant than the wild type. However, long-term photosynthesis, growth, and survival of plants were all compromised in the triple mutant at high temperature. The fad3-2 fad7-2 fad8 mutant is deficient in jasmonate synthesis and this hormone has been shown to mediate some aspects of thermotolerance; however, additional experiments demonstrated that a lack of jasmonate was not a major factor in the death of triple-mutant plants at high temperature. The results indicate that long-term thermotolerance requires a basal level of trienoic fatty acids. Thus, the success of genetic and molecular approaches to increase thermotolerance by reducing membrane unsaturation will be limited by countervailing effects that compromise essential plant functions at elevated temperatures.
Keywords: Arabidopsis, high temperature, lipid unsaturation, photosynthesis, thermotolerance
Introduction
The photosynthetic process depends on a highly organized complex of proteins and pigment molecules that are embedded in a unique lipid bilayer, the thylakoid. In all photosynthetic eukaryotes, the glycerolipid molecules that form the foundation of this membrane contain a very high level of trienoic fatty acids. Typically, more than two-thirds of the fatty acids present in the thylakoid membrane are 18:3 (α-linolenic acid) or a combination of 18:3 and 16:3 (Roughanic acid). The two major glycerolipids, monogalactosyl diacylglycerol and digalactosyl diacylglycerol, contain over 90% and 75% trienoic fatty acids, respectively (Douce and Joyard, 1982). The occurrence of such fatty acids as major components of thylakoid membranes is remarkable since they are highly reactive targets for active oxygen species and free radicals that are inevitable by products of oxygenic photosynthesis. For this reason, it has often been proposed that trienoic fatty acids are critical for photosynthetic function. However, during acclimation to high temperature, some plants show a decrease in the proportion of trienoic fatty acids in their membrane glycerolipids (Pearcy, 1978; Raison et al., 1982; Iba, 2002). A reduction of the level of unsaturated fatty acids in isolated thylakoid membranes by chemical hydrogenation also led to an increase in the heat tolerance of photosynthesis (Thomas et al., 1986). Thus, it is tempting to speculate that one of the strategies of plants to protect the photosynthetic apparatus from high-temperature damage is to decrease the proportion of highly unsaturated fatty acids in the thylakoid membrane. Studies of fatty acid-deficient Arabidopsis thaliana mutants (Hugly et al., 1989; Kunst et al., 1989a, 1989b; Wallis and Browse, 2002) and a tobacco transformant (Murakami et al., 2000) also support this hypothesis. Understanding the mechanism and details of this response will be important to cope with changes in plant agriculture and ecophysiology that will accompany climate change (Porter and Semenov, 2005; Friend, 2010; Long and Ort, 2010).
In Arabidopsis, there are two distinct pathways in leaf mesophyll cells for the biosynthesis of glycerolipids and the associated production of unsaturated fatty acids (Wallis and Browse, 2002; Benning, 2009). The prokaryotic pathway is located in the chloroplast inner envelope, whereas the eukaryotic pathway is located in the endoplasmic reticulum with a return flux of acyl lipids to the chloroplast. Three mutants, namely act1, fad5, and fad6, affected in the prokaryotic pathway, share a similar deficiency in highly unsaturated fatty acids. The act1 mutation reduces the activity of the first enzyme of the prokaryotic pathway, the glycerol-3-phosphate acyltransferase (Kunst et al., 1989a). This Arabidopsis mutant has extremely low levels of 16:3 (<1% of the total leaf fatty acids compared with 14% in the wild type) and higher levels of 18-carbon fatty acids. The FAD5 gene product is responsible for the desaturation of 16:0 fatty acid on monogalactosyl diacylglycerol (Kunst et al., 1989b). The fad5 mutant does not contain any 16:3 and has higher levels of 16:0. The fad6 mutant, affected in the prokaryotic 16:1/18:1 desaturase, contains elevated levels in 16:1 and 18:1 fatty acids and lower levels of polyunsaturated fatty acids: 37% 18:3 compared with 48% in the wild type and no detectable 16:3 (Hugly et al., 1989). The act1, fad5, and fad6 mutants exhibit slightly increased rates of photosynthetic electron transport and small increases in the thermal stability of their photosynthetic apparatus at elevated temperatures. Moreover, growth rates at high temperatures were increased, relative to wild-type controls, in fad5 (Kunst et al., 1989b) and act1 (Kunst et al., 1989a) but not in the fad6 mutant (Hugly et al., 1989).
Three Arabidopsis gene products (FAD3, FAD7, and FAD8) mediate the final desaturation of 16:2 and 18:2 fatty acids to 16:3 and 18:3 (McConn and Browse, 1996). The FAD3 gene encodes an endoplasmic reticulum desaturase; the FAD7 and FAD8 genes both encode chloroplast desaturase isozymes that recognize either 18:2 or 16:2 as a substrate attached to any chloroplast lipid. A mutation in one of these three genes results in no more than a partial reduction of the trienoic fatty acid content. Thus, the fad3-2 mutation only marginally reduces the desaturation level of the thylakoid galactolipids, while the fatty acid composition of fad8 is indistinguishable from the wild type. The fad7 mutant alleles result in temperature-dependent reductions in the 18:3 and 16:3 content in thylakoid lipids (Browse et al., 1986) and fad7 fad8 double-mutant plants have only 10–15% 16:3 and 18:3 at growth temperatures above 22°C, compared with approximately 60% 16:3 + 18:3 in wild-type plants (McConn and Browse, 1996).
Murakami et al. (2000) produced lines of transgenic tobacco (T25 and T23) in which the gene encoding the FAD7 desaturase was silenced. This tobacco transformant contained a lower level of trienoic fatty acids (25% 16:3 + 18:3 compared with 65% in wild-type tobacco leaves). These authors studied the effect of heat on photosynthesis and growth of T15, T23, and wild-type tobacco, and also carried out experiments on a fad7-1 fad8 double mutant of Arabidopsis that possesses a similar fatty acid deficiency. The photosynthetic activity of intact leaves pretreated at 40°C was higher in T15, T23, and fad7-1 fad8 double-mutant plants compared with their respective controls. Furthermore, plants of all three lines were considerably more tolerant of growth at high temperatures (47°C for tobacco, 36°C for Arabidopsis) compared with non-transgenic and wild-type controls.
In general, therefore, reductions in trienoic acids have been strongly correlated with improved performance of plants at high temperatures (Iba, 2002). To find out if this relationship continues to even lower levels of 16:3 and 18:3 fatty acids, this study investigated an Arabidopsis triple-mutant line, fad3-2 fad7-2 fad8 that contains less than 0.1% 16:3 + 18:3 fatty acids (McConn and Browse, 1996). The current study reports that short-term measurements of photosynthesis do indeed show enhanced thermotolerance in the triple mutant. However, growth of mutant plants is severely compromised at high temperatures indicating a specific requirement for trienoic fatty acids for survival of plants at high temperatures. Although reduced membrane unsaturation has been considered an important component of thermotolerance, these results indicate that there is a more complex relationship between trienoic fatty acid levels and plant responses to high temperature than previously thought.
Materials and methods
Plant material and growth conditions
The mutant lines of A. thaliana (L.) Heynh., used in this study, were derived from the Columbia wild type and have been described previously (McConn and Browse, 1996). Seeds were grown at 22°C (70% humidity) in continuous fluorescent illumination (100–150 μmol m−2 s−1) in soil irrigated with mineral nutrient solution. After 17 days, the seedlings were transferred to the experimental growth conditions.
Extraction and analysis of chlorophyll and lipids
Total chlorophyll was measured in 80% acetone. Fatty acid methyl esters were made from leaves or from extracts by trans-esterification in methanolic HCl and quantified by gas chromatography and flame ionization detection (Browse et al., 1986).
Chlorophyll fluorescence measurements
Fluorescence measurements were obtained using a pulse amplitude modulation fluorometer driven by the DA-100 data acquisition system software (model PAM 101; Walz, Effeltrich, Germany). The fluorometer was set up in a growth chamber and the probe was positioned above a thermoregulated cuvette at an angle that did not interrupt the incident illumination. Prior to experiments, leaves or plants were dark adapted for 1 h. Detached leaves were incubated at a given temperature in the dark or in the light. Then, the weak pulse measuring beam (0.02 μmol m−2 s−1) modulated at 1.6 kHz was switched on to determine Fo. A 1-s flash of saturating white light (2000 μmol m−2 s−1) gave the Fm. The parameter Fv/Fm = (Fm–Fo)/Fm was calculated from these data. Continuous white light (100 μmol m−2 s−1) was then switched on to record the fluorescence curve. During this period, 1-s flashes of saturating light were applied to measure Fm' (the prime refers to a state that is not dark-adapted). At the end of the light treatment, the quantum yield of steady-state PSII electron transport (ØII) as (Fm'–Fs)/Fm' was calculated following the method of Genty et al., 1989).
Chemical treatments
After heat treatment in the dark, 1-cm2 leaf discs were vacuum infiltrated at room temperature in a solution containing 0–150 mM hydroxylamine and 50 mM HEPES buffer (pH 7.5). Following infiltration, discs were incubated for 5 min before measurement of Fv/Fm.
A 0.01% (w/v) solution of methyl jasmonate in 0.01% Tween was sprayed directly on plants for 16 days beginning 3 days before the heat treatment started. This level of methyl jasmonate is within the physiological relevant range. Similar treatments have been shown to complement the male-sterile and defence phenotypes of the fad3-2 fad7-2 fad8 mutant (McConn and Browse, 1996; McConn et al., 1997; Vijayan et al., 1998; Stintzi et al., 2001).
Isolation of thylakoid membranes and photosynthetic electron transport measurements
Thylakoid membranes were isolated by grinding leaves in ice-cold 25 mM HEPES buffer (pH 7.8), 10 mM NaCl, 5 mM MgCl2, 0.35 M sorbitol, 10 mM EDTA, and 0.2% (w/v) bovine serum albumin. The homogenate was passed through four layers of Miracloth (Calbiochem, La Jolla, CA, USA) and centrifuged at 3000 g for 5 min at 4°C. The pellet was washed with ice-cold 10 mM HEPES buffer (pH 7.8), 5 mM NaCl, 2.5 mM MgCl2, 0.35 M sorbitol and 0.1% (w/v) bovine serum albumin and suspended in a small volume of washing buffer.
Isolated thylakoids were incubated in the dark for 10 min. at various temperatures from 25°C to 45°C prior to measurement of the oxygen evolution of whole-chain electron transfer, PSI, and PSII at 25°C in an Hansatech oxygen electrode in white saturating illumination (1200 μmol m−2 s−1). Whole-chain electron transport activities were assayed in 1 ml of a reaction mixture (25 mM TRIS pH 7.4, 100 mM sorbitol, 10 mM NaCl, 5 mM NH4Cl) using water as the electron donor, by monitoring the oxygen consumption by 1 mM methyl viologen. PSII-dependent electron transport used 100 μM 2-6-dichlorophenol indophenol as the electron acceptor. PSI-dependent oxygen evolution were recorded in the presence of 1 μM 3-(3-dichlorophenyl)1,1-dimethylurea to inhibit PSII activity and 0.1 mM N,N,N',N'-tetramethyl-p-phenylenediamine reduced with 1 mM ascorbate.
Results
Reduced high-temperature damage of PSII in the triple mutant
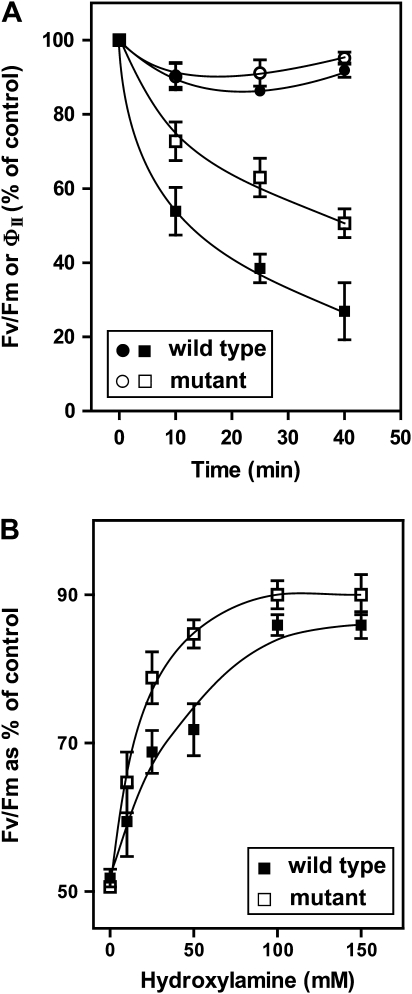
Photosynthesis is one of the most heat-sensitive functions of plant cells (Sharkey and Zhang, 2010). For most temperate C3 species, in vivo rates of photosynthesis begin to decline above 25–28°C and severe damage to the photosystems is often observed above 35–40°C. Importantly, heat damage is more severe in the dark and physiological levels of light have a protective effect (Kislyuk et al., 2008). To assess the effects of high temperatures on photosynthesis, the current study compared the time course of decline of photosynthetic quantum efficiency in leaves from wild-type and triple mutant fad3-2 fad7-2 fad8 Arabidopsis incubated at 40°C either in the dark or in the light. Two non-invasive chlorophyll fluorescence parameters were measured, Fv/Fm and ØII (Maxwell and Johnson, 2000). Fv/Fm is the maximal quantum yield of PSII photochemistry in dark-adapted leaves. This parameter describes the efficiency of electron transport inside PSII and has been shown to be linearly correlated with the quantum yield of light-limited O2 evolution and with the proportion of functional PSII reaction centres. ØII is the quantum yield of linear electron transfer measured at steady state under physiological light levels. This parameter also measures PSII quantum yield, but reflects the efficiency of the whole photosynthetic process (including PSI and CO2 assimilation) because PSII is coupled to downstream processes in the light (Genty et al., 1989). For plants grown at 22°C, The study centre has previously shown that there are no differences in Fv/Fm and ØII values between fad3-2 fad7-2 fad8 plants and wild-type controls (McConn and Browse, 1996; Routaboul et al., 2000). Fig. 1A shows that a 40-min incubation at 40°C in the light did not reduce ØII below 90% of the starting value in leaves of either the wild type or the mutant. In contrast, both wild-type and fad3-2 fad7-2 fad8 mutant photosynthetic capabilities, measured as Fv/Fm, significantly diminished when leaves were incubated at 40°C in the dark. However, the mutant tolerated high temperatures better than the wild type. To obtain a 50% decrease in Fv/Fm, it was necessary to incubate wild-type leaves at 40°C for only 15 min whereas leaves from the mutant required 40 min of heat treatment to reach the same level. These results indicate that, in the dark, high temperatures mainly affect PSII activity (Nash et al., 1985; Sharkey and Zhang, 2010; Toth et al., 2011).
Fig. 1.
Effect of high temperature on photosynthesis in wild-type and fad3-2 fad7-2 fad8 plants. (A) Leaves were incubated at 40°C and inhibition of photosynthesis was monitored in the dark by Fv/Fm (squares) and at a light intensity of 100 μmol quanta m−2 s−1 by ØII (circles). (B) Partial restoration of Fv/Fm in heat-damaged leaves by hydroxylamine. Data are shown as a percentage of controls not subject to heat treatment. Values are mean ± SE (n = 5).
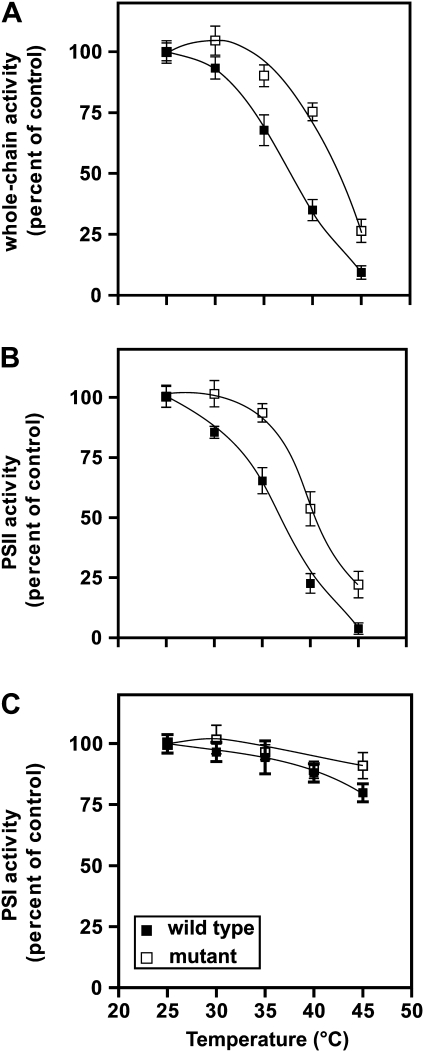
To confirm that the primary site of heat damage involved PSII, thylakoids from wild-type and mutant plants were isolated and incubated in the dark for 10 min at various temperatures (25–45°C) before measuring oxygen evolution at 25°C (Fig. 2A-C). Both whole-chain (Fig. 2A) and PSII-dependent electron transport (Fig. 2B) were markedly more tolerant to elevated temperature in the mutant compared with the wild type. The temperature resulting in half inactivation of PSII was 42°C for mutant thylakoids compared with 37°C for the wild type. In contrast, temperatures up to 45°C (Fig. 2C) had only a small effect on PSI electron transport in either wild-type or mutant thylakoids. Control thylakoids maintained in the dark and cold prior to assay retained high rates of PSII electron transport throughout the experiment and for several hours afterwards.
Fig. 2.
Profiles of high-temperature inactivation of whole-chain (A), PSII (B), and PSI (C) electron transport in wild-type and fad3-2 fad7-2 fad8 plants. Isolated thylakoids were preincubated at the designated temperatures in the dark for 10 min. Activities were measured at 25°C and expressed relative to those obtained with membranes preincubated in the dark at 4°C. The initial activities were: 197 ± 11 and 194 ± 12 μmol O2 h−1 mg−1 chlorophyll for wild-type and mutant whole-chain electron transport, 300 ± 20 and 282 ± 25 for wild-type and mutant PSII activities, and 276 ± 19 and 282 ± 19 for wild-type and mutant PSI activities, respectively. Values are mean ± SE (n = 3).
The disintegration of the protein complex responsible for oxygen evolution has been proposed to be the main cause of irreversible damage of PSII (Nash et al., 1985). To test for inhibition of the O2-evolving complex, detached leaves from wild-type and mutant plants were first incubated at 40°C, for 15 or 40 min, respectively, to obtain a similar half inhibition of Fv/Fm (see Fig. 1A). Then, leaves were infiltrated either with hydroxylamine solutions or with water as a control and Fv/Fm was measured again. Hydroxylamine is an artificial electron donor that bypasses any inhibition of the O2-evolving complex. While the control leaves exhibited a 50% reduction in Fv/Fm, as predicted from Fig. 1A, infiltration of leaves with 100 mM hydroxylamine substantially restored the Fv/Fm signal in both wild-type and mutant leaves. Hydroxylamine-treated leaves had Fv/Fm values that were 85–90% of the values in unheated leaves (Fig. 1B). It appears, therefore, that the absence of trienoic fatty acids partially protects the PSII O2-evolving complex from high-temperature damage.
Thermotolerance of PSII is strongly correlated to 16:3 content but not 18:3 content
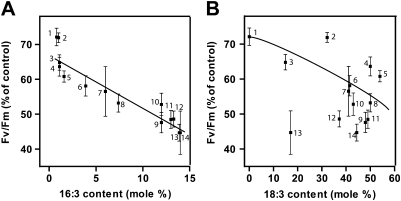
Clearly, these results are consistent with the broader, well-established finding that mutant and transgenic plants with reduced thylakoid unsaturation exhibit increased thermotolerance of PSII. To investigate this relationship more deeply, ten different mutants of Arabidopsis (together with wild-type controls) were grown at 22°C. Measurements of Fv/Fm at 25°C were made on individual leaves and these leaves were then incubated in the dark at 40°C for 20 min and returned to 25°C for 20 min before measuring Fv/Fm for a second time. The ratio of Fv/Fm after:before heat treatment, expressed as a percentage, was used as a quantitative measure of PSII thermotolerance. The fatty acid composition of each leaf was determined by gas chromatography.
Interestingly, thermotolerance across the 13 different lines used in this experiment was more strongly correlated with leaf 16:3 content (Fig. 3A) than to 18:3 content (Fig. 3B) or to the sum of 16:3 + 18:3, the average number of double bonds per glycerolipid molecule, or other measures of membrane unsaturation (data not shown). The most resistant mutants, namely fad3-2 fad7-2 fad8, fad7-2 fad8, fad5, fad6, and act1, contained less than 2% 16:3 compared with 12% in wild-type Arabidopsis. By contrast, the levels of 18:3 among these five mutants varied from 0 to 54%. The most thermosensitive lines, fab1, fad8, and the wild type, all contained 12–13% 16:3 in their leaf lipids together with 37–48% 18:3. The degree of linear correlation (r2) between PSII inactivation and 16:3 content was 0.88, while the r2 for 18:3 content was only 0.37.
Fig. 3.
Relationships between 16:3 content (A) and 18:3 content (B) and thermal tolerance. Fv/Fm was measured at 25°C on leaves of different lipid mutants after preincubation for 20 min at 40°C in the dark. 100% corresponds to a Fv/Fm value prior heat treatment. The data points represent: 1, fad3-2 fad7-2 fad8 triple mutant; 2, fad6; 3, fad7-2 fad8 double mutant; 4, fad5; 5, act1; 6, fad7-2; 7, fab2; 8, gly1; 9, wild type; 10, fad8; 11, fad4; 12, fab1; 13, fad2; 14, fad3. Values are mean ± SE (n = 15).
Decreased unsaturation in wild-type and fad3 fad7 fad8 plants at 33°C
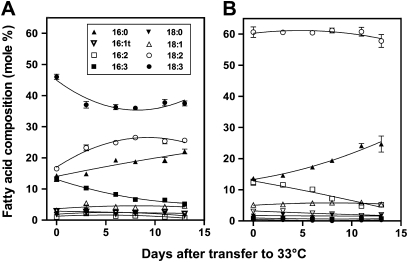
Previous studies have documented reductions in overall unsaturation of leaf lipids in desert and evergreen plants grown at high temperatures (Pearcy, 1978; Raison et al., 1982). Increasing temperature results in reduced unsaturation in Arabidopsi, also, and wild-type plants moved from the growth temperature of 22°C showed changes in leaf fatty acid composition over a period of 10 days (Falcone et al., 2004). The current study investigated the time course of changes in leaf fatty acid composition for plants transferred from 22°C to 33°C. High-temperature acclimation caused substantial decreases in the proportions of both 16:3 and 18:3 fatty acids in wild-type leaves, and corresponding increases in the amount of the less unsaturated 16:0 and 18:2 fatty acids (Fig. 4A). Wild-type plants contained only 5% 16:3 fatty acids after 13 days at 33°C. The changes observed are somewhat greater than those recorded for plants transferred to 29°C for 10 days (Falcone et al., 2004). Leaves of fad3 fad7 fad8 plants exhibited a heat-induced increase in the amount of 16:0 and a corresponding decrease in 16:2 fatty acids. However, there was almost no change in the extent of unsaturation of 18-carbon fatty acids in the mutant (Fig. 4B).
Fig. 4.
Changes in the fatty acid composition of leaves from wild-type (A) and fad3-2 fad7-2 fad8 (B) plants, after transfer to 33°C. Values are mean ± SE (n = 5).
Linolenic acid is essential for plant survival at 33°C
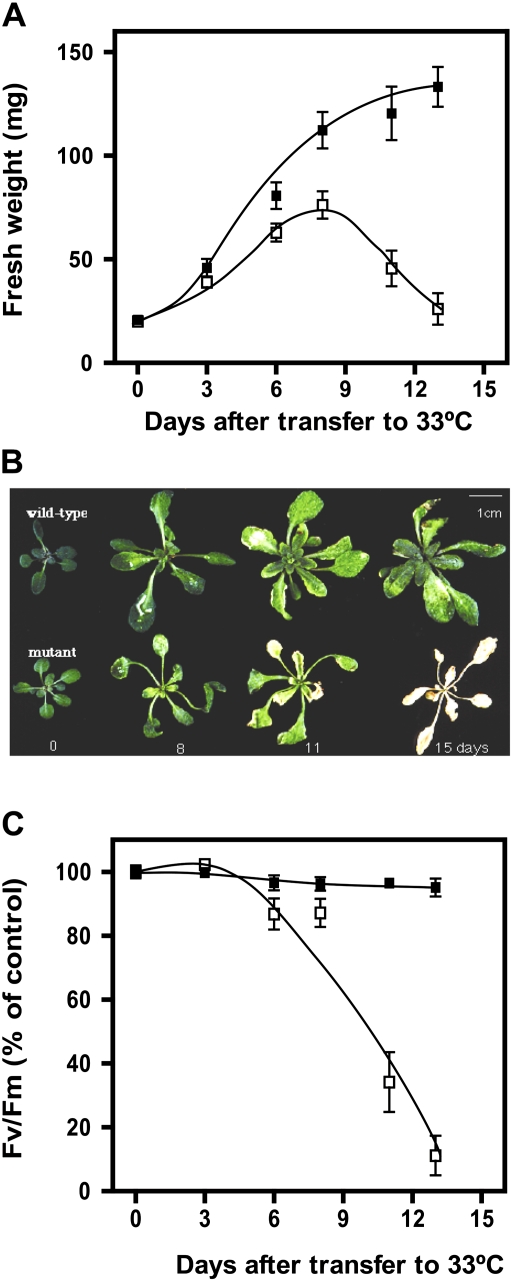
The results in Fig. 4 are consistent with a model in which plants acclimate to high temperatures, in part, by reducing thylakoid unsaturation in order to protect photosynthesis, and particularly PSII, from damage. Lipid mutants with reduced thylakoid unsaturation might be considered ‘pre-acclimated’ to the extent that PSII is consistently more thermotolerant in these mutants compared with the wild type (Hugly et al., 1989; Kunst et al., 1989a, 1989b; Murakami et al., 2000). For the fad5, act1, and fad7 fad8 mutants (Kunst et al., 1989a, 1989b; Murakami et al., 2000) but not fad6 (Hugly et al., 1989), increased thermotolerance of photosynthesis is associated with improved growth of the mutants, relative to the wild type, at temperatures above 30°C. To find out if the improved thermotolerance of photosynthesis in the fad3 fad7 fad8 mutant also provided for increased growth at high temperature, the current study monitored the growth, photosynthesis, and appearance of mutant and wild-type plants following transfer from 22°C to 33°C. During the first 6 days after transfer, mutant plants showed only a small decrease in growth rate, relative to the wild type (Fig. 5A). After 6 days, however, growth of mutant plants slowed rapidly, and at 8 days chlorosis and small areas of necrosis were visible on the leaves (Fig. 5B). Beyond 8 days, damage to leaves of the mutant plants became rapidly more severe (Fig. 5B) and the fresh weight of plants began to decline (Fig. 5A), presumably because of tissue dehydration. Thirteen days after transfer to 33°C, most of the fad3 fad7 fad8 plants were brown and dead (Fig. 5B). By contrast, wild-type plants remained healthy and continued to grow throughout the experiment. Fluorescence analysis was used to monitor the potential quantum yield of PSII in leaves of wild-type and mutant plants during the experiment (Fig. 5C). The value of Fv/Fm for the wild type declined by less than 5% after 13 days at 33°C. In the mutant, Fv/Fm declined rapidly after day 6, reflecting the visible damage observed in the plants.
Fig. 5.
The effects of high temperature on growth and photosynthesis of wild-type (filled square) and fad3-2 fad7-2 fad8 (open square) plants following transfer to 33°C. (A) Time course of aerial fresh-weight accumulation. (B) Photographs of representative plants. (C) PSII inhibition in wild-type (filled square) and fad3-2 fad7-2 fad8 (open square) plants. Detached leaves were cooled to 25°C for 1 h in the dark and Fv/Fm was measured at 25°C. In A and C, values are mean ± SE (n = 5).
Additional experiments at 33°C were conducted which included the fad3-2, fad7-2, and fad8 single mutants and the fad7-2 fad8 double mutant together with the fad3-2 fad7-2 fad8 mutants and the wild type. In these experiments, only the triple mutant plants showed the leaf necrosis and death seen in Fig. 5B. After 15 days at 33°C, leaves of the fad7-2 fad8 plants contained no 16:3 and only 6 ± 1% 18:3. These results indicate that death of fad3 fad7 fad8 plants at 33°C is caused by a deficiency in 18:3 synthesis, rather than by any other mutation in the genetic background of the triple mutant, and that a relatively low level of 18:3 (≤6%) is sufficient to rescue the phenotype.
Jasmonate deficiency does not cause death of plants at 33°C
fad3 fad7 fad8 mutant plants cannot synthesize jasmonate, because they lack the precursor, 18:3 (McConn and Browse, 1996). Jasmonate is an important chemical signal in plants with roles in insect and pathogen defence (Vijayan et al., 1998; Reymond et al., 2004; Browse, 2009), reproductive development (Feys et al., 1994; McConn and Browse, 1996), and other biological processes. Jasmonate has been shown to induce production of small heat-shock proteins (Hamilton and Coleman, 2001), which contribute to thermotolerance of PSII electron transport (Heckatorn et al., 1998), and to be necessary for basal thermotolerance in Arabidopsis plants exposed to 38°C (Clarke et al., 2009). Jasmonate is also known to have a role in stomatal control (Munemasa et al., 2011) and this may be important for limiting water loss at high temperatures.
This work considered the possibility that jasmonate is specifically required for survival of Arabidopsis at high temperatures. Application of jasmonate to fad3 fad7 fad8 plants and to other jasmonate-synthesis mutants has been demonstrated to complement the defence and sterility phenotypes (McConn and Browse, 1996; Vijayan et al., 1998). To test whether jasmonate could also rescue the triple mutants during exposure to 33°C, the experiment shown in Fig. 5 was repeated but with the plants being sprayed with a solution of 0.01% jasmonate each day, starting 3 days before transfer to 33°C. Application of jasmonate did not alter the outcome of the experiment: the fad3 fad7 fad8 mutant plants still died over a period of 10–20 days, while wild-type plants remained alive.
As an alternative test of a role for jasmonate in thermotolerance, this study performed experiments with two mutants, aos and opr3 (dde1) that are unable to synthesize jasmonate (Stintzi and Browse, 2000; Park et al., 2002), and the coi1 mutant that is deficient in all known responses to jasmonate (Feys et al., 1994). In these experiments, plants were scored for survival after 3 weeks at 33°C. Data from three replicate experiments are shown in Table 1. The opr3, aos, and coi1 plants all survived nearly as well as the wild type, with >90% of the plants alive at the end of heat treatments. Only the fad3 fad7 fad8 plants showed survival of less than 10% over the three experiments. It is concluded that the inability of fad3 fad7 fad8 plants to synthesize jasmonate does not cause this mutant to die at 33°C.
Table 1.
Survival of wild-type Arabidopsis and four jasmonate mutants at 33°C
| Survival Expt. 1 | Expt. 2 | Expt. 3 | Overall (%) | |
| Wild type | 10/10 | 13/13 | 26/26 | 100 |
| fad3 fad7 fad8 | 0/14 | 2/13 | 2/18 | 8.8 ± 3.7 |
| aos | 15/15 | 25/35 | 21/21 | 90.5 ± 7.8 |
| opr3 | 14/15 | 12/12 | 19/20 | 96.1 ± 1.6 |
| coi1 | 14/15 | 21/24 | 21/22 | 92.1 ± 1.9 |
The fad3 fad7 fad8 mutant was grown together with two other jasmonate-synthesis mutants, aos and opr3, the jasmonate-response mutant coi1, and the wild type. Plants were grown for 17 days at 22°C and then transferred to 33°C for 3 weeks. For each experiment, surviving/total plants are shown. The average percentage survival ± SE was calculated for the three experiments.
Discussion
Short-term increase in thermotolerance of photosynthesis
The increasing concentrations of greenhouse gases in the atmosphere and attendant global warming mean that it is important to understand the mechanisms of temperature acclimation and thermotolerance in plants (Porter and Semenov, 2005; Friend, 2010; Long and Ort, 2010). The extent of fatty acid unsaturation in plant membranes has long been considered a factor in plant temperature responses (Pearcy, 1978; Raison et al., 1982; Iba, 2002). In broad terms, fatty acid unsaturation (measured as the number of carbon–carbon double bonds per fatty acid or per glycerolipid molecule) is highest in plants grown at low temperatures and declines considerably as growth temperature is increased towards the upper limit for growth of a particular species. For example, in Arabidopsis, the average number of double bonds per glycerolipid molecule in leaves fell from 4.8 in plants grown at 17°C to 2.9 in plants grown at 36°C (Falcone et al., 2004). Following a shift from lower to higher temperature, levels of the most highly unsaturated fatty acids decline over time and there is a concomitant increase in fatty acids with fewer double bonds. For the current experiments with Arabidopsis, these changes are demonstrated by the data in Fig. 4A. A simple hypothesis from these general observations is that high membrane unsaturation is beneficial for photosynthesis and other plant processes at low temperature and that low unsaturation favours photosynthetic function at high temperatures.
The isolation of a series of Arabidopsis mutants with specific alterations in leaf fatty acid composition (Wallis and Browse, 2002) has provided an alternative means to investigate the relationship between membrane unsaturation and plant temperature responses. Five mutants that are substantially indistinguishable from the wild type when grown at 22°C all show symptoms of damage when grown at 2° to 6°C. The mutant lines are fab1, fad2, fad5 (previously fadB), fad6 (previously fadC), and the fad3 fad7 fad8 triple mutant (Hugly and Somerville, 1992; Routaboul et al., 2000; Wallis and Browse, 2002). Each of these mutants exhibits a distinct pattern of symptoms at low temperature, suggesting that different mechanisms of low-temperature damage are operating. In the fab1, fad5, fad6, and fad3 fad7 fad8 mutants, the chloroplast appears to be the primary site of low-temperature damage; however, comparisons of photosynthesis in wild-type and mutant plants at low temperatures indicate that photosynthesis in the mutants is not immediately affected, but instead is reduced progressively by long-term exposure to low temperatures. In the fad5, fad6, and fad3 fad7 fad8 mutants, poor growth at low temperatures has been related to reduced recovery from photoinhibition (Vijayan and Browse, 2002) and reduced capacity for thylakoid protein transport (Ma and Browse, 2006).
It may be tempting to consider changes in membrane unsaturation as providing a simple trade-off: reduced unsaturation compromises photosynthesis and plant growth at low temperatures, but allows improved performance at high temperatures. Experiments with Arabidopsis, as well as with other plants and cyanobacteria, indicate that the reality is not this simple. For example, it is true that estimates of photosynthetic thermotolerance indicate improved performance for the fad5 and fad6 mutants relative to the wild type (Hugly et al., 1989; Kunst et al., 1989a). However, mutants that exhibit no detrimental effects at low temperature, such as act1 and the fad7 fad8 double mutant, also show increased thermotolerance of photosynthesis as well as increased growth relative to the wild type at high temperatures (Kunst et al., 1989b; Murakami et al., 2000). In cyanobacteria, reductions in thylakoid unsaturation do not improve the thermotolerance of photosynthesis (Gombos et al., 1994; Wada et al., 1994).
In the short term, the fad3 fad7 fad8 mutant also shows improved photosynthetic parameters relative to the wild type at temperatures above 30°C (Figs. 1 and 2) and this reinforces the strong correlation observed between reduced thylakoid unsaturation and increased thermotolerance of photosynthesis in higher plants. The benefit of reduced thylakoid unsaturation has been attributed to improved thermotolerance of PSII, which is recognized as one of the most thermally sensitive processes in plants (Nash et al., 1985; Havaux and Tardy, 1997; Sharkey and Zhang, 2010; Toth et al., 2011). In leaves (Figs. 1 and 3) and thylakoids (Fig. 2) of fad3 fad7 fad8 plants grown at 22°C, PSII was partially protected from heat damage relative to wild-type controls. The artificial electron donor, hydroxylamine, substantially restored Fv/Fm of both wild-type and fad3 fad7 fad8 leaves incubated at 40°C. This result is consistent with previous results that indicate thermal damage to the O2-evolving complex, rather than to any other component of PSII (Nash et al., 1985; Toth et al., 2011). Interestingly, the current results indicate that this thermotolerance of the O2-evolving complex is closely correlated with the level of 16:3 fatty acid (Fig. 3), rather than to any other measure of membrane unsaturation. A previous study (Falcone et al., 2004) also found that three Arabidopsis mutants deficient in 16:3, fad5, fad6, and act1, had enhanced thermotolerance, relative to the wild type, when plants were grown at 17°C. When plants were grown at 29°C, thermotolerance of the three mutant lines did not change but thermotolerance of the wild type increased to be equal to that of the mutants, and this change was associated with a 67% decrease in the proportion of 16:3 in the total leaf lipids of wild-type plants. These results are consistent with the correlation identified in Fig. 3. The explanation for this intriguing observation remains to be elucidated, but may relate to the fact that 16:3 is predominantly found as an acyl component of monogalactosyl diacylglycerol, the major glycerolipid of the chloroplast thylakoid (Douce and Joyard, 1982). Additional research will be needed to determine whether and how the changes in fatty acid composition of this glycerolipid contribute to thermotolerance of PSII.
Trienoic fatty acids are required for plant survival at high temperature
Photosystem II and whole-chain electron transport in the fad3 fad7 fad8 mutant each has a 4–5°C increase in thermal tolerance over the wild type (Fig. 2). Although vegetative growth and development of fad3 fad7 fad8 plants is indistinguishable from the wild type at 22°C (McConn and Browse, 1996), after transfer to 33°C growth of mutant plants quickly slowed relative to wild-type controls (Fig. 5A). Leaves of mutant plants quickly showed signs of damage and most of the plants died within 10–20 days at 33°C (Fig. 5B, C). In contrast, and consistent with the observations of Murakami et al. (2000), this study found that the fad7-2 fad8 double mutant continued to grow at 33°C although leaves of this mutant contained only 6% 18:3 and no detectable 16:3, compared with 43% 18:3 + 16:3 in wild-type leaves. Taken together, these results indicate that there is a specific requirement for low levels of linolenic acid for survival of plants at high temperatures.
The plant hormone jasmonate has demonstrated roles in thermotolerance (Hamilton and Coleman, 2001; Clarke et al., 2009; Munemasa et al., 2011). The current study hypothesized that the inability of fad3 fad7 fad8 plants to synthesize jasmonate (McConn and Browse, 1996) might underlie the damage and death of these plants at 33°C (Fig. 5). However, application of exogenous jasmonate, which corrects other symptoms of jasmonate deficiency (McConn and Browse, 1996; McConn et al., 1997; Vijayan et al., 1998), did not reduce damage or improve survival of mutant plants at 33°C. Furthermore, other mutants deficient in jasmonate synthesis or perception exhibited high rates of survival during growth at 33°C (Table 1). These results indicate that jasmonate deficiency is not the principal factor contributing to the death of fad3 fad7 fad8 plants at 33°C.
In the fad7 fad8 mutant, both the chloroplast and extrachloroplast lipids contain low levels of 18:3 (McConn et al., 1994) that are absent from tissue of the fad3 fad7 fad8 mutants (McConn and Browse, 1996). For this reason, analysis of the mutants did not allow the determination of whether a deficiency in thylakoid 18:3 causes the thermosensitive phenotype of the fad3 fad7 fad8 mutant or, alternatively, if it is failure in another membrane compartment of the cell that causes the death of mutant plants. Further experiments will be required to address this question and to develop a more complete mechanistic understanding of the high-temperature phenotype of the fad3 fad7 fad8 mutant.
Acknowledgments
The authors thank Deirdre Fahy for assistance in preparing the figures. This work was supported by the US National Science Foundation (IBN-0084329), the US Department of Energy (DE-FG02-99ER20323), and the Agricultural Research Center at Washington State University.
References
- Benning C. Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annual Review of Cell and Developmental Biology. 2009;25:71–91. doi: 10.1146/annurev.cellbio.042308.113414. [DOI] [PubMed] [Google Scholar]
- Browse J. Jasmonate passes muster: a receptor and targets for the defense hormone. Annual Review of Plant Biology. 2009;60:183–205. doi: 10.1146/annurev.arplant.043008.092007. [DOI] [PubMed] [Google Scholar]
- Browse J, Mccourt P, Somerville C. A mutant of Arabidopsis deficient in c(18:3) and c(16:3) leaf lipids. Plant Physiology. 1986;81:859–864. doi: 10.1104/pp.81.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SM, Cristescu SM, Miersch O, Harren FJ, Wasternack C, Mur LA. Jasmonates act with salicylic acid to confer basal thermotolerance in. Arabidopsis thaliana. New Phytologist. 2009;182:175–187. doi: 10.1111/j.1469-8137.2008.02735.x. [DOI] [PubMed] [Google Scholar]
- Douce R, Joyard J. Plants galactolipids. The Biochemistry of Plants. 1982:331–332. [Google Scholar]
- Falcone DL, Ogas JP, Somerville CR. Regulation of membrance fatty acid composition by temperature in mutants of Arabidopsis with alternations in membrane lipid composition. BMC Plant Biology. 2004;4:17. doi: 10.1186/1471-2229-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male-sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. The Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend AD. Terrestrial plant production and climate change. Journal of Experimental Botany. 2010;61:1293–1309. doi: 10.1093/jxb/erq019. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochimica Et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Gombos Z, Wada H, Hideg E, Murata N. The unsaturation of membrane-lipids stabilizes photosynthesis against heat-stress. Plant Physiology. 1994;104:563–567. doi: 10.1104/pp.104.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton EW, Coleman JS. Heat-shock proteins are induced in unstressed leaves of Nicotiana attenuata (Solanaceae) when distant leaves are stressed. American Journal of Botany. 2001;88:950–955. [PubMed] [Google Scholar]
- Havaux M, Tardy F. Thermostability and photostability of photosystem II in leaves of the Chlorina-f2 barley mutant deficient in light-harvesting chlorophyll a/b protein complexes. Plant Physiology. 1997;113:913–923. doi: 10.1104/pp.113.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckatorn S, Downs C, Sharkey T, Coleman J. The small methionine rich heat-shock protein protects PSII electron transport during heat stress. Plant Physiology. 1998;116:439–444. doi: 10.1104/pp.116.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugly S, Somerville C. A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiology. 1992;99:197–202. doi: 10.1104/pp.99.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugly S, Kunst L, Browse J, Somerville C. Enhanced thermal tolerance of photosynthesis and altered chloroplast ultrastructure in a mutant of Arabidopsis deficient in lipid desaturation. Plant Physiology. 1989;90:1134–1142. doi: 10.1104/pp.90.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba K. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annual Review of Plant Biology. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- Kislyuk IM, Bubolo LS, Bykov OD, Kamentseva IE, Sherstneva OA. Protective and injuring action of visible light on photosynthetic apparatus in wheat plants during hyperthermia treatment. Russian Journal of Plant Physiology. 2008;55:613–620. [Google Scholar]
- Kunst L, Browse J, Somerville C. Altered chloroplast structure and function in a mutant of Arabidopsis deficient in plastid glycerol-3-phosphate acyltransferase activity. Plant Physiology. 1989a;90:846–853. doi: 10.1104/pp.90.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L, Browse J, Somerville C. Enhanced thermal tolerance in a mutant of Arabidopsis deficient in palmitic acid unsaturation. Plant Physiology. 1989b;91:401–408. doi: 10.1104/pp.91.1.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Ort DR. More than taking the heat: crops and global change. Current opinion in plant biology. 2010;3:241–248. doi: 10.1016/j.pbi.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Ma XY, Browse J. Altered rates of protein transport in Arabidopsis mutants deficient in chloroplast membrane unsaturation. Phytochemistry. 2006;67:1629–1636. doi: 10.1016/j.phytochem.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- McConn M, Browse J. The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. The Plant Cell. 1996;8:403–416. doi: 10.1105/tpc.8.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcconn M, Hugly S, Browse J, Somerville C. A mutation at the Fad8 locus of Arabidopsis identifies a second chloroplast [omega]-3 desaturase. Plant Physiology. 1994;106:1609–1614. doi: 10.1104/pp.106.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J. Jasmonate is essential for insect defense. Arabidopsis. Proceedings of the National Academy of Sciences, USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiology. 2011;155:553–561. doi: 10.1104/pp.110.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K. Trienoic fatty acids and plant tolerance of high temperature. Science. 2000;287:476–479. doi: 10.1126/science.287.5452.476. [DOI] [PubMed] [Google Scholar]
- Nash D, Miyao M, Murata N. Heat inactivation of oxygen evolution in photosystem-II particles and its acceleration by chloride depletion and exogenous manganese. Biochimica Et Biophysica Acta. 1985;807:127–133. [Google Scholar]
- Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R. A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. The Plant Journal. 2002;31:1–12. doi: 10.1046/j.1365-313x.2002.01328.x. [DOI] [PubMed] [Google Scholar]
- Pearcy R. Effect of growth temperature on the fatty acid composition of the leaf lipids in Atriplex lentiformis (Torr.) Wats. Plant Physiology. 1978;61:484–486. doi: 10.1104/pp.61.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JR, Semenov MA. Crop responses to climatic variation. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2005;360:2021–2035. doi: 10.1098/rstb.2005.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison JK, Roberts JKM, Berry JA. Correlations between the thermal-stability of chloroplast (thylakoid) membranes and the composition and fluidity of their polar lipids upon acclimation of the higher plant, Nerium oleander, to growth temperature. Biochimica et Biophysica Acta. 1982;688:218–228. [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE. A conserved transcript pattern in response to a specialist and a generalist herbivore. The Plant Cell. 2004;16:3132–3147. doi: 10.1105/tpc.104.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routaboul JM, Fischer SF, Browse J. Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiology. 2000;124:1697–1705. doi: 10.1104/pp.124.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Zhang R. High temperature effects on electron and proton circuits of photosynthesis. Journal of Integrative Plant Biology. 2010;52:712–722. doi: 10.1111/j.1744-7909.2010.00975.x. [DOI] [PubMed] [Google Scholar]
- Stintzi A, Browse J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proceedings of the National Academy of Sciences, USA. 2000;97:10625–10630. doi: 10.1073/pnas.190264497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE. Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proceedings of the National Academy of Sciences, USA. 2001;98:12837–12842. doi: 10.1073/pnas.211311098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PG, Dominy PJ, Vigh L, Mansourian AR, Quinn PJ, Williams WP. Increased thermal stability of pigment-protein complexes of pea thylakoids following catalytic hydrogenation of membrane lipids. Biochimica Et Biophysica Acta. 1986;849:131–140. [Google Scholar]
- Toth SZ, Nagy V, Puthur JT, Kovacs L, Garab G. The physiological role of ascorbate as photosystem II electron donor: protection against photoinactivation in heat-stressed leaves. Plant Physiology. 2011;156:382–392. doi: 10.1104/pp.110.171918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan P, Browse J. Photoinhibition in mutants of Arabidopsis deficient in thylakoid unsaturation. Plant Physiology. 2002;129:876–885. doi: 10.1104/pp.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan P, Shockey J, Levesque CA, Cook RJ, Browse J. A role for jasmonate in pathogen defense of. Arabidopsis. Proceedings of the National Academy of Sciences, USA. 1998;95:7209–7214. doi: 10.1073/pnas.95.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Gombos Z, Murata N. Contribution of membrane lipids to the ability of the photosynthetic machinery to tolerate temperature stress. Proceedings of the National Academy of Sciences, USA. 1994;91:4273–4277. doi: 10.1073/pnas.91.10.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JG, Browse J. Mutants of Arabidopsis reveal many roles for membrane lipids. Progress in Lipid Research. 2002;41:254–278. doi: 10.1016/s0163-7827(01)00027-3. [DOI] [PubMed] [Google Scholar]