Abstract
Programmed cell death (PCD) has been found to be induced after pollination both in papillar cells and in self-incompatible pollen in the olive (Olea europaea L.). Reactive oxygen species (ROS) and nitric oxide (NO) are known to be produced in the pistil and pollen during pollination but their contribution to PCD has so far remained elusive. The possible role of ROS and NO was investigated in olive pollen–pistil interaction during free and controlled pollination and it was found that bidirectional interaction appears to exist between the pollen and the stigma, which seems to regulate ROS and NO production. Biochemical evidence strongly suggesting that both O2˙− and NO are essential for triggering PCD in self-incompatibility processes was also obtained. It was observed for the first time that peroxynitrite, a powerful oxidizing and nitrating agent generated during a rapid reaction between O2˙− and NO, is produced during pollination and that this is related to an increase in protein nitration which, in turn, is strongly associated with PCD. It may be concluded that peroxynitrite mediates PCD during pollen–pistil interaction in Olea europaea L. both in self-incompatible pollen and papillar cells.
Keywords: Nitric oxide, Olea europaea L., peroxynitrite, programmed cell death, pollen–pistil interaction, reactive oxygen species
Introduction
In spite of the apparent paradox, cell death is crucial for the growth and development of eukaryotic cells because it maintains tissue and organ homeostasis (Van Breusegem and Dat, 2006). Programmed cell death (PCD) in plants is an active process leading to the selective elimination of unneeded or damaged cells during many developmental processes such as embryogenesis, tapetum degeneration, pollen selection due to self-incompatibility, organ senescence, and tracheary element differentiation and also during growth under stress conditions (Gechev et al., 2006; Rogers, 2006). It is genetically controlled and, in plants, includes a variety of types of cell death, although some of them share common morphological and biochemical features with animal cell apoptosis, such as DNA cleavage into internucleosomal fragments and shrinkage of the cytoplasm (Wang et al., 1996).
In recent years, a dual role for reactive oxygen and nitrogen species (ROS and RNS) has been recognized in plant biology (Mittler et al., 2004; Neill, 2005; del Rio and Puppo, 2009). The function of ROS is finely regulated by a ROS-producing and -detoxifying balance that adjusts their levels under different developmental or environmental conditions. ROS homeostasis may be cell-specific and even intracell-specific (Mittler et al., 2004). ROS and RNS were once considered to be toxic by-products of aerobic metabolism, leading to destructive modifications in proteins, DNA, and lipids, and that plants therefore developed a strong antioxidant system to remove any excess of ROS (Halliwell, 2006). Recently, however, they have been recognized as signalling molecules that fine-tune such processes in plant biology as defence, hormone signalling, and development (Mittler et al., 2004; del Rio et al., 2006; Gapper and Dolan, 2006). In addition, nitric oxide (NO) appears to play a key role as a signalling molecule in plants (Romero-Puertas and Delledonne, 2003; Delledonne, 2005; Neill, 2005). As a developmental regulator it promotes germination, leaf extension, and root growth, and delays leaf senescence and fruit ripening (Neill et al., 2003). It has recently been shown to be a key molecule that interacts with ROS in a number of ways, leading to cell death or signalling in response to different physiological and stress conditions (Clarke et al., 2000; de Pinto et al., 2002; Zaninotto et al., 2006). Recent genetic studies have demonstrated the crucial role of ROS at different stages of plant PCD in Arabidopsis thaliana (Mittler and Rizhsky, 2000; Lorrain et al., 2003), although initial evidence for this had already been obtained in cultured soybean cells (Levine et al., 1994), and in animals it has been well established that H2O2 and O2˙− co-operate with NO to induce PCD (Jabs, 1999; Van Breusegem and Dat, 2006). Our understanding of the genetic mechanisms that trigger and regulate plant PCD is, however, somewhat limited. This, together with the diversity of cell-death types described in plant biology, suggests that, despite some similarities, the signalling pathways may well be different.
Self-incompatibility is the most widespread mechanism in the prevention of inbreeding via the rejection of self-pollen and genetically related pollen (de Nettancourt, 1997) and it has been demonstrated that PCD occurs as a result of incompatible pollination (Rogers, 2006). The involvement of PCD in the rejection of self-incompatible pollen in an in vitro system in Papaver is the best characterized to date. It has been observed in this species that elongation of the pollen tube belonging to self-pollen is inhibited within minutes of its landing on the stigma, a process known as stigmal gametophytic SI (Franklin-Tong and Franklin, 2003). PCD has also been described in Pyrus pyrifolia, where pollen-tube growth is arrested in the style (stylar gametophytic SI) (Wang CL et al., 2009). More recently, Serrano et al. (2010) have demonstrated that PCD is involved in pollen selection in Olea europaea L., since self-incompatible pollen landing on the stigma triggers PCD. Self-incompatibility has been identified in most olive cultivars, including Picual, one of the most important for olive-oil production (Lavee et al., 1999; Moutier, 2000; Wu, 2002; Díaz et al., 2006). Nevertheless, difficulties in experimentation with this species (it flowers only once a year for a very short time) have meant that the olive tree has, in general, been studied from an agronomical point of view and that molecular and cell studies are very scarce.
It has been shown that stigmas from diverse angiosperms accumulate H2O2 and that pollen from a wide range of plant species produces NO (Prado et al., 2004; McInnis et al., 2006; Bright et al., 2009; Wang Y et al., 2009; Zafra et al., 2010; Wilkins et al., 2011). The involvement of ROS and NO in PCD caused by self-incompatibility has not been studied to date in an in vivo system, although very recently it has been shown in vitro that ROS and NO mediate PCD in pollen tubes during self-incompatibility responses in Papaver (Wilkins et al., 2011). In this study, the involvement of ROS and NO in PCD prompted by self-incompatibility in the olive was investigated using an in vivo system of controlled and freely pollinated flowers from Olea europaea, L.. The results reveal a ROS- and RNS-mediated interaction between pollen and pistil during pollination and a peroxynitrite-dependent PCD signalling pathway both in papillar cells and self-incompatible pollen grains after pollination.
Materials and methods
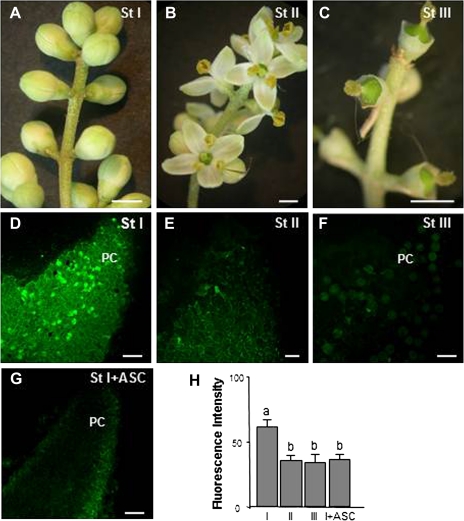
Perfect flowers, with both pistillate and staminate parts, were gathered periodically before and after pollination during the months of May and June 2007–2010 from Olea europaea L. cv. Picual trees growing in Granada and Córdoba (Spain). Within the phenologically mixed populations of flowers, care was taken to select exactly the different stages chosen for this study: stage I, white flower buds before pollination; stage II, open flowers after pollination; stage III, flowers after fertilization which had lost their petals and showed enlarged ovaries (Fig. 1A–C).
Fig. 1.
Hydrogen peroxide in olive pistils excised from freely pollinated flowers detected by confocal laser scanning microscope. (A, B, C) Stereomicroscope images of olive flowers before pollination (St I), during pollination (St II), and after fertilization (St III). (D, E, F, G) Detection of H2O2 by DCF-DA in stigmas at different stages. Green fluorescence corresponding to H2O2 is detected in papillar cells mainly at stage I. (G) Pistil at stage I treated with ascorbate (ASC), a H2O2 scavenger. (H) Histogram showing fluorescence quantified in total images of the stigmal lobules in arbitrary units using LAS AF Leica software. Different letters indicate significant difference at P <0.05 as determined by Duncan's multiple-range test. PC, papillar cells. (A, B, C) Bars=2 mm; (D, E, F, G) bars=50 μm. (This figure is available in colour at JXB online.)
For controlled pollination, white flower buds before pollination were either enclosed in bags for self-pollination (self-incompatible pollination) or emasculated and cross-pollinated by hand with pollen of cv. Arbequina (compatible pollination) before being enclosed in bags. Flowers inside the bags were collected for analysis when non-bagged flowers of reference inflorescences on the same branch reached stage III.
ROS, NO, and peroxynitrite detection by fluorescence microscopy
Reactive oxygen and nitrogen species were detected according to Rodriguez-Serrano et al. (2006). To detect nitric oxide, controlled and freely pollinated pistils excised from olive flowers at the three different stages studied were incubated for 1 h at 25 °C in darkness with 10 μM 4,5-diaminofluorescein diacetate (DAF-2 DA, Calbiochem, San Diego, CA, USA) in 10 mM TRIS-HCl (pH 7.4). The same method was used for the detection in pollen grains germinated in vitro. O2˙− and H2O2 were detected by incubating samples for 30 min at 37 °C with 10 μM dihydroethidium (DHE) and 25 μM 2′7′-dichlorofluorescein diacetate (DCF-DA) respectively, in 10 mM TRIS-HCl (pH 7.4). To detect peroxynitrite pistils excised from controlled and freely pollinated flowers, they were incubated for 1 h at 25 °C in darkness with 25 μM HKGreen-2, kindly provided by Dr Dan Yang (Sun et al., 2009). Controls of ROS, NO, and peroxynitrite detection were made by pre-incubating samples with 2 mM carboxy-2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) (a NO specific scavenger), 2 mM tetramethyl piperidinooxy (TMP) (an O2˙− scavenger), 2 mM ascorbate (ASC) (a H2O2 scavenger), and 2 mM epicatechin (a ONOO−-specific scavenger) (Pannala et al., 1997). All the samples were then washed, squashed, and their fluorescence examined under a confocal laser scanning microscope (Leica TCS SL, Leica Microsystems, Wetzlar, Germany). DAF-2 DA (excitation 495 nm and emission 515 nm) and DCF-DA (excitation 485 and emission 530 nm) probes emit green fluorescence, whereas DHE (excitation 490 nm and emission 520 nm) and HKGreen-2 (excitation 520 nm and emission 539 nm) emit red fluorescence. A total of nine pistils were analysed for each stage and fluorochrome in three independent experiments with three replicates every year for four years.
To check possible ROS and NO enzymatic sources, samples at stage III were studied after pre-incubation with 10 μM of diphenyliodonium (DPI) (a NADPH oxidase inhibitor), 10 mM of sodium azide (a peroxidase inhibitor), and 5 mM of NG-nitro-L-arginine methyl ester (L-NAME) (a NOS inhibitor), in the same way as with the scavengers. A total of six pistils were analysed with each inhibitor in two independent experiments with three replicates every year for four years. Fluorescence intensity was quantified taking into account the whole image of the stigma lobule.
Pollen–pistil interactions in vitro
Pollen grains isolated from olive trees were germinated in a medium containing 20% w/v sucrose, 0.01% w/v CaCl2, 0.01% w/v H3BO3, and 0.01% w/v KNO3 in a humid chamber at 30 °C for 3 h for ROS and NO detection, and for 18 h for RNA and protein extraction. Pollen grains were germinated in in vitro cultures both in the presence and absence of fresh receptive pistils.
After removing the pistils, pollen grains and pollen tubes were used to detect reactive species as described above for pistils. For RNA and protein studies pollen grains and pollen tubes were centrifuged before extraction. Germination in vitro included three independent experiments with three replicates each.
To test the influence of NO upon the development of stigma-cell death, 12 flower buds (stage I) were emasculated, sprayed with sodium nitroprusside (SNP), a NO donor, and enclosed in bags to avoid the arrival of pollen grains to their surface. The samples were collected when the flowers in the reference inflorescence reached stage III. Parallel control experiments were carried out with pistils not sprayed with SNP. ROS were detected in two independent experiments with 12 samples each.
Cell-death detection
Our group have already shown in experiments using the TUNEL reaction, DNA degradation analysis, and caspase-like activity that cell death detected by trypan blue in olive stigmal papillar cells and pollen grains corresponded to PCD (Serrano et al., 2010). Trypan-blue staining was used here because it is a very direct, simple, and rapid method to detect cells that are suffering cell death and allows a greater number of samples to be analysed compared with the techniques mentioned above. Fresh pistils were stained by boiling them in alcoholic lactophenol (96% ethanol:lactophenol, 1:1 v/v) containing 0.1 mg ml−1 trypan blue (Sigma) for 1 min, any excess of which was removed in a chloral hydrate solution (2.5 mg ml−1) at room temperature overnight. After staining, the ovary was removed and the stigma and style were squashed and studied under a Zeiss Axioplan microscope. To gauge the influence of O2˙−, NO, and ONOO− upon cell death, pollinated flowers (stage II) were sprayed with 2 mM tetramethylpiperidinooxy (TMP), 2 mM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), or 2 mM epicatechin and collected at stage III. A total of 12 flowers on four different branches were treated with each scavenger and the experiment was repeated twice, using the same number of flowers as control. A total of 1000 pollen grains were evaluated for each treatment (TMP, cPTIO, and epicatechin) to determine whether the differences found in the number of pollen grains positive to trypan-blue staining were significant. The significance of the differences was tested with a χ2 test.
Detection of nitrated proteins in pollen germinated in vitro
To detect possible differences in the number of nitrated proteins produced as a consequence of PCD, protein extracts from pollen germinated in vitro in the presence and absence of pistils of the same cultivar were used. To test the influence of peroxynitrite upon the nitration of pollen proteins, protein extracts from pollen germinated in vitro in the presence of 2 mM epicatechin were also obtained. Immunoblotting experiments were carried out with anti-nitrotyrosine antibody (Sigma). 100 mg of each sample was ground to a fine powder in liquid nitrogen using a mortar and pestle. Proteins were then extracted in a homogenization medium (50% v/v phenol, 0.45 M sucrose, 5 mM EDTA, 0.2% v/v 2-mercaptoethanol, and 50 mM TRIS-HCl, at pH 8.8) as described elsewhere (Hajduch et al., 2005). Protein concentration was determined using a protein assay from Bio-Rad (Hercules, CA) based on the modified procedure of Bradford (1976). Protein samples were loaded in 12% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Millipore Co., Bedford, MA, USA) following standard protocols (Sambrook and Russell, 2001). The membrane was stained with Ponceau red to check the equivalency in protein loading. Nitration was detected using the anti-nitrotyrosine antibody diluted 1:2000. Protein extracts were incubated with sodium dithionite 5 mM to ensure antibody specificity. Probing and detection of immunocomplexes were carried out as described for the ECL Plus detection system (Amersham Biosciences). This experiment was carried out twice, similar results being obtained on each occasion. The experimental procedures of the Supplementary figures are included in Appendix S1.
Results
ROS and NO presence throughout pollination
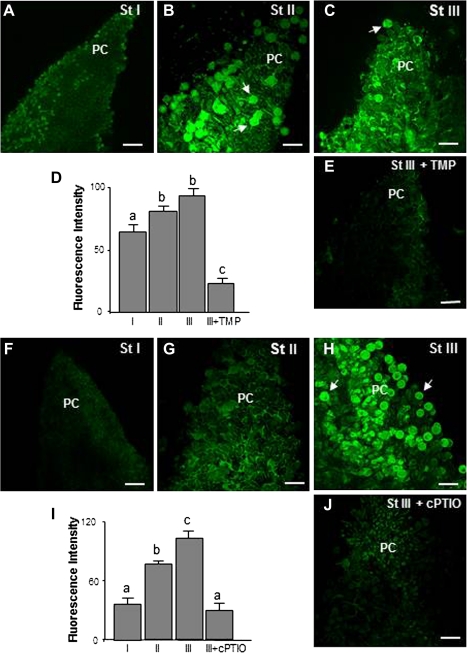
ROS and NO production during free pollination was analysed in pistils during three different stages: before pollination (white flower buds, stage I), after pollination (open flowers, stage II), and after fertilization (pistils with enlarged ovaries, stage III) (Fig. 1A, B, C). Since an in vivo system was used, it must be borne in mind that the time of flowering and the various stages depended to a great extent upon the environmental conditions, which is why the results of exogenous treatments were analysed not after any fixed time but when the flowers in the reference inflorescence reached the appropriate stage. All the experiments were repeated a significant number of times and similar results were obtained, thus proving that the environmental conditions had not affected the study. Observations with confocal laser scanning microscopy after pistil staining with specific fluorescent probes for ROS and NO (Figs 1, 2) revealed that changes in these molecules took place during the progamic phase (from pollination to fertilization) both in pollen and papillar cells. Fluctuations in H2O2 during the different stages were detected with DCF-DA. H2O2-dependent green fluorescence was mainly to be seen in the stigmal papillae of pistils during stage I (Fig. 1D, H) but decreased after pollination in the stigmal lobules during stages II and III (Fig. 1E, F, H). The level of fluorescence observed during stage II was due on the whole to the exudate present among papillar cells, whilst during stage III it was mainly due to pollen grains. Ascorbate was used as a negative control in stage I (Fig. 1G). DHE was used to visualize the superoxide anion (O2˙−). A green fluorescent signal was detected in the stigmal papillar cells of pistils during stage I (Fig. 2A), which increased during stages II and III, appearing not only in the papillar cells but also in the areas surrounding them containing exudate, where pollen grains were germinating. Most pollen grains and tubes showed fluorescent signal (Fig. 2B, C, D). TMP was used as a negative control for stage III (Fig. 2E). DAF-2DA was used to visualize NO. An increase in fluorescence of around 50% was observed after pollination (stage II), compared with fluorescence during stage I (Fig. 2G, I). Most striking was that, after fertilization (stage III), both the papillar cells and most of the pollen grains were fluorescent (close to 70%) (Fig. 2H, I). cPTIO was used as negative control in stage III (Fig. 2I, J).
Fig. 2.
Superoxide and NO detection in olive pistils excised from freely pollinated flowers by confocal laser scanning microscope. (A, B, C, E) Detection of O2˙− by DHE in pistils at different stages. Green fluorescence corresponding to O2˙− is visible during all three stages in papillar cells and pollen grains after pollination (arrows). (E) Pistil at stage III treated with TMP, an O2˙− scavenger. (F, G, H, J) Detection of NO by DAF2-DA in pistils at different stages. Green fluorescence corresponding to NO was detected both in papillar cells and pollen grains (arrows) at stages II and III. (J) Pistil at stage III treated with cPTIO, a NO scavenger. (D, I) Histograms showing fluorescence, quantified in total images of the stigmal lobules in arbitrary units using LAS AF Leica software. Different letters indicate significant difference at P <0.05 as determined by Duncan's multiple-range test. PC, papillar cells; pollen grains (arrows). Bars=50 μm. (This figure is available in colour at JXB online.)
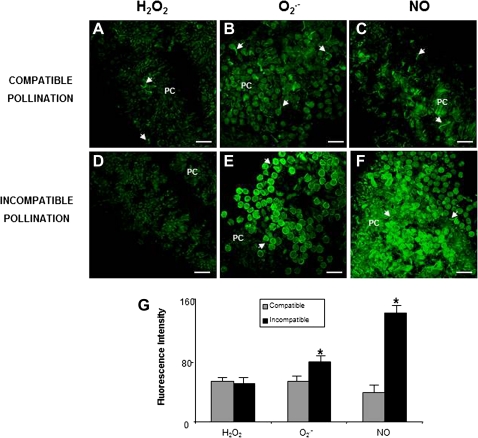
To establish clearly whether the fluctuations in ROS and NO in the papillar-cell fluorescence depended upon the kind of pollen landing on the stigma (compatible or incompatible), their production was analysed after controlled pollination with pollen of the same self-incompatible cultivar or with pollen of a compatible cultivar (Fig. 3). When fluorescence corresponding to H2O2 during stage III was analysed, a similar fluorescence was seen to occur after both compatible and incompatible pollination (Fig 3A, D, G). O2˙− -dependent green fluorescence increased by around 30% after incompatible pollination compared to compatible pollination (Fig. 3B, E, G). This increase was more obvious with NO-dependent green fluorescence, it being more than three times higher after incompatible pollination (Fig. 3C, F, G).
Fig. 3.
ROS and NO detected in olive pistils excised from controlled-pollinated flowers. Confocal laser scanning microscope images of stigmas from pistils at stage III. (A, B, C) Stigmas after compatible pollination. (A) H2O2 detection by DCF-DA. Faint fluorescence is observed. (B) Detection of O2˙− by DHE. Green fluorescence corresponding to O2˙− is visible only in some pollen tubes (arrows). (C) Detection of NO by DAF2-DA. Fluorescence detectable only in some pollen tubes (arrows). (D, E, F) Stigmas after incompatible pollination. (D) H2O2 detection by DCF-DA is similar to that in compatible pollination. (E) Detection of O2˙− by DHE. Papillar cells, pollen grains, and pollen tubes (arrows) show green fluorescence. (F) Detection of NO by DAF2-DA. Green fluorescence is visible in pollen grains and pollen tubes (arrows) and in papillar cells (PC). (G) Histogram showing relative fluorescence intensities quantified in total images of the stigmal lobules and corresponding to H2O2, O2˙−, and NO, reflecting differences between compatible and incompatible pollination. Asterisk indicates significant difference at P <0.05 according to Duncan's multiple-range test. Bars=50 μm. (This figure is available in colour at JXB online.)
To locate H2O2 and O2˙− more efficiently, DAB and NBT stainings were used in fresh pistils during free pollination. The pistils were observed directly under the microscope after they had been squashed and also fixed and embedded in Unicryl to obtain sections in which the presence of these molecules could be studied cytochemically. A brown staining corresponding to H2O2 was detected after DAB treatment in the stigmas during stage I (see Supplementary Fig. S1A, B at JXB online), which decreased after pollination during stages II and III (see Supplementary Fig. S1F, G, K, L at JXB online), confirming the results obtained with the fluorescent probes. In the sections, H2O2-dependent brown staining was visible in the cytoplasm of papillar cells at stage I (see Supplementary Fig. S2A at JXB online). O2˙− was present in the stigmas during stage I (see Supplementary Fig. S1C, D at JXB online). O2˙−-dependent staining remained after pollination during stages II and III (see Supplementary Fig. S1H, I, M, N at JXB online). O2˙− was located in the outer part of the papillar cells in the pistil sections, which supports the data obtained with the corresponding fluorescent dyes (see Supplementary Fig. S2B at JXB online). Furthermore, some pollen grains and tubes showed O2˙−-dependent staining (see Supplementary Fig S2B at JXB online). When no DAB or NBT was added to the incubation buffer no signal was visible (see Supplementary Fig. S1E, J, O at JXB online).
ROS and NO sources after free pollination
In the light of the fact that O2˙− and NO were the two main reactive species detected in papillar cells and pollen tubes in stages II and III, during which pollen–pistil interaction takes place, it was decided to study the enzymatic activities responsible for their production. When pistils at stage II were incubated with diphenyliodonium (DPI), and sodium azide, O2˙− -dependent fluorescence decreased during stage III both in papillar cells and pollen grains and tubes (Fig. 4A, B, C, F), suggesting that both NADPH oxidase and peroxidase activities could be involved in O2˙− production during free pollination in the olive. Furthermore, an increase in the expression of the different NADPH oxidase isoforms was observed in pollen grains germinated in the presence and absence of pistils (see Supplementary Fig. S3 at JXB online).
Fig. 4.
ROS and NO sources during free pollination. Confocal laser scanning microscope images of stigmas from pistils at stage III incubated with specific enzyme activity inhibitors. (A) Detection of O2˙− by the specific probe DHE in an untreated stigma. (B) Stigmas pretreated with the NADPH oxidase inhibitor DPI before O2˙− detection. (C) Detection of O2˙− by the specific probe DHE in a stigma pretreated with the peroxidase inhibitor sodium azide. (D) Detection of NO by DAF2-DA in an untreated stigma. (E) Stigma pretreated with the specific nitric oxide synthase inhibitor L-NAME before NO detection. (F, G) Histograms showing relative fluorescence intensities quantified in total images of the stigmal lobules and corresponding to O2˙− and NO, reflecting the effect of enzyme-activity inhibitors. Different letters indicate significant difference at P <0.05 according to Duncan's multiple-range test. PC, papillar cells; SR, subpapillar region. Pollen grains (arrows). Bars=50 μm. (This figure is available in colour at JXB online.)
In addition, after incubating the pistils during stage I with L-NAME, a nitric oxide synthase (NOS) inhibitor, NO-dependent fluorescence was reduced by about 50% in pollen grains in the stigma (Fig. 4D, E, G), suggesting that a NOS-like activity is involved in NO production during free pollination.
Pollen–pistil interaction controls ROS and NO production
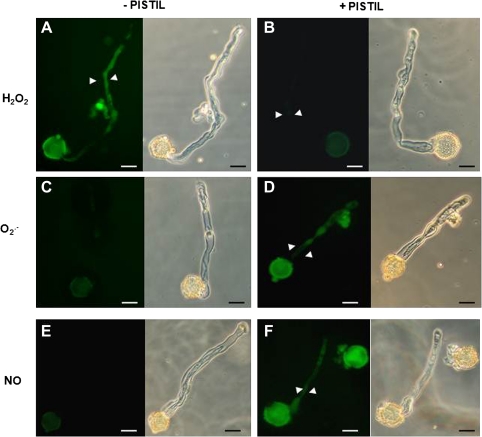
To check whether ROS and NO production in pollen were influenced by their interaction with the pistil, their accumulation was studied in pollen germinated in vitro in the presence and absence of pistils from the same cultivar. In the absence of pistils H2O2 was detected by DCF-DA both in pollen grains and tubes (Fig. 5A) but fluorescence clearly diminished when pollen grains were germinated in the presence of pistils (Fig. 5B). Staining with the fluorescent dye DHE showed that O2˙− was present in pollen grains but not in pollen tubes germinated in vitro in the absence of pistils (Fig. 5C). When pistils of the same cultivar were added to the germination medium, both pollen tubes and pollen grains showed an increase in O2˙− -dependent fluorescence (Fig. 5D). In addition, NO-dependent fluorescence was observed in pollen tubes only when the pollen grains were germinated in the presence of pistils (Fig. 5F). These results suggest that the presence of pistils modifies pollen ROS and NO accumulation, inhibiting H2O2 and inducing O2˙− and NO production.
Fig. 5.
Detection of ROS and NO in pollen grains germinated in vitro. Pollen grains were germinated in vitro in the presence or absence of receptive olive pistils of the same cultivar. (A) Detection of H2O2 by DCF-DA in pollen tubes (arrowheads) growing in the absence of pistils. (B) Detection of H2O2 in pollen grains and pollen tubes (arrowheads) growing in the presence of pistils. (C) Detection of O2˙− by DHE in pollen grains germinated in the absence of pistils. (D) Detection of O2˙− in pollen tubes (arrowheads) of pollen grains germinated in the presence of pistils. (E) Detection of NO by DAF2-DA in pollen grains germinated in the absence of pistils. (H) The same pollen grain under a light. (F) Detection of NO in the pollen tubes (arrowheads) of pollen grains germinated in the presence of pistils. The same pollen grain observed under a light microscope is shown in all cases. Bars=10 μm. (This figure is available in colour at JXB online.)
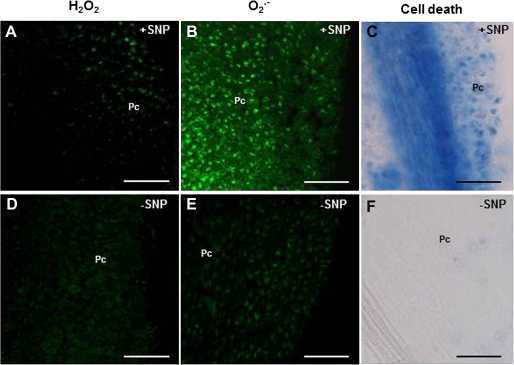
After checking the influence of the pistil ROS and NO production in pollen, it was decided to ascertain whether pollen arrival was responsible for the alterations in ROS content observed in the papillar cells. To this end, pistils in which pollination was avoided by emasculating and bagging the flower buds (stage I) were studied. Since pollen is known to produce NO, an external source of NO (SNP) was applied to these emasculated flowers during stage I to find out whether it was able to mimic the effect of pollen arrival. Flowers were bagged after SNP treatment to avoid the entrance of pollen. After SNP treatment, O2˙− was detected (Fig. 6B) and signs of cell death were found in the stigma during stage III (Fig. 6C). In addition, the level of H2O2 detected by DFC-DA was similar to that detected in pollinated pistils (Fig. 6A). When pistils from emasculated and bagged flower buds (stage I) were analysed at stage III without applying SNP, no fluorescence signal corresponding to H2O2 or O2˙− was observed (Fig. 6D, E), and no signs of cell death were found in papillar cells (Fig. 6F). These results highlight the fact that SNP mimicked the effect of pollination in papillar cells, thus supporting the idea of pollen–pistil cross-talk during pollination.
Fig. 6.
NO involvement in stigma development. (A, B, C) Flower buds were emasculated during stage I, sprayed with a NO donor (SNP), and bagged to avoid the arrival of pollen grains onto their surface, after which samples were collected at stage III. (D, E, F) Flower buds were emasculated and bagged during stage I without adding SNP, after which samples were collected at stage III. (A, D) H2O2 was detected by DCF-DA. (B, E) O2˙− was detected by DHE. (C, F) Cell death was detected by trypan blue staining. Pc, papillar cells. Bars=50 μm. (This figure is available in colour at JXB online.)
ROS and RNS are crucial for programmed cell death during pollination
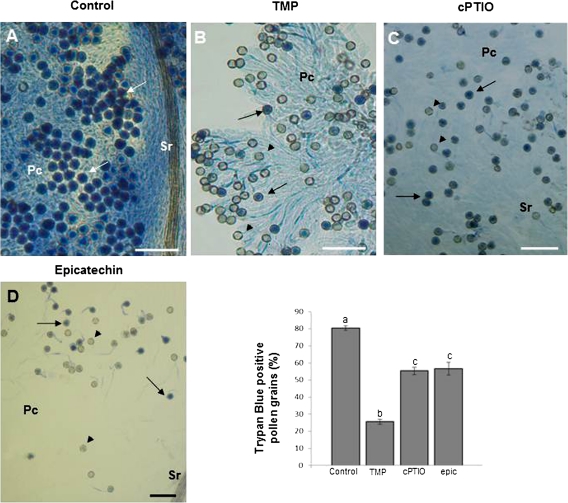
To characterize the possible contribution of ROS and RNS to PCD during free pollination in the olive, specific scavengers of these signalling molecules were applied directly to pistils during stage II, and cell death was then investigated at stage III by trypan-blue staining (Fig. 7). Practically all the stigma cells in untreated flowers were dead, as were a high percentage of the pollen grains germinating in them (80%). At the same stage, stigmas from pistils that had been sprayed with TMP during stage II showed a reduction in the staining of papillar cells with trypan blue as well as a 55% decrease in the number of pollen grains stained (Fig. 7A, B, E). Similarly, papillar cells remained practically unstained in stigmas sprayed with cPTIO, whereas there was a 30% decrease in the number of dead pollen grains compared with control samples (Fig. 7A, C, E). Papillar cells from stigmas sprayed with epicatechin were not stained with trypan blue and the number of pollen grains stained underwent a decrease of around 30%, similar to that observed with cPTIO (Fig. 7A, D, E). These results suggest that O2˙−, NO, and peroxynitrite are key signal molecules for PCD in papillar cells and self-incompatible pollen grains in the olive after free pollination.
Fig. 7.
ROS and RNS function in PCD after free pollination in olive trees. Flowers at stage II were sprayed with TMP (an O2˙− scavenger), cPTIO (a NO scavenger) or epicatechin (a ONOO− scavenger) and then collected at stage III, in which cell death was assayed by trypan-blue staining. (A) Stigma without pre-treatment. (B) Stigma after TMP pre-treatment. (C) Stigma after cPTIO pre-treatment. (D) Stigma after epicatechin treatment. (E) Histogram showing the number of dead pollen grains after the different pre-treatments. Different letters indicate significant difference at P <0.0001, tested with a χ2 test. Pc, papillar cells; Sr, subpapillar region. Blue pollen grains (arrows), non-stained pollen grains (arrowheads). Bars=100 μm. (This figure is available in colour at JXB online.)
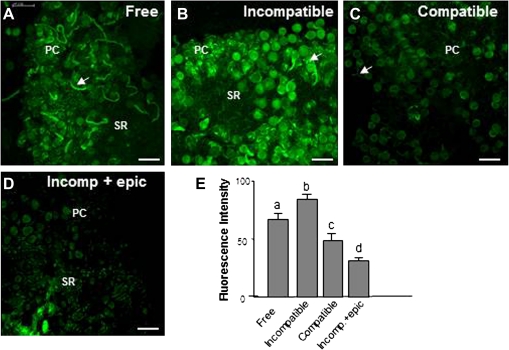
Since H2O2 levels fell significantly in pistils after pollination and O2˙− and NO coexisted in pollinated pistils the possible production of peroxynitrite (ONOO−) as a consequence of O2˙− and NO reaction was studied. The HKGreen-2 fluorescent probe was used to examine ONOO− production during stage III during free and controlled pollination (compatible and incompatible; Fig. 8). Fluorescence corresponding to ONOO− was detected in papillar cells, pollen grains, and pollen tubes during free pollination (Fig. 8A). A 20% increase in the signal was detected in controlled pollination with incompatible pollen (Fig. 8B, E) whilst ONOO−-dependent fluorescence was reduced by 30% after controlled pollination with compatible pollen (Fig. 8C, E) compared with free pollination. Epicatechin, a ONOO− scavenger, was used as a negative control, fluorescence intensity being reduced both in papillar cells and pollen during incompatible pollination (Fig. 8D, E).
Fig. 8.
Peroxynitrite detection by HKGreen-2 in controlled and freely pollinated pistils. Confocal laser scanning microscope images of stigmas from pistils at stage III. (A) Stigma after free pollination. Green fluorescence corresponding to ONOO− is detected mainly in pollen grains and pollen tubes. (B) Stigma after incompatible pollination. An increase in green fluorescence is detected in papillar cells, pollen grains, and pollen tubes (arrows) compared with freely pollinated pistils. (C) ONOO− detection after compatible pollination. Green fluorescence is reduced compared to freely pollinated pistils. (D) ONOO− detection in pistils after incompatible pollination treated with the ONOO− scavenger epicatechin. A reduction in green fluorescence is detected compared with incompatible pollinated pistils (B). (E) Histogram showing relative fluorescence intensities quantified in total images of the stigmal lobules and corresponding to peroxynitrite detection under different conditions. Different letters indicate significant difference at P <0.05 according to Duncan's multiple-range test. PC, papillar cells; SR, subpapillar region. Bars=50 μm. (This figure is available in colour at JXB online.)
Because ONOO− causes tyrosine nitration (Radi, 2004), its presence was also studied by immunocytochemistry with an anti-nitrotyrosine antibody to locate ONOO−-dependent nitration in pistil sections. Nitrotyrosines were detected after pollination in papillar cells and pollen (see Supplementary Fig. S4 at JXB online). When pistils were treated with TMP or cPTIO, a reduction in immunoreactivity was observed (see Supplementary Fig. S4A, B, C, E at JXB online). Furthermore, when nitrotyrosine detection and the TUNEL reaction were carried out in serial sections it was found that pollen containing the nitrotyrosine signal was also positive to TUNEL reaction, whereas the opposite was true with grains containing no nitrotyrosine signal (see Supplementary Fig. S4D at JXB online). It is likely, therefore, that ONOO−-dependent nitration is involved in PCD signalling taking place during incompatible pollination. These results affirm that there is a strong correlation between ONOO−-dependent nitration and PCD signalling that takes place during incompatible pollination.
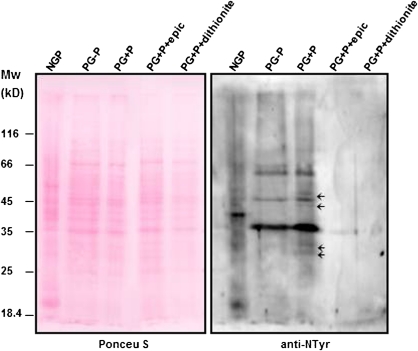
In addition, protein nitration was analysed in pollen extracts before and after germination. Western blot analysis using an anti-nitrotyrosine antibody revealed an increase in the number of nitrated proteins in germinated compared with non-germinated pollen (Fig. 9). When in vitro germination was carried out in the presence of pistils of the same self-incompatible olive cultivar four new nitrated proteins appeared, two of about 30 kDa and another two of about 50 kDa (Fig. 9), suggesting that pollen–pistil interactions are required to trigger the signalling pathway leading to PCD during olive pollination. These results were confirmed by the absence of nitrated proteins when epicatechin was applied in pollen grains germinating in the presence of Picual pistils (Fig. 9).
Fig. 9.
Tyrosine nitration during in vitro pollen germination. Proteins extracted from non-germinated pollen grains (NGP), germinated in vitro in the absence (PG-P) or presence (PG+P) of pistils of the same self-incompatible cultivar with epicatechine (PG+P+epic) or not (PG+P), and protein extracts incubated with sodium dithionite were subjected to protein-gel-blot analysis with an anti-nitrotyrosine antibody. The polyvinylidene difluoride membrane was stained with Ponceau red after protein transfer and the stained blot is shown as a loading control. Arrows indicate new bands that appear in the presence of olive pistils. (This figure is available in colour at JXB online.)
Discussion
It is accepted that some types of PCD in plants share features with that in animals, such as DNA fragmentation, cytochrome c leakage from mitochondria to the cytosol and caspase-3 activity (Krishnamurthy et al., 2000; van Doorn and Woltering, 2005; Reape and McCabe, 2008). One example of PCD in plants is the death of the pollen tube during self-incompatible interaction. Within this context, a Ca2+-dependent signalling pathway has been detected in Papaver, involving MAPK, cytochrome c, and caspase-3-like activity (Thomas et al., 2003, 2004, 2006; Wilkins et al., 2011). It has also been demonstrated that, after the in vitro germination of self-incompatible pollen of Pyrus pyrifolia, S-RNases induce mitochondria and nuclear DNA alterations (Wang CL et al., 2009) suggesting that PCD is involved in a self-incompatibility response in this species as well. Recently, Serrano et al. (2010) reported that PCD is activated in pollen and stigmal papillae during free pollination in vivo in olive trees (Olea europaea L.). Trypan-blue staining was used to detect the stages during which cell death occurred and, subsequently, TUNEL reaction, caspase-like enzyme activity, and DNA fragmentation showed that this cell death was in fact PCD. In this study it was also found that PCD began after pollination in papillar cells (irrespective of the type of pollen arriving at the stigma: compatible, incompatible or even sterile) and that about 80% of pollen undergoes PCD in freely pollinated pistils (Serrano et al., 2010).
During recent years a large body of evidence has suggested that ROS can activate cell-death programmes (Jabs, 1999; Van Breusegem and Dat, 2006). In plants, complementary data come from two different approaches: exogenous treatments with ROS or antioxidants, and transgenic plants with perturbed levels of ROS (Gechev and Hille, 2005; Zago et al., 2006; Gao et al., 2008). Experimental evidence exists to show the specificity in the signalling pathway of different ROS in their triggering of PCD (Gadjev et al., 2006; Gechev et al., 2006; Van Breusegem and Dat, 2006). Finely balanced concentrations of NO and ROS co-operate to trigger PCD (Delledonne et al., 2001; Zago et al., 2006). As far as is known, except for some very recent work carried out in Papaver pollen tubes in vitro (Wilkins et al., 2011), there are no published data on in vivo studies into the involvement of ROS and NO in self-incompatibility PCD. For this reason an analysis of H2O2, O2˙−, and NO production during free and controlled pollination in the olive tree was undertaken and it has been found for the first time that an interplay, both spatial and temporal, of O2˙− and NO is essential to the triggering of PCD both in papillar cells and self-incompatible pollen grains.
Firstly, a study into the presence of H2O2 during pollination was undertaken and it was found that H2O2 was present in stigmal papillae from pistils before pollination (Fig. 1). Within this context, it has been suggested that H2O2 may play some part in the defence of the stigma against pathogens before pollination (Carter and Thornburg, 2004; McInnis et al., 2006) and it may also be related to stigma receptivity in different species (Dafni and Maués, 1998). As has been reported elsewhere, after free pollination H2O2 decreases substantially in stigma lobules in the olive (Zafra et al., 2010). The reduction in H2O2 after pollen arrival suggests that it plays no direct role in triggering PCD in response to self-incompatibility in the olive in contrast to what occurs with HR in Arabidopsis (Delledonne et al., 2001).
Consequently, two other possible agents involved in PCD, NO, and O2˙−, were investigated (Fig. 2). It was found that papillar cells produced O2˙− before pollination (stage I) and its level increased during the pollination and fertilization processes (stages II and III). Furthermore, O2˙− was also observed in pollen grains and pollen tubes on the stigma. Papillar cells do not, however, produce NO before pollination, but they do so after the arrival of pollen and until the end of pollination. Furthermore, NO was found in pollen grains and tubes during free pollination. NO has in fact been found in pollen grains and tubes in experiments made in different species (Prado et al., 2004, 2008; McInnis et al., 2006; Bright et al., 2009; Zafra et al., 2010) and it has recently been suggested that it might function as an external signalling molecule, reducing H2O2 in Senecio squalidus and Arabidopsis thaliana stigmal papillae (McInnis et al., 2006). Thus this possibility was checked in our system and, when SNP was added to bag-emasculated flowers during stage I, H2O2 that was already present in stigmal papillae had disappeared from the pistils by stage III (Fig. 6), as it also had from pollinated pistils. This suggests that NO from pollen regulates ROS production in papillar cells. When pollination was impeded, no H2O2 was detected in the papillar cells of stigmas during stage III. This might be related to changes in receptivity due to alterations in H2O2 and peroxidase activity, which do not depend upon pollination, as has been described in various different species (Dafni and Maués, 1998; Honsho et al., 2007).
To discover whether the stigma might, in the same way, exert any influence upon pollen ROS and NO levels, ROS and NO production were analysed in pollen germinated in vitro both in the presence and absence of pistils (Fig. 5). The presence of H2O2 in germinating pollen tubes has been reported in Nicotiana and Papaver in in vitro systems (Potocky et al., 2007; Wilkins et al., 2011) and the same has been found in some olive pollen grains germinating in vivo (Zafra et al., 2010), but under our fluorescence-detection conditions this signal was only strong enough to be detected when pollen was germinated in vitro in the absence of pistils. In our assays, no clear differences were founded in vitro in ROS and NO in Picual pollen grains germinated in the presence of pistils of cv. Arbequina but, when the pistil added was from the same cultivar, H2O2 production was suppressed, indicating that H2O2 production is closely controlled when self-incompatible pollen comes into contact with the pistil. Nevertheless, the results of our experiments show clearly that the presence of self-incompatible pistils increased O2˙− and NO production in pollen grains and stimulated it in pollen tubes. They also indicate that O2˙− and NO may act as key signalling molecules in the interaction between incompatible pollen and pistils, this being confirmed in controlled pollinations, in which O2˙− and NO were mainly produced when incompatible pollen was used for pollination.
The induction of O2˙− observed in germinating pollen grains led us to search for a possible source of this particular ROS. Several mechanisms have been proposed to explain the generation of ROS by plants, but it would seem that a plasma membrane NADPH oxidase, analogous to that which generates the respiratory burst in mammalian phagocytes, is a likely source (Lamb and Dixon, 1997; Torres et al., 2002). In vivo assays showed a reduction of O2˙− in papillar cells, pollen grains, and tubes pretreated with DPI (Fig. 4), an inhibitor of the neutrophil NADPH oxidase, which blocks the plant oxidative burst (Doke and Ohashi, 1988), suggesting that this enzyme may well be a possible source of O2˙− in pollen. Semi-quantitative RT-PCR results showed an increase in NADPH oxidase 2 expression in germinating olive pollen in the presence of pistils of the same cultivar (see Supplementary Fig. S3 at JXB online), revealing the possible involvement of this isoform in self-incompatible O2˙− production. Furthermore, pretreatment with sodium azide, a peroxidase inhibitor, also resulted in a reduction in O2˙− production. NOS-like activity is one of the sources of NO since the treatment of pistils with L-NAME (Sandalio et al., 2008) drastically reduced NO production in both pollen and papillar cells. Alternative sources for both NO and O2˙− cannot be ruled out, i.e. the possible role of peroxisomes in ROS and NO production via xanthine oxidase and plant NOS-like activities respectively, which have been investigated in pollen and pistils of different species (Tezuka et al., 1997; Prado et al., 2004) and, in the same way, the involvement of nitrate reductase in the production of NO cannot be excluded, as has previously been suggested (Mittler et al., 2004; Neill et al., 2008).
Cell death during stage III in pistils that had previously been emasculated and bagged during stage I was checked to investigate further the involvement of pollen-dependent NO production in papillar PCD. In this case, no cell death was observed in stigmal papillar cells, confirming what has been previously reported (Serrano et al., 2010). Our results also showed that cell death appeared during stage III when emasculated bagged pistils had been pretreated during stage I with the NO donor SNP (Fig. 6). These results suggest that NO may play a role in papillar PCD. To confirm this, PCD was studied in vivo in pollen–pistil interactions taking place in the absence of NO. cPTIO eradicated PCD in papillar cells and reduced pollen PCD (Fig. 7). When pistils were pretreated with SNP during stage I and no pollen was allowed to land, both an accumulation of O2˙− and PCD were detected. Thus the possible involvement of O2˙− in PCD during pollen–pistil interaction was also investigated. The inhibition of superoxide accumulation by using TMP reduced PCD detected both in papillar cells and pollen grains by 55%, indicating that during pollen–pistil interaction both NO and O2˙− were required to activate PCD.
Our results showed that NO and O2˙− coexisted after pollination and both molecules were needed to activate PCD and so the possible role of ONOO− in self-incompatibility PCD was further investigated. NO and O2˙− rapidly form ONOO− in mammalian native immune cells such us macrophages and neutrophils in order to kill pathogens (Fang et al., 1997) and it is considered to be the main toxic reactive nitrogen species in animal cells (Stamler et al., 1992). Although high external concentrations of ONOO− (1 mM) have been reported to induce some necrotic lesions in Arabidopsis (Alamillo and Garcia-Olmedo, 2001), exposure to this high concentration does not seem to be essential to the induction of PCD (Delledonne et al., 2001; Romero-Puertas et al., 2007), probably due to the ability of plants to detoxify ONOO− under normal physiological conditions. Even so it is likely that ONOO− plays an important role in signalling functions in plants, since it was found that SIN-1, an ONOO− donor, induces genes related to defence against pathogenesis, such us PR-1 in tobacco leaves (Durner et al., 1998) and ONOO− induces protein nitration in soybean and tobacco (Delledonne et al., 2001; Saito et al., 2006). Furthermore, an increase in nitrated proteins during the progression of the HR has been shown in vivo, as well as an inhibition of the plant antioxidant system involved in ONOO− detoxification (Romero-Puertas et al., 2007). Experiments with HKGreen-2 showed that during free pollination, both papillar cells and pollen produce ONOO− during stage III (Fig. 8). PCD is induced in papillar cells after pollen landing irrespective of their origin (compatible, incompatible, and even sterile pollen; Serrano et al., 2010). No cell death was observed in papillar cells treated with epicatechin, indicating that ONOO− is involved in papillar cell PCD. In addition, ONOO− production was found in self-incompatible pollen, which undergoes PCD. Furthermore, when open flowers during stage II were treated with epicatechin, a reduction of about 30% was observed in pollen grains in the process of PCD (Fig. 7). These results suggest that ONOO− is a key signal molecule in the PCD of incompatible pollen. The increase in ONOO− during stage III reveals that O2˙− is reacting with NO, which could also explain why a parallel increase in H2O2 production was not observed.
In addition, when protein tyrosine nitration was looked into as being an effect of ONOO− reactivity (Radi, 2004; Szabo et al., 2007), nitration was found both in stigmal papillar cells and in pollen undergoing cell death. A positive nitrotyrosine signal was found in self-incompatible pollen undergoing PCD (see Supplementary Fig. S3 at JXB online). To confirm that protein nitration is important during pollen germination, pollen extracts before and after germination were studied in the presence and absence of pistils of the same cultivar. Immunoblotting revealed an increase in protein nitration in germinating pollen and four new nitrated proteins were detected when germination occurred in the presence of pistils of the same self-incompatible cultivar (Fig. 9). Tyrosine nitration decreased when TMP, cPTIO or epicatechin were applied, supporting the idea that the tyrosine nitration observed is ONOO−-dependent. Protein tyrosine nitration, the covalent attachment of a nitro (–NO2) group to the phenolic ring of Tyr, is considered to be a functionally important form of physiological NO-dependent post-translational modification (Schopfer et al., 2003). It has been shown that Tyr nitration can interfere with phosphorylation, a well-known signalling mechanism involved in a wide range of cell transduction pathways (Greenacre and Ischiropoulos, 2001; Schopfer et al., 2003). In particular, an important role in self-incompatibility has been shown for MAPK signalling via p56-MAPK, which participates in initiating the early PCD signalling cascade, leading to the death of the pollen tube (Li et al., 2007). Interestingly, a physiological concentration of ROS has been proposed in the enhancement of mammal sperm capacity by regulating phosphorylation pathways (Ford, 2004). In fact, in human sperm, superoxide regulates the phosphorylation of the Thr-Glu-Tyr motif in low-molecular-weight proteins (16–33 kDa), as well as the ERK pathway (de Lamirande and Gagnon, 2002). In addition, O2˙− may react with NO to produce ONOO−, which may go on to induce Tyr nitration of sperm proteins as well as Tyr phosphorylation (Herrero and Gagnon, 2001), suggesting an exciting parallel with sperm–egg interactions in mammal systems.
In conclusion, it would seem that there is sufficient evidence to assign a signalling role to ROS and RNS during pollen–pistil interactions in the olive, showing that reactive oxygen and nitrogen species are key components of pollen–stigma interaction leading papillar cells and self-incompatible pollen to PCD. These results reinforce the concept of a model of ROS/NO balance to decide cell fate, in particular, with regard to self-incompatibility. The link observed between Tyr nitration and PCD in self-incompatibility paves the way for future studies into possible interaction in nitration-phosphorylation signalling pathways.
Supplementary data
Supplementary data can be found at JXB online.
Appendix S1. Supplementary experimental procedures.
Supplementary Fig. S1. Detection by stereomicroscopy of ROS in olive pistils at stages I, II, and III.
Supplementary Fig. S2. Detection by light microscopy of ROS in olive pistils at stages I, II, and III.
Supplementary Fig. S3. NADPH oxidase expression during pollen germination.
Supplementary Fig. S4. Tyrosine nitration in in vivo pollen germination.
Acknowledgments
The authors acknowledge Dr D Yang for kindly providing HKGreen-2, Mrs R Luque and Mr F Yerón (Ingesar SL) for their valuable technical assistance, Mrs N de la Casa and Mr D Porcel for help with the confocal laser fluorescence microscopy (Technical Services of the University of Jaén and Granada, respectively) and Dr J Trout for revising our English text. This work was supported by ERDF-co-financed projects from the Spanish MEC (BFU2006-09876/BFI and BIO2008-04067). I Serrano received a research fellowship from the Ministry of Education.
References
- Alamillo JM, Garcia-Olmedo F. Effects of urate, a natural inhibitor of peroxynitrite-mediated toxicity, in the response of Arabidopsis thaliana to the bacterial pathogen Pseudomonas syringae. The Plant Journal. 2001;25:529–540. doi: 10.1046/j.1365-313x.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bright J, Hiscock SJ, James PE, Hancock JT. Pollen generates nitric oxide and nitrite: a possible link to pollen-induced allergic responses. Plant Physiology and Biochemistry. 2009;47:49–55. doi: 10.1016/j.plaphy.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Carter C, Thornburg RW. Is the nectar redox cycle a floral defense against microbial attack? Trends in Plant Science. 2004;9:320–324. doi: 10.1016/j.tplants.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ. NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. The Plant Journal. 2000;24:667–677. doi: 10.1046/j.1365-313x.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Dafni A, Maués MM. A rapid and simple procedure to determine stigma receptivity. Sexual Plant Reproduction. 1998;11:177–180. [Google Scholar]
- de Lamirande E, Gagnon C. The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Molecular Human Reproduction. 2002;8:124–135. doi: 10.1093/molehr/8.2.124. [DOI] [PubMed] [Google Scholar]
- de Nettancourt D. Incompatibility in angiosperms. Sexual Plant Reproduction. 1997;10:185–199. [Google Scholar]
- de Pinto MC, Tommasi F, De Gara L. Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco Bright-Yellow 2 cells. Plant Physiology. 2002;130:698–708. doi: 10.1104/pp.005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio LA, Puppo A. Reactive oxygen species in plant signaling. Springer-Verlag; 2009. [Google Scholar]
- del Rio LA, Sandalio LM, Corpas FJ, Palma JM, Barroso JB. Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiology. 2006;141:330–335. doi: 10.1104/pp.106.078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M. NO news is good news for plants. Current Opinion in Plant Biology. 2005;8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proceedings of the National Academy of Sciences, USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz A, Martín A, Rallo P, Barranco D, de la Rosa R. Self-incompatibility of ‘Arbequina’ and ‘Picual’ olive assessed by SSR markers. Journal of the American Society for Horticultural Science. 2006;131:250–255. [Google Scholar]
- Doke N, Ohashi Y. Involvement of an O2-generating system in the induction of necrotic lesions on tobacco leaves infected with tobacco mosaic virus. Physiological and Molecular Plant Pathology. 1988;32:163–175. [Google Scholar]
- Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proceedings of the National Academy of Sciences, USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Myllys V, Sandholm M. Resazurin reduction as a function of respiratory burst in bovine neutrophils. American Journal of Veterinary Research. 1997;58:601–607. [PubMed] [Google Scholar]
- Ford WC. Regulation of sperm function by reactive oxygen species. Human Reproduction Update. 2004;10:387–399. doi: 10.1093/humupd/dmh034. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong VN, Franklin FC. Gametophytic self-incompatibility inhibits pollen tube growth using different mechanisms. Trends in Plant Science. 2003;8:598–605. doi: 10.1016/j.tplants.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiology. 2006;141:436–445. doi: 10.1104/pp.106.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Xing D, Li L, Zhang L. Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta. 2008;227:755–767. doi: 10.1007/s00425-007-0654-4. [DOI] [PubMed] [Google Scholar]
- Gapper C, Dolan L. Control of plant development by reactive oxygen species. Plant Physiology. 2006;141:341–345. doi: 10.1104/pp.106.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. Journal of Cell Biology. 2005;168:17–20. doi: 10.1083/jcb.200409170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays. 2006;28:1091–1101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- Greenacre SAB, Ischiropoulos H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radical Research. 2001;34:541–581. doi: 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- Hajduch M, Ganapathy A, Stein JW, Thelen JJ. A systematic proteomic study of seed filling in soybean. Establishment of high-resolution two-dimensional reference maps, expression profiles, and an interactive proteome database. Plant Physiology. 2005;137:1397–1419. doi: 10.1104/pp.104.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero MB, Gagnon C. Nitric oxide: a novel mediator of sperm function. Journal of Andrology. 2001;22:349–356. [PubMed] [Google Scholar]
- Honsho C, Somsri S, Tetsumura T, Yamashita K, Yonemori K. Effective pollination period in durian (Durio zibethinus Murr.) and the factor regulating it. Scientia Horticulturae. 2007;111:193–196. [Google Scholar]
- Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochemical Pharmacology. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy PK, Mays JL, Bijur GN, Johnson GV. Transient oxidative stress in SH-SY5Y human neuroblastoma cells results in caspase dependent and independent cell death and tau proteolysis. Journal of Neuroscience Research. 2000;61:515–523. doi: 10.1002/1097-4547(20000901)61:5<515::AID-JNR6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Lavee S, Rallo L, Rapoport HF, Troncoso A. The floral biology of the olive. II. The effect of inflorescence load and distribution per shoot on fruit set and load. Scientia Horticulturae. 1999;82:181–192. [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Li S, Samaj J, Franklin-Tong VE. A mitogen-activated protein kinase signals to programmed cell death induced by self-incompatibility in Papaver pollen. Plant Physiology. 2007;145:236–245. doi: 10.1104/pp.107.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Vailleau F, Balague C, Roby D. Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends in Plant Science. 2003;8:263–271. doi: 10.1016/S1360-1385(03)00108-0. [DOI] [PubMed] [Google Scholar]
- McInnis SM, Desikan R, Hancock JT, Hiscock SJ. Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signalling crosstalk? New Phytologist. 2006;172:221–228. doi: 10.1111/j.1469-8137.2006.01875.x. [DOI] [PubMed] [Google Scholar]
- Mittler R, Rizhsky L. Transgene-induced lesion mimic. Plant Molecular Biology. 2000;44:335–344. doi: 10.1023/a:1026544625898. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Moutier N. Self-fertility and inter-compatibilities of sixteen olive varietes. Acta Horticulturae. 2000;586:209–212. [Google Scholar]
- Neill S. NO way to die-nitric oxide, programmed cell death and xylogenesis. New Phytologist. 2005;165:5–8. doi: 10.1111/j.1469-8137.2004.01267.x. [DOI] [PubMed] [Google Scholar]
- Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. Nitric oxide, stomatal closure and abiotic stress. Journal of Experimental Botany. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT. Nitric oxide signaling in plant. New Phytologist. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- Pannala A, Rice-Evans CA, Halliwell B, Singh S. Inhibition of peroxynitrite-mediated tyrosine nitration by catechin polyphenols. Biochemical and Biophysical Research Communications. 1997;232:164–168. doi: 10.1006/bbrc.1997.6254. [DOI] [PubMed] [Google Scholar]
- Potocky MA, Jones MA, Bezvoda R, Smirnoff N, Zarsky V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytologist. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- Prado AM, Colaco R, Moreno N, Silva AC, Feijo JA. Targeting of pollen tubes to ovules is dependent on nitric oxide (NO) signaling. Molecular Plant. 2008;1:703–714. doi: 10.1093/mp/ssn034. [DOI] [PubMed] [Google Scholar]
- Prado AM, Porterfield DM, Feijo JA. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development. 2004;131:2707–2714. doi: 10.1242/dev.01153. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proceedings of the National Academy of Sciences, USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reape TJ, McCabe PF. Apoptotic-like programmed cell death in plants. New Phytologist. 2008;180:13–26. doi: 10.1111/j.1469-8137.2008.02549.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, del Río LA, Sandalio LM. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) rootsImaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant, Cell and Environment. 2006;29:1532–1544. doi: 10.1111/j.1365-3040.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Rogers HJ. Programmed cell death in floral organs: how and why do flowers die? Annals of Botany. 2006;97:309–315. doi: 10.1093/aob/mcj051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Puertas MC, Delledonne M. Nitric oxide signaling in plant–pathogen interactions. IUBMB Life. 2003;55:579–583. doi: 10.1080/15216540310001639274. [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Laxa M, Matte A, Zaninotto F, Finkemeier I, Jones AM, Perazzolli M, Vandelle E, Dietz KJ, Delledonne M. S-nitrosylation of peroxiredoxin II E promotes peroxynitrite-mediated tyrosine nitration. The Plant Cell. 2007;19:4120–4130. doi: 10.1105/tpc.107.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Yamamoto-Katou A, Yoshioka H, Doke N, Kawakita K. Peroxynitrite generation and tyrosine nitration in defense responses in tobacco BY-2 cells. Plant and Cell Physiology. 2006;47:689–697. doi: 10.1093/pcp/pcj038. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Sandalio LM, Rodriguez-Serrano M, Romero-Puertas MC, Del Rio LA. Imaging of reactive oxygen species and nitric oxide in vivo in plant tissues. Methods in Enzymology. 2008;440:397–409. doi: 10.1016/S0076-6879(07)00825-7. [DOI] [PubMed] [Google Scholar]
- Schopfer FJ, Baker PR, Freeman BA. NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trends in Plant Science. 2003;28:646–654. doi: 10.1016/j.tibs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Serrano I, Pelliccione S, Olmedilla A. Programmed-cell-death hallmarks in incompatible pollen and papillar stigma cells of Olea europaea L. under free pollination. Plant Cell Reports. 2010;29:561–572. doi: 10.1007/s00299-010-0845-5. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Sun ZN, Wang HL, Liu FQ, Chen Y, Tam PKH, Yang D. BODIPY-based fluorescent probe for peroxynitrite detection and imaging in living cells. Organic Letters. 2009;11:1887–1890. doi: 10.1021/ol900279z. [DOI] [PubMed] [Google Scholar]
- Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nature Reviews Drug Discovery. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- Tezuka T, Tsuruhara A, Suzuki H, Takahashi SY. A connection between the self-incompatibility mechanism and the stress response in lily. Plant and Cell Physiology. 1997;38:107–112. [Google Scholar]
- Thomas S, Osman K, de Graaf BH, Shevchenko G, Wheeler M, Franklin C, Franklin-Tong N. Investigating mechanisms involved in the self-incompatibility response in Papaver rhoeas. Philosophical Transactions of the Royal Society. 2003;358:1033–1036. doi: 10.1098/rstb.2003.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SG, Franklin-Tong VE. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature. 2004;429:305–309. doi: 10.1038/nature02540. [DOI] [PubMed] [Google Scholar]
- Thomas SG, Huang S, Li S, Staiger CJ, Franklin-Tong VE. Actin depolymerization is sufficient to induce programmed cell death in self-incompatible pollen. Journal of Cell Biology. 2006;174:221–229. doi: 10.1083/jcb.200604011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proceedings of the National Academy of Sciences, USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiology. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ. Many ways to exit? Cell death categories in plants. Trends in Plant Science. 2005;10:117–122. doi: 10.1016/j.tplants.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Wang CL, Xu GH, Jiang XT, Chen G, Wu J, Wu HQ, Zhang SL. S-RNase triggers mitochondrial alteration and DNA degradation in the incompatible pollen tube of Pyrus pyrifolia in vitro. The Plant Journal. 2009;57:220–229. doi: 10.1111/j.1365-313X.2008.03681.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Li J, Bostock RM, Gilchrist DG. Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. The Plant Cell. 1996;8:375–391. doi: 10.1105/tpc.8.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen T, Zhang C, Hao H, Liu P, Zheng M, Baluska F, Samaj J, Lin J. Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes. New Phytologist. 2009;182:851–862. doi: 10.1111/j.1469-8137.2009.02820.x. [DOI] [PubMed] [Google Scholar]
- Wilkins KA, Bancroft J, Bosch M, Ings J, Smirnoff N, Franklin-Tong VE. Reactive oxygen species and nitric oxide mediate actin reorganization and programmed cell death in the self-incompatibility response of papaver. Plant Physiology. 2011;156:404–416. doi: 10.1104/pp.110.167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Collins G, Sedgley M. Sexual compatibility within and between olive cultivars. The Journal of Horticultural Science and Biotechnology. 2002;77:665–673. [Google Scholar]
- Zago E, Morsa S, Dat JF, Alard P, Ferrarini A, Inzé D, Delledonne M, Van Breusegem F. Nitric oxide- and hydrogen peroxide-responsive gene regulation during cell death induction in tobacco. Plant Physiology. 2006;141:404–411. doi: 10.1104/pp.106.078444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra A, Rodríguez-García MI, Alché JD. Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biology. 2010;10:36–50. doi: 10.1186/1471-2229-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninotto F, La Camera S, Polverari A, Delledonne M. Cross-talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiology. 2006;141:379–383. doi: 10.1104/pp.106.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.