Abstract
The development of eyes in Drosophila involves intricate epithelial reorganization events for accurate positioning of cells and proper formation and organization of ommatidial clusters. We demonstrate that Branchless (Bnl), the fibroblast growth factor ligand, regulates restructuring events in the eye disc primordium from as early as the emergence of clusters from a morphogenetic front to the cellular movements during pupal eye development. Breathless (Btl) functions as the fibroblast growth factor receptor to mediate Bnl signal, and together they regulate expression of DE-cadherin, Crumbs, and Actin. In addition, in the eye Bnl regulates the temporal onset and extent of retinal basal glial cell migration by activating Btl in the glia. We hypothesized that the Bnl functions in the eye are Hedgehog dependent and represent novel aspects of Bnl signaling not explored previously.
Keywords: cellular adhesion, cadherins, fibroblast growth factor (FGF) signaling, morphogenetic furrow, glial cell migration
The fibroblast growth factor (FGF) signaling pathway elicits a wide range of cellular processes ranging from mitogenesis, angiogenesis, cell proliferation, differentiation, and migration (Powers et al. 2000). Three FGF ligands (Pyramus, Thisbe, and Bnl) and two FGF receptors (Heartless [Htl] and Btl) (reviewed by Ornitz and Itoh 2001; Szebenyi and Fallon 1999) have been identified in Drosophila. Bnl functions as a chemoattractant to guide branch budding and outgrowth of the Btl-expressing tracheal cells (Klambt et al. 1992; Sutherland et al. 1996). Htl is essential for mesodermal patterning (Gisselbrecht et al. 1996) and during migration and morphogenesis of interface glial cells (Shishido et al. 1997). The ligands Pyramus and Thisbe mediate Htl function (Kadam et al. 2009). In Caenorhabditis elegans, the only FGF receptor, EGL-15, allows migration of sex myoblasts. In vertebrates, FGF promotes branching morphogenesis (Peters et al. 1994). FGF4 and FGF8 are essential for cell migration (reviewed in Huang and Stern 2005).
Cellular adhesion plays an integral role during migration as cells constantly replenish old junctions with new ones. During development of the Drosophila eye disc, a precise coordination of morphogenetic processes with other cellular events promotes proper patterning. Here, we show that FGF controls morphogenetic movements during larval and pupal Drosophila eye development by maintaining cellular adhesion and adherens junction (AJ) proteins. In addition, we demonstrate that Bnl from the eye controls proper migration of the retinal basal glial (RBG) cells into the eye disc.
Photoreceptor cells in the eye are born as a wave of differentiation called the morphogenetic furrow (MF) sweeps across the eye from the posterior to the anterior. The RBG cells originate from precursors in the optic stalk and migrate into the eye disc. This is closely coordinated with the onset of photoreceptor differentiation, and the RBGs terminate migration 3rd to 4th ommatidial columns posterior to the MF (Choi and Benzer 1994; Rangarajan et al. 1999; Silies et al. 2007). Pyramus, expressed by glia, stimulates glial proliferation and motility, whereas neuronally expressed Thisbe induces glial differentiation and terminates migration (Franzdottir et al. 2009). Htl is involved in mediating these functions (Franzdottir et al. 2009). Here we show nonautonomous Bnl/Btl signaling regulates RBG migration, where the RBGs express Btl and sense Bnl from the eye. Overall, this study indicates a coordinated role for Bnl/Btl signaling in eliciting differential responses during multiple stages of eye development determined by cell autonomy, given its requirement for proper formation of clusters in the larval eye to maintaining tissue architecture in pupal eye discs and its requirement during RBG migration.
Materials and Methods
The following fly stocks were used: y w ey-flp; FRT82B Ubi-GFP RpS3/TM6B Tb, y+, y w ey-flp; FRT80B Ubi-GFP RpS3/TM6B Tb, y+, y w ey-flp; ey-GAL4; FRT80B Ubi-GFP RpS3/TM6B Tb, y+, P{dpp-lacZ}, y w; FRT80B btlLG19/TM6B Tb, y+, y w; FRT82B bnl00857/TM6B Tb, y+, hhts2, UAS-cdc42N17, UAS-λbtl, and UAS-λhtl (Bloomington), y w; FRT82B bnlJR69/TM6B Tb, y+ (T. Liao), w;UAS > CD2 > y+>mCD8GFP/CyO; repo-GAL4, UAS-FLP/Tb (C. Klaembt). RNAi stocks: UAS-btlRNAi, UAS-bnlRNAi, UAS-dofRNAi, and UAS-htlRNAi (Vienna Drosophila Rnai Center [VDRC], Vienna, Austria).
For immunohistochemical analysis, the following primary antibodies were used: rat anti-BrdU (1:100; Abcam, Cambridge, MA), rabbit anti-cleaved Caspase 3 (1:500; Cell Signaling Technology, Beverly, MA), rat anti-Bnl (1:50; M. Krasnow), rat anti-Shg (1:500; V. Hartenstein), mouse anti-Dlg (1:100; Developmental Studies Hybridoma Bank [DSHB], Iowa City, IA), mouse anti-Crumbs (1:200; DSHB), mouse anti-DN-cadherin (1:200; DSHB), mouse anti-Repo (1:10; DSHB) and mouse anti-Armadillo (1:100; DSHB). The secondary antibodies were obtained from Jackson ImmunoResearch.
Quantification of apical cell surfaces in the MF was performed with the use of a published protocol (Corrigall et al. 2007). Mean apical cell surface area (μm2)/cell for mutant and wild-type tissue are given. The 2-tailed Student’s paired t-test was applied to all the quantifications.
Results and Discussion
Bnl/Btl signaling during Drosophila eye development
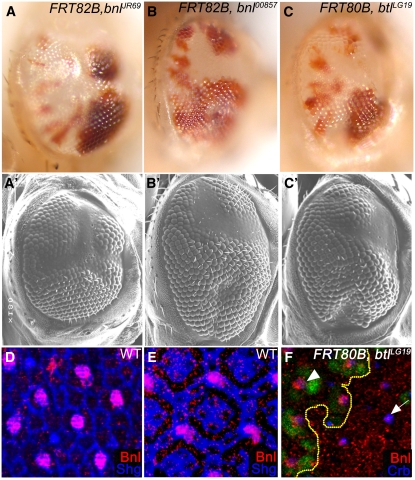
In a screen to identify mutations with patterning defects in the adult eye (Liao et al. 2006), we identified a loss-of-function allele of bnl, bnlJR69 that gives a “glossy-eye” phenotype (Figure 1, A and A′). This new allele fails to complement bnl00857, a well-characterized loss-of-function allele of bnl (Jarecki et al. 1999; Sutherland et al. 1996). bnl00857 clones in the eye also phenocopy the glossy-eye phenotype (Figure 1, B and B′). Loss of btl receptor function, induced by use of the btlLG19 allele, (Klambt et al. 1992; Reichman-Fried et al. 1994; Sutherland et al. 1996) also results in a similar phenotype (Figure 1, C and C′), whereas loss htl, does not phenocopy this effect (not shown). This represents a novel function for Bnl-mediated Btl signaling during Drosophila eye development not explored previously. Immunohistochemical studies reveal uniform low expression of Bnl protein in 3rd instar eye disc (data not shown). Whereas in the pupal eye discs aged approximately 28 hrs after pupal formation (APF), Bnl expression is restricted to the cone cells (Figure 1D) and later (~48 hrs APF) detected in the bristle cells (Figure 1E). btlLG19 clones in pupal eye disc (~28 hrs APF) have reduced Bnl protein expression (Figure 1F) residual, mislocalized staining is seen away from the tips of the cone cells, suggesting that ligand-receptor interaction promotes proper localization and stabilization of Bnl.
Figure 1 .
Bnl/Btl functions during Drosophila eye development. (A-C′) Loss of Bnl/Btl signaling affects eye development. Bright field images (A-C) containing somatic clones (marked by the absence of red pigmentation) and (A′-C′) the corresponding scanning electron micrographs with (A-A′) bnlJR69/bnlJR69, (B-B′) bnl00857/ bnl00857, and (C-C′) btlLG19/btlLG19. Wild-type facets are red and faceted, and the mutant tissue (white) appears glossy in both light and electron micrographs. Pupal eye disc stained with Bnl (red) protein shows elevated levels in (D) cone and (E) bristle cells, co-stained with anti-Shg (blue) antibody. (F) Somatic clones of btlLG19 (nongreen tissue) in pupal eye discs have reduced Bnl protein (red) expression in the cone cells (marked using Crumbs in blue).
Bnl signaling regulates cellular adhesion during Drosophila eye development
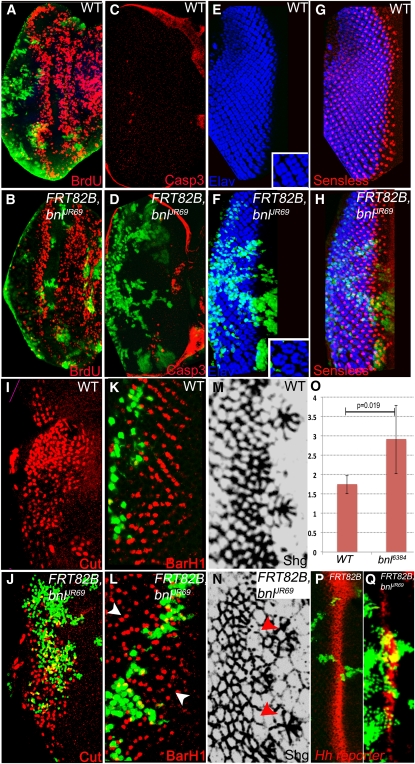
To investigate the developmental phenotype underlying Bnl dysfunction, somatic clones of mutant tissue were analyzed. bnl clones do not exhibit any defect or delay in G1-S transition (Figure 2, A and B) and early viability of the cells is unaffected (Figure 2, C and D). Cell fate specification markers are expressed in mutant cells (Figure 2, E−L). However, defects in ommatidial organization are seen using BarH1 antibody marking R1 and R6 photoreceptors that are normally organized in a linear pattern (Figure 2, K and L).
Figure 2 .
Bnl signaling regulates cell shape changes in the furrow. In all panels, eye discs from 3rd instar larvae are shown. Posterior is to the left. (A-B) Incorporation of 5-bromo-2′-deoxyuridine (BrdU; red) marks cells in S phase in 3rd instar larval eye disc. (A) Normal pattern of BrdU incorporation, where both the green and nongreen tissues are wild type. (B) Somatic clones of bnlJR69 (nongreen) show no defect in BrdU incorporation. (C-D) Assay for cell death using anti-cleaved Caspase 3 (red) antibody. (C) Wild-type and (D) eye discs containing somatic clones of bnlJR69 (non-green) show no difference in cell viability. (E-L) Loss of bnl function does not affect cell fate specification as visualized by expression of (E-F) Elav, marks photoreceptor neurons (blue), (G-H) Senseless is a marker for R8 (red), (I-J) Cut, marks cone cells (red), and (K-L) BarH1 is a marker for R1 and R6. Compare wild-type panels (E, G, I, and K) correspondingly with somatic clones of bnlJR69 (nongreen, F, H, J, and L). bnlJR69 clones show defects in ommatidial arrangement evident from organization of BarH1 expressing cells (L compared with K). Compared with (M) wild-type pattern of Shg (gray) expression at the MF, (N) bnlJR69 somatic clones shows loss in ommatidial organization (arrowheads). (O) Graphical representation of the mean surface area (μm2)/cell (shown on the y-axis) of the cells within the MF from wild-type and bnl00857 mutant clones (n = 6, P = 0.01). (P) Wild-type expression pattern of Hh reporter (red) is down-regulated (Q) in somatic clones of bnlJR69 (nongreen).
The earliest events in cluster formation involve morphogenetic changes and constriction of apical cell surfaces (Escudero et al. 2007; Ready et al. 1976; Wolff and Ready 1991). bnl mutant cells lose their ability to constrict their apical surfaces (Figure 2, M−O; numerically, at the morphogenetic furrow, wild-type cell surface/bnl mutant cell surface = 0.599, P = 0.01) and lose normal contacts between cells and form aberrant-shaped clusters (Figure 2N). Expression of the Hh activity-dependent reporter (dpp-lacZ), seen at high levels in the wild-type MF, is strongly reduced in bnlJR69 clones (Figure 2, P and Q), implying an interaction between Hh and FGF signaling in regulating cellular adhesion and morphogenesis during larval eye development. Hh signaling has been implicated in similar phenotypes and regulates Myosin II activity necessary for cellular rearrangement at the furrow (Corrigall et al. 2007, Escudero et al. 2007). It is therefore these early defects in proper rosette formation at the furrow (Ready et al. 1976; Wolff and Ready 1991) that are responsible for the improper organization of the ensuing cluster (Figure 2, M and N).
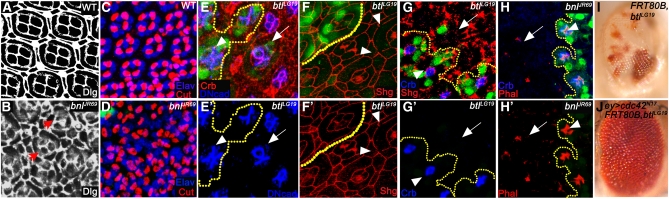
The next set of morphogenetic defects caused by FGF signaling is evident during pupal development, where a series of coordinated and precise cell movements lead to a well-patterned pupal epithelium (Cagan 1993; Cagan and Ready 1989; Ready et al. 1976). This regularity in the wild-type pattern is evident upon staining with septate junction marker Discs large (Figure 3A) (Woods and Bryant 1991). In bnl mutants the architecture of the ommatidial clusters is distorted, and instead of the regular hexagonal arrays, they often appear circular (Figure 3B). In addition, the Discs large staining in these clones is cytoplasmic (Figure 3B) and not localized to the membrane as in wild type (Figure 3A). The architectural defect in bnl mutants is not attributable to loss in neuronal or non-neuronal differentiation (Figure 3, C and D). However, bnl mutant clusters often contain improper number of cells (Figure 3B). More specifically, staining for DN-cadherin normally expressed at the interface between wild-type cone cells (Hayashi and Carthew 2004) revealed reduced numbers and altered arrangement of cone cells in btlLG19 mutant clusters (Figure 3, E and E′). The expression of Shotgun (DE-cadherin) in btl mutant clones is also fragmented and missing at several cell-cell contact points (Figure 3, F and F′). Crumbs protein, an apical marker found above AJs is observed at high levels in wild-type cells (Figure 3G) (Izaddoost et al. 2002). However, btl and bnl mutant tissues are devoid of Crumbs protein (Figure 3, G′ and H′) and they also lack Shotgun expression (Figure 3G′) and show defective actin organization (Figure 3H′). Small GTPases function downstream of many biological signals to regulate diverse cellular processes (Kuhn et al. 2000), including FGF (Wolf et al. 2002). We therefore investigated interactions with various activated and dominant-negative forms of small GTPases in btlLG19 clones. A dominant-negative version of cdc42 rescues the btlLG19 glossy-eye phenotype (Figure 3, I and J), suggesting that cdc42 functions as a negative downstream effector of Bnl signaling. Overall, the data here strongly suggest a central role for autonomous FGF signaling in the eye for the maintenance of proper cell-cell contact, junction stability and cell shape changes during retinal development which is achieved by modulating the levels of key cell adhesion and junctional proteins: DE-cadherin, Crumbs, and actin.
Figure 3 .
Bnl function maintains junctional proteins. In all panels, eye discs from pupal stages (48−50 hr APF) are shown. Compared with Dlg (gray) expression in wild type (A), bnlJR69 clones (B) show defects in cluster organization (arrowheads). Elav (blue) and Cut (red) expression level in wild type (C) and bnlJR69 (D) clones (nongreen) are comparable although cellular arrangement is disrupted in bnlJR69 mutants. (E and E′) Compared with DN-cadherin (blue) and Crumbs (red) expression in wild-type tissue (green, arrowhead), btlLG19 clones (nongreen) show defects in cone-cell architecture (arrow, n = 5). Compared with Shg (red) expression in wild-type tissue (F, green), btlLG19 clones (F′, nongreen) show disrupted Shg expression at cell−cell junctions (arrowheads, n = 4). (G and G′) Crumbs (blue) and Shg (red) expression is lost from btlLG19 mutant rhabdomere adherens junctions (AJ, nongreen, arrow, n = 5). Compare with surrounding wild-type rhabdomere AJ (green, arrowhead). (H and H′) Crumbs (blue) expression and phalloidin (red) staining are lost from bnlJR69 mutant rhabdomere AJ (nongreen, arrow, n = 10). Compare with surrounding wild-type rhabdomere AJ (green, arrowhead). (I) btlLG19 adult glossy-eye phenotype is rescued by (J) coexpressing a dominant-negative form of cdc42 (cdc42N17, n = 4).
FGF signaling regulates RBG cell migration
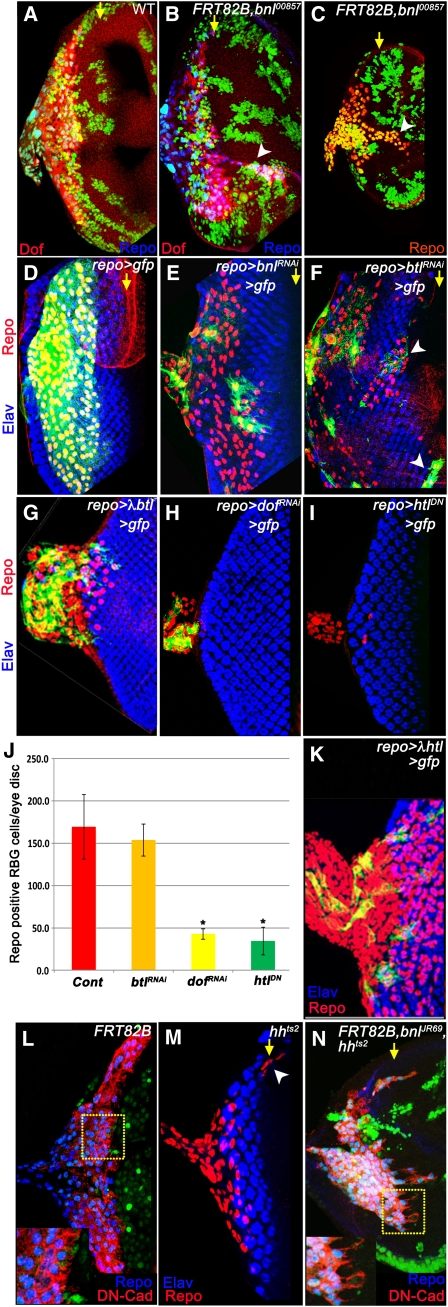
In contrast to the tissue autonomous defects in patterning discussed previously, loss of bnl function in the eye also causes defects in RBG migration (Figure 4, A and B). In eye discs containing bnl mutant clones, the RBG cells migrate ectopically to anterior regions of the eye beyond the MF (Figure 4B), not seen in similarly aged wild-type controls (Figure 4A). This migration defect is observed as early as in 2nd instar eye discs lacking bnl function (Figure 4C), earlier than RBG migration in wild types. bnl clones generated specifically in the glia do not show RBG migration defects (Figure 4, D and E). In contrast, btlRNAi expressed in the RBG cells causes the ectopic glial migration phenotype (Figure 4F) and when a constitutively active form of the receptor, λ-btl is expressed in glial cells, their migration is completely impaired and the RBGs are restricted to the optic stalk (Figure 4G).
Figure 4 .
Bnl/Btl signaling regulates RBG migration. In all panels except panel C, eye discs are from wandering 3rd instar larvae. Posterior is to the left. In panel C, the eye disc is from a mid 2nd instar larva. Yellow arrows mark the MF. Compared with RBG (Repo: blue and Dof: red) in (A) wild-type (green and nongreen tissues are normal), (B) eye discs containing bnl00857 clones have ectopically migrating RBGs (arrowhead B, compare with panel A). (C) 2nd instar eye discs containing bnl00857 somatic clones (nongreen) show precocious RBG (Repo: red) migration. (D) Control eye disc expressing GFP in the RBG cells (green; UAS > CD2 > y+>mCD8GFP/CyO; repo-GAL4, UAS-FLP/Tb; UAS-GFP; Repo: red) costained with Elav (blue) to mark photoreceptor cluster. RBG cell (Repo: red) clones expressing (E) bnlRNAi (green; UAS > CD2 > y+>mCD8GFP/CyO; repo-GAL4, UAS-FLP/Tb; UAS-bnlRNAi) show no defect in glial migration, (F) btlRNAi (green; UAS > CD2 > y+>mCD8GFP/CyO; repo-GAL4, UAS-FLP/Tb; UAS-btlRNAi) causes ectopic RBG migration (marked by arrowheads, n = 7) and (G) constitutively active btl (green; UAS > CD2 > y+>mCD8GFP/CyO; repo-GAL4, UAS-FLP/Tb; UAS-λ-btl) inhibits RBG migration (n = 5). Elav (blue) marks the photoreceptor clusters. RBG cells (Repo: red) clones expressing (H) dofRNAi (green; UAS > CD2 > y+>mCD8GFP/CyO; repo-GAL4, UAS-FLP/Tb; UAS-dofRNAi) or (I) htlDN (UAS > CD2 > y+>mCD8GFP/CyO; repo-GAL4, UAS-FLP/Tb; UAS-htlDN) show reduction in RBG number. (J) Quantification of RBG cell counts from control (n = 12), btlRNAi (n = 12), dofRNAi (n = 5, * indicates P < 0.001) and HtlDN (n = 12, * indicates P < 0.001). (K) Expressing constitutively active Htl (green; UAS > CD2 > y+>mCD8GFP/CyO; repo-GAL4, UAS-FLP/Tb; UAS-λ-Htl) causes expansion of RBG cell numbers. Elav (blue) marks photoreceptor clusters. Compared to RBG cells (Repo: blue and DN-cadherin: red) in (L) wild type (green and nongreen tissue are normal), eye discs from (M) hh mutants and (N) containing bnlJR69 and hh double mutant clones (nongreen) show ectopically migrating glia with long cytoplasmic extensions (small inset in N compared with control L).
These results suggest that Bnl expressed by the eye tissue activates nonautonomous Btl signaling in the glial cells to regulate the temporal onset and extent of RBG migration. Of importance, loss of bnl or btl function did not affect RBG proliferation or survival (Figure 4J). When analyzed for R cell axon migration in bnl mutant clones, no obvious defect in their growth and projection was apparent (not shown). Downstream-of-FGF (Dof) functions as a positive effector of FGF signaling (Battersby et al. 2003) and is expressed at high levels in RBG cells (Rangarajan et al. 1999). As reported previously, dof mutants generated in the glia causes reduction in RBG cell number, loss of differentiation, and lack of migration (Figure 4, H and J) (Franzdottir et al. 2009). These phenotypes are strikingly different from that observed in btl mutants (Figure 4F).
Given that htl functions as a FGF receptor and can activate signaling via dof, htl mutant clones generated in the glia phenocopied all dof mutant phenotypes (Figure 4, I and J) and expression of a constitutively active form htl (λ-htl) in the glial cells resulted in extensive proliferation of the RBG cells, without affecting their migration (Figure 4K) (Franzdottir et al. 2009). Given the phenotypic differences between bnl/btl and htl/dof backgrounds, we conclude that htl-mediated dof function is required earlier in RBG development to regulate proliferation, differentiation, and ability to initiate migration, whereas bnl/btl signaling functions later to limit RBGs from migrating precociously into the eye disc. This could be achieved by either functioning as an antagonist to an attractive signal or by directly signaling to inhibit their migration. A noncanonical mechanism of Hh function in the eye also renders similar, albeit weaker, glial migration defects (Figure 4, L and M) as observed in bnl mutants (Hummel et al. 2002) and in addition to the loss of hh reporter in bnl mutant clones (Figure 2Q), Bnl functions either upstream or in parallel to Hh in regulating RBG migration. Interestingly, eye discs containing hh and bnl double-mutant clones show ectopically migrating RBG cells (Figure 4N) with long cytoplasmic extensions not seen in wild-type RBG (Figure 4L) as observed by DN-cadherin expression.
Drosophila compound eye formation, comprising neuronal and non-neuronal cells in the eye disc proper and glia at the basal layer, is achieved by closely coordinating multiple developmental events. An important process involved is the migration of the RBG cells into the eye disc efficiently synchronized with the onset of differentiation and movement of the MF. The glial cells do not outpace the furrow. The coordination between the two independent processes, the initiation of neuronal development and glial migration, is intriguing. This study establishes that a single pathway involving Bnl and Btl is implicated in the emergence of clusters from the MF in the larval eye, controls the formation and stability of AJ, and as well controls the temporal onset and extent of RBG migration. In addition, the ligands Pyramus and Thisbe are also important for glial development, where Pyrmaus/Htl activation promotes proliferation and motility and Thisbe/Htl activation inhibits migration and promotes differentiation (Franzdottir et al. 2009). It is interesting how the relatively simple FGF/FGF receptor system in Drosophila, operating in a cell at a specific time, can generate differential responses determined by cell autonomy and ligand/receptor combinations. This study further highlights the importance of sequential receptor tyrosine kinase activation to achieve coordinated migration of all glial cells, as abnormal glial migration is a feature of many human diseases.
Acknowledgments
We thank M. Krasnow C. Klaembt, VDRC, Flybase, Bloomington, and DSHB for stocks and reagents and R. Nagaraj for helpful discussions. Supported by National Institutes of Health (NIH) grant R01-EY08152.
Literature Cited
- Battersby A., Csiszar A., Leptin M., Wilson R., 2003. Isolation of proteins that interact with the signal transduction molecule Dof and identification of a functional domain conserved between Dof and vertebrate BCAP. J. Mol. Biol. 329: 479–493 [DOI] [PubMed] [Google Scholar]
- Cagan R., 1993. Cell fate specification in the developing Drosophila retina. Dev. Suppl 19–28 [PubMed] [Google Scholar]
- Cagan R. L., Ready D. F., 1989. The emergence of order in the Drosophila pupal retina. Dev. Biol. 136: 346–362 [DOI] [PubMed] [Google Scholar]
- Choi K. W., Benzer S., 1994. Migration of glia along photoreceptor axons in the developing Drosophila eye. Neuron 12: 423–431 [DOI] [PubMed] [Google Scholar]
- Corrigall D., Walther R. F., Rodriguez L., Fichelson P., Pichaud F., 2007. Hedgehog signaling is a principal inducer of Myosin-II−driven cell ingression in Drosophila epithelia. Dev. Cell 13: 730–742 [DOI] [PubMed] [Google Scholar]
- Escudero L. M., Bischoff M., Freeman M., 2007. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev. Cell 13: 717–729 [DOI] [PubMed] [Google Scholar]
- Franzdottir S. R., Engelen D., Yuva-Aydemir Y., Schmidt I., Aho A., et al. , 2009. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature 460: 758–761 [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S., Skeath J. B., Doe C. Q., Michelson A. M., 1996. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 10: 3003–3017 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Carthew R. W., 2004. Surface mechanics mediate pattern formation in the developing retina. Nature 431: 647–652 [DOI] [PubMed] [Google Scholar]
- Huang P., Stern M. J., 2005. FGF signaling in flies and worms: more and more relevant to vertebrate biology. Cytokine Growth Factor Rev. 16: 151–158 [DOI] [PubMed] [Google Scholar]
- Hummel T., Attix S., Gunning D., Zipursky S. L., 2002. Temporal control of glial cell migration in the Drosophila eye requires gilgamesh, hedgehog, and eye specification genes. Neuron 33: 193–203 [DOI] [PubMed] [Google Scholar]
- Izaddoost S., Nam S. C., Bhat M. A., Bellen H. J., Choi K. W., 2002. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature 416: 178–183 [DOI] [PubMed] [Google Scholar]
- Jarecki J., Johnson E., Krasnow M. A., 1999. Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell 99: 211–220 [DOI] [PubMed] [Google Scholar]
- Kadam S., McMahon A., Tzou P., Stathopoulos A., 2009. FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development 136: 739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klambt C., Glazer L., Shilo B. Z., 1992. breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 6: 1668–1678 [DOI] [PubMed] [Google Scholar]
- Kuhn T. B., Meberg P. J., Brown M. D., Bernstein B. W., Minamide L. S., et al. , 2000. Regulating actin dynamics in neuronal growth cones by ADF/cofilin and rho family GTPases. J. Neurobiol. 44: 126–144 [PubMed] [Google Scholar]
- Liao T. S., Call G. B., Guptan P., Cespedes A., Marshall J., et al. , 2006. An efficient genetic screen in Drosophila to identify nuclear-encoded genes with mitochondrial function. Genetics 174: 525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Itoh N., 2001. Fibroblast growth factors. Genome Biol. 2: REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Werner S., Liao X., Wert S., Whitsett J., et al. , 1994. Targeted expression of a dominant negative FGF receptor blocks branching morphogenesis and epithelial differentiation of the mouse lung. EMBO J. 13: 3296–3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers C. J., McLeskey S. W., Wellstein A., 2000. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 7: 165–197 [DOI] [PubMed] [Google Scholar]
- Rangarajan R., Gong Q., Gaul U., 1999. Migration and function of glia in the developing Drosophila eye. Development 126: 3285–3292 [DOI] [PubMed] [Google Scholar]
- Ready D. F., Hanson T. E., Benzer S., 1976. Development of the Drosophila retina, a neurocrystalline lattice. Dev. Biol. 53: 217–240 [DOI] [PubMed] [Google Scholar]
- Reichman-Fried M., Dickson B., Hafen E., Shilo B. Z., 1994. Elucidation of the role of breathless, a Drosophila FGF receptor homolog, in tracheal cell migration. Genes Dev. 8: 428–439 [DOI] [PubMed] [Google Scholar]
- Shishido E., Ono N., Kojima T., Saigo K., 1997. Requirements of DFR1/Heartless, a mesoderm-specific Drosophila FGF-receptor, for the formation of heart, visceral and somatic muscles, and ensheathing of longitudinal axon tracts in CNS. Development 124: 2119–2128 [DOI] [PubMed] [Google Scholar]
- Silies M., Yuva Y., Engelen D., Aho A., Stork T., et al. , 2007. Glial cell migration in the eye disc. J. Neurosci. 27: 13130–13139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D., Samakovlis C., Krasnow M. A., 1996. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 87: 1091–1101 [DOI] [PubMed] [Google Scholar]
- Szebenyi G., Fallon J. F., 1999. Fibroblast growth factors as multifunctional signaling factors. Int. Rev. Cytol. 185: 45–106 [DOI] [PubMed] [Google Scholar]
- Wolf C., Gerlach N., Schuh R., 2002. Drosophila tracheal system formation involves FGF-dependent cell extensions contacting bridge-cells. EMBO Rep. 3: 563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T., Ready D. F., 1991. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development 113: 841–850 [DOI] [PubMed] [Google Scholar]
- Woods D. F., Bryant P. J., 1991. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 66: 451–464 [DOI] [PubMed] [Google Scholar]