Abstract
Background
Migraine is an independent risk factor for stroke. Mechanisms underlying this association are unclear. Familial hemiplegic migraine (FHM), a migraine subtype that also carries an increased stroke risk, is a useful model for common migraine phenotypes because of shared aura and headache features, trigger factors, and underlying glutamatergic mechanisms.
Methods and Results
Here, we show that FHM type 1 (FHM1) mutations in CaV2.1 voltage-gated Ca2+ channels render the brain more vulnerable to ischemic stroke. Compared to wild-type, two FHM1 mutant mouse strains developed earlier onset of anoxic depolarization and more frequent peri-infarct depolarizations, associated with rapid expansion of infarct core on diffusion-weighted MRI and larger perfusion deficits on laser speckle flowmetry. Cerebral blood flow required for tissue survival was higher in the mutants, leading to infarction with milder ischemia. As a result, mutants developed larger infarcts and worse neurological outcomes after stroke, which were selectively attenuated by a glutamate receptor antagonist.
Conclusions
We propose that enhanced susceptibility to ischemic depolarizations akin to spreading depression predisposes migraineurs to infarction during mild ischemic events, thereby increasing the stroke risk.
Keywords: stroke, spreading depression, migraine, calcium channels
Migraine is the most common neurological condition affecting young to middle-age adults. Up to a third of migraineurs experience transient neurological symptoms called aura. Migraine, particularly with aura, is associated with increased stroke risk both during and between attacks, especially in women1–4. The biological basis for this association is unknown. Stroke risk is also increased in familial hemiplegic migraine (FHM), a monogenic migraine subtype with hemiplegic auras in addition to the common aura forms5. FHM is a useful model for common migraine with aura because of shared clinical features and trigger factors, and because two-thirds of FHM patients and their first degree relatives also have attacks of common migraine with or without aura6, 7. Neuronal network hyperexcitability and enhanced glutamate release have been implicated in both FHM and common forms of migraine8.
FHM type 1 (FHM1) is caused by mutations in the CACNA1A gene, which encodes the pore-forming α1A subunit of neuronal CaV2.1 voltage-gated Ca2+ channels9. Presynaptic CaV2.1 channels are major regulators of excitatory neurotransmitter release. FHM1 mutant channels open with smaller depolarizations and stay open longer10, which augments presynaptic Ca2+ entry and glutamate release thereby enhancing brain excitability11. Transgenic mice expressing the human R192Q or S218L FHM1 mutation show an increased susceptibility to spreading depression, the electrophysiological substrate for migraine, and display characteristic clinical features of FHM such as transient hemiplegia12–14. Glutamatergic mechanisms and hyperexcitability have been implicated in the pathogenesis of common forms of migraine as well15, 16. Genetic support for this link was recently obtained in a genome-wide association study identifying the astrocyte elevated gene 1 (AEG1), encoding a regulator of glial glutamate transporter EAAT2, as the first migraine gene16.
Glutamate excitotoxicity also plays a pivotal role in the pathogenesis of stroke. We, therefore, hypothesized that genetic mutations conferring cerebral hyperexcitability and migraine susceptibility increase the vulnerability to ischemic stroke as one mechanism to explain the migraine-stroke association, and tested this using CaV2.1 S218L and R192Q transgenic mouse models of FHM1. The results reveal electrophysiological and hemodynamic mechanisms that accelerate hyperacute stroke evolution and worsen ischemic outcome in FHM1 mutants, and suggest a pivotal role for enhanced glutamatergic transmission in increasing the vulnerability to ischemic stroke in susceptible migraineurs.
Methods
Experimental animals
Experimental procedures were approved by the institutional review board. A total of 267 male and female mice were used. Transgenic knockin Cacna1a migraine mouse models homozygous (HOM) or heterozygous (HET) for R192Q or S218L FHM1 mutations were generated by a gene targeting approach14. The R192Q mutant strain was compared to C57BL6/J, backcrossed for 10 generations. The S218L mutants were compared to their wild type (WT) littermates. Because stroke risk is highest in young adult migraineurs, mice were studied between 2–6 months of age. All experiments were carried out with the investigators blinded, and confirmatory genotyping was done.
Systemic physiological monitoring
Arterial pH, pO2, pCO2, and blood pressure were measured via a femoral artery catheter under isoflurane anesthesia (2.5% induction, 1.5% maintenance, in 70% N2O and 30% O2; Supplemental Table 1). Rectal temperature was controlled at 37°C during ischemia, and intermittent monitoring continued for 6 hours in a subset of mice.
Transient filament occlusion of the middle cerebral artery (fMCAO)
A nylon monofilament was inserted into internal via the external carotid artery followed by reperfusion after 30 or 60 minutes, under isoflurane anesthesia (2.5% induction, 1.5% maintenance, in 70% N2O and 30% O2) and laser Doppler monitoring. Mice were placed in a temperature-controlled incubator with easy access to food and water. Neurological outcomes were scored 24 hours after reperfusion, using a five-point scale: 0, normal; 1, forepaw monoparesis; 2, circling to left; 3, falling to left; 4, no spontaneous walking and depressed consciousness; 5, death. Infarct volume was calculated by integrating the infarct area in ten 1 mm-thick 2,3,5-triphenyltetrazolium chloride (TTC)-stained coronal sections. Infarct volumes were calculated by subtracting the volume of ipsilateral non-infarcted tissue from contralateral hemisphere. Ischemic swelling volumes were calculated by subtracting the volume of contralateral hemisphere from the volume of ipsilateral hemisphere. Glutamatergic mechanisms were tested by administering MK-801 (1mg/kg, intraperitoneal; Sigma, St Louis, MO) 15 minutes before fMCAO.
MRI
Apparent diffusion coefficient (ADC) maps were acquired under isoflurane anesthesia with a 9.4-Tesla MRI scanner (Bruker Biospin, Inc., Billerica, MA) 30 and 60 minutes after fMCAO (TR/TE=3000/27 ms, b=154 & 1294 s/mm2, 180•180 μm2 in-plan resolution, 1 mm slice thickness, NA=8). Mean and standard deviation of ADC in cortex, striatum, hippocampus and thalamus were extracted from the normal hemisphere, and thresholds defined as mean minus 2 standard deviations to calculate lesion volumes. Normal systemic physiological parameters were confirmed under simulated MRI conditions in a separate group of mice (not shown).
Electrophysiological recordings
After fMCAO, isoflurane-anesthetized mice were intubated, ventilated, and femoral artery catheterized for blood pressure and blood gas monitoring. Two intracortical glass micropipettes were placed and extracellular recordings (depth 250 μm) started within 15 minutes after the onset of ischemia and continued for approximately 2 hours.
Receptor autoradiography
The density and distribution of glutamate and GABAA receptors and glutamate re-uptake sites were assessed on 10 μm frozen sections using tritium-sensitive storage phosphor screens (GE Healthcare) as described17.
Laser speckle-flowmetry of transient distal middle cerebral artery occlusion (dMCAO)
Spontaneously breathing mice (S218L HOM) were anesthetized with isoflurane as above, femoral artery catheterized for blood pressure and gas measurements. Mice were placed in a stereotaxic frame, a temporal burr hole (2 mm diameter) was drilled above the zygomatic arch, and distal middle cerebral artery was occluded using a microvascular clip for 60 minutes. Cortical perfusion was imaged during dMCAO using laser speckle flowmetry through intact skull18. Cerebral blood flow (CBF) changes were calculated for each pixel relative to pre-ischemic baseline, and the area of cortex with residual CBF ≤30% was determined by thresholding. Neurological outcomes and infarcts were assessed 48 hours later as described above. In addition, CBF threshold for tissue viability was estimated by superimposing the images of CBF and infarct. In the R192Q HOM strain, mice were intubated and ventilated to ensure normal arterial blood gas values, precluding survival for neurological and infarct assessment in this strain.
Anatomical analysis of the circle of Willis and pial collaterals
Mice were transcardially perfused with carbon black. Diameter of cerebral arteries, patency of the posterior communicating artery, and the number of pial arterial anastomoses between the anterior, posterior and middle cerebral arteries and their distance from midline were determined.
Absolute resting CBF
Mice were anesthetized with α-chloralose (50 mg/kg) and ventilated. Femoral artery and external jugular vein were cannulated. Arterial blood was withdrawn continuously (0.3 mL/min). One μCi of N-isopropyl-[methyl-1,3-14C]-p-iodoamphetamine was injected in 0.1 mL saline over 10 seconds. Twenty seconds after injection, animal was decapitated and the blood withdrawal terminated simultaneously. The brain was removed, frozen and dissected. CBF was calculated from the radioactivity in tissue and blood measured by liquid scintillation spectrometry.
Statistical Analysis
Data were analyzed using SPSS (v11.0), and presented as mean ± standard error, or median [interquartile range]. We used chi-square test to compare proportions, ANOVA to compare mean values of continuous measures according to genotypes, and general linear models for repeated measures to compare mean values over time according to genotypes. Comparisons of disability scores were performed by using non-parametric rank tests, cumulative peri-infarct depolarization (PID) incidence by log rank test, and PID frequency vs. the area of CBF deficit by Pearson correlation test. P values are two-tailed, and P<0.05 was considered statistically significant.
Results
Enhanced susceptibility to anoxic and peri-infarct depolarizations
Anoxic depolarization is characterized by a sudden loss of membrane ionic gradients, uncontrolled glutamate release and cell swelling, triggered by the failure of Na+/K+ ATPase under ischemic conditions. We found significantly earlier onset of anoxic depolarization in FHM1 mutants after fMCAO, by monitoring its vasoconstrictive effect on cerebral vasculature as previously described (Fig. 1a, b)18, 19. Importantly, the magnitude of CBF reduction in the ischemic core did not differ among groups in this fMCAO model, eliminating the possibility that faster anoxic depolarization rates were due to more severe ischemia (residual CBF 10–17% of baseline in both S218L and R192Q; P=0.634 and 0.599, respectively, data not shown).
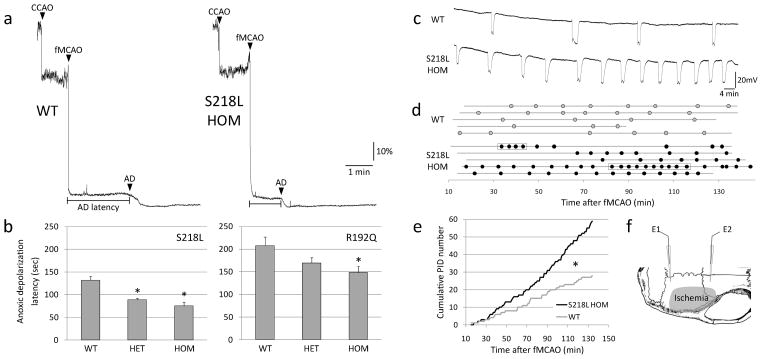
Figure 1.
Faster anoxic depolarization rates and more frequent peri-infarct depolarizations in FHM1 mutant mice. a) Representative laser Doppler tracings show CBF reduction upon common carotid artery occlusion (CCAO) followed by fMCAO. A further decline in CBF marks the onset of anoxic depolarization (AD), and is due to the vasoconstrictive effect of tissue depolarization on ischemic microvasculature18. Scale bars: vertical 10%, horizontal 1 min. b) The latency to AD was shorter in S218L and R192Q mutants (p<0.001 and p=0.035, respectively), with a trend for allele-dosage effect. *p<0.05 vs. WT. c) Representative electrophysiological tracings show more frequent PIDs in S218L HOM compared to WT. Scale bars: vertical 20 mV, horizontal 4 min. d) PIDs (round symbols shown as a function of time) occurred in S218L HOM more frequently and sometimes in clusters (rectangular boxes). Horizontal lines indicate the time of onset and end of electrophysiological recordings in each mouse (n=5 each). When calculating the average PID frequency, these minor differences in recording duration were taken into account. e) Pooled cumulative PID numbers as a function of time after fMCAO was more than doubled in S218L HOM (*p<0.001; n=5 each). f) Experimental setup showing two intracortical glass micropipettes (E1, E2) placed outside the ischemic territory to detect PIDs after fMCAO. Shaded area indicates typical distribution of CBF deficit after fMCAO.
PIDs are recurrent propagating depolarization waves akin to spreading depression that exacerbate the metabolic mismatch in penumbra and promote infarct growth during hyperacute stroke18–21. We reasoned that FHM1 mutations that enhance spreading depression susceptibility13 might also facilitate the occurrence of PIDs. Using intracortical microelectrode recordings during fMCAO, we indeed found a two-fold increase in the frequency of PIDs in mutants over WT (5.3±1.3 vs. 2.6±0.3 PIDs/h, P=0.028; Fig. 1c–e). In the mutants, PIDs sometimes occurred in clusters, possibly reflecting circling around the ischemic core (see below)22, but otherwise did not differ from WT in terms of their durations and amplitudes (not shown). Altogether, these data suggest that genetically enhanced susceptibility to spreading depression11–14 facilitates the occurrence of anoxic depolarization and PIDs during acute stroke as a novel mechanism to explain increased stroke vulnerability in migraineurs.
Rapid growth of hyperacute ischemic core on MRI
To assess whether enhanced susceptibility to anoxic and peri-infarct depolarizations accelerate the hyperacute stroke evolution in FHM1 mutants, we performed serial diffusion-weighted MRI during fMCAO. Reduced ADC values on diffusion-weighted MRI reflect anoxic depolarization, loss of transmembrane ionic gradients, and cell swelling (i.e., ischemic core). We found that the ADC lesion volumes expanded more rapidly in FHM1 mutant strains compared to WT controls (Fig. 2). Although larger ADC lesion volumes were primarily due to more severe cortical involvement, the hyperacute lesion also encompassed hippocampus and thalamus in S218L mutants.
Figure 2.
FHM1 mutant mice show accelerated lesion growth on MRI during hyperacute stroke. a) ADC lesion (i.e., ischemic core with restricted water diffusion, purple) was larger on diffusion-weighted MR images in S218L (left panel) and R192Q (right panel) mutants compared to WT during the hyperacute phase after fMCAO. b) ADC lesion volumes shown as a function of time after fMCAO were 40–50% larger in S218L and R192Q HOM compared to WT as early as 30 minutes after fMCAO suggesting faster growth of ischemic core (p=0.019 and p=0.018, respectively). The difference remained significant at 60 minutes. Vertical and horizontal error bars reflect the standard errors for total ADC lesion volume and timing of MRI scans, respectively. c) Thirty minutes after stroke onset, enlarged ADC lesion volumes in S218L HOM and R192Q HOM were primarily due to more severe cortical involvement (p=0.015 and p=0.023, respectively), although S218L HOM mutants showed hyperacute ADC changes in the hippocampus and thalamus as well (P=0.018 and P=0.117, respectively). Of note, the average regional ADC values in the center of ischemic core did not significantly differ between FHM1 mutants and their WT controls, suggesting that cytotoxic cell swelling is complete in ischemic core in all groups (60±7% vs. 56±6% in R192Q WT and HOM, and 57±5% vs. 57±6% of contralateral hemisphere in S218L WT and HOM, respectively, 60 minutes after stroke onset). *p<0.05 vs. WT.
Larger perfusion deficit during hyperacute stroke
Ischemic depolarizations compromise residual CBF within the territory supplied by the occluded artery via vasoconstrictive (i.e., inverse) neurovascular coupling18, 19, as a major determinant of outcome in cerebral ischemia. Using laser speckle flowmetry, we found larger cortical perfusion deficits after dMCAO in FHM1 mutants (Fig. 3a, b; only S218L shown), associated with an increased frequency of PIDs (0.9±0.3 vs. 4.6±1.2 PID/h in WT and S218L HOM, respectively; Fig. 3c) which circled around the hypoperfused core in 38% of S218L mutants, but not in the WT (Movies 1 and 2)22. In fact, higher PID frequencies were associated with larger cortical CBF deficits (Fig. 3d), as well as bigger infarcts (Fig. 3e, f) and worse neurological outcomes (deficit score 1 [1–1] vs. 0 [0–0.25] in S218L HOM and WT mice respectively; P=0.019). These data suggest that ischemic depolarizations adversely influence the perfusion deficits and exacerbate the metabolic and O2 supply-demand mismatch in FHM1 mutants, in part via vasoconstrictive (i.e., inverse) neurovascular coupling as an additional hemodynamic mechanism for infarct growth18, 19, 23.
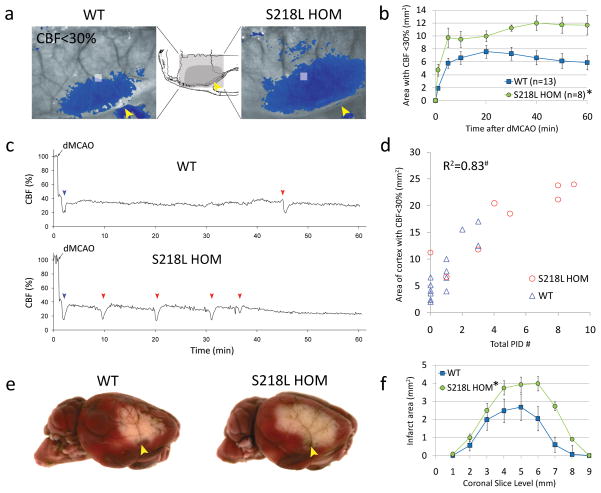
Figure 3.
FHM1 mutant mice develop larger areas of CBF deficit during dMCAO because of increased susceptibility to ischemic depolarizations. a) Representative laser speckle contrast images show the area of cortex with ≤30% residual CBF compared to pre-ischemic baseline (blue pixels), 60 min after dMCAO in WT and S218L HOM. Similar data were obtained using the R192Q strain (n=6 mutant and 6 WT; data not shown). Imaging was performed over the right hemisphere (light gray shaded rectangle in the inset), through intact skull. Arrowheads indicate clip occlusion. b) The area of CBF deficit expanded rapidly in S218L HOM throughout the 60 minute dMCAO (*p=0.004). c) Representative tracings show cortical blood flow reductions in penumbra (measured within the gray squares shown in ‘a’) after dMCAO. Anoxic depolarization triggers the first peri-infarct depolarization (blue arrowheads) marking a second abrupt reduction in perfusion. Each subsequent peri-infarct depolarization (red arrowheads) causes a characteristic blood flow transient. d) The frequency of PIDs was higher in S218L HOM, and correlated with the area of hypoperfused cortex 60 minutes after dMCAO (#p<0.001). Each symbol represents the PID frequency in individual mice. e) Representative TTC-stained whole brains show enlarged infarcts in S218L HOM 48 hours after 60 min dMCAO. f) The area of infarcts in 1 mm-thick coronal slices (0=anterior, 9=posterior) were larger in S218L HOM compared to WT (*p=0.016 S218L HOM vs. WT for infarct areas). Integrated total infarct volumes were also larger in the mutants (19±3 vs. 11±2 mm3, respectively; p=0.008).
Importantly, we found no difference in absolute resting CBF values between WT and R192Q HOM mice in cortex, striatum and cerebellum using [14C]iodoamphetamine method (Supplemental Table 2), indicating that differences in pre-ischemic resting CBF did not influence our measurements. This was also confirmed in WT and S218L HOM mice under isoflurane anesthesia using the correlation time values obtained by laser speckle imaging, which allow direct comparison of resting CBF among groups of mice (data not shown).24, 25 Moreover, the incidence of incomplete circle of Willis, diameter of its major branches, and the number and location of pial arterial anastomoses did not differ between WT and S218L HOM mice, suggesting that developmental differences in cerebrovascular anatomy did not contribute to worse perfusion deficits in the mutant (Supplemental Fig. 1; Supplemental Table 3).
Higher CBF threshold for tissue survival
In order to determine the critical tissue perfusion level below which infarction ensued (i.e., viability threshold), we calculated the regional CBF at the infarct margin by spatially co-registering the laser speckle perfusion map during dMCAO with the infarct that developed 48 hours later (Fig. 4). We found that cortical tissue in S218L HOM mutants required a higher CBF level for survival compared to WT mice (42±3% vs. 35±2% of baseline CBF, respectively; P=0.048). These data underscore the importance of parenchymal mechanisms, such as neuronal hyperexcitability and ischemic depolarizations, as the main cause for increased vulnerability to ischemic stroke in FHM1 mutants, independent of the severity of CBF deficit.
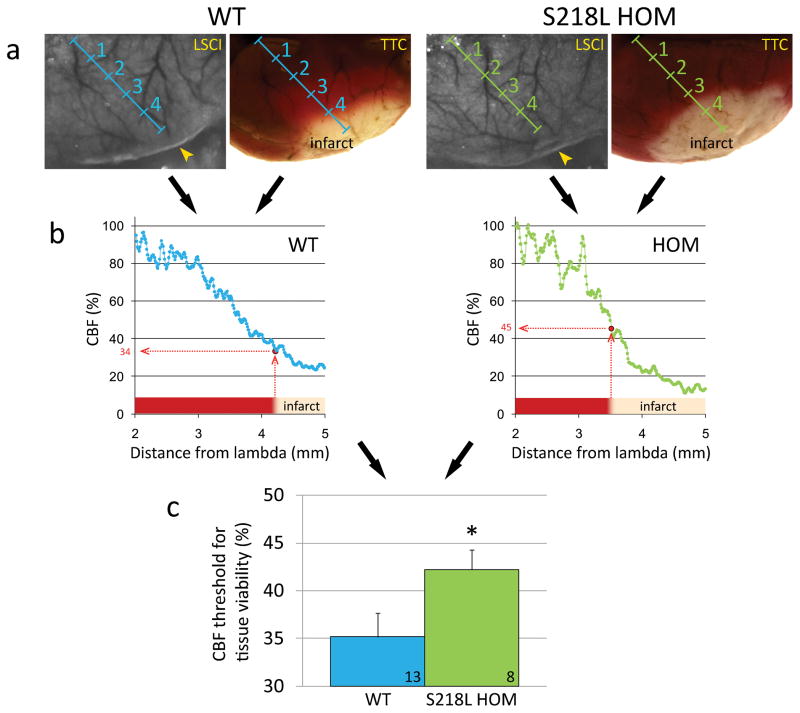
Figure 4.
Elevated blood flow threshold for tissue survival in FHM1 mutant mice. a) Representative laser speckle contrast images (LSCI) during dMCAO (left) and TTC-stained brain showing the infarct 48 hours after 60 min dMCAO (right) are shown for WT and S218L HOM mice. Imaging field was positioned as shown in Figure 3a. Images were spatially co-registered using surface landmarks. Line profiles (blue and green oblique lines, labeled in mm) were drawn between lambda and the clip occluding the middle cerebral artery branch (yellow arrowheads). b) For each animal, cortical blood flow (CBF) was plotted along these line profiles as a function of distance from lambda using laser speckle images, and the blood flow level corresponding to the infarct edge was determined (red dotted lines). This value represented the CBF threshold for viability, below which the tissue infarcted in each mouse. c) The average viability threshold was significantly higher in S218L HOM mutants compared to WT controls (p=0.048) indicating that FHM1 mutant brains are more vulnerable to ischemia and require higher blood flow to survive. Numbers of mice are shown on each bar. *p<0.05 vs. WT.
Worse stroke outcomes
Enhanced susceptibility to anoxic and peri-infarct depolarizations and accelerated hyperacute infarct growth with more severe CBF deficits translated into worse stroke outcomes in FHM1 mutants. Transient fMCAO for 1 hour produced larger infarcts in both S218L and R192Q mutant mice compared to their WT controls (Fig. 5a). Larger infarcts predominantly reflected more severe cortical involvement in both mutants (more than 70% of total infarct volume); however, the incidence of hippocampal or thalamic infarction also tended to be higher in the S218L mutant (present in 33% of S218L HET mice compared to 13% of WT; P=0.1; data not shown), consistent with higher incidence of subcortical infarction observed on MRI in this strain (see above). Functional outcomes, assessed using a combined death and neurological disability score as a clinically relevant endpoint, were worse in mutants compared to WT (Table 1). Indeed, mortality rate was significantly higher in the S218L mutants, reaching 100% in the HOM within 24 hours after stroke onset (Supplemental Fig. 2a). The timing of death after stroke was variable (12±3 hours after stroke onset), and not associated with overt seizure activity. Immediate postmortem examination revealed two-fold larger infarcts in S218L HOM when compared to WT mice sacrificed at the same time point of death of each mutant after 60 min fMCAO (95±15 vs. 44±7 mm3, respectively, n=5 and 4; p=0.031), suggesting that selection bias due to high mortality in the mutants diminished the strain differences in outcome. These data were excluded from the overall comparisons among genotypes (Fig. 5) because of variable time of death. Ischemic brain swelling tended to be more severe in the mutants in proportion to the actual infarct volume, and might have contributed to the high mortality in the S218L mutants.
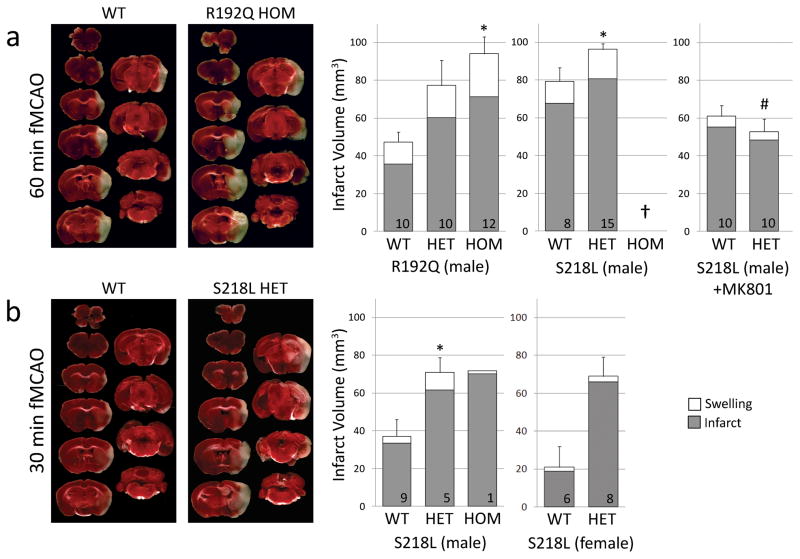
Figure 5.
FHM1 mutant mice develop larger infarcts after experimental stroke selectively attenuated by NMDA receptor antagonist MK-801. Left panel shows representative infarcts (unstained white tissue) 24 hours after fMCAO. Right panel shows infarct and ischemic swelling volumes (grey and white bars, respectively). a) After 60 minute fMCAO, infarcts were larger in both R192Q and S218L mutants compared to WT (p=0.007 and p=0.019, respectively). One of 9 WT, 8 of 23 S218L HET, and all 8 S218L HOM (†) mice died within 24 hours (Table 1), and were excluded from infarct volume analysis. Because genetic backgrounds and infarct volumes significantly differed between WT controls of the two mutant strains, we did not directly compare S218L and R192Q mutants in this study. MK-801 (1 mg/kg, intraperitoneally 15 minutes before fMCAO) significantly reduced infarct volume in S218L HET mutants (P<0.001 vs. untreated S218L HET shown in ‘a’) but not in the WT (P=0.061 vs. untreated WT shown in ‘a’). Therefore, MK-801 was more efficacious in FHM1 mutants (P=0.026 for infarct reduction by MK-801 between WT and FHM1 mutants). As a result, after MK-801, infarct and swelling volumes were comparable between WT and S218L HET mice (P=0.367), as were neurological outcomes (Table 1). b) Thirty minute fMCAO also resulted in larger infarcts in male and female S218L mutants compared to WT (p=0.028). Despite shorter ischemia, 3 of 4 S218L HOM mutants died within 24 hours and were again excluded from infarct volume analysis; the data from the only surviving HOM mutant are shown. There was no mortality in WT and HET groups after 30 min fMCAO. Numbers of mice are shown on each bar. Standard error bars and p values refer to total volume (i.e., infarct plus swelling). *p<0.05 vs. WT, #p<0.05 vs. untreated S218L HET after 60 min fMCAO.
Table 1.
Death and neurological disability after transient fMCAO in S218L mutant mice.
| Experiment | 60 min fMCAO* | 60 min fMCAO + MK-801†# | 30 min fMCAO‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender, Genotype | Male WT | Male HET | Male HOM | Male WT | Male HET | Male WT | Male HET | Male HOM | Female WT | Female HET |
| N | 9 | 23 | 8 | 9 | 10 | 9 | 5 | 4 | 6 | 8 |
| Mortality | 11% | 35% | 100% | 0% | 10% | 0% | 0% | 75% | 0% | 0% |
| Functional outcome score | 2 [2–3] | 3 [2–5] | 5 [5–5] | 2 [1–2] | 2 [1–2] | 1 [1–2] | 2 [1–2] | 5 [4–5] | 1 [0.3–1] | 2 [1–2] |
Functional outcome scores are shown as median [interquartile range]. 0 = best, 5 = worst, see Methods for the scoring system.
p<0.001 and p=0.008 for the effect of genotype on mortality and functional outcome score, respectively.
p=0.353 and p=0.757 for the effect of genotype on mortality and functional outcome score, respectively.
p=0.303 and p=0.315 for the effect of MK-801 on mortality and functional outcome score, respectively, in WT, and p=0.022 and p=0.009 for the effect of MK-801 on mortality and functional outcome score, respectively, in the S218L HET, compared to the untreated group.
p<0.001 and p=0.086 for the effect of genotype on mortality and functional outcome score in males, respectively, and p=0.02 for the effect of genotype on functional outcome score in females, after 30 min fMCAO. In the R192Q mutant strain, the mortality rate was 17%, 0% and 14% in WT, HET and HOM, respectively; neurological disability was not studied in this mutant strain.
To mimic transient ischemic attacks and circumvent the high mortality rate in S218L HOM mice, we subjected this mutant strain to 30 minutes of fMCAO. With shorter duration of ischemia, we did not detect overt infarcts in 27% of WT mice using TTC staining, whereas all S218L HET and HOM mutant mice developed conspicuous territorial infarcts (p=0.077). Selective ischemic changes in scattered neurons were nevertheless present upon histological examination of brains without an overt infarct (not shown). Infarct volumes were once again larger in the S218L mutants compared to WT (Fig. 5b). The volume of subcortical infarction, limited to the striatum in this shorter ischemia model, was also larger in the S218L HET compared to WT (17±4 and 5±1 mm3, respectively, in males, P=0.002; 16±3 and 4±2 mm3, respectively in females, p=0.013). Because classical, sporadic and FHM are more prevalent in women of reproductive age,5, 26–29 and because susceptibility to spreading depression is higher in female FHM1 mutant mice compared to males,13, 30 we also studied female mice and found an even more striking increase in infarct volumes in S218L HET compared to WT (Fig. 5b). Despite the shorter ischemia duration, mortality was still high in the S218L HOM mutants (75%), but all HET mutants survived for at least 24 hours and showed a trend for worse functional outcome compared to WT (P=0.086; Table 1). To assess long term tissue and neurological outcome (2 weeks) in FHM1 mutants we subjected female S218L HET and WT mice to 30 min fMCAO. However, we observed more than 60% mortality in the mutants predominantly between 24 and 96 hours, which precluded outcome comparisons between the mutant and WT strains at this late time point (Supplemental Fig. 2b). Altogether, these data indicate that mice expressing FHM1 mutations are particularly susceptible to infarction when challenged by cerebral arterial occlusion.
Enhanced neuroprotective efficacy of glutamate receptor antagonist MK-801
FHM1 mutations enhance glutamate release11, and glutamate plays a pivotal role in spreading depression and ischemic depolarizations, as well as in excitotoxic cell death, mainly via N-methyl-D-aspartate (NMDA) subtype of receptors. Therefore, we tested whether enhanced glutamatergic activity in FHM1 mutants is responsible for their vulnerability to infarction. Pre-ischemic treatment with the NMDA receptor inhibitor MK-801 abolished the differences in stroke phenotype between genotypes. MK-801 reduced infarct volume by 45% in S218L HET compared to only 23% in WT (Fig. 5a), and functional outcome was improved only in S218L mutants (Table 1).
As an important control, we also examined the density of glutamate and GABAA binding sites in the FHM1 mutant and WT mice using quantitative in vitro autoradiography, and did not find overt differences (Supplemental Fig. 3; Supplemental Table 4). These data are consistent with recent proteomics analysis of cortical synapses in this mutant31, and suggest that enhanced susceptibility to ischemic depolarizations in FHM1 mutants is unlikely to reflect changes in neurotransmitter receptors and reuptake mechanisms.
Discussion
Our data provide a novel mechanism to explain the higher incidence of ischemic stroke in migraineurs. Two genetic mouse models expressing FHM1 mutations were at risk to develop large infarcts and worse neurological outcomes after transient focal cerebral ischemia. Consistent with the higher stroke risk in women compared to men with migraine with aura, we found more striking increases in infarct volume in female mutants compared to males. Faster AD rates, more frequent PIDs and enhanced neuroprotective efficacy of NMDA-antagonist MK-801 in the mutants implicated glutamatergic neuronal hyperexcitability as one mechanism, and larger perfusion defects linked to ischemic depolarizations implicated vasoconstrictive neurovascular coupling as another. Therefore, neuronal and vascular mechanisms together render migraineurs more vulnerable to cerebral infarction upon ischemia.
The data have clinical implications. In susceptible migraineurs, increased sensitivity to ischemia may predispose to strokes during mild ischemic events, which remain clinically silent or only manifest as transient ischemic attacks in non-migraineurs. Moreover, elevated CBF threshold for viability32 as a sign of increased vulnerability to ischemia, may promote rapid infarct expansion into tissue with milder perfusion deficits, diminish salvageable tissue at risk (i.e., ischemic penumbra), and shorten the therapeutic window of acute stroke interventions in migraineurs. Lastly, higher mortality among the S218L mutants may have implications for malignant infarcts, large supratentorial strokes characterized by progressive loss of consciousness over 48 hours with up to 80% mortality if untreated33. To date, there has been no reliable predictor for malignant infarction, and history of migraine with aura (or its genetic determinants) may be one such marker increasing the risk of stroke progression, and in case of large territorial infarcts, the risk of death, as has recently been reported for hemorrhagic stroke in migraineurs34.
Mechanisms of ischemic vulnerability in FHM1 mice
Biological mechanisms underlying the association between migraine and stroke are unknown, although in both diseases dynamic interactions among the constituents of the neurovascular unit are important to the pathophysiology. At the onset of cerebral ischemia the gradual failure of Na+, K+-ATPase causes a gradual rise in extracellular K+ and loss of neuronal membrane potential until a critical threshold for initiation of anoxic depolarization is reached35, 36. FHM1 mutations shift the CaV2.1 channel opening voltage to more negative membrane potentials so that channels open with smaller depolarizations triggering glutamate release, which can explain faster anoxic depolarization onset in the mutants. Glutamate is also critical for PIDs, which are spreading depolarization waves triggered in ischemic penumbra. Therefore, enhanced release in ischemic penumbra can also explain higher PID frequencies in FHM1 mutants11. Delayed CaV2.1 channel inactivation may further exacerbate the excitotoxicity by prolonging the Ca2+ influx and glutamate release during PIDs in penumbra. Moreover, PIDs exacerbate the metabolic mismatch in penumbra by stimulating O2 and glucose consumption, and by worsening tissue perfusion via vasoconstrictive (i.e., inverse) neurovascular coupling18, 19, 23, particularly when they occur with high frequency and in clusters as observed in FHM1 mutants19, 37–39. With each PID, more of the penumbra is incorporated into the core, accounting for concentric infarct growth over time22, 40–42. PIDs do occur frequently in human brain after ischemic or hemorrhagic stroke and head trauma, and appear to worsen patient outcomes similar to experimental stroke38, 43–45. Hence, migraine with aura may be a risk factor for increased occurrence of PIDs and worse outcomes in human stroke, as recently suggested in subarachnoid hemorrhage46.
Association between migraine and stroke
Our data in FHM1 mutant mice support shared genetic risk factors enhancing susceptibility to spreading depression as one mechanism to explain migraine-stroke association. Shared genetic factors enhancing susceptibility to migraine and stroke have been described, such as NOTCH3 mutations in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)47–50. Indeed, NOTCH3 mutations, despite being exclusively expressed in vascular smooth muscle cells, augment susceptibility to spreading depression51. Mutations associated with FHM2 and FHM3 are also predicted to enhance neuronal excitability and spreading depression susceptibility possibly via glutamatergic mechanisms52. Glutamatergic mechanisms and hyperexcitability are implicated in common forms of migraine as well, by recent studies linking the AEG1 encoding a regulator of glial glutamate transporter EAAT2, and the KCNK18 encoding the TRESK potassium channel, to migraine15, 16. Vascular mechanisms (e.g. endothelial dysfunction) have also been implicated in increasing stroke risk in migraineurs53, 54. Indeed, functional Cav2.1 channel expression has been reported in renovascular smooth muscle cells55, 56, but whether FHM1 mutations alter cerebrovascular physiology is not known. Together with parenchymal mechanisms that enhance vulnerability to perfusion deficits, vascular mechanisms might further augment stroke risk in migraineurs. For example, highly focal and mild ischemic vascular events such as microembolism57 may trigger spreading depression more readily in migraineurs highly susceptible to ischemic depolarizations, providing a possible explanation for the origin of a subset of migraine auras.
In summary, a monogenic determinant of migraine with aura increases stroke vulnerability via glutamatergic mechanisms that enhance susceptibility to ischemic depolarizations akin to spreading depression, and accelerate stroke evolution. Hence, our data put FHM1 mutations among the shared genetic determinants of migraine with aura and stroke. More work is needed to extrapolate these data to other monogenic syndromes, as well as to the more common and genetically more complex forms of migraine with aura, and determine whether targeting hyperexcitability and spreading depression, such as migraine prophylaxis58, confers ischemic protection in susceptible mouse strains or in migraineurs.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by the American Heart Association (09GRNT2060416, 10SDG2610275), National Institutes of Health (NS061505, NS055104, NS35611, NS057556, DA024235, DA026108), Deane Institute for Integrative Research in Stroke and Atrial Fibrillation; Netherlands Organization for Scientific Research (903-52-291 and Vici 918.56.602; Spinoza 2009), EU “EUROHEAD” grant (LSHM-CT-2004-504837), the Centre for Medical Systems Biology in the framework of the Netherlands Genomics Initiative, Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2009-0066654), and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers).
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: Systematic review and meta-analysis of observational studies. BMJ. 2005;330:63. doi: 10.1136/bmj.38302.504063.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: Systematic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruit MC, van Buchem MA, Hofman PA, Bakkers JT, Terwindt GM, Ferrari MD, Launer LJ. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291:427–434. doi: 10.1001/jama.291.4.427. [DOI] [PubMed] [Google Scholar]
- 4.Scher AI, Gudmundsson LS, Sigurdsson S, Ghambaryan A, Aspelund T, Eiriksdottir G, van Buchem MA, Gudnason V, Launer LJ. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301:2563–2570. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, Russell MB. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain. 2002;125:1379–1391. doi: 10.1093/brain/awf132. [DOI] [PubMed] [Google Scholar]
- 6.Terwindt GM, Ophoff RA, Haan J, Vergouwe MN, van Eijk R, Frants RR, Ferrari MD. Variable clinical expression of mutations in the p/q-type calcium channel gene in familial hemiplegic migraine. Dutch migraine genetics research group. Neurology. 1998;50:1105–1110. doi: 10.1212/wnl.50.4.1105. [DOI] [PubMed] [Google Scholar]
- 7.Ducros A, Denier C, Joutel A, Cecillon M, Lescoat C, Vahedi K, Darcel F, Vicaut E, Bousser MG, Tournier-Lasserve E. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med. 2001;345:17–24. doi: 10.1056/NEJM200107053450103. [DOI] [PubMed] [Google Scholar]
- 8.Moskowitz MA, Bolay H, Dalkara T. Deciphering migraine mechanisms: Clues from familial hemiplegic migraine genotypes. Ann Neurol. 2004;55:276–280. doi: 10.1002/ana.20035. [DOI] [PubMed] [Google Scholar]
- 9.Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the ca2+ channel gene cacnl1a4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 10.Tottene A, Pivotto F, Fellin T, Cesetti T, van den Maagdenberg AM, Pietrobon D. Specific kinetic alterations of human cav2.1 calcium channels produced by mutation s218l causing familial hemiplegic migraine and delayed cerebral edema and coma after minor head trauma. J Biol Chem. 2005;280:17678–17686. doi: 10.1074/jbc.M501110200. [DOI] [PubMed] [Google Scholar]
- 11.Tottene A, Conti R, Fabbro A, Vecchia D, Shapovalova M, Santello M, van den Maagdenberg AM, Ferrari MD, Pietrobon D. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in ca(v)2.1 knockin migraine mice. Neuron. 2009;61:762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 12.van den Maagdenberg AM, Pizzorusso T, Kaja S, Terpolilli N, Shapovalova M, Hoebeek FE, Barrett CF, Gherardini L, van de Ven RC, Todorov B, Broos LA, Tottene A, Gao Z, Fodor M, De Zeeuw CI, Frants RR, Plesnila N, Plomp JJ, Pietrobon D, Ferrari MD. High cortical spreading depression susceptibility and migraine-associated symptoms in ca(v)2.1 s218l mice. Ann Neurol. 2010;67:85–98. doi: 10.1002/ana.21815. [DOI] [PubMed] [Google Scholar]
- 13.Eikermann-Haerter K, Dilekoz E, Kudo C, Savitz SI, Waeber C, Baum MJ, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest. 2009;119:99–109. doi: 10.1172/JCI36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, van de Ven RC, Tottene A, van der Kaa J, Plomp JJ, Frants RR, Ferrari MD. A cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–710. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 15.Lafreniere RG, Cader MZ, Poulin JF, Andres-Enguix I, Simoneau M, Gupta N, Boisvert K, Lafreniere F, McLaughlan S, Dube MP, Marcinkiewicz MM, Ramagopalan S, Ansorge O, Brais B, Sequeiros J, Pereira-Monteiro JM, Griffiths LR, Tucker SJ, Ebers G, Rouleau GA. A dominant-negative mutation in the tresk potassium channel is linked to familial migraine with aura. Nat Med. 2011;16:1157–1160. doi: 10.1038/nm.2216. [DOI] [PubMed] [Google Scholar]
- 16.Anttila V, Stefansson H, Kallela M, Todt U, Terwindt GM, Calafato MS, Nyholt DR, Dimas AS, Freilinger T, Muller-Myhsok B, Artto V, Inouye M, Alakurtti K, Kaunisto MA, Hamalainen E, de Vries B, Stam AH, Weller CM, Heinze A, Heinze-Kuhn K, Goebel I, Borck G, Gobel H, Steinberg S, Wolf C, Bjornsson A, Gudmundsson G, Kirchmann M, Hauge A, Werge T, Schoenen J, Eriksson JG, Hagen K, Stovner L, Wichmann HE, Meitinger T, Alexander M, Moebus S, Schreiber S, Aulchenko YS, Breteler MM, Uitterlinden AG, Hofman A, van Duijn CM, Tikka-Kleemola P, Vepsalainen S, Lucae S, Tozzi F, Muglia P, Barrett J, Kaprio J, Farkkila M, Peltonen L, Stefansson K, Zwart JA, Ferrari MD, Olesen J, Daly M, Wessman M, van den Maagdenberg AM, Dichgans M, Kubisch C, Dermitzakis ET, Frants RR, Palotie A. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat Genet. 2011;42:869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayata C, Ayata G, Hara H, Matthews RT, Beal MF, Ferrante RJ, Endres M, Kim A, Christie RH, Waeber C, Huang PL, Hyman BT, Moskowitz MA. Mechanisms of reduced striatal nmda excitotoxicity in type i nitric oxide synthase knock-out mice. J Neurosci. 1997;17:6908–6917. doi: 10.1523/JNEUROSCI.17-18-06908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- 19.Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, Nagafuji T, Ninomiya M, Nakamura H, Dunn AK, Graf R. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- 20.Hartings JA, Rolli ML, Lu XC, Tortella FC. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: Relation to infarct growth and neuroprotection. J Neurosci. 2003;23:11602–11610. doi: 10.1523/JNEUROSCI.23-37-11602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopwood SE, Parkin MC, Bezzina EL, Boutelle MG, Strong AJ. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J Cereb Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura H, Strong AJ, Dohmen C, Sakowitz OW, Vollmar S, Sue M, Kracht L, Hashemi P, Bhatia R, Yoshimine T, Dreier JP, Dunn AK, Graf R. Spreading depolarizations cycle around and enlarge focal ischaemic brain lesions. Brain. 2010;133:1994–2006. doi: 10.1093/brain/awq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreier JP, Windmüller O, Petzold G, Lindauer U, Einhäupl KM, Dirnagl U. Ischemia caused by inverse coupling between neuronal activation and cerebral blood flow in rats. In: Tomita M, Kanno I, Hamel E, editors. Brain activation and cbf control. Amsterdam: Elsevier; 2002. pp. 487–492. [Google Scholar]
- 24.Shin HK, Jones PB, Garcia-Alloza M, Borrelli L, Greenberg SM, Bacskai BJ, Frosch MP, Hyman BT, Moskowitz MA, Ayata C. Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain. 2007;130:2310–2319. doi: 10.1093/brain/awm156. [DOI] [PubMed] [Google Scholar]
- 25.Ayata C, Dunn AK, Gursoy OY, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–755. doi: 10.1097/01.WCB.0000122745.72175.D5. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991;44:1147–1157. doi: 10.1016/0895-4356(91)90147-2. [DOI] [PubMed] [Google Scholar]
- 27.Bille BS. Migraine in school children. A study of the incidence and short-term prognosis, and a clinical, psychological and electroencephalographic comparison between children with migraine and matched controls. Acta paediatrica. 1962;136:1–151. [PubMed] [Google Scholar]
- 28.Eriksen MK, Thomsen LL, Olesen J. Implications of clinical subtypes of migraine with aura. Headache. 2006;46:286–297. doi: 10.1111/j.1526-4610.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen LL, Kirchmann M, Bjornsson A, Stefansson H, Jensen RM, Fasquel AC, Petursson H, Stefansson M, Frigge ML, Kong A, Gulcher J, Stefansson K, Olesen J. The genetic spectrum of a population-based sample of familial hemiplegic migraine. Brain. 2007;130:346–356. doi: 10.1093/brain/awl334. [DOI] [PubMed] [Google Scholar]
- 30.Eikermann-Haerter K, Baum MJ, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C. Androgenic suppression of spreading depression in familial hemiplegic migraine type 1 mutant mice. Ann Neurol. 2009;66:564–568. doi: 10.1002/ana.21779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klychnikov OI, Li KW, Sidorov IA, Loos M, Spijker S, Broos LA, Frants RR, Ferrari MD, Mayboroda OA, Deelder AM, Smit AB, van den Maagdenberg AM. Quantitative cortical synapse proteomics of a transgenic migraine mouse model with mutated ca(v)2.1 calcium channels. Proteomics. 2010;10:2531–2535. doi: 10.1002/pmic.200900733. [DOI] [PubMed] [Google Scholar]
- 32.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 33.Huttner HB, Schwab S. Malignant middle cerebral artery infarction: Clinical characteristics, treatment strategies, and future perspectives. Lancet Neurol. 2009;8:949–958. doi: 10.1016/S1474-4422(09)70224-8. [DOI] [PubMed] [Google Scholar]
- 34.Kurth T, Kase CS, Schurks M, Tzourio C, Buring JE. Migraine and risk of haemorrhagic stroke in women: Prospective study. BMJ. 2010:c3659. doi: 10.1136/bmj.c3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vyskocil F, Kritz N, Bures J. Potassium-selective microelectrodes used for measuring the extracellular brain potassium during spreading depression and anoxic depolarization in rats. Brain Res. 1972;39:255–259. doi: 10.1016/0006-8993(72)90802-5. [DOI] [PubMed] [Google Scholar]
- 36.Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular k+ and h+ at critical levels of brain ischemia. Stroke. 1977;8:51–57. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]
- 37.Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- 38.Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, Brinker G, Dreier JP, Woitzik J, Strong AJ, Graf R. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- 39.Back T, Kohno K, Hossmann KA. Cortical negative dc deflections following middle cerebral artery occlusion and kcl-induced spreading depression: Effect on blood flow, tissue oxygenation, and electroencephalogram. J Cereb Blood Flow Metab. 1994;14:12–19. doi: 10.1038/jcbfm.1994.3. [DOI] [PubMed] [Google Scholar]
- 40.Higuchi T, Takeda Y, Hashimoto M, Nagano O, Hirakawa M. Dynamic changes in cortical nadh fluorescence and direct current potential in rat focal ischemia: Relationship between propagation of recurrent depolarization and growth of the ischemic core. J Cereb Blood Flow Metab. 2002;22:71–79. doi: 10.1097/00004647-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Busch E, Gyngell ML, Eis M, Hoehn-Berlage M, Hossmann KA. Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: Contribution to lesion growth assessed by diffusion-weighted nmr and biochemical imaging. J Cereb Blood Flow Metab. 1996;16:1090–1099. doi: 10.1097/00004647-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Takano K, Latour LL, Formato JE, Carano RA, Helmer KG, Hasegawa Y, Sotak CH, Fisher M. The role of spreading depression in focal ischemia evaluated by diffusion mapping. Ann Neurol. 1996;39:308–318. doi: 10.1002/ana.410390307. [DOI] [PubMed] [Google Scholar]
- 43.Strong AJ, Hartings JA, Dreier JP. Cortical spreading depression: An adverse but treatable factor in intensive care? Curr Opin Crit Care. 2007;13:126–133. doi: 10.1097/MCC.0b013e32807faffb. [DOI] [PubMed] [Google Scholar]
- 44.Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosche B, Graf R, Ernestus RI, Dohmen C, Reithmeier T, Brinker G, Strong AJ, Dreier JP, Woitzik J. Recurrent spreading depolarizations after subarachnoid hemorrhage decreases oxygen availability in human cerebral cortex. Ann Neurol. 2010;67:607–617. doi: 10.1002/ana.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dreier JP, Kremer C, Lammers G, Lohmann F, Hansen HC, Valdueza JM. Migraine and delayed ischaemic neurological deficit after subarachnoid haemorrhage in women: A case-control study. Eur J Neurol. 2007;14:1363–1368. doi: 10.1111/j.1468-1331.2007.01980.x. [DOI] [PubMed] [Google Scholar]
- 47.Scher AI, Terwindt GM, Verschuren WM, Kruit MC, Blom HJ, Kowa H, Frants RR, van den Maagdenberg AM, van Buchem M, Ferrari MD, Launer LJ. Migraine and mthfr c677t genotype in a population-based sample. Ann Neurol. 2006;59:372–375. doi: 10.1002/ana.20755. [DOI] [PubMed] [Google Scholar]
- 48.Stam AH, Haan J, van den Maagdenberg AM, Ferrari MD, Terwindt GM. Migraine and genetic and acquired vasculopathies. Cephalalgia. 2009;29:1006–1017. doi: 10.1111/j.1468-2982.2009.01940.x. [DOI] [PubMed] [Google Scholar]
- 49.Pezzini A, Grassi M, Del Zotto E, Giossi A, Monastero R, Dalla Volta G, Archetti S, Zavarise P, Camarda C, Gasparotti R, Magoni M, Camarda R, Padovani A. Migraine mediates the influence of c677t mthfr genotypes on ischemic stroke risk with a stroke-subtype effect. Stroke. 2007;38:3145–3151. doi: 10.1161/STROKEAHA.107.491506. [DOI] [PubMed] [Google Scholar]
- 50.Schurks M, Zee RY, Buring JE, Kurth T. Interrelationships among the mthfr 677c>t polymorphism, migraine, and cardiovascular disease. Neurology. 2008;71:505–513. doi: 10.1212/01.wnl.0000316198.34558.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eikermann-Haerter K, Yuzawa I, Dilekoz E, Joutel A, Moskowitz MA, Ayata C. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy syndrome mutations increase susceptibility to spreading depression. Ann Neurol. 2011;69:413–418. doi: 10.1002/ana.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Maagdenberg AM, Haan J, Terwindt GM, Ferrari MD. Migraine: Gene mutations and functional consequences. Curr Opin Neurol. 2007;20:299–305. doi: 10.1097/WCO.0b013e3281338d1f. [DOI] [PubMed] [Google Scholar]
- 53.Tietjen EG. Migraine and ischaemic heart disease and stroke: Potential mechanisms and treatment implications. Cephalalgia. 2007;27:981–987. doi: 10.1111/j.1468-2982.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 54.Bigal ME, Kurth T, Hu H, Santanello N, Lipton RB. Migraine and cardiovascular disease: Possible mechanisms of interaction. Neurology. 2009;72:1864–1871. doi: 10.1212/WNL.0b013e3181a71220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansen PB, Poulsen CB, Walter S, Marcussen N, Cribbs LL, Skott O, Jensen BL. Functional importance of l- and p/q-type voltage-gated calcium channels in human renal vasculature. Hypertension. 2011;58:464–470. doi: 10.1161/HYPERTENSIONAHA.111.170845. [DOI] [PubMed] [Google Scholar]
- 56.Hansen PB, Jensen BL, Andreasen D, Friis UG, Skott O. Vascular smooth muscle cells express the alpha(1a) subunit of a p-/q-type voltage-dependent ca(2+)channel, and it is functionally important in renal afferent arterioles. Circ Res. 2000;87:896–902. doi: 10.1161/01.res.87.10.896. [DOI] [PubMed] [Google Scholar]
- 57.Nozari A, Dilekoz E, Sukhotinsky I, Stein T, Eikermann-Haerter K, Liu C, Wang Y, Frosch MP, Waeber C, Ayata C, Moskowitz MA. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann Neurol. 2010;67:221–229. doi: 10.1002/ana.21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–661. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.