Abstract
Aims
Survival preferences, ascertained from time-trade-off utilities, have not been studied in heart failure patients who designate a ‘do not resuscitate’ (DNR) status. Therefore, the aim of this study was to determine the association of heart failure patients' resuscitation preferences with survival preferences and mortality in the ESCAPE trial.
Methods and results
We analysed the association of resuscitation orders at 1 month with time-trade-off utilities and 6-month mortality. There were 26 and 349 patients with a DNR order and Full Code order, respectively. DNR patients were older, had more coronary artery disease, hypertension, renal impairment, and poorer exercise capacity than Full Code patients. DNR patients also experienced longer hospitalization and higher 6-month mortality. In multivariate analysis, DNR preference was associated with 10-fold higher odds of willingness to trade survival time (lower time-trade-off utility) in favour of improved quality of life [odds ratio 10.33, 95% confidence interval (CI) 1.65–64.80]. DNR preference was the best predictor of mortality (χ2 26.12, P < 0.0001, hazard ratio 6.88, 95% CI 3.28–14.41), despite adjustment for known predictors including brain natriuretic peptide.
Conclusions
Heart failure patients' requests to forgo resuscitation may signify more than simply ‘what-if’ directives for emergency care. DNR decisions may reflect preferences for intervention to enhance quality rather than prolong survival, which is particularly important as these patients have high early mortality.
Keywords: Resuscitation, Heart failure, Preferences, Quality of life
Introduction
Patient-centred care has been endorsed by the US Institute of Medicine as one of six essential dimensions of an optimal healthcare system.1 Patient-centred care is ‘responsive to individual patient preferences, needs, and values and ensures that patient values guide all clinical decisions’. However, there are no consistent recommendations for determination of the preferences of heart failure (HF) patients for quality vs. length of survival.2
Patient preferences are particularly relevant in the care of HF patients due to the wide array of therapeutic choices in even the most advanced stages of disease. Advanced HF patients and caregivers commonly face choices regarding continuing or discontinuing life-sustaining interventions.3 These choices are complex, as patients incorporate multiple factors in decision-making, including burden of therapy, type of outcome, and the likelihood of the outcome.4 A previous analysis of the ESCAPE trial reported that a substantial percentage of HF patients had a low survival preference, i.e. they were willing to trade nearly all remaining survival for better perceived health.5 Unfortunately, HF patient preferences for end-of-life care are commonly misperceived or not assessed by physicians and caregivers.6,7
In clinical practice, patient preference regarding cardiopulmonary resuscitation (CPR) is commonly assessed at the time of hospital admission in part due to requirements of the Patient Self-Determination Act.8 However, formal assessment of patient preferences for quality vs. duration of life is not common in clinical practice.9 Although about a quarter of critically ill, hospitalized patients express a ‘do not resuscitate’ (DNR) preference, there are few studies of HF patients that explore the relationship of a DNR preference to other preferences for care.10 Further, while the presence of a written DNR order has been associated with higher mortality in a study of hospitalized HF patients, the reasons for this association have not been explored.11
The time-trade-off utility is a research tool that quantifies a person's preference for improved quality rather than extended duration of life; it represents the ‘value’ (from 0 to 1) that a person places on his/her current health state.12 In this analysis of the ESCAPE trial, we sought to determine if: (i) patient preference to forgo resuscitation (i.e. DNR preference) would predict survival preference, i.e. lower time-trade-off utility; and (ii) whether DNR preference would predict higher 6-month mortality after adjustment for known prognostic markers.
Methods
Patient population
The ESCAPE trial was a randomized, multicentre trial enrolling 433 patients hospitalized with advanced HF from January 2000 to November 2003. The design, primary endpoints, inclusion/exclusion criteria, and results of the ESCAPE trial have been published previously.13 Patients hospitalized with severe symptomatic HF despite treatment were assigned to receive clinical assessment or pulmonary artery catheter (PAC)-guided therapy. Patients were evaluated at 7–14 days, and 1, 2, 3, and 6 months after discharge. Data were collected on clinical status, medications, exercise, and quality of life measurements.
Descriptive statistics
Patient characteristics and outcomes were compared using χ2 test for categorical variables and t-test for continuous variables. Where specific distributional assumptions for these tests were violated, the Fisher's exact and Wilcoxon signed rank tests, respectively, were used.
Resuscitation orders
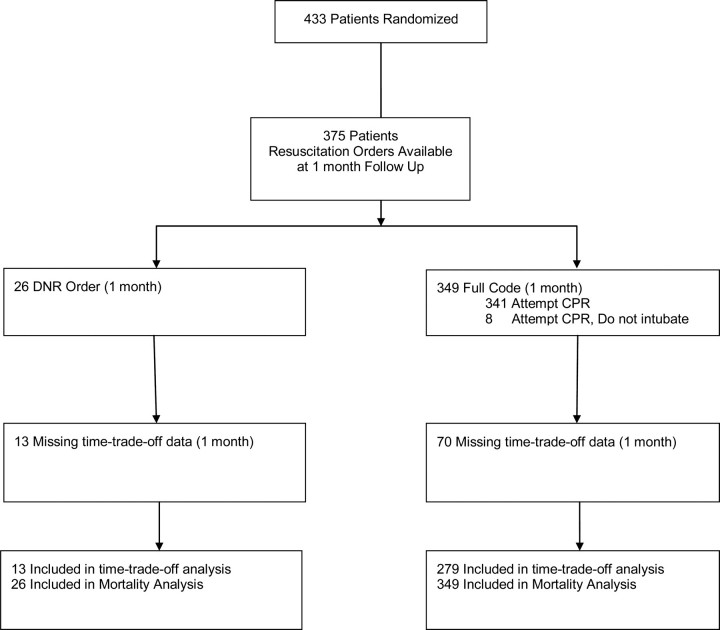
Orders for CPR were recorded by study co-ordinators at randomization and at 1, 2, 3, and 6 months; we classified patients' resuscitation preference based on their stated preference at 1 month in order to correspond with the time-trade-off endpoint, also at 1 month. The range of possible resuscitation orders included (i) ‘attempt CPR’; (ii) ‘attempt CPR but do not intubate’; or (iii) ‘do not attempt CPR’. Because of the clinically and ethically problematic nature of limited CPR,14 for the purposes of this study, we defined a priori ‘DNR’ as an order to avoid all forms of CPR. We defined ‘Full Code’ as any physician order for CPR; therefore, we included orders to ‘attempt CPR’ or ‘attempt CPR but do not intubate’. We only included patients that had explicit resuscitation orders ascertained by the study co-ordinator; no imputation of missing resuscitation orders was performed (Figure 1). There was no information on how study co-ordinators ascertained resuscitation orders, whether through chart abstraction or patient interview.
Figure 1.
Flow diagram of the study. CPR, cardiopulmonary resuscitation; DNR, do not resuscitate.
Survival preference (time-trade-off) endpoint
The time-trade-off instrument was verbally administered to patients by study co-ordinators at baseline, 1, 2, 3, and 6 months.5 Because a previous study from the ESCAPE trial reported a stabilization of time-trade-off preferences after hospital discharge (at 1 month and thereafter), we chose an endpoint of time-trade-off at 1 month after randomization.5 The initial question was ‘Would you prefer living 2 years in your current state of health or living 1 day in excellent health?’ An answer of 1 day, equated to a utility of nearly zero, would end the script. An answer of 2 years would be followed by the next choice, between living ‘2 years in your current state of health or living 1 year 11 months in excellent health?’ After further choices, the number of months (≤24 months) in excellent health that the subject considered equivalent in value to 24 months of survival in current health was recorded, and this ratio was the utility (between 0 and 1). The number of months at the indifference point subtracted from 24 yielded the number of months that the patient would be willing to trade, i.e. the duration of time-trade-off.
At 1 month, there were 292 time-trade-off observations available for modelling. The time-trade off values (range: 0–24 months) had a bimodal distribution; patients were willing to trade all or none of their remaining survival time (hypothetical 24 month survival time). Thus, based on the distribution of time-trade-off values, we modelled the time-trade-off endpoint as a dichotomous variable rather than as a continuous variable. We called this variable ‘half time-trade-off’, which corresponded to willingness to trade ≥12 months of hypothetical 24-month survival. In addition, we felt that ‘half-time-trade-off’, indicating a patient's willingness to give up greater than half of remaining (hypothetical) survival time, was a significant threshold that may be of relevance to clinicians who treat advanced HF patients.
Modelling survival preference (time-trade-off)
Covariates that predicted HF patient mortality15 or had been previously associated with time-trade-off in ESCAPE5 were considered for multivariable logistic regression modelling of ‘half-time-trade-off’ with forward and backward stepwise selection methods. In addition to resuscitation status, 10 covariates were included as candidates for stepwise modelling: occurrence of in-hospital resuscitation (mechanical ventilation or CPR), Minnesota Living With Heart Failure (MLWHF) score (1 month follow-up), patient-reported global health (visual analogue scale), number of hospitalizations in the previous 6 months, discharge sodium and blood urea nitrogen (BUN), 6 min walk distance at discharge, age, depression (MLWHF question 17, 1 month follow-up), and beta-blocker prescription at discharge. The variable, discharge brain natriuretic peptide (BNP; per doubling), was excluded from the list of candidates for modelling this endpoint due to excessive missing data. Forward and backward selections of covariates from the final candidates were performed with a model inclusion criteria of P = 0.25. No imputation methods were applied to missing data, and only full cases were included in modelling. Interaction terms were not tested.
Mortality endpoint
An endpoint committee blinded to treatment allocation adjudicated all hospitalization and mortality events.16 In order to examine the effect of resuscitation preference at 1 month on mortality, deaths prior to 1 month were censored in this analysis. Among patients with known resuscitation preference at 1 month, a total of 55 post-1 month deaths were observed during the follow-up period.
Modelling mortality
We utilized a proportional hazards model and included as covariates four mortality predictors from a previously published analysis of ESCAPE.15 These multivariate predictors were discharge BNP (per doubling), in-hospital cardiac resuscitation, discharge BUN, and discharge sodium. We also attempted to utilize a multivariable discharge score derived from the latter analysis, but this was aborted due to excessive missing data. Only complete cases were included in modelling, and interaction terms were not tested.
Results
Patient characteristics (baseline and in-hospital)
At 1 month follow-up, resuscitation preference was available for 375 patients. Twenty-six patients had DNR status and 349 were Full Code (Figure 1). DNR patients were older with a higher prevalence of coronary artery disease, hypertension, and renal impairment (as measured by serum creatinine) (Table 1). Among DNR patients, there was a non-significant trend towards white ethnicity and less depression. There were no differences in baseline HF medications, left ventricular ejection fraction, quality of life measures (MLWHF, global health status, breathing, depression), or implantable cardiac defibrillator (ICD) therapy between the two groups. We consistently deleted New York Heart Association (NYHA) class from these analyses, because it is inconsistently obtained; by definition, patients with resting symptoms are class IV. DNR patients had markedly lower exercise capacity (6 min walk distance) than Full Code patients [median (interquartile range; IQR) 0 ft (0, 356) vs. 369 ft (0, 714), P = 0.002, non-parametric]. Median 6 min walk distance for DNR patients was 0 ft because 12 of 23 DNR patients (3 patients had missing walk data) were too ill to walk. The median distance for those DNR patients who did walk was 356 ft. DNR patients had longer initial hospitalization [median (IQR): 10 day (5, 16) vs. 6 (4, 10), P = 0.015]. There were no significant differences in cause of death or inotrope/vasodilator utilization (at discharge) between the two groups.
Table 1.
Baseline characteristics of patients according 1-month resuscitation orders
| Baseline factor | DNR group (n = 26) | Full Code group (n = 349) | P-value |
|---|---|---|---|
| Age, median (IQR), years | 64 (53–72) | 56 (48–66) | 0.02 |
| Male gender, % | 65 | 74 | 0.36 |
| Minority, no. (%) | 6 (23) | 141 (40) | 0.08 |
| Aetiology, ischaemic, no. (%) | 19 (73) (n = 26) | 167 (48) (n = 347) | 0.01 |
| Implantable defibrillator, no. (%) | 5 (20) (n = 25) | 101 (29) (n = 348) | 0.33 |
| Hypertension, no. (%) | 17 (68) (n = 25) | 161 (46) (n = 348) | 0.04 |
| Diabetes, no. (%) | 10 (40) (n = 25) | 113 (33) (n = 348) | 0.44 |
| Heart rate, median (IQR), b.p.m. | 80 (70, 90) (n = 25) | 81 (70, 92) (n = 344) | 0.45 |
| Systolic blood pressure, median (IQR), mmHg | 112 (98, 120) (n = 25) | 104 (92, 116) (n = 345) | 0.12 |
| LV ejection fraction, median (IQR), % | 20 (15, 25) (n = 24) | 20 (15, 25) (n = 342) | 0.40 |
| Urea nitrogen, median (IQR), mg/dLa | 37 (17, 50) (n = 25) | 28 (19, 41) (n = 345) | 0.19 |
| Creatinine, median (IQR), mg/dLb | 1.7 (1.1, 2.0) (n = 26) | 1.3 (1.1, 1.8) (n = 347) | 0.04 |
| ACE inhibitor, no. (%) | 21 (81) | 275 (79) | 0.81 |
| Beta-blocker, no. (%) | 14 (54) (n = 26) | 224 (64) (n = 348) | 0.28 |
| 6 min walk distance, median (IQR), ft | 0 (0, 356) (n = 23) | 369 (0, 714) (n = 316) | 0.002 |
| MLWHF scorec | 74 (66, 92) (n = 26) | 76 (64, 86) (n = 340) | 0.71 |
| Patient global health VASd | 46 (30, 70) (n = 26) | 42 (30, 60) (n = 343) | 0.52 |
| Breathing VASe | 50 (40, 75) (n = 11) | 50 (30, 60) (n = 164) | 0.46 |
| Depression, no. (%)f | 0.07 | ||
| 0 | 5 (19) | 39 (12) | |
| 1 | 6 (23) | 48 (14) | |
| 2 | 3 (12) | 43 (13) | |
| 3 | 5 (19) | 60 (18) | |
| 4 | 0 (0) | 66 (19) | |
| 5 | 7 (27) (n = 26) | 84 (25) (n = 340) | |
| Length of initial hospitalization, median (IQR), days | 10 (5, 16) | 6 (4, 10) (n = 347) | 0.015 |
| Inotrope or vasodilator on discharge, no. (%) | 3 (12) | 16 (5) | 0.14 |
ACE, angiotensin-converting enzyme; DNR, do not resuscitate; IQR, interquartile range; LV, left ventricular; MLWHF, Minnesota Living With Heart Failure; NYHA, New York Heart Association; VAS, visual analogue scale.
aSI conversion factor: to convent urea nitrogen to mmol/L multiply by 0.357.
bSI conversion factor: to convert creatinine to μmol/L, multiply by 88.4.
cMLWHF questionnaire (0–105), with lower scores indicating better quality of life.
dGlobal health VAS (0–100) is scored so that higher scores indicate higher function. Scores were truncated at 72 due to non-linearity.
eBreathing VAS (0–100), with higher scores indicating higher function.
fDepression, MLWHF question 17 (0–5), 0 = no depression, 5 = very depressed.
Comparing the Full Code vs. DNR group, there was no difference in non-pulmonary artery catheterization procedures (P = 0.17 for comparison) or in mean number of major cardiovascular procedures per patient (P = 0.13). Of 26 patients who had a DNR order at 1 month, 19 patients also had a DNR order at baseline (‘DNR/DNR’) (Table 2). Six patients were Full Code at baseline and subsequently became DNR at 1 month (‘Full Code/DNR’). Among the ‘DNR/DNR’ cohort, there were two left heart catheterizations, two cardioversions, one percutaneous intervention, and one permanent pacemaker implantation. Among the ‘Full Code/DNR group’, there was one ICD implantation, one left heart catheterization, two cardioversions, one mechanical ventilation, and one permanent pacemaker implantation.
Table 2.
In-hospital procedures among patients with DNR orders at 1 month, stratified by baseline resuscitation status
| Procedure | DNR baseline/DNR 1 month (n = 19) | Full code baseline/DNR 1 month (n = 6) |
|---|---|---|
| ICD implantation, no. (%) | 0 (0) | 1 (17) |
| CABG, no. (%) | 0 (0) | 0 (0) |
| Left heart catheterization, no. (%) | 2 (11) | 1 (17) |
| Cardiopulmonary resuscitation, no. (%) | 0 (0) | 1 (17) |
| Cardioversion, no. (%) | 2 (11) | 2 (33) |
| Intra-aortic balloon pump, no. (%) | 0 (0) | 0 (0) |
| Left ventricular assist device, no. (%) | 0 (0) | 0 (0) |
| Mechanical ventilation, no. (%) | 0 (0) | 1 (17) |
| PCI, no. (%) | 1 (5) | 0 (0) |
| Permanent pacemaker, no. (%) | 1 (5) | 1 (17) |
CABG, coronary artery bypass graft; DNR, do not resuscitate; ICD, implantable cardiac defibrillator; PCI, percutaneous coronary intervention.
Missing data
One-month time-trade-off data were absent for 13 DNR patients (Table 3). For these patients, systolic blood pressure, 6 min walk distance, and global health assessment were lower, and length of stay was longer compared with DNR patients not missing time-trade-off data. Among Full Code patients, 70 patients had missing 1-month time-trade-off data. For these patients, 6 min walk distance was shorter than for Full Code patients not missing time-trade-off data.
Table 3.
Characterization of patients with missing time-trade-off data at 1 month, stratified by 1-month resuscitation orders
| Baseline factor | DNR, missing TTO (n = 13) | DNR, TTO available (n = 13) | Full Code, missing TTO (n = 70) | Full Code, TTO available (n = 279) |
|---|---|---|---|---|
| Age, median (IQR), years | 62 (54, 68) | 65 (53, 72) | 58 (49, 68) | 56 (46, 65) |
| Male gender, no. (%) | 10 (77) | 7 (54) | 49 (70) | 208 (75) |
| Minority (%) | 3 (23) | 3 (23) | 31 (44) | 110 (39) |
| Ischaemic aetiology of HF, no. (%) | 9 (69) | 10 (77) | 34 (49) | 133 (48) (n = 277) |
| Implantable defibrillator, no. (%) | 4 (31) | 1 (8) (n = 12) | 19 (27) | 82 (30) (n = 278) |
| Hypertension, no. (%) | 7 (54) | 10 (83) (n = 12) | 41 (59) | 120 (43) (n = 278) |
| Diabetes, no. (%) | 6 (46) | 4 (33) (n = 12) | 26 (37) | 87 (31) (n = 278) |
| Heart rate, median (IQR), b.p.m. | 81 (68, 95) | 78 (70, 87) (n = 12) | 83 (66, 90) | 81 (70, 92) (n = 274) |
| Systolic blood pressure, median, (IQR), mmHg | 98 (94, 102) | 120 (113, 131) n = 12 | 101 (90, 112) (n = 70) | 105 (94, 116) (n = 275) |
| LV ejection fraction, median (IQR), % | 20 (15, 23) (n = 12) | 20 (15, 28) (n = 12) | 20 (15, 22) (n = 69) | 20 (15, 25) (n = 273) |
| Urea nitrogen, median (IQR), mg/dLa | 35 (29, 47) | 40 (15, 55) (n = 12) | 31 (24, 46) (n = 69) | 27 (19, 40) (n = 276) |
| Creatinine, median (IQR) mg/dLb | 1.7 (1.5, 2.0) | 1.7 (0.9, 2.0) | 1.4 (1.1, 1.9) (n = 69) | 1.3 (1.0, 1.7) (n = 278) |
| ACE inhibitor, no. (%) | 11 (85) | 10 (77) | 53 (76) | 222 (80) |
| Beta-blocker, no. (%) | 6 (46) | 8 (62) | 39 (56) (n = 70) | 185 (67) (n = 278) |
| 6 min walk, median, ft | 0 (0, 138) (n = 11) | 25 (0, 378) (n = 12) | 192 (0, 590) (n = 63) | 418 (14, 840) (n = 253) |
| MLHF scorec | 74 (67, 92) (n = 13) | 74 (62, 83) (n = 13) | 77 (66, 85) (n = 67) | 76 (64, 87) (n = 273) |
| Patient global health VASd | 40 (30, 60) | 50 (30, 70) | 40 (25, 60) (n = 66) | 40 (30, 60) (n = 277) |
| Breathing VASe | 50 (45, 60) (n = 5) | 50 (40, 75) (n = 6) | 50 (40, 60) (n = 26) | 50 (30, 70) (n = 138) |
| Depression, no. (%)f | ||||
| 0 | 2 (67) | 2 (22) | 8 (25) | 53 (20) |
| 1 | 0 (0) | 1 (11) | 4 (13) | 45 (17) |
| 2 | 0 (0) | 0 (0) | 4 (13) | 50 (19) |
| 3 | 0 (0) | 2 (22) | 7 (22) | 51 (19) |
| 4 | 0 (0) | 0 (0) | 4 (13) | 30 (11) |
| 5 | 1 (33) (n = 3) | 4 (44) (n = 9) | 5 (16) (n = 32) | 39 (15) (n = 268) |
| Length of initial hospitalization, median (IQR), days | 14 (8, 20) | 7 (4, 11) | 8 (5, 12) (n = 69) | 6 (4, 9) (n = 278) |
| Inotrope or vasodilator on discharge, No. (%) | 2 (15) | 1 (8) | 6 (9) | 10 (4) |
ACE, angiotensin-converting enzyme; DNR, do not resuscitate; HF, heart failure; IQR, interquartile range; LV, left ventricular; MLWHF, Minnesota Living With Heart Failure; NYHA, New York Heart Association; TTO, time-trade-off; VAS, visual analogue scale.
aSI conversion factor: to convent urea nitrogen to mmol/L multiply by 0.357.
bSI conversion factor: to convert creatinine to μmol/L, multiply by 88.4.
cMLWHF questionnaire (0–105), with lower scores indicating better quality of life.
dGlobal health VAS (0–100) is scored so that higher scores indicate higher function. Scores were truncated at 72 due to non-linearity.
eBreathing VAS (0–100), with higher scores indicating higher function.
fDepression, MLWHF question 17 (0–5), 0 = no depression, 5 = very depressed.
Missing time-trade off data were not due to loss to follow-up. At 1 month, there was only one patient lost to follow-up, and this occurred in the Full Code group. However, for DNR and Full Code patients with missing time-trade-off data, the mode of contact for 1-month follow-up was more frequently via telephone call when compared with DNR and Full Code patients not missing data.
Unadjusted outcomes by resuscitation preference (Table 4)
Table 4.
Six-month unadjusted outcomes according to resuscitation orders at 1 month follow-up
| Outcome | DNR group (n = 26) | CPR group (n = 349) | P-value |
|---|---|---|---|
| Time-trade-off at 1 month, months willing to tradea | 12 (0, 24) (n = 13) | 1 (0, 6) (n = 279) | 0.03 |
| Post-1 month rehospitalization, no. (%) | 7 (41) (n = 17) | 149 (52) (n = 284) | 0.79* |
| Post-1-month death, no. (%) | 12 (46) (n = 26) | 43 (12) (n = 349) | <0.0001* |
CPR, cardiopulmonary resuscitation; DNR, do not resuscitate.
aTime-trade-off (0–24) indicates months of survival time in current health that patient is willing to ‘trade’ for excellent health.
*P-value from log-rank test.
DNR patients had a median willingness to trade 12 of 24 months [IQR (0, 24)] of theoretical survival time compared with 1 month [IQR (0, 6), P = 0.03] for Full Code patients. DNR patients did not differ in 6-month rehospitalization rate (P = 0.79, log-rank test), but had a higher 6-month mortality (46% vs. 12%, P <0.0001, log-rank test).
Survival preference (time-trade-off) model (adjusted)
At 1 month, time-trade-off data were available for 13 of 26 DNR status patients and 279 of 349 Full Code patients (Figure 1). Seven of 13 (54%) DNR patients expressed a desire for ‘half time-trade-off’ (willingness to trade ≥12 months of 24 month survival) compared with 60 of 279 (22%) Full Code patients (P = 0.007, χ2). Covariates that were also predictive of ‘half time-trade-off’ in univariate analysis were: quality of life (MLWHF), global symptom score, and depression (MLWHF, question 17). Factors that were not predictive included in-hospital resuscitation, discharge BNP, total hospitalizations (previous 6 months), discharge sodium and BUN, 6 min walk distance (discharge), age, and beta-blocker (discharge). In multivariate analysis, DNR preference, quality of life (MLWHF), and global symptom score were all predictive of ‘half time-trade-off’ but not depression (Table 5). The overall model was moderately discriminating for ‘half time-trade-off’, with a c-statistic of 0.769. After quality of life, DNR preference was the second most explanatory predictor variable and was associated with 10-fold higher odds of ‘half time-trade-off’ [odds ratio (OR) 10.33, 95% CI 1.65–64.80].
Table 5.
Logistic regression model for predicting half time-trade-off at 1-month follow-up
| Factor | Odds ratio | 95% Wald CI | Wald χ2 | P-value |
|---|---|---|---|---|
| DNR preference at 1 month | 10.33 | 1.65–64.80 | 6.21 | 0.01 |
| Quality of life at 1 month (MLWHF), each pointa | 1.04 | 1.02–1.05 | 17.08 | <0.0001 |
| Patient global health VAS, each pointb | 0.98 | 0.96–0.99 | 4.55 | 0.03 |
C-Index = 0.769, n = 277, events = 65.
Model selected by forward and backward stepwise selection from full candidate set which included the above, along with depression (MLWHF question17 at 1 month).
DNR, do not resuscitate, MLWHF, Minnesota Living With Heart Failure; VAS, visual analogue scale.
aMLHF questionnaire (0–105), with lower scores indicating better quality of life.
bGlobal health VAS (0–100), with higher scores indicating higher function. Scores were truncated at 72 due to non-linearity.
Mortality model (adjusted)
In a multivariate model, DNR preference, BNP, occurrence of in-hospital CPR, discharge BUN, and discharge serum sodium were associated with 6-month mortality (Table 6). DNR preference was the most explanatory variable [χ2 26.12, P < 0.0001, hazard ratio (HR) 6.88, 95% CI 3.28–14.41].
Table 6.
Proportional hazards model for predicting 6-month mortalitya
| Factor | χ2 | P-value | HR | HR 95% CI |
|---|---|---|---|---|
| DNR preference at 1 month | 26.12 | <.0001 | 6.88 | 3.28–14.41 |
| Log of BNP (per doubling) at discharge | 6.56 | 0.01 | 1.28 | 1.06–1.54 |
| BUN at discharge | 3.03 | 0.082 | 1.23 | 0.97–1.55 |
| Serum sodium at discharge | 1.48 | 0.22 | 0.96 | 0.91–1.02 |
| In-hospital cardiopulmonary resuscitation | 5.48 | 0.019 | 3.15 | 1.21–8.24 |
C-Index = 0.743, n = 228, events = 42.
BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CI, confidence interval; DNR, do not resuscitate; HR, hazard ratio.
aDeaths prior to 1 month are censored.
Discussion
There were several important findings from our study. First, patients' DNR preferences were associated with lower preferences for survival, i.e. lower time-trade-off utilities. While this finding may seem intuitive, it expands the possible significance of a patient's stated DNR preference. DNR preference may be interpreted widely by healthcare providers, from simply an advance care directive in the event of a cardiopulmonary arrest to preferences for end-of-life care directed at relieving symptoms. In our analysis it appears that DNR preferences may reflect a willingness to trade longevity for better perceived health. The second important finding was that DNR preference was highly predictive of 6-month mortality despite adjustment for previously validated mortality predictors including BNP.
This is the first study of a HF-specific cohort to link HF patients' DNR preferences directly with preferences for improved quality of life over extended survival. Our findings are congruent to those of the SUPPORT study which was comprised of a mixed cohort of critically ill patients (∼1/3 acute HF diagnosis).17 In SUPPORT, patients who expressed a DNR preference reported greater preferences for quality of life as measured by time-trade-off utilities. Other studies have measured time-trade-off in HF patients. Stevenson et al., in a previous analysis of the ESCAPE trial, reported a largely dichotomous distribution of time-trade-off in which most HF patients were either willing to trade almost none or almost all of their remaining survival time to feel better.5 Other factors which have been previously associated with greater patient preferences for quality of life include: Duke Activity status, exercise capacity (6 min walk distance, peak oxygen consumption), global health status, NYHA class, quality of life, depression, dyspnoea, and elevation of jugular venous pressure.5,18,19
To our knowledge, this is the first study to report in a HF cohort that a patient preference had more prognostic value for mortality than markers of disease severity. Previous studies in non-HF populations have demonstrated higher mortality in patients with expressed DNR preferences or a written DNR order.19 This higher mortality might be partially explained by disease severity; Medicare patients who received DNR orders have more co-morbidities, older age, non-black race, and more functional impairment.11,20 Similarly, in our study, DNR patients were older, had more co-morbidities, poorer exercise capacity, longer index hospitalization, and tended to be non-black. Previous studies have also reported that minority patients have preferences for more intensive end-of-life care and have lower preferences for DNR status.21 However, adjustment for disease severity did not fully explain the higher mortality of DNR patients in our study and in others. In the SUPPORT study, hospitalized patients with a DNR preference had nearly two-fold higher mortality despite adjustment for patient prognosis and other characteristics.7 Further, in our analysis, the excess hazard for mortality for the DNR patients was found despite adjustment for all multivariate mortality predictors [discharge BNP (per doubling), in-hospital cardiac resuscitation, discharge BUN, and discharge sodium] from a previous analysis of the ESCAPE trial.15
Besides higher disease severity, DNR orders have been associated with disparities in evidence-based HF care which might explain higher mortality in these patients. In a large Worcester, MA population cohort, HF patients with in-hospital DNR orders received less intensive care with fewer quality measures such as assessment of left ventricular function, angiotensin-converting enzyme inhibitor/ angiotensin receptor blocker use, and non-pharmacological counselling.11 Unlike the Worcester study, DNR and Full Code patients in our study had no differences in evidence-based HF medication use, ICD therapy, and major cardiac procedures; yet, in our study, the adjusted HR for death in DNR patients was nearly seven-fold greater than for Full Code patients. Our findings regarding DNR patients' preferences for quality of life might explain higher mortality rates and disparities in care that have been reported in the literature, i.e. patients' underlying care preferences may be directing their physicians to deliver care that is aimed primarily at symptom control. In addition, DNR status may be an independent marker of disease severity that is not explained by other conventional HF prognostic factors. It is also possible that DNR status is a marker of depression or a ‘death wish’, though we found no evidence that DNR patients in our study were more depressed than Full Code patients.
What is the potential impact of this research on clinicians who care for HF patients? We propose that a patient's DNR preference may be a clue regarding his or her preferences for life-sustaining interventions and thus could serve as a stepping stone to a more comprehensive discussion about goals of care. As a corollary, designations of ‘Full Code’ should be carefully reviewed with patients who express preferences for improving quality of life more than survival. While some patients may be amenable to frank discussions about end-of-life care, we know that such discussions between physicians and patients are rare.9 From the standpoint of new therapy development, it is ironic that the patients most likely to contribute mortality endpoints to a trial in advanced HF may be those to whom mortality is less important than the impact of a therapy to improve quality of life.
Patient preferences are often assumed incorrectly by physicians,10 who then base their recommendations on these assumptions, rather than on direct elicitation and application of those preferences. Therefore, physicians caring for advanced HF patients need an understanding of factors that may identify patients for whom extension of life is not the priority. Such patients might benefit from referral to hospice or palliative therapies such as intravenous inotropes which can improve symptoms but shorten survival.22 Conversely, patients who prefer CPR or express other preferences for extended survival might be appropriate candidates for high-burden therapies such as mechanical circulatory support or heart transplantation. It is important to caution that a patient's expressed preference to forgo resuscitation is not synonymous with a desire to forgo life-prolonging treatment; however, our findings do suggest that the two preferences may be closely related.
There are several limitations in our study. First, the sample size was modest. However, the event rate was high, reflecting the mortality risk and co-morbidities in the study population. Further, the strength of the associations we observed was strong. Secondly, we do not have specific information on how study co-ordinators across different study sites ascertained resuscitation orders; we do not know whether the co-ordinators performed chart abstraction or conducted patient interviews. Though site-to-site variability in assessment of resuscitation orders could impact the validity and precision of such assessments, we have no reason to believe that Full Code and DNR patients were assessed differently, thus we do not anticipate any impact on the results and conclusions. We do know that these data were prospectively collected in ‘real-time’ by study co-ordinators who were working closely with bedside clinicians. Thirdly, there were missing 1-month time-trade-off data on 13 of 26 DNR patients; however, none of the 13 patients was actually lost to follow-up. Further, only 1 of the 13 DNR patients with missing data died prior to 1 month follow-up, suggesting that the missing time-trade-off data were not attributable to excess mortality. However, it does appear that both the DNR patients, and to a lesser extent the Full Code patients, with missing time-trade-off data may have had greater illness severity than those patients not missing data. Thus, we speculate that our findings may have actually underestimated the association of DNR orders with higher mortality. Fourthly, our ability to understand dynamic patient preferences is limited by the cross-sectional nature of our analysis; however, we believe our choice to study time-trade-off preferences at the 1-month time point was supported by the findings of a previous ESCAPE substudy.5 Fifthly, in the context of a clinical trial of advanced HF therapy that included randomization to a PAC, it is unclear if the patients had similar characteristics to those in unselected, community-based HF populations. We suspect that the low prevalence of DNR status patients in our study (7%) compared with 23% reported among HF patients in the SUPPORT study reflects the differences in study populations, era (the SUPPORT study was in the 1990s), and timing of ascertainment of resuscitation status. In SUPPORT, 40% of hospitalized HF patients preferring DNR status subsequently changed their resuscitation preference 2 months after discharge.10 Still, an advantage of this study is that patients all had rigorously defined ‘advanced HF’ and were well characterized with respect to both clinical characteristics and survival preferences.
In summary, HF patients' DNR preferences strongly predicted preferences for better perceived health over prolongation of life and identified patients at higher risk of early mortality. By soliciting and responding to this commonly assessed preference in combination with measures of quality of life, we hope that clinicians can develop a clearer understanding of HF patients' preferences in order to deliver more patient-centred care. No short-hand designation will ever substitute for direct communication with patients. However, we believe that our findings, if confirmed, might encourage HF physicians to expand upon resuscitation questions and to assist patients in medical decision-making that is increasingly complex as multiple options are available to alter the quality of life and timing of death.
Funding
National Heart, Lung, and Blood Institute (N01-HV-98177); Duke Clinical Research Institute, Durham, NC, USA.
Conflict of interest: none declared.
Authors' contributions: S.D. and R.M.C. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
The authors wish to thank Linda Shaw, MS, and Karen Pieper, MS, for statistical assistance. This work was supported in part by the Department of Veterans Affairs. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Committee on Quality of Health Care in America IoM. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. [PubMed] [Google Scholar]
- 2.Adams K, Lindenfeld J, Arnold JM, Baker D, Barnard DH, Baughman KL, Boehmer JP, Deedwania P, Dunbar SB, Elkayam U, Gheorghiade M, Howlett JG, Konstam MA, Kronenberg MW, Massie BM, Mehra M, Miller AB, Moser DK, Patterson JK, Rodeheffer RJ, Sackner-Bernstein J, Silver MA, Starling RC, Stevenson LW, Wagoner LE. HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:e1–e119. [Google Scholar]
- 3.Fried T, O'Leary J. Using the experiences of bereaved caregivers to inform patient- and caregiver-centered advance care planning. J Gen Intern Med. 2008;23:1602–1607. doi: 10.1007/s11606-008-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson LW, Hellkamp AS, Leier CV, Sopko G, Koelling T, Warnica JW, Abraham WT, Kasper EK, Rogers JG, Califf RM, Schramm EE, O'Connor CM. Changing preferences for survival after hospitalization with advanced heart failure. J Am Coll Cardiol. 2008;52:1702–1708. doi: 10.1016/j.jacc.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulmasy DP, Terry PB, Weisman CS, Miller DJ, Stallings RY, Vettese MA, Haller KB. The accuracy of substituted judgments in patients with terminal diagnoses. Ann Intern Med. 1998;128:621–629. doi: 10.7326/0003-4819-128-8-199804150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Phillips RS, Wenger NS, Teno J, Oye RK, Youngner S, Califf R, Layde P, Desbiens N, Connors AF, Lynn J. Choices of seriously ill patients about cardiopulmonary resuscitation: correlates and outcomes. Am J Med. 1996;100:128–137. doi: 10.1016/s0002-9343(97)89450-8. [DOI] [PubMed] [Google Scholar]
- 8.Arnold J, Liu P, Demers C, Dorian P, Giannetti N, Haddad H, Heckman G, Howlett J, Ignaszewski A, Johnstone D, Jong P, McKelvie R, Moe G, Parker J, Rao V, Ross H, Sequeira E, Svendsen A, Teo K, Tsuyuki R, White M. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: diagnosis and management. Can J Cardiol. 2006;22:23–45. doi: 10.1016/s0828-282x(06)70237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heffner JE, Barbieri C. End-of-life care preferences of patients enrolled in cardiovascular rehabilitation programs. Chest. 2000;117:1474–1481. doi: 10.1378/chest.117.5.1474. [DOI] [PubMed] [Google Scholar]
- 10.Krumholz HM, Phillips RS, Hamel MB, Teno JM, Bellamy P, Broste SK, Califf RM, Vidaillet H, Davis RB, Muhlbaier LH, Connors AF, Jr, Lynn J, Goldman L. Resuscitation preferences among patients with severe congestive heart failure: results from the SUPPORT project. Circulation. 1998;98:648–655. doi: 10.1161/01.cir.98.7.648. [DOI] [PubMed] [Google Scholar]
- 11.Chen JLT, Sosnov J, Lessard D, Goldberg RJ. Impact of do-not-resuscitation orders on quality of care performance measures in patients hospitalized with acute heart failure. Am Heart J. 2008;156:78–84. doi: 10.1016/j.ahj.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsevat J, Dawson NV, Wu AW, Lynn J, Soukup JR, Cook EF, Vidaillet H, Phillips RS for the HI. Health values of hospitalized patients 80 years or older. JAMA. 1998;279:371–375. doi: 10.1001/jama.279.5.371. [DOI] [PubMed] [Google Scholar]
- 13.The EIaESC. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 14.Sanders A, Schepp M, Baird M. Partial do-not-resuscitate orders: a hazard to patient safety and clinical outcomes? Crit Care Med. 2011;39:14–18. doi: 10.1097/CCM.0b013e3181feb8f6. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55:872–878. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah MR, O'Connor CM, Sopko G, Hasselblad V, Califf RM, Stevenson LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness (ESCAPE): design and rationale. Am Heart J. 2001;141:528–535. doi: 10.1067/mhj.2001.113995. [DOI] [PubMed] [Google Scholar]
- 17.Tsevat J, Cook EF, Green ML, Matchar DB, Dawson NV, Broste SK, Wu AW, Phillips RS, Oye RK, Goldman L. Health values of the seriously ill. Ann Intern Med. 1995;122:514–520. doi: 10.7326/0003-4819-122-7-199504010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Havranek EP, McGovern KM, Weinberger J, Brocato A, Lowes BD, Abraham WT. Patient preferences for heart failure treatment: utilities are valid measures of health-related quality of life in heart failure. J Card Fail. 1999;5:85–91. doi: 10.1016/s1071-9164(99)90030-1. [DOI] [PubMed] [Google Scholar]
- 19.Havranek EP, Simon TA, L'Italien G, Smitten A, Brett Hauber A, Chen R, Lapuerta P. The relationship between health perception and utility in heart failure patients in a clinical trial: results from an OVERTURE substudy. J Card Fail. 2004;10:339–343. doi: 10.1016/j.cardfail.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Wenger NS, Pearson ML, Desmond KA, Harrison ER, Rubenstein LV, Rogers WH, Kahn KL. Epidemiology of do-not-resuscitate orders. Disparity by age, diagnosis, gender, race, and functional impairment. Arch Intern Med. 1995;155:2056–2062. [PubMed] [Google Scholar]
- 21.Barnato A, Anthony D, Skinner J, Gallagher P, Fisher E. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med. 2009;24:695–701. doi: 10.1007/s11606-009-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felker GM, O'Connor CM. Inotropic therapy for heart failure: an evidence-based approach. Am Heart J. 2001;142:393–401. doi: 10.1067/mhj.2001.117606. [DOI] [PubMed] [Google Scholar]