Abstract
(See the editorial commentary by Grijalva and Griffin on pages 355–7.)
Background. Infection with influenza virus increases the risk for developing pneumococcal disease. The A/H1N1 influenza pandemic in autumn 2009 provided a unique opportunity to evaluate this relationship.
Methods. Using weekly age-, state-, and cause-specific hospitalizations from the US State Inpatient Databases of the Healthcare Cost and Utilization Project 2003–2009, we quantified the increase in pneumococcal pneumonia hospitalization rates above a seasonal baseline during the pandemic period.
Results. We found a significant increase in pneumococcal hospitalizations from late August to mid-December 2009, which corresponded to the timing of highest pandemic influenza activity. Individuals aged 5–19 years, who have a low baseline level of pneumococcal disease, experienced the largest relative increase in pneumococcal hospitalizations (ratio, 1.6 [95% confidence interval {CI}, 1.4–1.7]), whereas the largest absolute increase was observed among individuals aged 40–64 years. In contrast, there was no excess disease in the elderly. Geographical variation in the timing of excess pneumococcal hospitalizations matched geographical patterns for the fall pandemic influenza wave.
Conclusions. The 2009 influenza pandemic had a significant impact on the rate of pneumococcal pneumonia hospitalizations, with the magnitude of this effect varying between age groups and states, mirroring observed variations in influenza activity.
Clinicians have long recognized that influenza infections increase the risk for developing secondary bacterial disease, particularly with Streptococcus pneumoniae (pneumococcus) [1]. Numerous epidemiological studies, including reports from the 1918 influenza pandemic, have provided evidence for this relationship during both pandemic and interpandemic periods [1–21], and this association has recently been strengthened by experimental findings [22].
In a typical interpandemic season, influenza virus activity sharply spikes in the winter and early spring, with the timing and severity of epidemics varying between years. In contrast, pneumococcal disease has a broader winter peak that does not change substantially from year to year [23], although it does exhibit increased incidence during the influenza period. A recent study estimates that, on average, 4.5%–6% of invasive pneumococcal pneumonia can be attributed to influenza [6]. Given that the incidence of disease caused by both of these pathogens typically peaks in midwinter, it can be difficult to isolate the effect of influenza on pneumococcus from the effects of other viral agents, such as respiratory syncytial virus [4], environmental conditions [23], or increased contacts around the holidays [24].
Influenza activity increased substantially during the autumn of 2009 in the United States due to the emergence of a novel influenza A/H1N1 pandemic virus [25] with high infection rates among school-aged children. There was considerable variability in the timing of this autumn wave, and some regions also experienced a first wave in the early summer. This unusual temporal pattern for influenza activity provides a unique opportunity to observe the interaction between influenza and pneumococcal disease in the absence of other seasonal factors. Given the large increase in influenza activity in autumn, we expected that there would be a corresponding increase in pneumococcal disease incidence among the affected age groups. To further understand the relationship between these pathogens and to help inform planning for future pandemics, we sought to quantify the population-wide impacts of the 2009 influenza pandemic on the age-specific incidence of pneumococcal disease hospitalizations across the United States.
METHODS
Data Sources and Extraction
We obtained weekly hospitalization data from the State Inpatient Databases of the Healthcare Cost and Utilization Project, maintained by the Agency for Healthcare Research and Quality, through an active collaboration. This database contains all hospital discharge records from community hospitals in participating states [26]; we focused on the period 2003–2009 to include the pandemic period in 2009 and have enough historical years to create a baseline for pneumococcus, while excluding years immediately following the introduction of pneumococcal conjugate vaccine (PCV7) in the United States. Admissions after week 50 in 2009 were excluded from the analyses because the discharge data were incomplete for these weeks. Cases were identified by the presence of the relevant diagnostic codes listed anywhere in the patient’s record, including pneumococcal pneumonia (International Classification of Diseases, Ninth Revision [ICD-9], code 481), pneumococcal septicemia (code 038.2), or influenza (codes 487–488). We also considered a control bacterial outcome that should not be associated with influenza activity, septicemia caused by Escherichia coli (code 38.42).
Weekly time series were created based on date of hospital admission for each disease outcome and age category (0–4, 5–19, 20–39, 40–64, and ≥65 years). Midyear population size estimates for each state and age group were obtained from the US Census Bureau. For these analyses, we used data from 30 states for which we had available data for 2003–2009 at the time of writing (Arizona, California, Colorado, Georgia, Iowa, Illinois, Kansas, Kentucky, Massachusetts, Maryland, Maine, Michigan, Minnesota, Missouri, North Carolina, Nebraska, New Jersey, Nevada, Ohio, Oregon, Rhode Island, South Carolina, South Dakota, Tennessee, Utah, Virginia, Vermont, Washington, Wisconsin, and West Virginia), covering a population of approximately 190 million individuals. All analyses were performed with SAS software, version 9.2 (SAS Institute, Cary, North Carolina).
Calculation of Excess Pneumococcal Pneumonia Hospitalization Rates
We calculated the excess pneumococcal pneumonia hospitalization incidence using 2 complementary approaches. In the first approach, we set a weekly seasonal baseline for pneumococcal hospitalizations using the prepandemic period 2003–2008 and measured increases above this baseline during the fall of 2009 (late August to mid-December). This approach allows simple comparisons of the observed weekly incidence in 2009 with the average weekly incidence from the previous 6 years. In the second approach, we used a regression model in which weekly influenza hospitalizations were explicitly included as a covariate, which allowed us to evaluate whether increases in pneumococcal pneumonia were associated with increases in influenza incidence and also allows comparisons between pandemic and epidemic seasons.
For the first approach, we set the weekly baseline for the period July 2003 to week 14 of 2009 using Poisson regression: Y/N = exp(b0 + bi*weeki), where Y/N is the weekly incidence of pneumococcal pneumonia, pneumococcal septicemia, or E. coli septicemia hospitalizations (per 100 000 population) and weeki is a dummy variable for week of the year (i = 1, 2,.. 52). The baseline was calculated separately for each age group, and the 95% prediction interval was calculated with adjustments for overdispersion of the count data, as described elsewhere [27]. Weekly excess disease was calculated as the difference between observed and baseline for weeks in which the incidence exceeded the upper limit of the prediction interval for 2 consecutive weeks. The total excess for the fall of 2009 was the sum of the weekly excess estimates for weeks 34–50 when pandemic activity was most intense.
For the second approach, we calculated the incidence of pneumococcal disease attributable to influenza in each July–June respiratory year by fitting a Poisson regression model to the period week 26 of 2003 to week 50 in December 2009 [6, 28]. Specifically, the model was:
where Y/N is the weekly incidence of pneumococcal pneumonia, time is a running index for time weeki is a dummy variable for week of the year, pandemic period is a dummy variable for weeks 15–50 in 2009, and influenza is the weekly rate of influenza hospitalizations (unlagged) for each state. The interaction term allows the effect of influenza to change during the pandemic period to account for artifactual increases in influenza-specific coding. Akaike information criteria were used for model fitting—the above model was superior to a model that did not include influenza terms or to a model that used sine and cosine terms in place of the weeki dummy variable.
The incidence of pneumococcal disease associated with influenza was calculated as the difference between the predicted incidence from the full model and the predicted incidence when the influenza and pandemic period variables were set to 0. To estimate the error for these estimates, we fit the model to 1000 bootstrapped data sets (8-week overlapping blocks sampled with replacement within each age category) [29, 30]; the median and 2.5th and 97.5th percentiles of the bootstrapped estimates are presented.
Timing of the Fall Wave of the 2009 Pandemic
We created weekly time series for pneumococcal pneumonia and influenza (among 5–64-year-olds, the age group most affected by the pandemic) for each state and created a pneumococcal pneumonia baseline for each state as described above. Excess cases of pneumococcal pneumonia were calculated as observed minus baseline for each week. We then determined the timing of influenza incidence and excess pneumococcal pneumonia incidence in the 2009 pandemic period in each state by calculating the “center of gravity,” defined as the average of the week number (34–50) weighted by the number of influenza hospitalizations or excess pneumococcal pneumonia hospitalizations in that week [31, 32]. This provides estimates of the midpoint of the pandemic and pneumococcus waves, which are less prone to stochastic noise than measures of peak timing (Supplementary Figure 1).
RESULTS
The baseline rates of pneumococcal pneumonia hospitalizations in our database varied between age groups with the highest rates in the elderly and the lowest rates among 5–19-year-olds (Table 1). These age patterns are consistent with those reported elsewhere for the post–conjugate vaccine period in the United States [33].
Table 1.
Pneumococcal Pneumonia Hospitalizations Attributable to Influenza, by Age and Season, US.
| Age Group |
|||||
| <5 years | 5–19 years | 20–39 years | 40–64 years | ≥65 years | |
| Total pneumococcal pneumonia hospitalizations/100 000a | 10.97 (10.56–12.15) | 2.80 (2.66–3.18) | 5.49 (4.82–5.90) | 20.46 (19.19–22.01) | 73.34 (68.14–78.83) |
| Pneumococcal pneumonia hospitalizations/100 000 attributed to influenzab | |||||
| Calculated from baseline method | |||||
| Autumn 2009 | 0.00 (−.40 to .40) | 0.47 (.30–.63) | 0.52 (.34–.69) | 1.25 (.88–1.62) | 0.00 (−2.14 to 2.14) |
| Calculated from regression model | |||||
| Autumn 2009 | 0.04 (−.68 to .88) | 0.46 (−.03 to 1.50) | 0.47 (−.03 to 1.61) | 0.92 (−.10 to 3.10) | −0.80 (−4.75 to 1.01) |
| 2003–2004 | 0.72 (−.02 to 2.96) | 0.24 (.00–.75) | 0.69 (.01–2.08) | 1.81 (.04–5.76) | 7.24 (.13–23.89) |
| 2004–2005 | 0.42 (−.16 to 1.66) | 0.15 (.00–.54) | 0.42 (.01–1.30) | 1.17 (.04–3.61) | 4.35 (.12–14.75) |
| 2005–2006 | 0.31 (−.15 to 1.12) | 0.12 (.00–.36) | 0.31 (.02–.83) | 0.85 (.05–2.16) | 3.04 (.11–8.28) |
| 2006–2007 | 0.19 (−.04 to .67) | 0.07 (.00–.21) | 0.18 (.02–.47) | 0.49 (.04–1.28) | 1.89 (.17–4.84) |
| 2007–2008 | 0.53 (−.21 to 1.82) | 0.21 (.01–.64) | 0.56 (.02–1.73) | 1.47 (.06–4.08) | 5.01 (.25–14.46) |
| 2008–2009 | 0.07 (−.74 to .60) | 0.14 (.00–.51) | 0.18 (−.12 to .55) | 0.62 (−.16 to 1.67) | 1.34 (−1.13 to 5.36) |
| Estimated excess pneumococcal pneumonia cases for entire United States in Autumn 2009 | |||||
| Calculated from baseline method | 0 (0–85) | 289 (189–390) | 432 (288–577) | 1255 (879–1630) | 0 (0–845) |
| Calculated from regression model | 8 (0–187) | 286 (0–932) | 393 (0–1347) | 923 (0–3112) | 0 (0–399) |
Mean pneumococcal pneumonia hospitalizations per 100 000 population for each July–June period from 2003/2004 to 2008/2009. Numbers in parentheses indicate range across seasons.
Excess pneumococcal pneumonia hospitalizations per 100 000 attributable to influenza. Numbers in parentheses indicate the 95% confidence interval.
Magnitude of the Increase in Pneumococcal Pneumonia Hospitalizations During the 2009 Pandemic
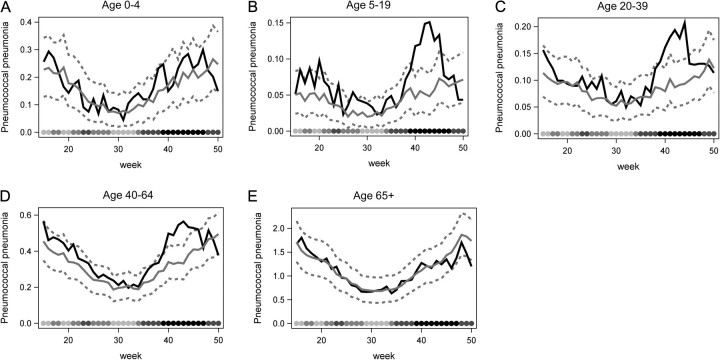
Significant increases in weekly rates of hospitalization for pneumococcal pneumonia were evident among individuals aged >5 years and <65 years during the autumn influenza wave in 2009, compared with a seasonal baseline generated from the previous 6 years (Figure 1, Table 1). No detectable increase among young children or the elderly was observed (Figure 1, Table 1), consistent with these age groups being largely spared from the 2009 influenza pandemic (Supplementary Figure 1). Following these increases in pneumococcal pneumonia in the autumn, the incidence rates declined to baseline levels, or in some instances below baseline levels, by December 2009, consistent with the timing of the retreat of pandemic activity (Figure 1). We also evaluated changes in the rate of hospitalizations for pneumococcal septicemia (which is not mutually exclusive with pneumococcal pneumonia) and found increases similar to those seen for pneumococcal pneumonia (Supplementary Figure 2).
Figure 1.
Weekly pneumococcal pneumonia hospitalizations per 100 000 in 2009 (black solid line) compared with 2003–2008 baseline 95% prediction interval (gray solid and dashed lines) for age groups (A) 0–4, (B) 5–19, (C) 20–39, (D) 40–64, and (E) ≥65 years. Weeks 15–50 (April–December) of the 2009 calendar year are shown, with the baseline calculated from the simple empirical approach. The shaded circles on the bottom of each panel indicate the number of influenza hospitalization in each week across all states and age groups, with darker shades indicating more hospitalizations.
In terms of the relative magnitude of excess pneumococcal pneumonia hospitalizations in autumn 2009, the 5–19-year-old age group exhibited the largest increase with a 3-fold spike over baseline at week 43 (late October). This age group had the lowest baseline incidence of pneumococcal pneumonia hospitalizations of any age group (Table 1). Across the entire autumn pandemic period (late August to mid-December), the rate of pneumococcal pneumonia hospitalization in this age group was elevated by 1.6-fold above baseline (95% confidence interval [CI], 1.4–1.7). Less pronounced increases were observed among young and middle-aged adults, with a 1.4-fold increase (95% CI, 1.3–1.5) among the 20–39-year-olds and a 1.2-fold increase among the 40–64-year-olds (95% CI, 1.2–1.3). In contrast, children <5 years (ratio, 1.1 [95% CI, 1.0–1.2]) and seniors (ratio, 0.9 [95% CI, .9–1.0]) did not exhibit a significant autumn elevation of pneumococcal pneumonia.
In absolute terms, the 40–64-year-old population had the greatest increase above baseline during the pandemic (Table 1), with an excess of 1.25 (95% CI, .88–1.62) pneumococcal pneumonia hospitalizations per 100 000 population (Table 1). Younger age groups (with lower baseline rates) experienced a moderate excess of 0.47 (95% CI, .30–.63) hospitalizations per 100 000 in 5–19-year-olds, and 0.52 (95% CI, .34–.69) hospitalizations per 100 000 in 20–39-year-olds. In total, we estimate that there was an excess of 1976 95% (95% CI, 1031–2921) pneumococcal pneumonia hospitalizations across all age groups in the United States during the pandemic period (Table 1).
Ruling out Possible Confounders
We also considered whether the apparent increase in pneumococcal disease could simply have been explained by an increase in testing for bacteremia during the pandemic period. To evaluate this possibility, we examined the incidence of E. coli septicemia, which should not be affected by influenza. While there were short-term increases of E. coli septicemia throughout the year, these spikes did not become more frequent during the pandemic period, and there were no sustained increases of ≥2 weeks that suggest systematic testing biases (Supplementary Figure 3). In contrast, pneumococcal septicemia demonstrated sustained increases during the pandemic period. This supports our conclusion that the increase in pneumococcal disease was not an artifact of changes in testing during the pandemic.
Exploring Geographical Heterogeneity in the Timing of the Autumn Pandemic Wave
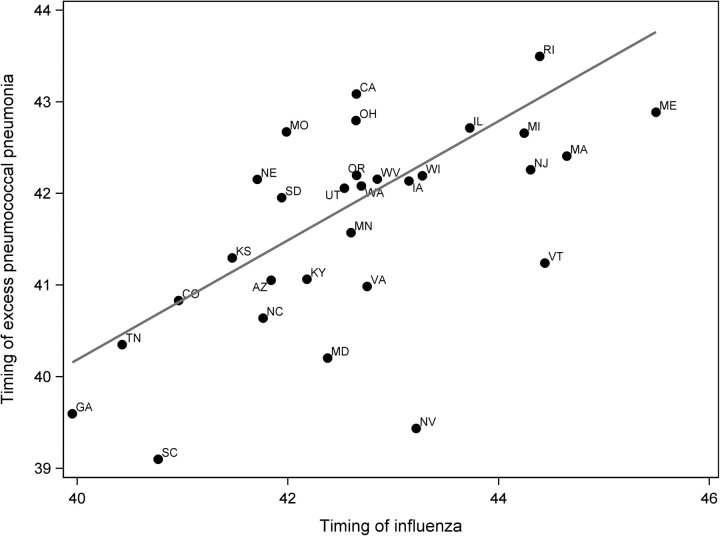
We next evaluated whether the pronounced heterogeneity in the timing of the autumn pandemic wave across the United States affected the timing of the increase of pneumococcal pneumonia hospitalizations above baseline. The timing of the increase in pneumococcal pneumonia in the autumn did vary with the timing of the influenza peak, with later pneumococcal pneumonia excesses occurring in states with later influenza peaks (Figure 2, correlation between timing of peak influenza and excess pneumococcal activity, R2 = 0.43; P < .001), further supporting the link between these 2 diseases.
Figure 2.
Timing of influenza compared with the timing of excess pneumococcal pneumonia in fall of 2009 among 5–64-year-olds. Timing is defined as the average of the week number (34–50 of 2009) weighted by the number of influenza hospitalizations or excess pneumococcal pneumonia hospitalizations in that week. Excess pneumococcal pneumonia hospitalizations for each state were calculated using the simple empirical baseline method. R2 = 0.43 from a linear regression weighted by population size of each state (P < .001).
Comparison of the Pneumococcal Pneumonia Increase in 2009 With Those Seen During Recent Influenza Seasons
We next sought to compare the increases in pneumococcal pneumonia hospitalizations in the autumn period of 2009 (late August to mid-December) with the increases seen during a typical influenza season. To do this, we fit a regression model that included influenza hospitalizations as a covariate and controlled for baseline seasonal variations (Supplementary Figure 4). We found that among the 5–19-year-olds, the influenza-attributable increase in pneumococcal pneumonia rates in the autumn of 2009 was higher than in any of the previous 6 influenza seasons (Table 1). Among those aged 20–40 and 40–64 years, however, the influenza-attributable increases in 2009 were comparable in magnitude to those observed for the 2007–2008 influenza season and the severe A/H3N2/Fujian influenza season of 2003–2004. For the entire baseline period, 4.5%–7.7% of pneumococcal pneumonia hospitalizations were attributable to influenza (varying between age groups). The model estimates of excess pneumococcal pneumonia hospitalizations attributable to influenza during the pandemic period were comparable to the estimates generated by the cruder prepandemic baseline approach (Table 1).
DISCUSSION
We have demonstrated that the 2009 H1N1 influenza pandemic period was associated with a significant increase in pneumococcal pneumonia hospitalizations among older children and young to middle-aged adults, but there was no such increase among persons over the age of 65, consistent with the pandemic sparing of seniors (Supplementary Figure 1). The magnitude of the increase in disease incidence among the middle-aged adults was comparable to what has been seen in other recent severe seasonal influenza seasons. However, there was an unusual increase among the 5–19-year-old population, which has relatively low baseline levels of pneumococcal disease. Variations between states in the timing of pneumococcal pneumonia increases and variations between age groups in the magnitude of these increases matched the observed variation in influenza pandemic dynamics, further reinforcing the interaction between these 2 pathogens.
For the 20–39 and 40–64-year age groups, the magnitude of the increase in pneumococcal pneumonia during the pandemic was comparable to that seen during the most severe influenza epidemic in recent years, the influenza A/H3N2/Fujian-dominated 2003–2004 season [34]. This suggests that although the timing of the 2009 pandemic was unusual and the age distribution of influenza cases was unusually shifted toward children and middle-aged adults [35], the effect on bacterial disease was not substantially worse in this age group than in the most severe interpandemic influenza seasons. By contrast, the 5–19-year-olds, who typically have a low baseline incidence of pneumococcal pneumonia hospitalizations, experienced 2–3 times more excess hospitalizations during the pandemic period compared with any of the previous 6 influenza seasons. This unusual increase in pneumococcal pneumonia incidence in school-aged children could be explained by the high influenza attack rate in this age group during the pandemic [36, 37]. Immunity from prior experience with influenza A/H1N1 viruses in childhood had led to a relative sparing of the elderly [35, 38]; this agreed with our observation that this age group had no significant increase in pneumococcal pneumonia during the pandemic period. Additionally, children <5 years did not show a significant increase in pneumococcal pneumonia during the pandemic, reflecting the lower impact of the influenza pandemic in this age group (Supplementary Figure 1)
Despite the clear relationship between influenza and pneumococcus reported in this study and by others [6], influenza still explains only a small percentage of all invasive pneumococcal disease incidence. Recent estimates from the Centers for Disease Control and Prevention suggest that, overall, just 4.5%–6% of all invasive pneumococcal pneumonia cases can be attributed to influenza, and our estimates largely agree with these figures. Clearly, then, other risk factors can play an important role in determining susceptibility to pneumococcal pneumonia. Environmental risk factors [23], social behaviors [24], and other viruses [2] could influence pneumococcal seasonality patterns. However, given that pneumococcal seasonal incidence was remarkably similar between baseline prepandemic years, and given the consistency in geographic and age patterns in the observed influenza and pneumococcal pneumonia time series in 2009, influenza is the most likely cause of the increase in pneumococcal pneumonia hospitalizations during the pandemic period.
There was a decrease in hospitalization rates in our database in most age categories and syndromes toward the end of 2009. We used a discharge database, so if a patient was admitted toward the end of 2009 but discharged in 2010, that patient would not be registered in the database until the 2010 data becomes available. On the basis of hospitalizations for all causes, we note that 97% of patients admitted before week 50 were discharged by the end of 2009 and thus included in our analysis (with substantially lower rates of 2009 discharge in those admitted after week 50, hence the week 50 cutoff in our study). Additionally, the mean length of hospital stay in 2009 for pneumonia and septicemia was 5.3 days and 8.8 days, respectively. Thus, the decreases seen late in the pandemic period might represent a real drop in pneumococcal disease incidence.
There have been suggestions that influenza pandemics or epidemics may be associated with a change in the distribution of pneumococcal serotypes causing disease. For instance, during the 1918 pandemic, specific serotypes increased as causes of disease [10], and an epidemic of serotype 5 and serogroup 12 coincided with the influenza epidemic of 1968–1969. Unfortunately, we did not have serotype data available to explore this possibility.
It is not clear from our data, which ended in mid-December 2009 due to incomplete information for the end of the year, whether the increase in pneumococcal hospitalizations in the fall would be offset by a sustained decrease in pneumococcal pneumonia for the remainder of the 2009–2010 winter period, consistent with the unusual absence of influenza. For the over-65 population, which did not have an increase in pneumococcal disease above baseline in the fall, this could result in a net decline in the amount of disease for the 2009–2010 season. These short-term trends should be explored when more data become available.
One limitation of our study is the reliance on ICD-9 disease codes, which is subject to bias if there are changes in diagnostic testing or changes in coding practice during the pandemic. We tested for such biases by looking at changes in the incidence of E. coli septicemia, a bacterial infection that should be unaffected by the pandemic. We saw no evidence of systematic, sustained increases in E. coli incidence over baseline during the pandemic period compared with the rest of the year, suggesting that there was not an increase in testing for septicemia. However, we cannot exclude the possibility that clinicians were more aware of the possibility for pneumococcal coinfections during the pandemic and thus were more likely to diagnose pneumococcal pneumonia. Additionally, there were clearly increases in ICD-9 coding for influenza, even during the summer of 2009 when influenza activity was lower. Due to this issue, we allowed the effect of influenza in our model to vary during the 2009 pandemic period compared with the prepandemic years. The 2 statistical approaches that we employed—the baseline method and the attribution model—gave similar results, suggesting that this was a valid modification to the model. The similarity between the estimates from these 2 approaches also supports the validity of both statistical approaches, which have been used previously [6, 28].
We focused on pneumococcal pneumonia hospitalizations, the most common pneumococcal-specific ICD-9 diagnosis in our State Inpatient Discharge data set. This is perhaps a more sensitive but less specific diagnostic criterion than “invasive pneumonia,” which is frequently reported and requires the isolation of pneumococcus from a normally sterile site such as blood or cerebrospinal fluid. The similar patterns seen with pneumococcal septicemia diagnoses (more specific but less sensitive than pneumococcal pneumonia [39]) suggest that the results are not sensitive to the diagnostic criteria (Supplementary Figure 2).
It has been suggested that because of the risk for secondary bacterial diseases during a pandemic, stockpiling antibacterial, as well as antiviral, medications would be prudent public health policy [18, 40]. The 2009 pandemic was not as severe as had been initially feared, and as we show here, the number of bacterial infections was not much above what one would expect in a typical winter season. However, given a more severe pandemic, a rapid rise in subsequent bacterial infections does have the potential to cause major public health problems, and having sufficient antibacterial drugs might have the potential to prevent some severe disease cases. Also, the new formulation of the pneumococcal conjugate vaccine, which promises to further reduce disease incidence and transmission of highly invasive serotypes [41, 42], could help to reduce the impacts of future pandemics.
In summary, the findings of this study provide further support for the link between influenza and pneumococcal disease. During the autumn 2009 pandemic, we observed a significant spike in pneumococcal pneumonia hospitalizations, which temporally and spatially corresponded to the peak influenza pandemic periods. In young and middle-aged adults, the magnitude of the increase in pneumococcal pneumonia hospitalizations was comparable to what has been seen in previous years, while the increase was unusually high in school-aged children.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
This research was conducted in the context of the MISMS Study, an ongoing international collaborative effort to understand influenza epidemiological and evolutionary patterns, led by the Fogarty International Center, National Institutes of Health (http://www.origem.info/misms/index.php).
Financial support.
The MISMS study is funded by the International Influenza Unit, Office of Global Affairs, US Department of Health and Human Services. L. S. acknowledges support from the RAPIDD program (Research And Policy for Infectious Diseases Dynamics) funded by the Fogarty International Center, National Institutes of Health (NIH), and the Department of Homeland Security.
Potential conflicts of interest.
L. S. has received research support from Pfizer for an observational study of pneumococcal vaccine program benefits. All other authors report no conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–82. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodges R, MacLeod C. Epidemic pneumococcal pneumonia. Am J Epidemiol. 1946;44:231–6. [PubMed] [Google Scholar]
- 3.O'Brien KL, Walters MI, Sellman J, et al. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin Infect Dis. 2000;30:784–9. doi: 10.1086/313772. [DOI] [PubMed] [Google Scholar]
- 4.Techasaensiri B, Techasaensiri C, Mejías A, McCracken GH, Ramilo O. Viral coinfections in children with invasive pneumococcal disease. Pediatr Infect Dis J. 2010;29:519–23. doi: 10.1097/INF.0b013e3181cdafc2. [DOI] [PubMed] [Google Scholar]
- 5.Kim PE, Musher DM, Glezen WP, Barradas MC, Nahm WK, Wright CE. Association of invasive pneumococcal disease with season, atmospheric conditions, air pollution, and the isolation of respiratory viruses. Clin Infect Dis. 1996;22:100–6. doi: 10.1093/clinids/22.1.100. [DOI] [PubMed] [Google Scholar]
- 6.Walter ND, Taylor TH, Shay DK, et al. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis. 2010;50:175–83. doi: 10.1086/649208. [DOI] [PubMed] [Google Scholar]
- 7.Mufson MA, Kruss DM, Wasil RE, Metzger WI. Capsular types and outcome of bacteremic pneumococcal disease in the antibiotic era. Arch Intern Med. 1974;134:505–10. [PubMed] [Google Scholar]
- 8.Martín-Loeches I, Sanchez-Corral A, Diaz E, et al. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A (H1N1) virus. Chest. 2011;139:555–62. doi: 10.1378/chest.10-1396. [DOI] [PubMed] [Google Scholar]
- 9.Jansen AG, Sanders E, Van der Ende A, Van Loon A, Hoes A, Hak E. Invasive pneumococcal and meningococcal disease: association with influenza virus and respiratory syncytial virus activity? Epidemiol Infect. 2008;136:1448–54. doi: 10.1017/S0950268807000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klugman KP, Chien Y-W, Madhi SA. Pneumococcal pneumonia and influenza: a deadly combination. Vaccine. 2009;27:C9–14. doi: 10.1016/j.vaccine.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Ampofo K, Herbener A, Blaschke AJ, et al. Association of 2009 pandemic influenza A (H1N1) infection and increased hospitalization with parapneumonic empyema in children in Utah. Pediatr Infect Dis J. 2010;29:905–9. doi: 10.1097/INF.0b013e3181df2c70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Infection (CDC) Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–4. [PubMed] [Google Scholar]
- 13.Isais F, Dimatactac F, Llorin R, Chow A, Leo Y. Streptococcus pneumoniae bacteraemia in a young man with pandemic infuenza A (H1N1) 2009. Ann Acad Med Singapore. 2010;39:338–40. [PubMed] [Google Scholar]
- 14.Lucas S. Predictive clinicopathological features derived from systematic autopsy examination of patients who died with A/H1N1 influenza infection in the UK 2009-10 pandemic. Health Technol Assess. 2011;14:83–114. doi: 10.3310/hta14550-02. [DOI] [PubMed] [Google Scholar]
- 15.Mauad T, Hajjar LA, Callegari GD, et al. Lung pathology in fatal novel human influenza A (H1N1) Infection. Am J Respir Crit Care Med. 2010;181:72–9. doi: 10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]
- 16.Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One. 2009;4:e8540. doi: 10.1371/journal.pone.0008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brundage J, Shanks G. Deaths from bacterial pneumonia during 1918–19 influenza pandemic. Emerg Infect Dis. 2008;14:1193–9. doi: 10.3201/eid1408.071313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6:303–12. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien Y-W, Klugman KP, Morens DM. Bacterial pathogens and death during the 1918 influenza pandemic. N Engl J Med. 2009;361:2582–3. doi: 10.1056/NEJMc0908216. [DOI] [PubMed] [Google Scholar]
- 20.Chien Y-W, Klugman KP, Morens DM. Efficacy of whole-cell killed bacterial vaccines in preventing pneumonia and death during the 1918 influenza pandemic. J Infect Dis. 2010;202:1639–48. doi: 10.1086/657144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Questions and answers: 2009 H1N1 and pneumococcal disease in the news. 2011. http://www.cdc.gov/h1n1flu/vaccination/qa_pneumococcal_disease.htmAccessed 27 July 2011. [Google Scholar]
- 22.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis. 2010;202:1287–95. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowell S, Whitney C, Wright C, Rose C, Schuchat A. Seasonal patterns of invasive pneumococcal disease. Emerg Infect Dis. 2003;9:573–9. doi: 10.3201/eid0905.020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter ND, Taylor TH, Dowell SF, Mathis S, Moore MR. Holiday spikes in pneumococcal disease among older adults. N Engl J Med. 2009;361:2584–5. doi: 10.1056/NEJMc0904844. [DOI] [PubMed] [Google Scholar]
- 25.Jhung MA, Swerdlow D, Olsen SJ, et al. Epidemiology of 2009 pandemic influenza A (H1N1) in the United States. Clin Infect Dis. 2011;52:S13–26. doi: 10.1093/cid/ciq008. [DOI] [PubMed] [Google Scholar]
- 26.Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality. Rockville, MD: 2011. www.hcup-us.ahrq.gov/sidoverview.jspAccessed 15 July 2011. [PubMed] [Google Scholar]
- 27.Farrington CP, Andrews NJ, Beale AD, Catchpole MA. A statistical algorithm for the early detection of outbreaks of infectious disease. J R Stat Soc Ser A Stat Soc. 1996;159:547–63. [Google Scholar]
- 28.Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. mBio. 2011;2:e00309–1. doi: 10.1128/mBio.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller D. Bootstrap 101: obtain robust confidence intervals for any statistic. Montreal: SAS SUGI 29; 2004. pp. 193–29. http://www2.sas.com/proceedings/sugi29/193-29.pdfAccessed July 2011. [Google Scholar]
- 30.Rockhill B, Weinberg CR, Newman B. Population attributable fraction estimation for established breast cancer risk factors: considering the issues of high prevalence and unmodifiability. Am J Epidemiol. 1998;147:826–33. doi: 10.1093/oxfordjournals.aje.a009535. [DOI] [PubMed] [Google Scholar]
- 31.Pitzer VE, Viboud C, Simonsen L, et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science. 2009;325:290–4. doi: 10.1126/science.1172330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viboud C, Bjørnstad ON, Smith DL, Simonsen L, Miller MA, Grenfell BT. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science. 2006;312:447–51. doi: 10.1126/science.1125237. [DOI] [PubMed] [Google Scholar]
- 33.Pilishvili T, Lexau C, Farley Monica M, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC) Seasonal influenza resources. 2011. www.cdc.gov/fluAccessed April 2011. [Google Scholar]
- 35.Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–9. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 36.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–8. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 37.Shrestha SS, Swerdlow DL, Borse RH, et al. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010) Clin Infect Dis. 2011;52:S75–82. doi: 10.1093/cid/ciq012. [DOI] [PubMed] [Google Scholar]
- 38.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 39.Guevara RE, Butler JC, Marston BJ, Plouffe JF, File TM, Breiman RF. Accuracy of ICD-9-CM codes in detecting community-acquired pneumococcal pneumonia for incidence and vaccine efficacy studies. Am J Epidemiol. 1999;149:282–9. doi: 10.1093/oxfordjournals.aje.a009804. [DOI] [PubMed] [Google Scholar]
- 40.Gupta R, George R, Nguyen-Van-Tam J. Bacterial pneumonia and pandemic influenza planning. Emerg Infect Dis. 2008;14:1187–92. doi: 10.3201/eid1408.070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flasche S, Van Hoek AJ, Sheasby E, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8:e1001017. doi: 10.1371/journal.pmed.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinberger D, Harboe Z, Flasche S, Scott J, Lipsitch M. Prediction of serotypes causing invasive pneumococcal disease in unvaccinated and vaccinated populations. Epidemiology. 2011;22:199–207. doi: 10.1097/EDE.0b013e3182087634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.